Abstract
Drug-induced immune thrombocytopenia (DITP) is a challenging clinical problem that is under-recognized, difficult to diagnose and associated with severe bleeding complications. DITP may be caused by classic drug-dependent platelet antibodies (eg, quinine); haptens (eg, penicillin); fiban-dependent antibodies (eg, tirofiban); monoclonal antibodies (eg, abciximab); autoantibody formation (eg, gold); and immune complex formation (eg, heparin). A thorough clinical history is essential in establishing the diagnosis of DITP and should include exposures to prescription medications, herbal preparations and even certain foods and beverages. Clinical and laboratory criteria have been established to determine the likelihood of a drug being the cause of thrombocytopenia, but these criteria can only be applied retrospectively. The most commonly implicated drugs include quinine, quinidine, trimethoprim/sulfamethoxazole and vancomycin. We propose a practical approach to the diagnosis of the patient with suspected DITP. Key features are: the presence of severe thrombocytopenia (platelet nadir <20 × 109/L); bleeding complications; onset 5 to 10 days after first drug exposure, or within hours of subsequent exposures or after first exposure to fibans or abciximab; and exposure to drugs that have been previously implicated in DITP reactions. Treatment involves stopping the drug(s), administering platelet transfusions or other therapies if bleeding is present and counselling on future drug avoidance. The diagnosis can be confirmed by a positive drug re-challenge, which is often impractical, or by demonstrating drug-dependent platelet reactive antibodies in vitro. Current test methods, which are mostly flow cytometry-based, must show drug-dependence, immunoglobulin binding, platelet specificity and ideally should be reproducible across laboratories. Improved standardization and accessibility of laboratory testing should be a focus of future research.
Thrombocytopenia caused by drugs is a particularly vexing clinical problem. It is common, yet under-recognized, difficult to diagnose and associated with severe bleeding complications. Many drugs have been associated with the development of thrombocytopenia; however, the syndrome of drug-induced immune thrombocytopenia (DITP) is an idiosyncratic drug reaction that occurs with only several drugs. DITP is caused by drug-dependent platelet antibodies that produce platelet clearance by the reticuloendothelial system, often resulting in severe thrombocytopenia and mucocutaneous bleeding.
DITP typically presents 5 to 10 days after beginning daily exposure to a drug, or within hours after re-exposure to a drug that has been taken occasionally for a period of time. Platelet counts are usually less than 20 × 109/L, the onset of thrombocytopenia is rapid, and bleeding symptoms frequently occur. DITP can resemble primary immune thrombocytopenia (ITP) [1]; however, differentiating these syndromes is important to avoid unnecessary treatments and prevent future exposures to the drug [2]. Recent studies have better defined the characteristics of drug-dependent platelet antibodies, which have led to a better understanding of the pathogenesis of this disorder [3]. DITP frequently occurs in hospitalized patients who are taking multiple medications and have a number of other comorbidities; thus relating the thrombocytopenia to a particular drug is often challenging. The lack of an accessible diagnostic test for DITP means that timely laboratory confirmation is generally not possible and providers must rely on a careful clinical to make the diagnosis. In this review, we summarize the mechanisms of DITP, the clinical features and laboratory tests, and we present a practical approach to the management of the patient with suspected DITP. We highlight areas that require further research starting with a case.
Case Presentation
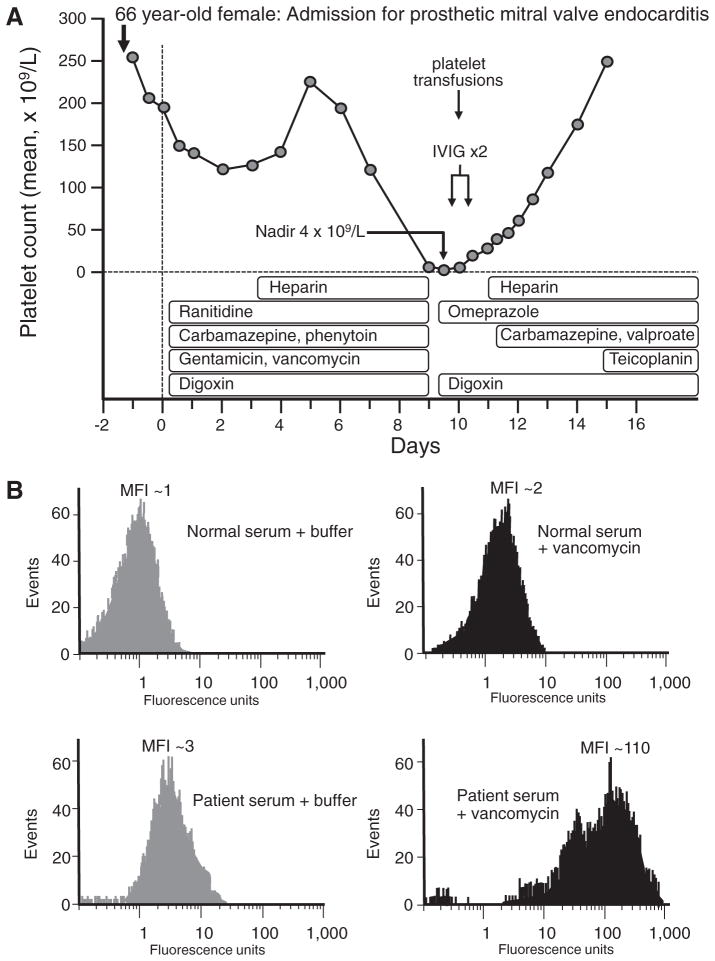
A 66-year-old woman presented with seizures secondary to prosthetic mechanical mitral valve endocarditis complicated by atrial fibrillation and cerebral microabscesses. She underwent emergency mitral valve replacement, and was started on multiple medications on the day of surgery: carbamazepine, phenytoin, gentamicin, vancomycin, ranitidine, digoxin; unfractionated heparin (UFH) prophylaxis was started on postoperative day 3 [4]. On postoperative day 9, severe thrombocytopenia occurred (platelet count, 4 × 109/L), with petechiae. Review of the serial platelet counts revealed that the platelet count began to fall on postoperative day 6 (from 220 × 109/L on day 5 to 190 × 109/L on day 6). This was compatible with DITP that could be explained by any of the following six drugs: carbamazepine, phenytoin, gentamicin, vancomycin, ranitidine, digoxin, with 2 of them (carbamazepine, vancomycin) meeting criteria for drugs that were known to have been implicated in DITP reactions by clinical and laboratory features (drug-dependent antibodies) [5]. In contrast, heparin-induced thrombocytopenia (HIT) was not a plausible diagnosis, for several reasons: (a) the onset of thrombocytopenia began only 3 days after starting UFH (eg, too soon to be explained by heparin-induced immunization), and (b) HIT is not characterized by such severe thrombocytopenia and mucocutaneous bleeding (rather, HIT is a prothrombotic syndrome). The management of this patient was to substitute potentially implicated drugs with non-cross reactive alternatives (eg, teicoplanin instead of gentamicin/vancomycin; omeprazole instead of ranitidine) as well as discontinuation of carbamazepine and phenytoin; in contrast, digoxin (judged most unlikely to cause DITP) was continued. In addition, high-dose intravenous immune globulin (IVIG) 2 g/kg administered over 2 days and platelet transfusions were administered. Within 1 day, the platelet count rose to 20 × 109/L and continued to rise steadily (Fig 1). Testing the patient’s serum for drug-dependent antibodies by flow cytometry revealed the presence of vancomycin-dependent antibodies, and thus carbamazepine was restarted along with valproic acid. The platelet count recovered completely. Based upon clinical and laboratory features, the diagnosis was vancomycin-induced DITP.
Fig 1.
Illustrative case presentation of drug-induced immune thrombocytopenia. A, Clinical picture (See text for details). B, Results of patient serum and drug-dependent antibody binding by flow cytometry. IVIG = intravenous immune globulin. MFI, mean fluorescence intensity. Reproduced with permission from: Warkentin TE. Thrombocytopenia caused by platelet destruction, hypersplenism, or hemodilution. Adapted from Elsevier 2013: 1895–1912.
Thrombocytopenia Caused by Drugs
Many drugs can cause thrombocytopenia either by non-immune or immune mechanisms. Non-immune thrombocytopenia results in suppression of platelet production by general myelotoxicity (eg, chemotherapy), dose-dependent myelosuppression (eg, linezolid) or interference with specific megakaryocyte function (eg, bortezomib). Immune-mediated thrombocytopenia results in accelerated platelet destruction by drug-dependent platelet antibodies that cause platelet clearance (eg, quinine and quinidine) or platelet activation (eg, heparin). Drug-dependent megakaryocyte antibodies may also cause immune-mediated suppression of platelet production.
Non-Immune Suppression of Platelet Production
Platelet production depends on functional megakaryocytes in the bone marrow. Non-immune thrombocytopenia caused by drugs results from a loss of cellularity within the bone marrow and an impairment of megakaryocyte proliferation and maturation, thereby leading to a decrease in platelet production. The myelosuppression is dose-dependent and often occurs slowly over the course of several weeks [6]. Chemotherapeutic compounds, including antimetabolites, cytotoxic agents, and alkylating agents are directly toxic to hematopoietic cells.
Reversible dose-dependent myelosuppression is associated with certain antibiotics including linezolid, a synthetic oxazolidinone with activity against vancomycin and penicillin resistant gram positive bacteria. Linezolid-induced thrombocytopenia tends to occur with prolonged treatment (>2 weeks) [7–9] and at high serum concentrations [10]. Platelets are typically more severely affected than other cell lines. Neither linezolid-dependent anti-platelet antibodies nor a specific megakaryocyte effect of the drug has been demonstrated [7].
Bortezomib is a potent and reversible proteasome inhibitor used for the treatment of multiple myeloma. It is commonly associated with the development of transient thrombocytopenia [11]. Although the precise mechanism remains uncertain, bortezomib may interfere with platelet release from megakaryocytes, rather than causing a general cytotoxic effect on hematopoietic progenitor cells [12]. Thiazide diuretics, tolbutamide, and antivirals have also been associated thrombocytopenia which may be due to suppression of megakaryocytes.
Immune-Mediated Platelet Destruction
DITP is a unique clinical syndrome characterized by severe thrombocytopenia, bleeding and drug-dependent platelet antibodies. DITP can be caused by prescription medications, herbal products and certain foods and beverages [13]. Quinine, and its structural isomer quinidine, is classically associated with severe DITP (platelet count nadir, <10 × 109/L) [14,15]. It is approved for the treatment of uncomplicated malaria caused by the parasite Plasmodium falciparum, and is also used off label for the treatment of leg cramps. A review of reports submitted to the Food and Drug Administration Adverse Event Reporting System from April 2005 to October 1, 2008, found 38 US cases of serious adverse events associated with quinine. As a result, the Food and Drug Administration has issued warnings against unapproved uses of quinine (http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm218202.htm). Quinine is also found in certain beverages including tonic water and Dubonnet aperitif [13].
Several theories have been proposed to explain the development of immune thrombocytopenia in the presence of certain drugs (Table 1): (1) Classic drug-dependent platelet antibodies (quinine-type); (2) hapten-induced antibodies (eg, penicillin); (3) fiban-dependent antibodies (eg, tirofiban); (4) Fab-binding monoclonal antibodies (eg, abciximab); (5) drug-induced autoantibody formation (eg, gold); and (6) immune complex formation (eg, heparin).
Table 1.
Mechanisms of the development of drug-dependent platelet antibodies
| Mechanism | Description | Examples of Drugs |
|---|---|---|
| Classic drug-dependent platelet antibodies | Non-covalent binding of drug to platelet glycoproteins creates a neoepitope and allows antibody binding that marks the platelets for destruction by the reticuloendothelial system | Quinine, quinidine |
| Hapten-induced antibodies | Covalent binding of drug to platelet proteins induced an immune response and antibody production | Penicillin |
| Fiban-dependent antibodies | Binding of drug to the RGD sequence of GPIIbIIIa creates a neoepitope that enhances the affinity of anti-platelet antibodies | Tirobifan, eptifibatide |
| Fab-binding monoclonal antibodies | Monoclonal antibodies bind to platelet surface proteins and become targets of naturally occurring antibodies that lead to platelet destruction | Abciximab |
| Autoantibody production | Drug exposure leads to autoantibody production, which is independent of the drug. | Gold |
| Platelet-localizing immune complexes | Drug binds PF4, forming large complexes on platelet surfaces to which IgG binds via its Fab moieties, with subsequent interactions of IgG Fc with platelet Fcγ, leading to platelet activation and release of procoagulant microparticles (similar mechanisms result in monocyte activation) | Heparin |
RGD sequence = arginine-glycine-aspartic acid. PF4, platelet factor 4.
Drug-Dependent Antibodies
Severe thrombocytopenia that occurs within 5 to 10 days of beginning daily administration of a new drug may be the result of classic drug-dependent antibodies. Quinine binds to platelet glycoproteins (GPs) allowing platelet-reactive antibodies to bind tightly to circulating platelets only when soluble drug is present. The targets of these antibodies are often on platelet GPIIbIIIa or GPIbIX. Many quinine-dependent antibodies appear to be naturally-occurring; normally, these antibodies have a weak affinity for platelet epitopes, but in the presence of the drug their affinity for platelet targets increases leading to immune-mediated destruction [16]. Alternatively, the association of quinine with a target platelet proteins can induce a conformational neoepitope in the GP that can provoke an immune response. The fiexible nature of the target plexin/semaphorin/integrin domain of GPIIIa may conform to a denatured state [17] allowing quinine to bind to and stabilize the denatured conformation of the platelet receptor [18]. Antibody-bound platelets are rapidly cleared by the reticuloendothelial system.
Hapten-Induced Antibodies
Haptens are drugs and other small molecules (<2–5 kDa) that are typically not immunogenic on their own but induce an immune response when linked covalently to a larger carrier protein [19]. The resulting antibodies recognize the protein where the “hapten” is attached. Penicillin can form a covalent linkage with proteins on red blood cells and platelets through its reactive β-lactam ring. The antibodies observed in patients treated with large doses of penicillin often caused immune hemolytic anemia [20] but may also cause immune thrombocytopenia when the hapten links to platelet membrane GPs [21].
Fiban-Dependent Antibodies
Fibans (tirofiban and eptifibatide) are medications that bind to the arginine-glycine-aspartic acid (RGD) recognition site on GPIIbIIIa and prevent the formation of platelet thrombi by competitively inhibiting fibrinogen binding. These agents are commonly during percutaneous coronary interventions. Severe thrombocytopenia occurs in 0.1% to 2% of patients treated with fibans often within hours of the first exposure to the drug [22,23]. The mechanism of thrombocytopenia is thought to be the formation of a neoepitope on GPIIbIIIa, which becomes the target of naturally-occurring antibodies or antibodies induced by prior exposure to the drug [23]. In addition to causing platelet clearance, eptifibatide-dependent anti-platelet antibodies have been shown to induce platelet activation through platelet FcγRIIa receptor binding, which may explain the occurrence of thrombosis in some patients with eptifibatide-thrombocytopenia [24].
Fab-Binding Monoclonal Antibodies
The antiplatelet agent abciximab is a chimeric Fab fragment (human-murine) that is specific for GPIIIa. It blocks the binding of fibrinogen to the RGD sequence on GPIIbIIIa [25]. It is commonly used in high-risk coronary angioplasty procedures [26]. Abciximab itself does not cause thrombocytopenia because it lacks an Fc domain; however, thrombocytopenia can occur in up to 12% of patients after repeat exposure and 2% of patients on first exposure [27] due to the presence of naturally-occurring antibodies that recognize the murine elements of the drug [28].
Severe acute thrombocytopenia has been reported following the administration of rituximab, a chimeric anti-CD20 antibody that targets B. Rituximab is commonly used to treat B cell lymphoproliferative diseases and autoimmune conditions including ITP [29]. Possible mechanisms include the formation of circulating immune complexes causing platelet lysis by complement activation [30–32] or direct binding to the CD20 antigen on the surface of platelets [30,32,33]. Thrombocytopenia is often preceded by infusion-related symptoms and cytokine secretion and may present with features of disseminated intravascular coagulation.
Drug-Induced Autoantibody Formation
Certain drugs can induce the formation of drug-independent platelet autoantibodies. These autoantibodies can bind to platelet antigens even in the absence of the drug and the resultant thrombocytopenia can persist after the drug is discontinued. Gold, L-dopa, procainamide and sulphonamides have been associated with this type of reaction [16]. Recently, alemtuzumab, a humanized anti-CD52 monoclonal antibody was associated with the development of ITP in 6 (2.8%) of 216 patients with multiple sclerosis [34]. Thrombocytopenia developed between 19 and 39 months from first exposure and serological studies for anti-platelet antibodies were inconclusive. The mechanism for autoantibody production is unknown, but may be caused by altered processing of platelet GPs.
Formation of Immune Complexes
Immune complex formation is well described following treatment with UFH and less commonly, low molecular weight heparin in the syndrome called heparin-induced thrombocytopenia (HIT). While in circulation, heparin binds to platelet factor 4 (PF4) and forms an antigenic structure. Antibodies bind to the PF4/heparin complex by their Fab portion and to the surface of platelets and monocytes by their Fc portion causing intense platelet activation and release of procoagulant microparticles [35,36]. A similar syndrome has recently been described following exposure to protamine used to reverse the effects of heparin after cardiac surgery [37–39]. Protamine binds heparin to form antigenic complexes that are immunogenic in mice and humans. These complexes bind IgG antibodies which activate platelets via FcγRIIa leading to thrombocytopenia and potentially thrombosis [38,39]. The mechanism underlying HIT is distinct from other forms of DITP and the clinical presentation is characterized by thrombosis rather than bleeding [40] (Table 2).
Table 2.
Differences between classic (quinine-type) DITP and HIT
| DITP | HIT | |
|---|---|---|
| Onset of thrombocytopenia | 5–10 days | 5–10 days (classic) |
| Median platelet count nadir | 10 × 109/L | 60 × 109/L |
| Clinical manifestation | Bleeding | Thrombosis |
| In vitro diagnostic testing | Flow cytometry (or other method) demonstrating patient IgG binding to washed platelets only in the presence of the drug | Functional test demonstrating activation of washed platelets by patient serum in the presence of pharmacologic heparin concentrations (peak platelet activation at 0.1– 0.3 IU/mL). |
| Treatment | Stop the drug | Stop the heparin and start an alternate anticoagulant |
| Persistence of drug-induced platelet antibodies | Indefinitely | Median detectability, 50 to 80 days |
| Re-exposure after confirmed diagnosis | Never | Possible (pending non- detectability of platelet- activating antibodies) |
Immune-mediated suppression of platelet production
Immune thrombocytopenia in DITP may result from antibodies that bind to megakaryocytes resulting in impaired platelet production. Eptifibatide-dependent platelet antibodies have been shown to impair viability of megakaryopoietic cells in an in vitro culture assay [41]. Similarly, quinine-dependent antibodies can bind megakaryocytes leading to the induction of apoptosis and reduced proplatelet formation [42]. These findings suggest that drug-dependent antibodies may affect the number of functional megakaryocytes available for platelet release and interfere with their capacity to produce platelets.
Establishing the Diagnosis of DITP
Clinical History
A thorough clinical history is essential in establishing the diagnosis of DITP. A complete drug history should be obtained from any patient presenting with thrombocytopenia including prescription medications, herbal preparations and even certain foods and beverages. DITP is frequently overlooked as a cause of thrombocytopenia and the syndrome may be clinically indistinguishable from primary ITP. Moreover, DITP can occur because of occult drug exposures. Reddy et al describe 5 patients with persistent thrombocytopenia who were presumed to have relapsing ITP [43]. They received multiple treatments including splenectomy before the use of quinine tablets to self-treat leg cramps was uncovered. In one report, severe thrombocytopenia after knee replacement surgery was found to be caused by vancomycin that was contained in the prosthesis cement [44]. Recurrent thrombocytopenia after ingestion of walnuts has been confirmed by a walnut re-challenge and the demonstration of walnut (Juglans regia)–dependent platelet antibodies in vitro [45]. Other foods and natural products with definite evidence for causing DITP are cow’s milk, cranberry juice, sesame seeds (in tahini), African bean (Lupinus termis), and Jui [13] (Table 3). These reports highlight the importance of a careful and detailed history of drug exposures including their temporal relationship with the development of the thrombocytopenia.
Table 3.
Foods, beverages and natural products associated with definite evidence* for the development of drug-induced immune thrombocytopenia [13]
| Substance | Platelet nadir | Bleeding | Confirmation |
|---|---|---|---|
| Cow’s milk | 5 × 109/L | Yes | Re-challenge |
| Cranberry juice | 1 × 109/L | Yes | DDAbs |
| Jui | 0 | Yes | Re-challenge |
| Lupinus termis bean | 10 × 109/L | Not reported | Re-challenge |
| Sesame seeds | 6 × 109/L | Yes | Re-challenge |
| Walnut (Juglans regia) [46] | 4 × 109/L | Yes | Re-challenge and DDAbs |
Definite evidence = Ingestion of the substance preceded the thrombocytopenia and platelet count recovered once the substance was stopped; candidate substance was the only agent ingested or other agents were continued or re-introduced and platelet count remained normal; other etiologies of thrombocytopenia were excluded; confirmation in vivo with a re-challenge or in vitro with the demonstration of drug-dependent platelet antibodies (DDAbs).
Certain unique clinical features can help distinguish DITP from other causes of thrombocytopenia. Particular attention should be paid to the severity of thrombocytopenia, the presence of bleeding, the timing and rapidity of the onset of thrombocytopenia and the implicated drug. Thrombocytopenia is typically severe with platelet counts typically below 20 × 109/L. Evidence of hemostatic impairment is almost always present and patients can present with petechiae, oral mucous membrane blood blisters, serious gastrointestinal bleeding or intracranial hemorrhage. Deaths from bleeding have been reported [46]. The onset of thrombocytopenia occurs 5 to 10 days after first drug exposure with classic DITP reactions and more rapidly (within hours) following fiban and abciximab. Rarely, the development of thrombocytopenia after abciximab can be delayed for up to 8 days, since platelet-bound abciximab may persist for up to 2 weeks after treatment [47]. The most commonly implicated drugs in DITP reactions include quinine, quinidine, trimethoprim/sulfamethoxazole and vancomycin (Table 4); however, any drug should be considered if the timing and clinical presentation fits.
Table 4.
List of drugs implicated in drug-induced immune thrombocytopenia reactions from various sources (definite evidence)
| Hackett [48] | George [46] | Reese [49] | Arnold [5] | |
|---|---|---|---|---|
| Implicated drugs | Acetaminophen | Quinidine | Abciximab | Quinine |
| Allylisopropylcarbamide | Quinine | Acetaminophen | Quinidine | |
| Alprenolol | Rifampin | Amiodarone | Trimethoprim-Sulfamethoxazole | |
| Chlorothiazide | Trimethoprim-sulfamethoxazole | Ampicillin | Vancomycin | |
| Digitoxin | Methyldopa | Carbamazepine | Penicillin | |
| Digoxin | Acetaminophen | Eptifibatide | Rifampin | |
| Levamisole | Digoxin | Ethambutol | Carbamazepine | |
| Methicillin | Danazol | Haloperidol | Ceftriaxone | |
| Methyldopa | Diclofenac | Ibuprofen | Ibuprofen | |
| Novobiocin | Aminoglutethimide | Irinotecan | Mirtazapine | |
| Organic arsenicals | Amphotericin B | Naproxen | Oxaliplatin | |
| Oxprenolol | Aminosalicylic acid | Oxaliplatin | Suramin | |
| Para-aminosalicylic acid | Oxprenolol | Phenytoin | Abciximab | |
| Quinidine | Vancomycin | Piperacillin | Tirofiban | |
| Quinine | Levamisole | Quinidine | Eptifibatide | |
| Rifampicin | Meclofenamate | Quinine | Heparin | |
| Stibophen | Diatrizoate meglumine-diatrizoate sodium | Ranitidine | ||
| Sulfathiazole | Amiodarone | Rifampin | ||
| Sulfisoxazole | Nalidixic acid | Simvastatin | ||
| Acetylsalicylic acid | Cimetidine | Sulfisoxazole | ||
| Co-trimoxazole | Chlorothiazide | Tirofiban | ||
| Desipramine | Diatrizoate Meglumine | Trimethoprim-sulfamethoxazole | ||
| Diazepam | Interferon-α | Valproic acid | ||
| Diphenylhydantoin | Sulfasalazine | Vancomycin | ||
| Ethambutol | ||||
| Iopanoic acid | ||||
| Sulfisoxazole | ||||
| Tamoxifen | ||||
| Thiothixene | ||||
| Naphazoline | ||||
| Amrinone | ||||
| Lithium | ||||
| Diazepam | ||||
| Haloperidol | ||||
| Alprenolol | ||||
| Tolmetin | ||||
| Nitroglycerin | ||||
| Minoxidil | ||||
| Diazoxide | ||||
| Chlorpromazine | ||||
| Isoniazid | ||||
| cephalothin | ||||
| Difiuormethylornithine | ||||
| Piperacillin | ||||
| Diethylstilbestrol | ||||
| Methicillin | ||||
| Deferoxamine | ||||
| Novobiocin |
Clinical Criteria for DITP
To help establish the likelihood that a particular drug was the cause of the thrombocytopenia, several clinical scoring systems have been developed. In 1982 Hackett et al proposed the following criteria: (1) Thrombocytopenia developed while the patient is taking the drug, resolved once the drug is stopped and did not recur while the patient was off the drug; (2) other causes of thrombocytopenia were excluded; (3) the thrombocytopenia recurred upon re-administration of the drug; and (4) an in vitro test for drug-dependent platelet antibodies was positive [48]. A positive re-challenge or positive laboratory test was sufficient to confirm the diagnosis. The authors identified 24 drugs from published reports that were implicated in DITP using these criteria (Table 4). In 1998, George et al used similar clinical criteria to establish levels of evidence for DITP drugs [47] and identified 48 drugs with a definite association (Table 4). This list has been updated regularly and is available online (www.ouhsc.edu/platelets). These criteria have been helpful for developing lists of implicated drugs, but are less helpful for the clinician faced with a patient with suspected DITP since they can only be applied retrospectively.
Laboratory Criteria for DITP
The detection of drug-dependent platelet antibodies in vitro can confirm the diagnosis of DITP. Challenges with the implementation of DITP testing have included a wide variety of techniques used over the years, the lack of standardization, and the lack of validation across a range of drugs.
The characteristics of a positive in vitro test proposed by Hackett included one measure of sensitivity-that the patient’s serum plus test drug produce a measurable effect in test platelets, and two measures of specificity-that neither non-implicated drugs nor serum from non-thrombocytopenic controls produce the effect. A variety of tests have been developed based on these principles; however, they have often been difficult to interpret due the lack of validity and reproducibility. Thus, by consensus among a group of experts, Arnold et al proposed the following criteria for the assessment of the quality of DITP laboratory test methods and results from published reports, called the “DITP criteria”: (1) Drug (or drug metabolite) was required for the reaction in vitro; (2) Immunoglobulin binding was demonstrated; (3) Two or more laboratories obtained positive results on separate occasions; and (4) Platelets were the target of immunoglobulin binding. The laboratory diagnosis of DITP was considered definite when all criteria were met; and probable when positive results were reported by only one laboratory instead of two [5].
Test Methods That Met Laboratory Criteria
Several assays can measure the ability of antibody (in serum or plasma) to bind platelets in the presence of the drug or its metabolite. Sera from affected patients with no drug added and sera from patients who did not develop thrombocytopenia after drug exposure can be used as negative controls [50]. Test methods [51] that met validity criteria were flow cytometry, platelet suspension immunofluorescence test, enzyme immunoassays, radiolabeled anti-globulin-based assays, and GP-specific assays (Table 5). Flow cytometry is most commonly used because of its sensitivity and ability to provide a quantitative result. Some laboratories use assays that detect binding of antibodies to whole platelets using enzyme-conjugated or fluorescent-tagged secondary antibodies. A number of older tests have been used throughout the years to evaluate the effect of specific drugs on platelets, which are mainly of historical interest (Table 6).
Table 5.
Test methods used to evaluate drugs suspected of causing DITP that met all validity criteria [5]
| Method | Description |
|---|---|
| Flow cytometry | Quantification of patient antibody bound to platelets using fluorescent-labeled antiglobulin processed on a flow cytometer, with the ability for simultaneous detection of different antibody classes. |
| Platelet suspension immunofluorescence test | Semi-quantitative measurement by light microscopy of drug-dependent antibody bound to platelets using fluorescent-labeled antiglobulin. |
| Monoclonal antibody immobilization of platelet antigens (MAIPA) | Antibody detection is based on tri-molecular complexes formed by binding of glycoprotein- specific monoclonal antibodies to intact platelets with bound human antibody. Following lysis of the platelet membrane, glycoprotein-specific human antibody is measured using enzyme-conjugated anti-globulin in an enzyme immunoassay. |
| Antigen-capture ELISA (ACE; modified ACE) | Similar to MAIPA assay, but glycoprotein-specific monoclonal antibody is used to capture human antibody bound to the specific platelet protein after lysis of the platelets. This allows for routine processing and storage of lysates for future testing with various monoclonal antibodies. |
| Enzyme immunoassay | Enzyme-conjugated antiglobulin is used to detect platelet-bound IgG on platelets adherent to microtitre wells. |
| Platelet antiglobulin test | Modification of the enzyme immunoassay in which radiolabeled antiglobulin is used to detect the human antibody bound to target platelets. |
| Immunoprecipitation | Measurement of antibody bound to 125Iodine- radiolabeled platelet proteins which are identified by their mobility following gel electrophoresis. |
| Immunoblot assay | Measurement of human antibody binding to platelet proteins previously separated by gel electrophoresis and transferred to a membrane. Antibody binding may be limited by destruction of epitopes on the denatured proteins during electrophoresis. |
Table 6.
Historical techniques previously used to evaluate the effect of drugs on platelets
| Test | Description of methods |
|---|---|
| Patch test | Topical application of the drug to the patient’s skin. The test was considered positive if local purpura developed [52]. |
| Antiglobulin consumption assay (serum bindable IgG) | A fixed amount of antiglobulin was incubated with test platelets. Residual antiglobulin was measured by complement lysis of IgG-coated erythrocytes or by quantification of radiolabeled antiglobulin bound to IgG-coated beads [53]. |
| Platelet factor 3 (PF3) test (“immune injury” test) | Normal citrated platelet rich plasma was incubated with drug and patient serum. Increased PF3 activity, indicated by a shortening of the Russell’s viper venom (Stypven) time, was considered positive [54]. This assay was refined with a neutralization [55] or IgG isolation step [56]. |
| Clot retraction inhibition test | Visual inspection of a blood clot formed in solution in the presence of test serum with and without drug. Inhibition of clot retraction was considered positive indicating the lack of functional platelets as a result of antibody interference [57]. |
| Complement fixation test | Incubation of patient serum, drug, test platelets and a source of complement. Residual complement activity was measured by lysis of IgG-sensitized sheep red blood cells [58]. |
| Platelet agglutination test | Patient serum was incubated with test platelets in the presence of the drug. Clumping in the test tube was assessed visually and graded semi-quantitatively from 0–4 after a 2-hour incubation at 37°C [59]. |
| Platelet lysis test | 51Cr-labeled platelets were incubated with test serum (or IgG), normal platelets, and a source of complement with and without drug. Percentage platelet lysis was calculated [60,61]. |
Combining Clinical and Laboratory Criteria to Identify Implicated Drugs
To narrow the list of drugs implicated in DITP, Reese et al proposed a classification system that incorporated clinical criteria and results of laboratory testing [49]. The authors cross-referenced an online list of drugs with a US national voluntary-reporting database and DITP test results from a reference laboratory database. Using this strategy, the authors identified 24 drugs (Table 4). More recently, Arnold et al identified 16 drugs that met both clinical criteria and laboratory criteria from published reports which were felt to have the strongest evidence for causing DITP [4]. Quinine, quinidine, trimethoprim/sulfamethoxazole, vancomycin, penicillin, rifampin, carbamazepine, ceftriaxone, ibuprofen, mirtazapine and suramin; the GPIIbIIIa inhibitors abciximab, tirofiban and eptifibatide; and heparin (Table 4).
Approach to management of the patient with suspected DITP
Diagnosis
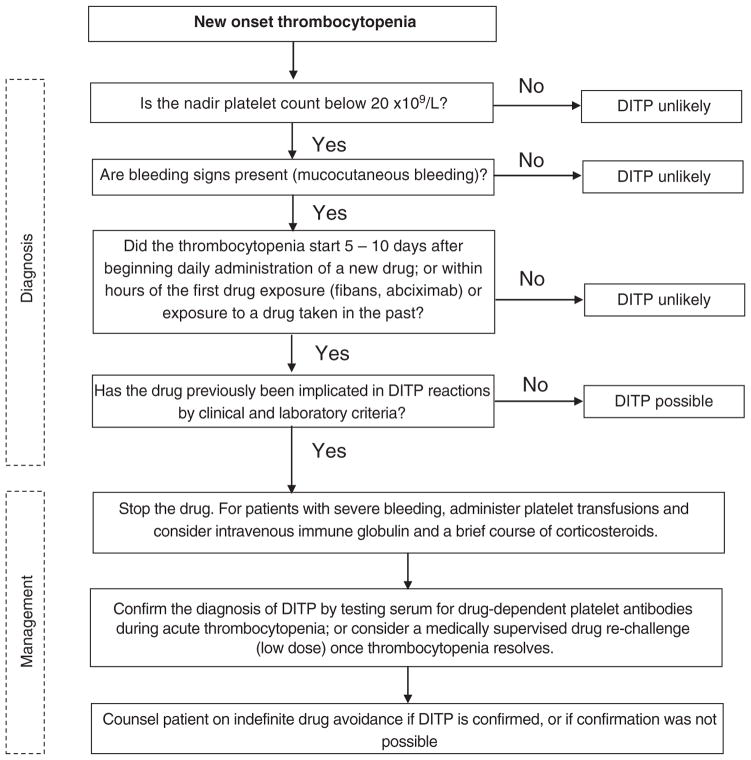
We propose an approach to the diagnosis and management of the patient with new-onset thrombocytopenia in whom DITP is suspected (Fig 2). The diagnosis should be considered in any patient who develops severe thrombocytopenia (nadir platelet count less than 20 × 109/L) with bleeding complications that begins 5 to 10 days after starting daily administration of a new drug, or within hours of the first exposure to certain drugs such as fibans and abciximab or within hours of subsequent drug exposures. Other causes of thrombocytopenia should be sought in patients without these clinical features. If the implicated drug has been previously shown to be associated with DITP (Table 4), then the diagnosis is more likely; however, DITP should be considered with any drug. Positive laboratory testing demonstrating drug-dependent platelet reactive antibodies can confirm the diagnosis, but a negative test does not rule it out. Recurrence of thrombocytopenia with drug re-exposure can also confirm the diagnosis. A drug re-challenge, starting with low doses of drug, may be considered when common drugs such as acetaminophen [62] are implicated and results of laboratory testing are negative or unavailable. This approach may expose patients to bleeding risk and thus should only be done under careful supervision.
Fig 2.
Approach to the diagnosis and management of a patient with new-onset thrombocytopenia in whom drug-induced immune thrombocytopenia is suspected.
Management
The most important aspect of management is to immediately stop the drug. Platelet counts typically begin to recover within 1 to 2 days. Platelet transfusions may be required to treat patients with severe thrombocytopenia and bleeding and other supportive measures including high dose IVIG and a brief course of corticosteroids may be reasonable. Drug-dependent platelet antibodies can persist for many years; thus, patients with a confirmed diagnosis should be counselled to avoid future exposures to the drug [63]. Counselling on drug avoidance is less certain for patients in whom the diagnosis cannot be confirmed.
Future directions
Accessible and reliable laboratory testing would help establish the diagnosis of DITP and provide guidance for clinicians and patients on the need for drug avoidance. However, testing is only available in a few reference laboratories and test methods have not been standardized [64]. Reasons for this are: (1) no single method has been universally accepted, although flow cytometry is commonly used; (2) assays must be specifically developed for each drug, some of which cannot be solubilized at neutral pH; (3) DITP may be due to metabolites of the drug which are not always available for use as a reagent in the test; and (4) other platelet antibodies can confound the measurement of drug-dependent antibodies. The International Society of Thrombosis and Hemostasis is working towards standardizing laboratory testing for the detection of drug-dependent platelet antibodies for the most commonly implicated drugs.
Acknowledgments
We thank Jo-Ann I. Sheppard for preparing figure 1. This study was supported by a grant from the Canadian Institutes for Health Research (grant #89897) and Canadian Blood Services awarded to D. Arnold.
Footnotes
Conflict of interest statement: Dr Warkentin has served as consultant and/or has received honoraria for speaking on behalf of companies that manufacture low-molecular-weight heparin (Pfizer Canada, Sanofi-Aventis), heparin-coated grafts (W. L. Gore), heparin-like molecules (Paringenix), and non-heparin anticoagulants for management of HIT (Canyon Pharmaceuticals, GlaxoSmithKline). His institution has received funding from GlaxoSmithKline, Instrumentation Laboratories, as well as from the Heart & Stroke Foundation of Ontario for research related to HIT. He has also received royalties from Informa for a book, entitled Heparin-Induced Thrombocytopenia. He receives compensation for medicolegal consultation and testimony regarding thrombocytopenic disorders including HIT. None of the other authors have any conflict of interest to disclose relevant to this manuscript.
References
- 1.Kojouri K, Perdue JJ, Medina PJ, George JN. Occult quinine-induced thrombocytopenia. J Okla State Med Assoc. 2000;93:519–21. [PubMed] [Google Scholar]
- 2.Neylon AJ, Saunders PW, Howard MR, Proctor SJ, Taylor PR. Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: a prospective study of a population-based cohort of 245 patients. Br J Haematol. 2003;122:966–74. doi: 10.1046/j.1365-2141.2003.04547.x. [DOI] [PubMed] [Google Scholar]
- 3.Aster RH, Curtis BR, McFarland JG, Bougie DW. Drug-induced immune thrombocytopenia: pathogenesis, diagnosis, and management. J Thromb Haemost. 2009;7:911–8. doi: 10.1111/j.1538-7836.2009.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warkentin TE. Thrombocytopenia caused by platelet destruction, hypersplenism, or hemodilution. In: Hoffman R, Benz EJJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, editors. Hematology: basic principles and practice. Philadelphia: Churchill Livingstone Elsevier; 2013. pp. 1895–912. [Google Scholar]
- 5.Arnold DM, Kukaswadia S, Nazi I, Esmail A, Dewar L, Smith JW, et al. A systematic evaluation of laboratory testing for drug-induced immune thrombocytopenia. J Thromb Haemost. 2013;11:169–76. doi: 10.1111/jth.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wazny LD, Ariano RE. Evaluation and management of drug-induced thrombocytopenia in the acutely ill patient. Pharmacotherapy. 2000;20:292–307. doi: 10.1592/phco.20.4.292.34883. [DOI] [PubMed] [Google Scholar]
- 7.Gerson SL, Kaplan SL, Bruss JB, Le V, Arellano FM, Hafkin B, et al. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother. 2002;46:2723–6. doi: 10.1128/AAC.46.8.2723-2726.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attassi K, Hershberger E, Alam R, Zervos MJ. Thrombocytopenia associated with linezolid therapy. Clin Infect Dis. 2002;34:695–8. doi: 10.1086/338403. [DOI] [PubMed] [Google Scholar]
- 9.Priziola JL, Smythe MA, Dager WE. Drug-induced thrombocytopenia in critically ill patients. Crit Care Med. 2010;38:S145–54. doi: 10.1097/CCM.0b013e3181de0b88. [DOI] [PubMed] [Google Scholar]
- 10.Hiraki Y, Tsuji Y, Hiraike M, Misumi N, Matsumoto K, Morita K, et al. Correlation between serum linezolid concentration and the development of thrombocytopenia. Scand J Infect Dis. 2012;44:60–4. doi: 10.3109/00365548.2011.608712. [DOI] [PubMed] [Google Scholar]
- 11.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–22. [PubMed] [Google Scholar]
- 12.Lonial S, Waller EK, Richardson PG, Jagannath S, Orlowski RZ, Giver CR, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106:3777–84. doi: 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royer DJ, George JN, Terrell DR. Thrombocytopenia as an adverse effect of complementary and alternative medicines, herbal remedies, nutritional supplements, foods, and beverages. Eur J Haematol. 2010;84:421–9. doi: 10.1111/j.1600-0609.2010.01415.x. [DOI] [PubMed] [Google Scholar]
- 14.Brinker AD, Beitz J. Spontaneous reports of thrombocytopenia in association with quinine: clinical attributes and timing related to regulatory action. Am J Hematol. 2002;70:313–7. doi: 10.1002/ajh.10148. [DOI] [PubMed] [Google Scholar]
- 15.Warkentin TE. Think of HIT. Hematology Am Soc Hematol Educ Program. 2006:408–14. doi: 10.1182/asheducation-2006.1.408. [DOI] [PubMed] [Google Scholar]
- 16.Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357:580–7. doi: 10.1056/NEJMra066469. [DOI] [PubMed] [Google Scholar]
- 17.Dill KA, Shortle D. Denatured states of proteins. Annu Rev Biochem. 1991;60:795–825. doi: 10.1146/annurev.bi.60.070191.004051. [DOI] [PubMed] [Google Scholar]
- 18.Li R. A hypothesis that explains the heterogeneity of drug-induced immune thrombocytopenia. Blood. 2010;115:914. doi: 10.1182/blood-2009-09-242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weltzien HU, Moulon C, Martin S, Padovan E, Hartmann U, Kohler J. T cell immune responses to haptens. Structural models for allergic and autoimmune reactions. Toxicology. 1996;107:141–51. doi: 10.1016/0300-483x(95)03253-c. [DOI] [PubMed] [Google Scholar]
- 20.Garratty G. Immune cytopenia associated with antibiotics. Transfus Med Rev. 1993;7:255–67. doi: 10.1016/s0887-7963(93)70145-5. [DOI] [PubMed] [Google Scholar]
- 21.Salamon DJ, Nusbacher J, Stroupe T, Wilson JH, Hanrahan JB. Red cell and platelet-bound IgG penicillin antibodies in a patient with thrombocytopenia. Transfusion. 1984;24:395–8. doi: 10.1046/j.1537-2995.1984.24585017827.x. [DOI] [PubMed] [Google Scholar]
- 22.Aster RH. Immune thrombocytopenia caused by glycoprotein IIb/IIIa inhibitors. Chest. 2005;127:53S–9S. doi: 10.1378/chest.127.2_suppl.53S. [DOI] [PubMed] [Google Scholar]
- 23.Bougie DW, Wilker PR, Wuitschick ED, Curtis BR, Malik M, Levine S, et al. Acute thrombocytopenia after treatment with tirofiban or eptifibatide is associated with antibodies specific for ligand-occupied GPIIb/IIIa. Blood. 2002;100:2071–6. [PubMed] [Google Scholar]
- 24.Gao C, Boylan B, Bougie D, Gill JC, Birenbaum J, Newman DK, et al. Eptifibatide-induced thrombocytopenia and thrombosis in humans require FcgammaRIIa and the integrin beta3 cytoplasmic domain. J Clin Invest. 2009;119:504–11. doi: 10.1172/JCI36745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Artoni A, Li J, Mitchell B, Ruan J, Takagi J, Springer TA, et al. Integrin beta3 regions controlling binding of murine mAb 7E3: implications for the mechanism of integrin alphaIIbbeta3 activation. Proc Natl Acad Sci U S A. 2004;101:13114–20. doi: 10.1073/pnas.0404201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The EPIC. Investigation. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994;330:956–61. doi: 10.1056/NEJM199404073301402. [DOI] [PubMed] [Google Scholar]
- 27.Dery JP, Braden GA, Lincoff AM, Kereiakes DJ, Browne K, Little T, et al. Final results of the ReoPro readministration registry. Am J Cardiol. 2004;93:979–84. doi: 10.1016/j.amjcard.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 28.Curtis BR, Swyers J, Divgi A, McFarland JG, Aster RH. Thrombocytopenia after second exposure to abciximab is caused by antibodies that recognize abciximab-coated platelets. Blood. 2002;99:2054–9. doi: 10.1182/blood.v99.6.2054. [DOI] [PubMed] [Google Scholar]
- 29.Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146:25–33. doi: 10.7326/0003-4819-146-1-200701020-00006. [DOI] [PubMed] [Google Scholar]
- 30.Otrock ZK, Mahfouz RA, Oghlakian GO, Salem ZM, Bazarbachi A. Rituximab-induced acute thrombocytopenia: a report of two cases. Haematologica. 2005;90(Suppl):ECR23. [PubMed] [Google Scholar]
- 31.Thachil J, Mukherje K, Woodcock B. Rituximab-induced haemorrhagic thrombocytopenia in a patient with hairy cell leukaemia. Br J Haematol. 2006;135:273–4. doi: 10.1111/j.1365-2141.2006.06299.x. [DOI] [PubMed] [Google Scholar]
- 32.Ram R, Bonstein L, Gafter-Gvili A, Ben-Bassat I, Shpilberg O, Raanani P. Rituximab-associated acute thrombocytopenia: an under-diagnosed phenomenon. Am J Hematol. 2009;84:247–50. doi: 10.1002/ajh.21372. [DOI] [PubMed] [Google Scholar]
- 33.Pamuk GE, Donmez S, Turgut B, Demir M, Vural O. Rituximab-induced acute thrombocytopenia in a patient with prolymphocytic leukemia. Am J Hematol. 2005;78:81. doi: 10.1002/ajh.20218. [DOI] [PubMed] [Google Scholar]
- 34.Cuker A, Coles AJ, Sullivan H, Fox E, Goldberg M, Oyuela P, et al. A distinctive form of immune thrombocytopenia in a phase 2 study of alemtuzumab for the treatment of relapsing-remitting multiple sclerosis. Blood. 2011;118:6299–305. doi: 10.1182/blood-2011-08-371138. [DOI] [PubMed] [Google Scholar]
- 35.Warkentin TE, Hayward CP, Boshkov LK, Santos AV, Sheppard JA, Bode AP, et al. Sera from patients with heparin-induced thrombocytopenia generate platelet-derived microparticles with procoagulant activity: an explanation for the thrombotic complications of heparin-induced thrombocytopenia. Blood. 1994;84:3691–9. [PubMed] [Google Scholar]
- 36.Lee DH, Warkentin TE, Denomme GA, Hayward CP, Kelton JG. A diagnostic test for heparin-induced thrombocytopenia: detection of platelet microparticles using flow cytometry. Br J Haematol. 1996;95:724–31. doi: 10.1046/j.1365-2141.1996.d01-1944.x. [DOI] [PubMed] [Google Scholar]
- 37.Singla A, Sullivan MJ, Lee G, Bartholomew J, Kapadia S, Aster RH, et al. Protamine-induced immune thrombocytopenia. Transfusion. 2013 doi: 10.1111/trf.12112. http://dx.doi.org/10.1111/trf.12112. [DOI] [PMC free article] [PubMed]
- 38.Bakchoul T, Zollner H, Amiral J, Panzer S, Selleng S, Kohlmann T, et al. Anti-protamine-heparin antibodies: incidence, clinical relevance and pathogenesis. Blood. 2013;121:2821–7. doi: 10.1182/blood-2012-10-460691. [DOI] [PubMed] [Google Scholar]
- 39.Lee GM, Welsby IJ, Phillips BB, Ortel TL, Arepally GM. High incidence of antibodies to protamine and protamine/heparin complexes in patients undergoing cardio-pulmonary bypass. Blood. 2013;121:2828–35. doi: 10.1182/blood-2012-11-469130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warkentin TE. Drug-induced immune-mediated thrombocytopenia—from purpura to thrombosis. N Engl J Med. 2007;356:891–3. doi: 10.1056/NEJMp068309. [DOI] [PubMed] [Google Scholar]
- 41.Greinacher A, Fuerll B, Zinke H, Mullejans B, Kruger W, Michetti N, et al. Megakaryocyte impairment by eptifibatide-induced antibodies causes prolonged thrombocytopenia. Blood. 2009;114:1250–3. doi: 10.1182/blood-2009-02-203034. [DOI] [PubMed] [Google Scholar]
- 42.Perdomo J, Yan F, Ahmadi Z, Jiang XM, Stocker R, Chong BH. Quinine-induced thrombocytopenia: drug-dependent GPIb/IX antibodies inhibit megakaryocyte and proplatelet production in vitro. Blood. 2011;117:5975–86. doi: 10.1182/blood-2010-10-314310. [DOI] [PubMed] [Google Scholar]
- 43.Reddy JC, Shuman MA, Aster RH. Quinine/quinidine-induced thrombocytopenia: a great imitator. Arch Intern Med. 2004;164:218–20. doi: 10.1001/archinte.164.2.218. [DOI] [PubMed] [Google Scholar]
- 44.O’Donnell E, Shepherd C, Neff A. Immune thrombocytopenia from vancomycin in orthopedic cement. Am J Hematol. 2007;82:1122. doi: 10.1002/ajh.20916. [DOI] [PubMed] [Google Scholar]
- 45.Achterbergh R, Vermeer HJ, Curtis BR, Porcelijn L, Aster RH, Deenik W, et al. Thrombocytopenia in a nutshell. Lancet. 2012;379:776. doi: 10.1016/S0140-6736(11)61643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.George JN, Raskob GE, Shah SR, Rizvi MA, Hamilton SA, Osborne S, et al. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Intern Med. 1998;129:886–90. doi: 10.7326/0003-4819-129-11_part_1-199812010-00009. [DOI] [PubMed] [Google Scholar]
- 47.Curtis BR, Divgi A, Garritty M, Aster RH. Delayed thrombocytopenia after treatment with abciximab: a distinct clinical entity associated with the immune response to the drug. J Thromb Haemost. 2004;2:985–92. doi: 10.1111/j.1538-7836.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- 48.Hackett T, Kelton JG, Powers P. Drug-induced platelet destruction. Semin Thromb Hemost. 1982;8:116–37. doi: 10.1055/s-2007-1005047. [DOI] [PubMed] [Google Scholar]
- 49.Reese JA, Li X, Hauben M, Aster RH, Bougie DW, Curtis BR, et al. Identifying drugs that cause acute thrombocytopenia: an analysis using 3 distinct methods. Blood. 2010;116:2127–33. doi: 10.1182/blood-2010-03-276691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Von DA, Curtis BR, Bougie DW, McFarland JG, Ahl S, Limbu I, et al. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007;356:904–10. doi: 10.1056/NEJMoa065066. [DOI] [PubMed] [Google Scholar]
- 51.Curtis BR, McFarland JG. Detection and identification of platelet antibodies and antigens in the clinical laboratory. Immunohematology. 2009;25:125–35. [PubMed] [Google Scholar]
- 52.Freedman AL, Barr PS, Brody EA. Hemolytic anemia due to quinidine: observations on its mechanism. Am J Med. 1956;20:806–16. doi: 10.1016/0002-9343(56)90164-4. [DOI] [PubMed] [Google Scholar]
- 53.Kelton JG, Moore J, Gauldie J, Neame PB, Hirsh J, Tozman E. The development and application of a serum assay for platelet-bindable IgG (S-PBIgG) J Lab Clin Med. 1981;98:272–9. [PubMed] [Google Scholar]
- 54.Horowitz HI, Rappaport HI, Young RC, Fujimoto MM. Change in platelet factor 3 as a means of demonstrating immune reactions involving platelets: Its use as a test for Quinidine-induced thrombocytopenia. Transfusion. 1965;5:336–43. doi: 10.1111/j.1537-2995.1965.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 55.Karpatkin S, Siskind GW. In vitro detection of platelet antibody in patients with idiopathic thrombocytopenic purpura and systemic lupus erythematosus. Blood. 1969;33:795–812. [PubMed] [Google Scholar]
- 56.Garg SK, Sarker CR. Aspirin-induced thrombocytopenia on an immune basis. Am J Med Sci. 1974;267:129–32. doi: 10.1097/00000441-197402000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Young RC, Nachman RL, Horowitz HI. Thrombocytopenia due to digitoxin. Demonstration of antibody and mechanisms of action. Am J Med. 1966;41:605–14. doi: 10.1016/0002-9343(66)90222-1. [DOI] [PubMed] [Google Scholar]
- 58.Aster RH, Cooper HE, Singer DL. Simplified complement fixation test for the detection of platelet antibodies in human serum. J Lab Clin Med. 1964;63:161–72. [PubMed] [Google Scholar]
- 59.Hamilton HE, Sheets RF. Sulfisoxazole-induced thrombocytopenic purpura. Immunologic mechanism as cause. JAMA. 1978;239:2586–7. doi: 10.1001/jama.239.24.2586. [DOI] [PubMed] [Google Scholar]
- 60.Aster RH, Enright SE. A platelet and granulocyte membrane defect in paroxysmal nocturnal hemoglobinuria: usefulness for the detection of platelet antibodies. J Clin Invest. 1969;48:1199–210. doi: 10.1172/JCI106084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cimo PL, Pisciotta AV, Desai RG, Pino JL, Aster RH. Detection of drug-dependent antibodies by the 51Cr platelet lysis test: documentation of immune thrombocytopenia induced by diphenylhydantoin, diazepam, and sulfisoxazole. Am J Hematol. 1977;2:65–72. doi: 10.1002/ajh.2830020109. [DOI] [PubMed] [Google Scholar]
- 62.Bougie D, Aster R. Immune thrombocytopenia resulting from sensitivity to metabolites of naproxen and acetaminophen. Blood. 2001;97:3846–50. doi: 10.1182/blood.v97.12.3846. [DOI] [PubMed] [Google Scholar]
- 63.George JN, Aster RH. Drug-induced thrombocytopenia: pathogenesis, evaluation, and management. Hematology Am Soc Hematol Educ Program. 2009:153–8. doi: 10.1182/asheducation-2009.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu GG, Kaplan C, Curtis BR, Pearson HA. Report on the 14th International Society of Blood Transfusion Platelet Immunology Workshop. Vox Sang. 2010;99:375–81. doi: 10.1111/j.1423-0410.2010.01348.x. [DOI] [PubMed] [Google Scholar]