Abstract
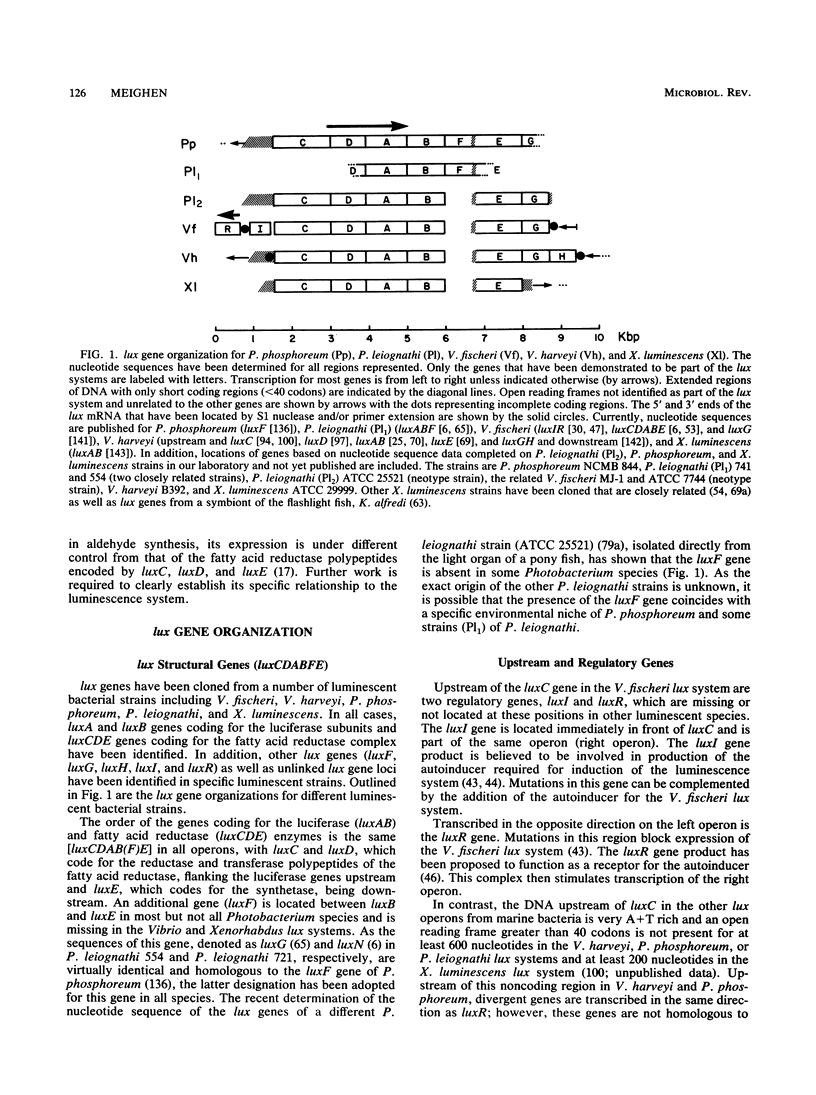
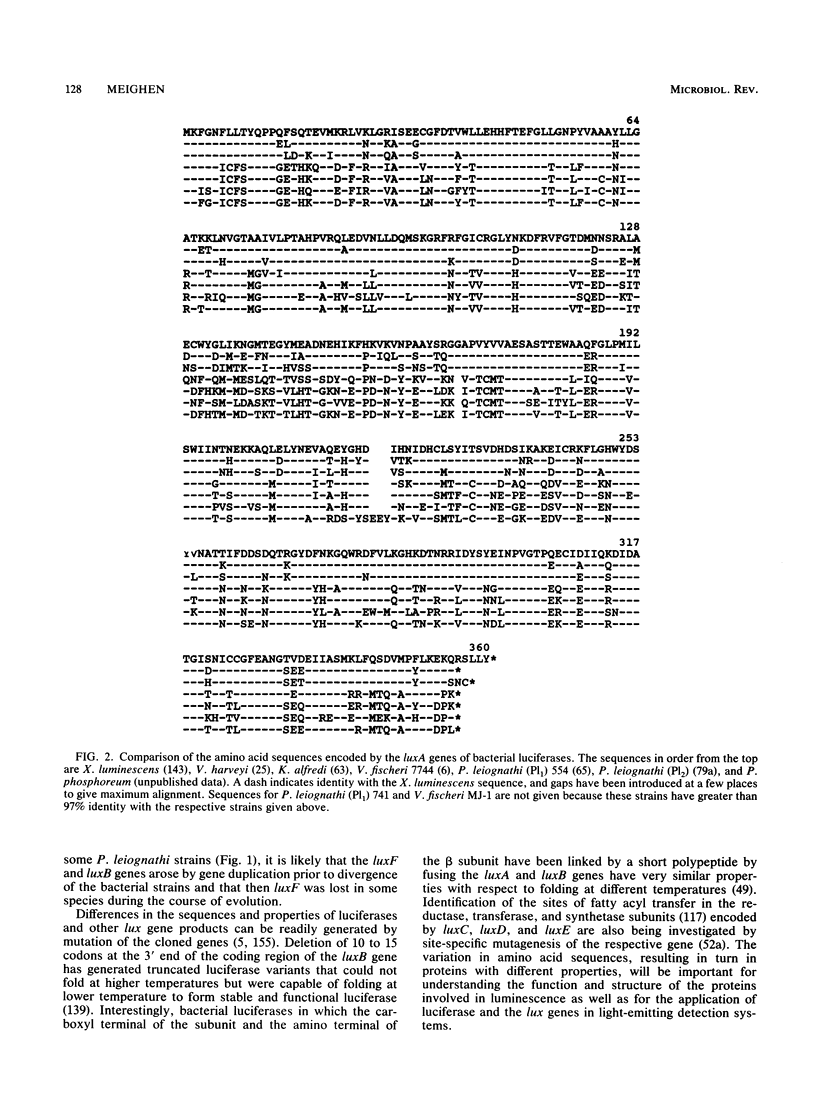
The cloning and expression of the lux genes from different luminescent bacteria including marine and terrestrial species have led to significant advances in our knowledge of the molecular biology of bacterial bioluminescence. All lux operons have a common gene organization of luxCDAB(F)E, with luxAB coding for luciferase and luxCDE coding for the fatty acid reductase complex responsible for synthesizing fatty aldehydes for the luminescence reaction, whereas significant differences exist in their sequences and properties as well as in the presence of other lux genes (I, R, F, G, and H). Recognition of the regulatory genes as well as diffusible metabolites that control the growth-dependent induction of luminescence (autoinducers) in some species has advanced our understanding of this unique regulatory mechanism in which the autoinducers appear to serve as sensors of the chemical or nutritional environment. The lux genes have now been transferred into a variety of different organisms to generate new luminescent species. Naturally dark bacteria containing the luxCDABE and luxAB genes, respectively, are luminescent or emit light on addition of aldehyde. Fusion of the luxAB genes has also allowed the expression of luciferase under a single promoter in eukaryotic systems. The ability to express the lux genes in a variety of prokaryotic and eukaryotic organisms and the ease and sensitivity of the luminescence assay demonstrate the considerable potential of the widespread application of the lux genes as reporters of gene expression and metabolic function.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almashanu S., Musafia B., Hadar R., Suissa M., Kuhn J. Fusion of LuxA and LuxB and its expression in E. coli, S. cerevisiae and D. melanogaster. J Biolumin Chemilumin. 1990 Apr-Jun;5(2):89–97. doi: 10.1002/bio.1170050204. [DOI] [PubMed] [Google Scholar]
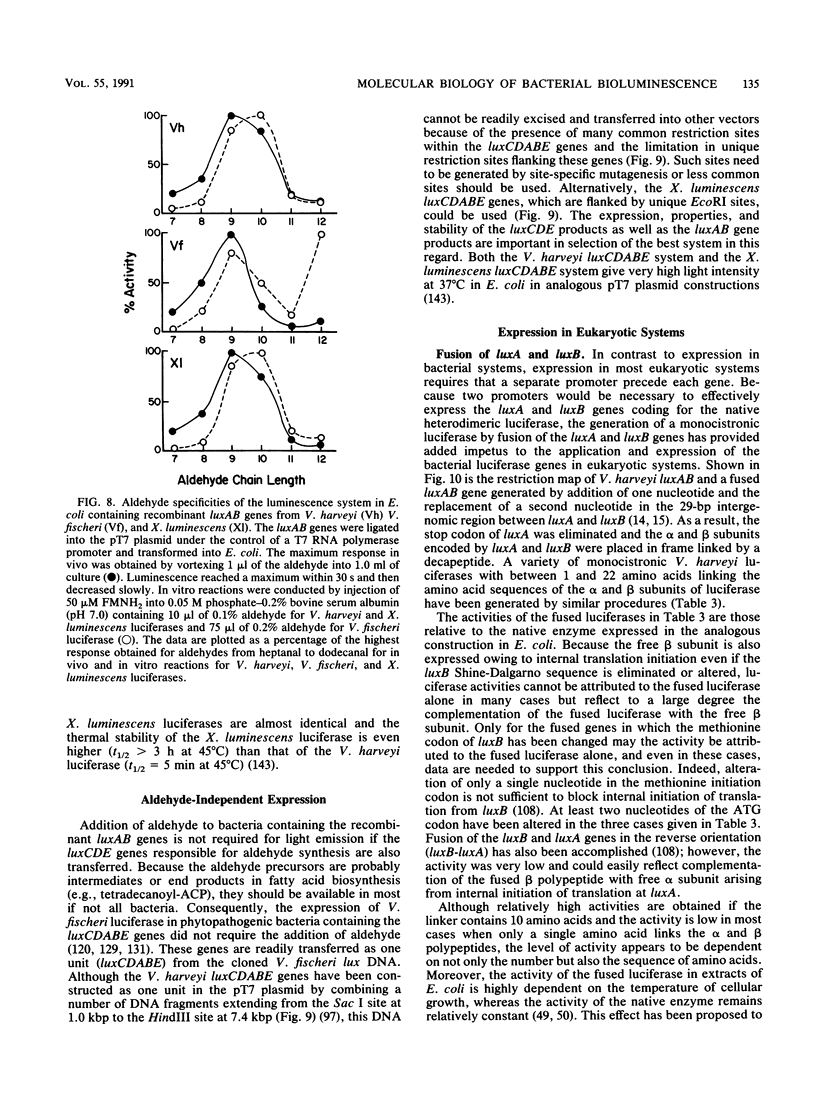
- Balaguer P., Térouanne B., Eliaou J. F., Humbert M., Boussioux A. M., Nicolas J. C. Use of glucose-6-phosphate dehydrogenase as a new label for nucleic acid hybridization reactions. Anal Biochem. 1989 Jul;180(1):50–54. doi: 10.1016/0003-2697(89)90085-7. [DOI] [PubMed] [Google Scholar]
- Baldwin T. O., Berends T., Bunch T. A., Holzman T. F., Rausch S. K., Shamansky L., Treat M. L., Ziegler M. M. Cloning of the luciferase structural genes from Vibrio harveyi and expression of bioluminescence in Escherichia coli. Biochemistry. 1984 Jul 31;23(16):3663–3667. doi: 10.1021/bi00311a014. [DOI] [PubMed] [Google Scholar]
- Baldwin T. O., Devine J. H., Heckel R. C., Lin J. W., Shadel G. S. The complete nucleotide sequence of the lux regulon of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence. J Biolumin Chemilumin. 1989 Jul;4(1):326–341. doi: 10.1002/bio.1170040145. [DOI] [PubMed] [Google Scholar]
- Baldwin T. O., Treat M. L., Daubner S. C. Cloning and expression of the luxY gene from Vibrio fischeri strain Y-1 in Escherichia coli and complete amino acid sequence of the yellow fluorescent protein. Biochemistry. 1990 Jun 12;29(23):5509–5515. doi: 10.1021/bi00475a014. [DOI] [PubMed] [Google Scholar]
- Baldwin T. O., Ziegler M. M., Powers D. A. Covalent structure of subunits of bacterial luciferase: NH2-terminal sequence demonstrates subunit homology. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4887–4889. doi: 10.1073/pnas.76.10.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Baumann L., Woolkalis M. J., Bang S. S. Evolutionary relationships in vibrio and Photobacterium: a basis for a natural classification. Annu Rev Microbiol. 1983;37:369–398. doi: 10.1146/annurev.mi.37.100183.002101. [DOI] [PubMed] [Google Scholar]
- Belas R., Mileham A., Cohn D., Hilman M., Simon M., Silverman M. Bacterial bioluminescence: isolation and expression of the luciferase genes from Vibrio harveyi. Science. 1982 Nov 19;218(4574):791–793. doi: 10.1126/science.10636771. [DOI] [PubMed] [Google Scholar]
- Bioluminescence and chemiluminescence literature--Lux, Luc and Phot genes. J Biolumin Chemilumin. 1990 Apr-Jun;5(2):141–152. doi: 10.1002/bio.1170050210. [DOI] [PubMed] [Google Scholar]
- Boivin R., Chalifour F. P., Dion P. Construction of a Tn5 derivative encoding bioluminescence and its introduction in Pseudomonas, Agrobacterium and Rhizobium. Mol Gen Genet. 1988 Jul;213(1):50–55. doi: 10.1007/BF00333397. [DOI] [PubMed] [Google Scholar]
- Boylan M. O., Pelletier J., Dhepagnon S., Trudel S., Sonenberg N., Meighen E. A. Construction of a fused LuxAB gene by site-directed mutagenesis. J Biolumin Chemilumin. 1989 Jul;4(1):310–316. doi: 10.1002/bio.1170040143. [DOI] [PubMed] [Google Scholar]
- Boylan M., Graham A. F., Meighen E. A. Functional identification of the fatty acid reductase components encoded in the luminescence operon of Vibrio fischeri. J Bacteriol. 1985 Sep;163(3):1186–1190. doi: 10.1128/jb.163.3.1186-1190.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan M., Miyamoto C., Wall L., Graham A., Meighen E. Lux C, D and E genes of the Vibrio fischeri luminescence operon code for the reductase, transferase, and synthetase enzymes involved in aldehyde biosynthesis. Photochem Photobiol. 1989 May;49(5):681–688. doi: 10.1111/j.1751-1097.1989.tb08441.x. [DOI] [PubMed] [Google Scholar]
- Boylan M., Pelletier J., Meighen E. A. Fused bacterial luciferase subunits catalyze light emission in eukaryotes and prokaryotes. J Biol Chem. 1989 Feb 5;264(4):1915–1918. [PubMed] [Google Scholar]
- Byers D. M., Bognar A., Meighen E. A. Differential regulation of enzyme activities involved in aldehyde metabolism in the luminescent bacterium Vibrio harveyi. J Bacteriol. 1988 Feb;170(2):967–971. doi: 10.1128/jb.170.2.967-971.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D. M., Meighen E. A. Acyl-acyl carrier protein as a source of fatty acids for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6085–6089. doi: 10.1073/pnas.82.18.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D., Meighen E. Vibrio harveyi aldehyde dehydrogenase. Partial reversal of aldehyde oxidation and its possible role in the reduction of fatty acids for the bioluminescence reaction. J Biol Chem. 1984 Jun 10;259(11):7109–7114. [PubMed] [Google Scholar]
- Campbell A. K. Living light: biochemistry, applications. Essays Biochem. 1989;24:41–81. [PubMed] [Google Scholar]
- Cao J. G., Meighen E. A. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989 Dec 25;264(36):21670–21676. [PubMed] [Google Scholar]
- Carmi O. A., Stewart G. S., Ulitzur S., Kuhn J. Use of bacterial luciferase to establish a promoter probe vehicle capable of nondestructive real-time analysis of gene expression in Bacillus spp. J Bacteriol. 1987 May;169(5):2165–2170. doi: 10.1128/jb.169.5.2165-2170.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W., Hastings J. W. Mutationally altered bacterial luciferase. Implications for subunit functions. Biochemistry. 1972 Aug 29;11(18):3359–3370. doi: 10.1021/bi00768a008. [DOI] [PubMed] [Google Scholar]
- Cohn D. H., Mileham A. J., Simon M. I., Nealson K. H., Rausch S. K., Bonam D., Baldwin T. O. Nucleotide sequence of the luxA gene of Vibrio harveyi and the complete amino acid sequence of the alpha subunit of bacterial luciferase. J Biol Chem. 1985 May 25;260(10):6139–6146. [PubMed] [Google Scholar]
- Cohn D. H., Ogden R. C., Abelson J. N., Baldwin T. O., Nealson K. H., Simon M. I., Mileham A. J. Cloning of the Vibrio harveyi luciferase genes: use of a synthetic oligonucleotide probe. Proc Natl Acad Sci U S A. 1983 Jan;80(1):120–123. doi: 10.1073/pnas.80.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colepicolo P., Cho K. W., Poinar G. O., Hastings J. W. Growth and luminescence of the bacterium Xenorhabdus luminescens from a human wound. Appl Environ Microbiol. 1989 Oct;55(10):2601–2606. doi: 10.1128/aem.55.10.2601-2606.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delong E. F., Steinhauer D., Israel A., Nealson K. H. Isolation of the lux genes from Photobacterium leiognathi and expression in Escherichia coli. Gene. 1987;54(2-3):203–210. doi: 10.1016/0378-1119(87)90488-4. [DOI] [PubMed] [Google Scholar]
- Devine J. H., Shadel G. S., Baldwin T. O. Identification of the operator of the lux regulon from the Vibrio fischeri strain ATCC7744. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5688–5692. doi: 10.1073/pnas.86.15.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V., Greenberg E. P. Control of Vibrio fischeri luminescence gene expression in Escherichia coli by cyclic AMP and cyclic AMP receptor protein. J Bacteriol. 1985 Oct;164(1):45–50. doi: 10.1128/jb.164.1.45-50.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V., Greenberg E. P. Control of Vibrio fischeri lux gene transcription by a cyclic AMP receptor protein-luxR protein regulatory circuit. J Bacteriol. 1988 Sep;170(9):4040–4046. doi: 10.1128/jb.170.9.4040-4046.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V., Ray J. M. Requirement for autoinducer in transcriptional negative autoregulation of the Vibrio fischeri luxR gene in Escherichia coli. J Bacteriol. 1989 Jun;171(6):3549–3552. doi: 10.1128/jb.171.6.3549-3552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V. Regulation of luminescence by cyclic AMP in cya-like and crp-like mutants of Vibrio fischeri. J Bacteriol. 1989 Feb;171(2):1199–1202. doi: 10.1128/jb.171.2.1199-1202.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A., Burlingame A. L., Eberhard C., Kenyon G. L., Nealson K. H., Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981 Apr 28;20(9):2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- Eberhard A. Inhibition and activation of bacterial luciferase synthesis. J Bacteriol. 1972 Mar;109(3):1101–1105. doi: 10.1128/jb.109.3.1101-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A., Widrig C. A., McBath P., Schineller J. B. Analogs of the autoinducer of bioluminescence in Vibrio fischeri. Arch Microbiol. 1986 Oct;146(1):35–40. doi: 10.1007/BF00690155. [DOI] [PubMed] [Google Scholar]
- Eckstein J. W., Cho K. W., Colepicolo P., Ghisla S., Hastings J. W., Wilson T. A time-dependent bacterial bioluminescence emission spectrum in an in vitro single turnover system: energy transfer alone cannot account for the yellow emission of Vibrio fischeri Y-1. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1466–1470. doi: 10.1073/pnas.87.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Nucleotide sequence of the regulatory locus controlling expression of bacterial genes for bioluminescence. Nucleic Acids Res. 1987 Dec 23;15(24):10455–10467. doi: 10.1093/nar/15.24.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Simon M., Silverman M. Measuring gene expression with light. Science. 1985 Mar 15;227(4692):1345–1347. doi: 10.1126/science.2983423. [DOI] [PubMed] [Google Scholar]
- Escher A., O'Kane D. J., Lee J., Szalay A. A. Bacterial luciferase alpha beta fusion protein is fully active as a monomer and highly sensitive in vivo to elevated temperature. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6528–6532. doi: 10.1073/pnas.86.17.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
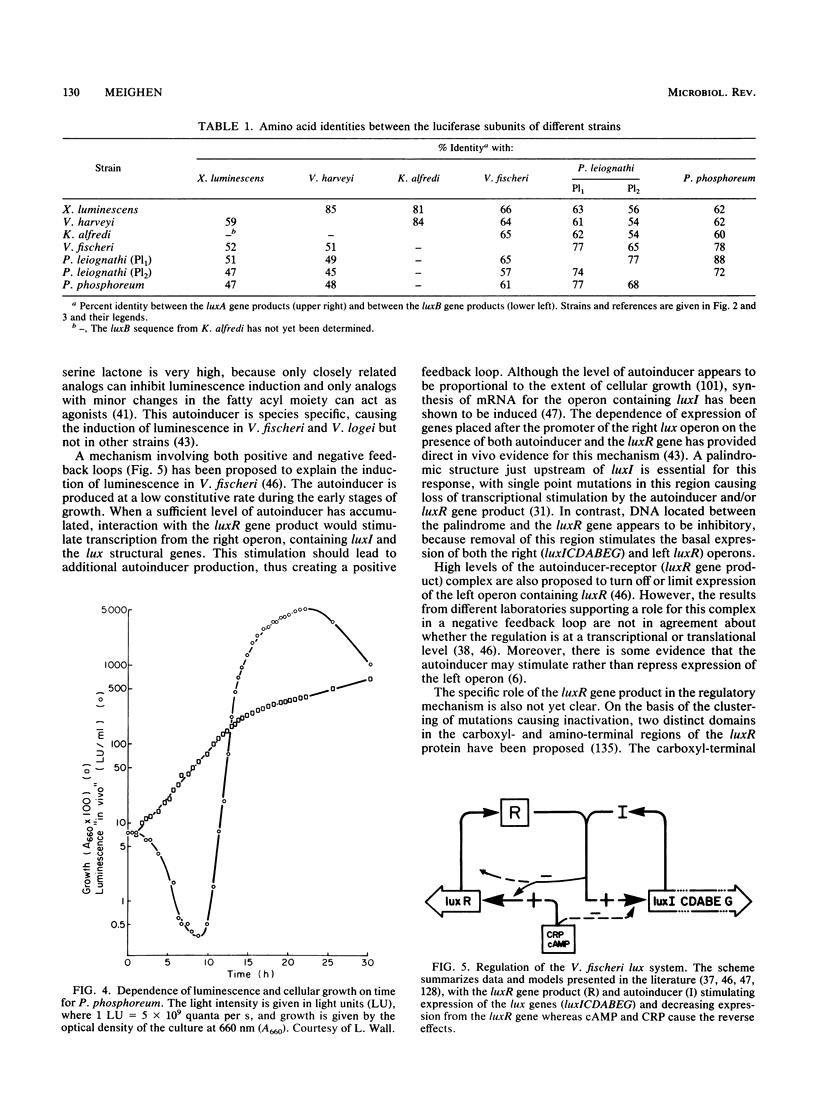
- Evans J. F., McCracken S., Miyamoto C. M., Meighen E. A., Graham A. F. In vitro synthesis of subunits of bacterial luciferase in an Escherichia coli system. J Bacteriol. 1983 Jan;153(1):543–545. doi: 10.1128/jb.153.1.543-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Jorgensen J. H., Grimont P. A., Akhurst R. J., Poinar G. O., Jr, Ageron E., Pierce G. V., Smith J. A., Carter G. P., Wilson K. L. Xenorhabdus luminescens (DNA hybridization group 5) from human clinical specimens. J Clin Microbiol. 1989 Jul;27(7):1594–1600. doi: 10.1128/jcm.27.7.1594-1600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran D. R., Brown W. M. Nucleotide sequence of the LuxA and LuxB genes of the bioluminescent marine bacterium Vibrio fischeri. Nucleic Acids Res. 1988 Jan 25;16(2):777–777. doi: 10.1093/nar/16.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackman S., Anhalt M., Nealson K. H. Cloning, organization, and expression of the bioluminescence genes of Xenorhabdus luminescens. J Bacteriol. 1990 Oct;172(10):5767–5773. doi: 10.1128/jb.172.10.5767-5773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. C., Reese C. P., Hastings J. W. Mobilization of cloned luciferase genes into Vibrio harveyi luminescence mutants. Arch Microbiol. 1986 Jan;143(4):325–329. doi: 10.1007/BF00412797. [DOI] [PubMed] [Google Scholar]
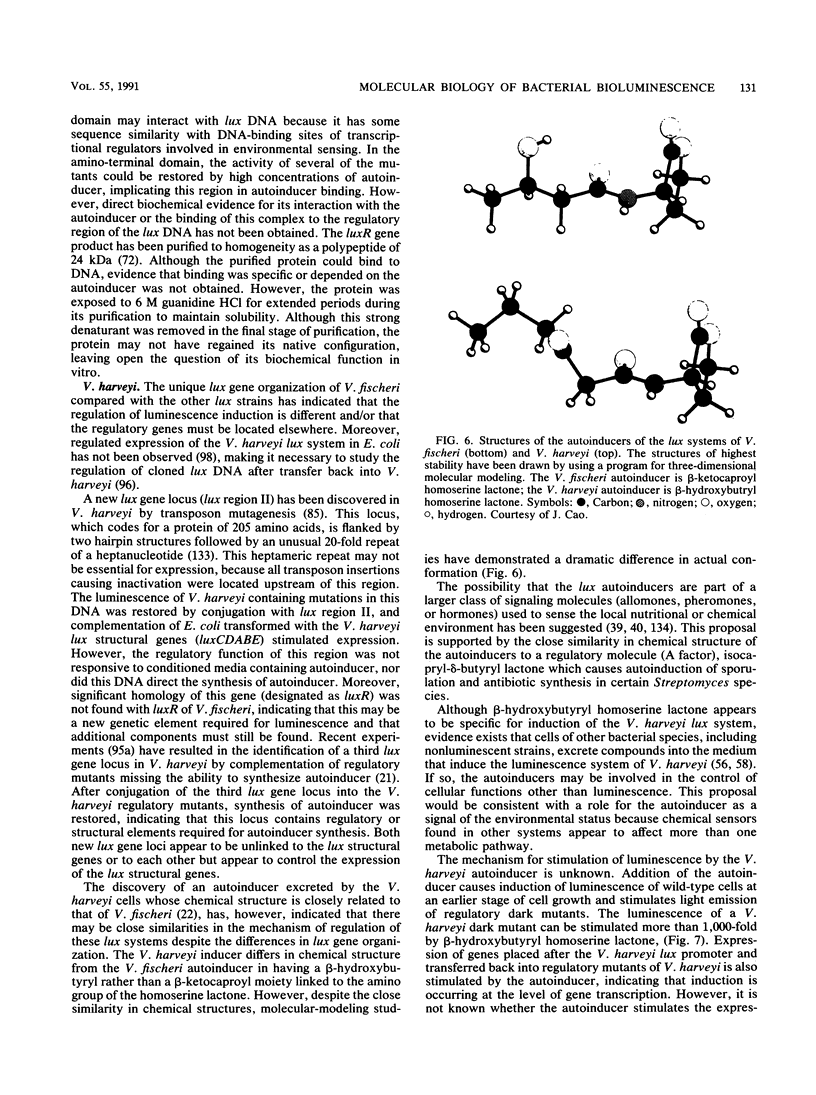
- Hastings J. W., Potrikus C. J., Gupta S. C., Kurfürst M., Makemson J. C. Biochemistry and physiology of bioluminescent bacteria. Adv Microb Physiol. 1985;26:235–291. doi: 10.1016/s0065-2911(08)60398-7. [DOI] [PubMed] [Google Scholar]
- Haygood M. G. Relationship of the luminous bacterial symbiont of the Caribbean flashlight fish, Kryptophanaron alfredi (family Anomalopidae) to other luminous bacteria based on bacterial luciferase (luxA) genes. Arch Microbiol. 1990;154(5):496–503. doi: 10.1007/BF00245234. [DOI] [PubMed] [Google Scholar]
- Hirooka T., Rogowsky P. M., Kado C. I. Characterization of the virE locus of Agrobacterium tumefaciens plasmid pTiC58. J Bacteriol. 1987 Apr;169(4):1529–1536. doi: 10.1128/jb.169.4.1529-1536.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illarionov B. A., Blinov V. M., Donchenko A. P., Protopopova M. V., Karginov V. A., Mertvetsov N. P., Gitelson J. I. Isolation of bioluminescent functions from Photobacterium leiognathi: analysis of luxA, luxB, luxG and neighboring genes. Gene. 1990 Jan 31;86(1):89–94. doi: 10.1016/0378-1119(90)90117-a. [DOI] [PubMed] [Google Scholar]
- Jablonski E., DeLuca M. Purification and properties of the NADH and NADPH specific FMN oxidoreductases from Beneckea harveyi. Biochemistry. 1977 Jun 28;16(13):2932–2936. doi: 10.1021/bi00632a020. [DOI] [PubMed] [Google Scholar]
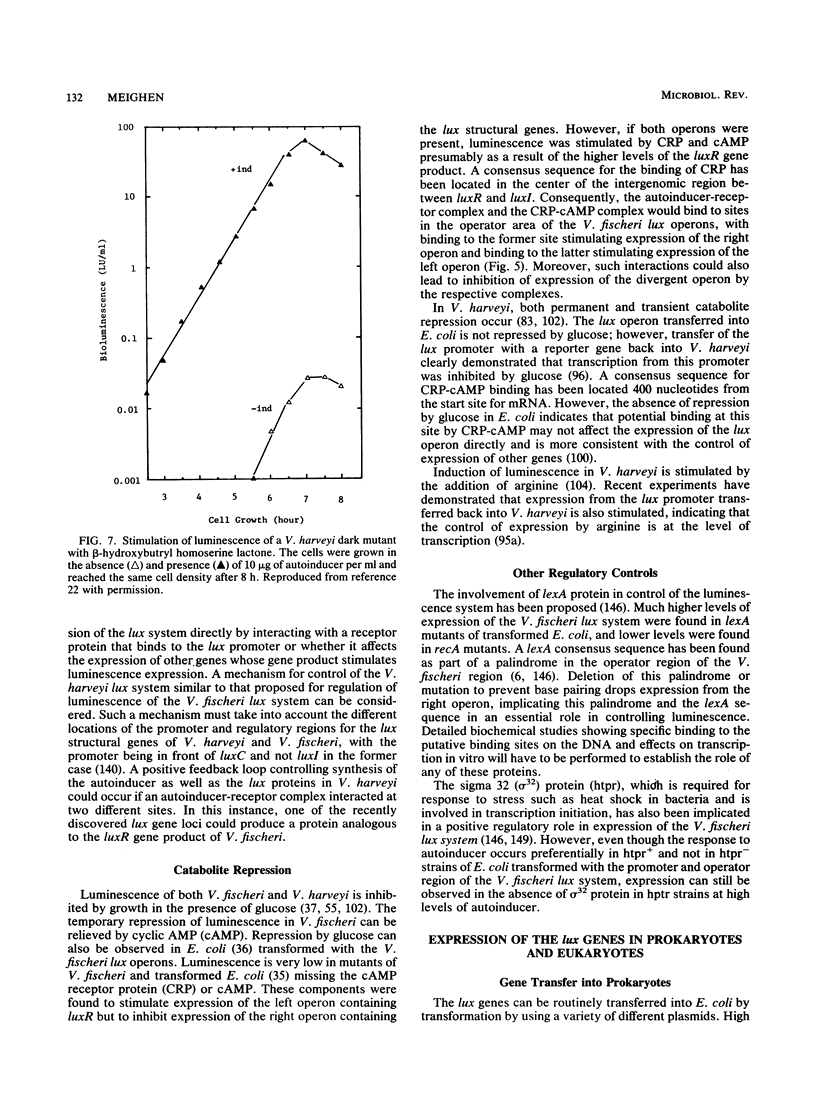
- Jassim S. A., Ellison A., Denyer S. P., Stewart G. S. In vivo bioluminescence: a cellular reporter for research and industry. J Biolumin Chemilumin. 1990 Apr-Jun;5(2):115–122. doi: 10.1002/bio.1170050207. [DOI] [PubMed] [Google Scholar]
- Johnston T. C., Hruska K. S., Adams L. F. The nucleotide sequence of the luxE gene of Vibrio harveyi and a comparison of the amino acid sequences of the acyl-protein synthetases from V. harveyi and V. fischeri. Biochem Biophys Res Commun. 1989 Aug 30;163(1):93–101. doi: 10.1016/0006-291x(89)92103-7. [DOI] [PubMed] [Google Scholar]
- Johnston T. C., Rucker E. B., Cochrum L., Hruska K. S., Vandegrift V. The nucleotide sequence of the luxA and luxB genes of Xenorhabdus luminescens HM and a comparison of the amino acid sequences of luciferases from four species of bioluminescent bacteria. Biochem Biophys Res Commun. 1990 Jul 31;170(2):407–415. doi: 10.1016/0006-291x(90)92106-a. [DOI] [PubMed] [Google Scholar]
- Johnston T. C., Thompson R. B., Baldwin T. O. Nucleotide sequence of the luxB gene of Vibrio harveyi and the complete amino acid sequence of the beta subunit of bacterial luciferase. J Biol Chem. 1986 Apr 15;261(11):4805–4811. [PubMed] [Google Scholar]
- Kaplan H. B., Greenberg E. P. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985 Sep;163(3):1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. B., Greenberg E. P. Overproduction and purification of the luxR gene product: Transcriptional activator of the Vibrio fischeri luminescence system. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6639–6643. doi: 10.1073/pnas.84.19.6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp M. Expression of bacterial luciferase genes from Vibrio harveyi in Bacillus subtilis and in Escherichia coli. Biochim Biophys Acta. 1989 Jan 23;1007(1):84–90. doi: 10.1016/0167-4781(89)90134-6. [DOI] [PubMed] [Google Scholar]
- Kirchner G., Roberts J. L., Gustafson G. D., Ingolia T. D. Active bacterial luciferase from a fused gene: expression of a Vibrio harveyi luxAB translational fusion in bacteria, yeast and plant cells. Gene. 1989 Sep 30;81(2):349–354. doi: 10.1016/0378-1119(89)90195-9. [DOI] [PubMed] [Google Scholar]
- Koncz C., Martini N., Mayerhofer R., Koncz-Kalman Z., Körber H., Redei G. P., Schell J. High-frequency T-DNA-mediated gene tagging in plants. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8467–8471. doi: 10.1073/pnas.86.21.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C., Olsson O., Langridge W. H., Schell J., Szalay A. A. Expression and assembly of functional bacterial luciferase in plants. Proc Natl Acad Sci U S A. 1987 Jan;84(1):131–135. doi: 10.1073/pnas.84.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kricka L. J. Clinical and biochemical applications of luciferases and luciferins. Anal Biochem. 1988 Nov 15;175(1):14–21. doi: 10.1016/0003-2697(88)90354-5. [DOI] [PubMed] [Google Scholar]
- Kurose K., Inouye S., Sakaki Y., Tsuji F. I. Bioluminescence of the Ca2+-binding photoprotein aequorin after cysteine modification. Proc Natl Acad Sci U S A. 1989 Jan;86(1):80–84. doi: 10.1073/pnas.86.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge W. H., Fitzgerald K. J., Koncz C., Schell J., Szalay A. A. Dual promoter of Agrobacterium tumefaciens mannopine synthase genes is regulated by plant growth hormones. Proc Natl Acad Sci U S A. 1989 May;86(9):3219–3223. doi: 10.1073/pnas.86.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legocki R. P., Legocki M., Baldwin T. O., Szalay A. A. Bioluminescence in soybean root nodules: Demonstration of a general approach to assay gene expression in vivo by using bacterial luciferase. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9080–9084. doi: 10.1073/pnas.83.23.9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini J. A., Boylan M., Soly R. R., Graham A. F., Meighen E. A. Cloning and expression of the Photobacterium phosphoreum luminescence system demonstrates a unique lux gene organization. J Biol Chem. 1988 Oct 5;263(28):14308–14314. [PubMed] [Google Scholar]
- Martin M., Showalter R., Silverman M. Identification of a locus controlling expression of luminescence genes in Vibrio harveyi. J Bacteriol. 1989 May;171(5):2406–2414. doi: 10.1128/jb.171.5.2406-2414.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighen E. A., Bartlet I. Complementation of subunits from different bacterial luciferases. Evidence for the role of the beta subunit in the bioluminescent mechanism. J Biol Chem. 1980 Dec 10;255(23):11181–11187. [PubMed] [Google Scholar]
- Meighen E. A., MacKenzie R. E. Flavine specificity of enzyme-substrate intermediates in the bacterial bioluminescent reaction. Structural requirements of the flavine side chain. Biochemistry. 1973 Apr 10;12(8):1482–1491. doi: 10.1021/bi00732a003. [DOI] [PubMed] [Google Scholar]
- Meighen E. A., Nicoli M. Z., Hastings J. W. Functional differences of the nonidentical subunits of bacterial luciferase. Properties of hybrids of native and chemically modified bacterial luciferase. Biochemistry. 1971 Oct 26;10(22):4069–4073. doi: 10.1021/bi00798a009. [DOI] [PubMed] [Google Scholar]
- Michaliszyn G. A., Wing S. S., Meighen E. A. Purification and properties of a NAD(P)H:flavin oxidoreductase from the luminous bacterium, Beneckea harveyi. J Biol Chem. 1977 Nov 10;252(21):7495–7499. [PubMed] [Google Scholar]
- Miyamoto C. M., Boylan M., Graham A. F., Meighen E. A. Organization of the lux structural genes of Vibrio harveyi. Expression under the T7 bacteriophage promoter, mRNA analysis, and nucleotide sequence of the luxD gene. J Biol Chem. 1988 Sep 15;263(26):13393–13399. [PubMed] [Google Scholar]
- Miyamoto C. M., Graham A. D., Boylan M., Evans J. F., Hasel K. W., Meighen E. A., Graham A. F. Polycistronic mRNAs code for polypeptides of the Vibrio harveyi luminescence system. J Bacteriol. 1985 Mar;161(3):995–1001. doi: 10.1128/jb.161.3.995-1001.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto C. M., Graham A. F., Meighen E. A. Nucleotide sequence of the LuxC gene and the upstream DNA from the bioluminescent system of Vibrio harveyi. Nucleic Acids Res. 1988 Feb 25;16(4):1551–1562. doi: 10.1093/nar/16.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto C. M., Meighen E. A., Graham A. F. Transcriptional regulation of lux genes transferred into Vibrio harveyi. J Bacteriol. 1990 Apr;172(4):2046–2054. doi: 10.1128/jb.172.4.2046-2054.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto C., Byers D., Graham A. F., Meighen E. A. Expression of bioluminescence by Escherichia coli containing recombinant Vibrio harveyi DNA. J Bacteriol. 1987 Jan;169(1):247–253. doi: 10.1128/jb.169.1.247-253.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H. Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch Microbiol. 1977 Feb 4;112(1):73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Eberhard A., Hastings J. W. Catabolite repression of bacterial bioluminescence: functional implications. Proc Natl Acad Sci U S A. 1972 May;69(5):1073–1076. doi: 10.1073/pnas.69.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Hastings J. W. Bacterial bioluminescence: its control and ecological significance. Microbiol Rev. 1979 Dec;43(4):496–518. doi: 10.1128/mr.43.4.496-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Platt T., Hastings J. W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970 Oct;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum A., Cohen A. Use of a bioluminescence gene reporter for the investigation of red-dependent and gam-dependent plasmid recombination in Escherichia coli K12. J Mol Biol. 1988 Sep 20;203(2):391–402. doi: 10.1016/0022-2836(88)90007-1. [DOI] [PubMed] [Google Scholar]
- Olsson O., Escher A., Sandberg G., Schell J., Koncz C., Szalay A. A. Engineering of monomeric bacterial luciferases by fusion of luxA and luxB genes in Vibrio harveyi. Gene. 1989 Sep 30;81(2):335–347. doi: 10.1016/0378-1119(89)90194-7. [DOI] [PubMed] [Google Scholar]
- Olsson O., Nilsson O., Koncz C. Novel monomeric luciferase enzymes as tools to study plant gene regulation in vivo. J Biolumin Chemilumin. 1990 Apr-Jun;5(2):79–87. doi: 10.1002/bio.1170050203. [DOI] [PubMed] [Google Scholar]
- Ow D. W., DE Wet J. R., Helinski D. R., Howell S. H., Wood K. V., Deluca M. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science. 1986 Nov 14;234(4778):856–859. doi: 10.1126/science.234.4778.856. [DOI] [PubMed] [Google Scholar]
- Park S. F., Stirling D. A., Hulton C. S., Booth I. R., Higgins C. F., Stewart G. S. A novel, non-invasive promoter probe vector: cloning of the osmoregulated proU promoter of Escherichia coli K12. Mol Microbiol. 1989 Aug;3(8):1011–1023. doi: 10.1111/j.1365-2958.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Prasher D. C., McCann R. O., Longiaru M., Cormier M. J. Sequence comparisons of complementary DNAs encoding aequorin isotypes. Biochemistry. 1987 Mar 10;26(5):1326–1332. doi: 10.1021/bi00379a019. [DOI] [PubMed] [Google Scholar]
- Prasher D. C., O'Kane D., Lee J., Woodward B. The lumazine protein gene in Photobacterium phosphoreum is linked to the lux operon. Nucleic Acids Res. 1990 Nov 11;18(21):6450–6450. doi: 10.1093/nar/18.21.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A., Riendeau D., Meighen E. Purification of the acyl coenzyme A reductase component from a complex responsible for the reduction of fatty acids in bioluminescent bacteria. Properties and acyltransferase activity. J Biol Chem. 1983 Apr 25;258(8):5233–5237. [PubMed] [Google Scholar]
- Rodriguez J. F., Rodriguez D., Rodriguez J. R., McGowan E. B., Esteban M. Expression of the firefly luciferase gene in vaccinia virus: a highly sensitive gene marker to follow virus dissemination in tissues of infected animals. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1667–1671. doi: 10.1073/pnas.85.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogowsky P. M., Close T. J., Chimera J. A., Shaw J. J., Kado C. I. Regulation of the vir genes of Agrobacterium tumefaciens plasmid pTiC58. J Bacteriol. 1987 Nov;169(11):5101–5112. doi: 10.1128/jb.169.11.5101-5112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Nealson K. H. A luminous bacterium that emits yellow light. Science. 1977 Apr 22;196(4288):432–434. doi: 10.1126/science.850787. [DOI] [PubMed] [Google Scholar]
- Schauer A., Ranes M., Santamaria R., Guijarro J., Lawlor E., Mendez C., Chater K., Losick R. Visualizing gene expression in time and space in the filamentous bacterium Streptomyces coelicolor. Science. 1988 May 6;240(4853):768–772. doi: 10.1126/science.3363358. [DOI] [PubMed] [Google Scholar]
- Schmetterer G., Wolk C. P., Elhai J. Expression of luciferases from Vibrio harveyi and Vibrio fischeri in filamentous cyanobacteria. J Bacteriol. 1986 Jul;167(1):411–414. doi: 10.1128/jb.167.1.411-414.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T. M., Kopecky K., Nealson K. H. Bioluminescence of the insect pathogen Xenorhabdus luminescens. Appl Environ Microbiol. 1989 Oct;55(10):2607–2612. doi: 10.1128/aem.55.10.2607-2612.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz D. W., Yarus M. A simple and sensitive in vivo luciferase assay for tRNA-mediated nonsense suppression. J Bacteriol. 1990 Feb;172(2):595–602. doi: 10.1128/jb.172.2.595-602.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel G. S., Devine J. H., Baldwin T. O. Control of the lux regulon of Vibrio fischeri. J Biolumin Chemilumin. 1990 Apr-Jun;5(2):99–106. doi: 10.1002/bio.1170050205. [DOI] [PubMed] [Google Scholar]
- Shakin S. H., Liebhaber S. A. Destabilization of messenger RNA/complementary DNA duplexes by the elongating 80 S ribosome. J Biol Chem. 1986 Dec 5;261(34):16018–16025. [PubMed] [Google Scholar]
- Shaw W. C., Addy M., Dummer P. M., Ray C., Frude N. Dental and social effects of malocclusion and effectiveness of orthodontic treatment: a strategy for investigation. Community Dent Oral Epidemiol. 1986 Feb;14(1):60–64. doi: 10.1111/j.1600-0528.1986.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H., Morise H. The aldehyde content of luminous bacteria and of an "aldehydeless" dark mutant. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4666–4669. doi: 10.1073/pnas.71.12.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter R. E., Martin M. O., Silverman M. R. Cloning and nucleotide sequence of luxR, a regulatory gene controlling bioluminescence in Vibrio harveyi. J Bacteriol. 1990 Jun;172(6):2946–2954. doi: 10.1128/jb.172.6.2946-2954.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slock J., VanRiet D., Kolibachuk D., Greenberg E. P. Critical regions of the Vibrio fischeri luxR protein defined by mutational analysis. J Bacteriol. 1990 Jul;172(7):3974–3979. doi: 10.1128/jb.172.7.3974-3979.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soly R. R., Mancini J. A., Ferri S. R., Boylan M., Meighen E. A. A new lux gene in bioluminescent bacteria codes for a protein homologous to the bacterial luciferase subunits. Biochem Biophys Res Commun. 1988 Aug 30;155(1):351–358. doi: 10.1016/s0006-291x(88)81092-1. [DOI] [PubMed] [Google Scholar]
- Sugihara J., Baldwin T. O. Effects of 3' end deletions from the Vibrio harveyi luxB gene on luciferase subunit folding and enzyme assembly: generation of temperature-sensitive polypeptide folding mutants. Biochemistry. 1988 Apr 19;27(8):2872–2880. doi: 10.1021/bi00408a031. [DOI] [PubMed] [Google Scholar]
- Swartzman A., Kapoor S., Graham A. F., Meighen E. A. A new Vibrio fischeri lux gene precedes a bidirectional termination site for the lux operon. J Bacteriol. 1990 Dec;172(12):6797–6802. doi: 10.1128/jb.172.12.6797-6802.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzman E., Miyamoto C., Graham A., Meighen E. Delineation of the transcriptional boundaries of the lux operon of Vibrio harveyi demonstrates the presence of two new lux genes. J Biol Chem. 1990 Feb 25;265(6):3513–3517. [PubMed] [Google Scholar]
- Szittner R., Meighen E. Nucleotide sequence, expression, and properties of luciferase coded by lux genes from a terrestrial bacterium. J Biol Chem. 1990 Sep 25;265(27):16581–16587. [PubMed] [Google Scholar]
- Thompson E. M., Nagata S., Tsuji F. I. Cloning and expression of cDNA for the luciferase from the marine ostracod Vargula hilgendorfii. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6567–6571. doi: 10.1073/pnas.86.17.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur S., Hastings J. W. Evidence for tetradecanal as the natural aldehyde in bacterial bioluminescence. Proc Natl Acad Sci U S A. 1979 Jan;76(1):265–267. doi: 10.1073/pnas.76.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur S., Kuhn J. The transcription of bacterial luminescence is regulated by sigma 32. J Biolumin Chemilumin. 1988 Apr-Jun;2(2):81–93. doi: 10.1002/bio.1170020205. [DOI] [PubMed] [Google Scholar]
- Ulitzur S. The regulatory control of the bacterial luminescence system--a new view. J Biolumin Chemilumin. 1989 Jul;4(1):317–325. doi: 10.1002/bio.1170040144. [DOI] [PubMed] [Google Scholar]
- Wall L. A., Byers D. M., Meighen E. A. In vivo and in vitro acylation of polypeptides in Vibrio harveyi: identification of proteins involved in aldehyde production for bioluminescence. J Bacteriol. 1984 Aug;159(2):720–724. doi: 10.1128/jb.159.2.720-724.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. V., Lam Y. A., Seliger H. H., McElroy W. D. Complementary DNA coding click beetle luciferases can elicit bioluminescence of different colors. Science. 1989 May 12;244(4905):700–702. doi: 10.1126/science.2655091. [DOI] [PubMed] [Google Scholar]
- Xi L., Cho K. W., Herndon M. E., Tu S. C. Elicitation of an oxidase activity in bacterial luciferase by site-directed mutation of a noncatalytic residue. J Biol Chem. 1990 Mar 15;265(8):4200–4203. [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., Helinski D. R., DeLuca M. Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7870–7873. doi: 10.1073/pnas.82.23.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]