Abstract
Background
Sexually transmitted infection with the human papillomavirus (hpv) is responsible for a significant burden of human cancers involving the cervix, anogenital tract, and oropharynx. Studies in the United States and Europe have demonstrated an alarming increase in the frequency of hpv-positive oropharyngeal cancer, but the same direct evidence does not exist in Canada.
Methods
Using the London Health Sciences Centre pathology database, we identified tonsillar cancers diagnosed between 1993 and 2011. Real-time polymerase chain reaction was then used on pre-treatment primary-site biopsy samples to test for dna from the high-risk hpv types 16 and 18. The study cohort was divided into three time periods: 1993–1999, 2000–2005, and 2006–2011.
Results
Of 160 tumour samples identified, 91 (57%) were positive for hpv 16. The total number of tonsillar cancers significantly increased from 1993–1999 to 2006–2011 (32 vs. 68), and the proportion of cases that were hpv-positive substantially increased (25% vs. 62%, p < 0.002). Those changes were associated with a marked improvement in 5-year overall survival (39% in 1993–1999 vs. 84% in 2006–2011, p < 0.001). When all factors were included in a multivariable model, only hpv status predicted treatment outcome.
Interpretation
The present study is the first to provide direct evidence that hpv-related oropharyngeal cancer is increasing in incidence in a Canadian population. Given the long lag time between hpv infection and clinically apparent malignancy, oropharyngeal cancer will be a significant clinical problem for the foreseeable future despite vaccination efforts.
Keywords: Human papillomavirus, oropharyngeal cancer, epidemiology, hnscc
1. INTRODUCTION
Historically, head-and-neck squamous cell carcinoma has been known to strike elderly patients with a prolonged history of tobacco and alcohol abuse. After decades of public health initiatives, smoking rates have declined markedly since the 1960s1. Paralleling that decline, rates of head-and-neck squamous cell carcinoma have declined significantly. The exception has been the rate of oropharyngeal cancers (opcs), particularly those involving the tonsils and the base of the tongue2. In the United States and Europe, opc has been shown to be dramatically on the rise because of newfound associations with the human papillomavirus (hpv)3,4.
Because of changes in sexual practices, rates of sexually transmitted infections have risen steadily over the last several decades5. Indeed, hpv-related opc has been specifically linked to increased numbers of oral sex partners, especially in men6. Up to 80% of sexually active individuals acquire an hpv infection at some point in life, often in their late teens and early twenties; however, these infections for the most part clear spontaneously7,8. A fraction result in the integration of viral dna into the genome of the host’s cells. This integrated dna can induce cancers years—often decades—later. Indeed, most patients with hpv-related opc are diagnosed in their fifties and sixties9,10. The contributors to this delay between infection and development of cancer are not clearly understood, but because of it and because of the increasing rates of sexually transmitted infections, it is likely that rates of hpv-related opc will continue to rise dramatically. This formidable trend has been called a “slow epidemic,” and it holds profound implications for health care resources. It also highlights the need for new treatments for this subset of patients.
Although there is indirect evidence for this phenomenon in Canada2,11,12, it has not been confirmed with hpv-tested samples. The goal of the present study was to provide the first direct evidence of this dramatic trend in Canada.
2. METHODS
2.1. Study Population
Study approval was obtained from the University of Western Ontario Research Ethics Board. A retrospective search of the London Health Sciences Centre pathology database was performed for three time intervals: 1993–1999, 2000–2005, and 2006–2011. All available pathology reports were reviewed to identify samples representing pre-treatment tonsillar biopsy specimens of squamous cell cancer. Hematoxylin and eosin slides were reviewed by study pathologists (BW, KK) to confirm the presence of tumour. For samples meeting the criteria, a retrospective chart review extracted patient data, including age at diagnosis, use of tobacco and alcohol, American Joint Committee on Cancer TNM stage, treatment regimen, and post-treatment follow-up information.
2.2. Analysis of Pathology Samples
Deparaffinization, dna extraction, and detection of high-risk hpv types 16 and 18 by multiplex real-time polymerase chain reaction (pcr) was performed as previously described13. Standard curves were produced from 10-times serial dilutions of CaSki cell genomic dna. CaSki cell dna was used as a positive control for hpv 16, and HeLa cell genomic dna was used as the positive control for hpv 18.
2.3. Statistical Analysis
The statistical analysis used the R system (The R Foundation for Statistical Computing, Vienna, Austria), including the survival and mass packages. For all analyses, a p value of 0.05 or less was considered statistically significant. Patient variables were compared using the Fisher exact test. Changes in the proportion of total and hpv-positive patients over the three time intervals were compared using the Cochran–Armitage test. Recurrence-free survival was defined as the time from completion of treatment to local, regional, or distant recurrence. Survival curves were created using the Kaplan–Meier method. The Cox proportional hazards model was used to estimate the relative hazard of mortality or recurrence during the follow-up period and to perform univariable and multivariable analyses. Variables identified in the univariable model with a value of p < 0.1 were selected for inclusion in the multivariable model.
3. RESULTS
A search of the pathology database yielded 260 samples. Review of the pathology reports revealed that 224 samples represented pre-treatment tonsillar cancer biopsies, of which 184 were able to be retrieved. Sufficient tissue for hpv testing was available from 174 samples. Patient data for 14 patients were inadequate, including 7 from 1993–1999 (all hpv-negative samples) and 3 from each of the sequential time intervals (2000–2005: 2 of 3 hpv-positive; 2006–2011: 2 of 3 hpv-positive). Among the remaining 160 samples, hpv 16 was detected in 91 (57%); no sample was positive for hpv 18.
3.1. HPV-Related OPC Occurs More Often in Young Nonsmokers with Advanced Nodal Disease
Table i summarizes patient demographics, tumour staging, and treatment details by hpv status. Consistent with other reports, hpv-positive tumours were associated with younger nonsmokers with advanced nodal disease14.
TABLE I.
Patient factors by positivity or negativity for the human papillomavirus (hpv)
| Characteristic |
Patient group (n)
|
p Valuea | ||
|---|---|---|---|---|
| Overall | hpv − | hpv + | ||
| Age | ||||
| <60 Years | 91 | 25 | 66 | <0.001 |
| ≥60 Years | 69 | 44 | 25 | |
| Sex | ||||
| Male | 125 | 50 | 75 | 0.18 |
| Female | 35 | 19 | 16 | |
| T Classification | ||||
| 1 | 41 | 14 | 27 | 0.42 |
| 2 | 61 | 26 | 35 | |
| 3 | 40 | 19 | 21 | |
| 4 | 18 | 10 | 8 | |
| N Classification | ||||
| 0 | 33 | 23 | 10 | 0.004 |
| 1 | 24 | 11 | 13 | |
| 2 | 86 | 29 | 57 | |
| 3 | 17 | 6 | 11 | |
| Overall stage | ||||
| i | 8 | 6 | 2 | 0.015 |
| ii | 16 | 11 | 5 | |
| iii | 27 | 13 | 14 | |
| iv | 109 | 39 | 70 | |
| Smoking | ||||
| Never | 30 | 4 | 26 | <0.001 |
| 1–9 py | 11 | 4 | 7 | |
| 10–19 py | 21 | 6 | 15 | |
| >20 py | 88 | 50 | 38 | |
| Unknown | 10 | 5 | 5 | |
| Alcoholic drinks | ||||
| <21 Weekly | 109 | 42 | 67 | 0.17 |
| ≥21 Weekly | 44 | 24 | 20 | |
| Unknown | 7 | 3 | 4 | |
| Treatment | ||||
| crt | 97 | 28 | 69 | <0.001 |
| Radiation | 50 | 34 | 16 | |
| Induction+crt | 6 | 3 | 3 | |
| Surgery+crt | 5 | 4 | 1 | |
| tors+nd | 2 | 0 | 2 | |
By Fisher exact test.
py = pack–years; crt = chemoradiation; tors = trans-oral robotic surgery; nd = neck dissection.
3.2. HPV-Positive OPC Is on the Rise in Southwestern Ontario
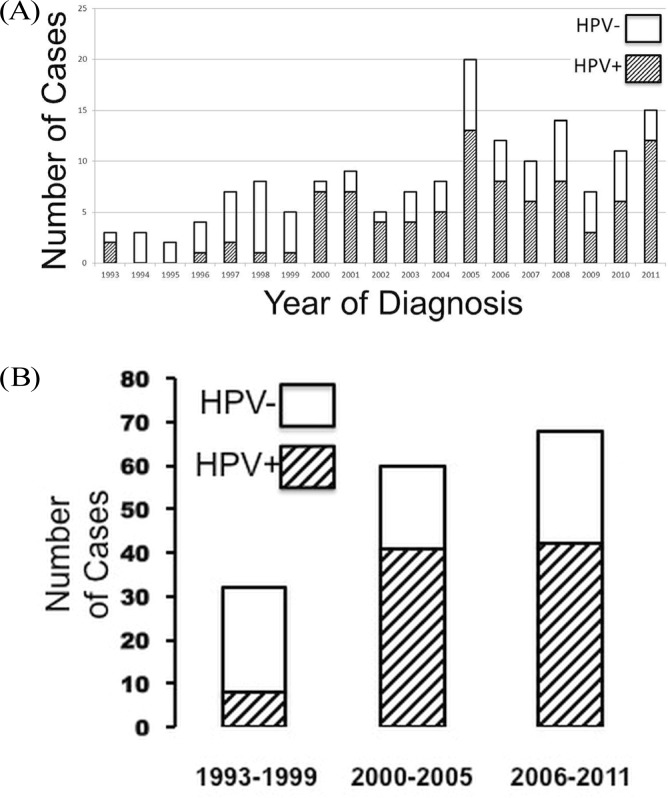
We observed a significant increase in the number of total and hpv-related tonsillar cancers over the sequential time intervals (Figure 1, Table ii, p < 0.002). In contrast, the number of hpv-negative cases remained stable (p > 0.05). This change was associated with the change in patient demographics to younger patients with advanced nodal disease. From the first time interval to the second and third, we observed a significant change in treatment regimens, with most patients receiving concurrent chemoradiation instead of radiation alone (Table ii, p < 0.001).
FIGURE 1.
Proportion of tonsillar carcinomas positive and negative for the human papillomavirus (hpv) (A) by year of diagnosis and (B) during three sequential time intervals.
TABLE II.
Patient factors and human papillomavirus (hpv) status by time interval
| Characteristic |
Patients by time interval (n)
|
p Valuea | ||||
|---|---|---|---|---|---|---|
| Overall | 1993–1999 | 2000–2005 | 2006–2011 | |||
| Age | <60 Years | 91 | 11 | 35 | 45 | 0.01 |
| ≥60 Years | 69 | 21 | 25 | 23 | ||
| Sex | Male | 125 | 24 | 46 | 55 | 0.77 |
| Female | 35 | 8 | 14 | 13 | ||
| T Classification | 1 | 41 | 9 | 12 | 20 | 0.84 |
| 2 | 61 | 11 | 26 | 24 | ||
| 3 | 40 | 9 | 16 | 15 | ||
| 4 | 18 | 3 | 6 | 9 | ||
| N Classification | 0 | 33 | 9 | 11 | 13 | 0.015 |
| 1 | 24 | 11 | 5 | 8 | ||
| 2 | 86 | 9 | 37 | 40 | ||
| 3 | 17 | 3 | 7 | 7 | ||
| Overall stage | i | 8 | 3 | 2 | 3 | 0.02 |
| ii | 16 | 3 | 5 | 8 | ||
| iii | 27 | 12 | 7 | 8 | ||
| iv | 109 | 14 | 46 | 49 | ||
| Smoking | Never | 30 | 3 | 10 | 17 | 0.65 |
| 1–9 py | 11 | 2 | 5 | 4 | ||
| 10–19 py | 21 | 6 | 6 | 9 | ||
| >20 py | 88 | 20 | 34 | 34 | ||
| Unknown | 10 | 1 | 5 | 4 | ||
| Alcoholic drinks | <21 Weekly | 109 | 19 | 38 | 52 | 0.12 |
| ≥21 Weekly | 44 | 13 | 18 | 13 | ||
| Unknown | 7 | 0 | 4 | 3 | ||
| Treatment | crt | 97 | 4 | 46 | 47 | 0.001 |
| Radiation alone | 50 | 25 | 14 | 11 | ||
| Induction+crt | 6 | 0 | 0 | 6 | ||
| Surgery+crt | 5 | 3 | 0 | 2 | ||
| tors+nd | 2 | 0 | 0 | 2 | ||
| hpv | Negative | 69 | 24 | 19 | 26 | 0.0001 |
| Positive | 91 | 8 | 41 | 42 | ||
By Fisher exact test.
py = pack–years; crt = chemoradiation; tors = trans-oral robotic surgery; nd = neck dissection.
3.3. Survival for Tonsillar Cancer Improved Over Each Sequential Time Interval, Partially Because of Changes in the Prevalence of HPV-Positive Disease
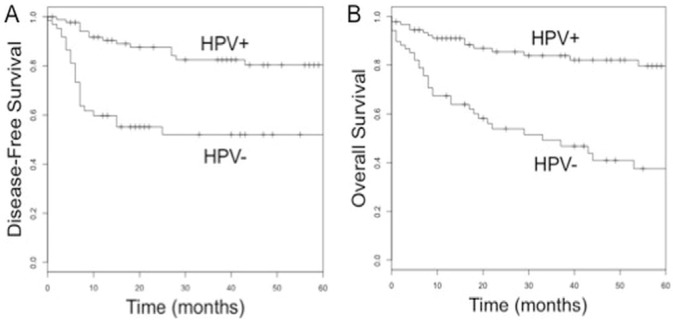
Compared with hpv-negative patients, patients positive for hpv experienced markedly improved recurrence-free and 5-year overall survival (recurrence-free survival: 82% vs. 53%; overall survival: 83% vs. 37%; p < 0.0001; Figure 2). Because most patients received either radiation or concurrent chemoradiation, we excluded the 13 patients who received alternative treatment regimens, leaving 147 patients for analysis.
FIGURE 2.
(A) Disease-free and (B) overall survival for tumours positive and negative for the human papillomavirus (hpv).
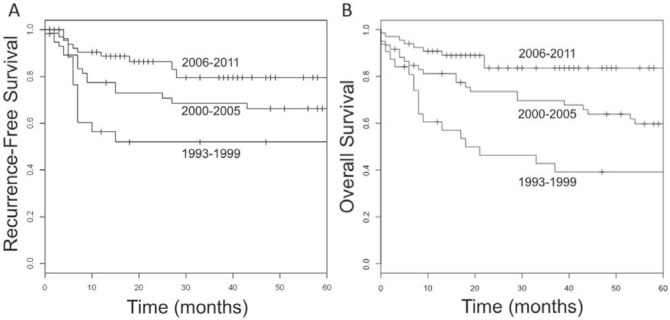
Improved survival was noted for each sequential time interval (Figure 3, p < 0.001). Univariable analysis over the entire period revealed that age greater than 60 years, tobacco and alcohol use, hpv-negative status, radiation alone, and receiving treatment in the 1993–1999 interval were each associated with a poorer outcome (Table iii, p < 0.05). When all factors reaching p < 0.1 were included in a multivariable model, only hpv status predicted treatment outcome (Table iii).
FIGURE 3.
(A) Recurrence-free and (B) overall survival during three sequential time intervals.
TABLE III.
Univariable and multivariable predictors of recurrence-free and overall survival
| Variable |
Univariable analysis
|
Multivariable analysis
|
|||||
|---|---|---|---|---|---|---|---|
| hr | 95% cia | p Valueb | hr | 95% cia | p Valueb | ||
| Recurrence-free survival | |||||||
| Age | ≥60 vs. <60 Years | 2.13 | 1.10 to 4.10 | 0.02 | 0.31 | 0.63 to 2.73 | 0.48 |
| Sex | Male vs. female | 1.14 | 0.52 to 2.50 | 0.74 | |||
| T stage | 3+4 vs. 1+2 | 0.99 | 0.51 to 1.95 | 0.98 | |||
| N stage | N2/3 vs. N1/0 | 0.69 | 0.37 to 1.29 | 0.25 | |||
| Smoking | >10 py vs. <10 py or unknown | 1.96 | 1.00 to 3.86 | 0.05 | 1.41 | 0.67 to 2.98 | 0.36 |
| Alcoholic drinks | ≥ 21 vs. <21 weekly and unknown | 2.05 | 1.05 to 4.00 | 0.03 | 1.68 | 0.83 to 3.38 | 0.15 |
| hpv | Positive vs. negative | 0.25 | 0.13 to 0.49 | <0.001 | 0.37 | 0.17 to 0.81 | 0.01 |
| Treatment | rt vs. crt | 2.78 | 1.46 to 5.28 | 0.002 | 2.15 | 0.95 to 4.89 | 0.06 |
| Time interval | 2000–2011 vs. 1993–1999 | 0.46 | 0.23 to 0.92 | 0.03 | 1.47 | 0.60 to 3.60 | 0.4 |
| Overall survival | |||||||
| Age | ≥60 vs. <60 | 2.49 | 1.41 to 4.40 | 0.002 | 1.51 | 0.81 to 2.83 | 0.2 |
| Sex | Male vs. female | 1.64 | 0.88 to 3.04 | 0.12 | |||
| T stage | 3+4 vs. 1+2 | 1.43 | 0.83 to 2.46 | 0.20 | |||
| N stage | N2/3 vs. N1/0 | 0.69 | 0.41 to 1.17 | 0.17 | |||
| Smoking | >10 py vs. <10 py or unknown | 1.79 | 1.01 to 3.17 | 0.05 | 1.07 | 0.57 to 2.00 | 0.84 |
| Alcoholic drinks | ≥21 vs. <21 weekly or unknown | 2.25 | 1.3 to 3.9 | 0.004 | 1.79 | 1.00 to 3.22 | 0.05 |
| hpv | Positive vs. negative | 0.21 | 0.11 to 0.39 | <0.001 | 0.29 | 0.14 to 0.58 | <0.001 |
| Treatment | rt vs. crt | 2.09 | 1.21 to 3.61 | 0.008 | 1.03 | 0.50 to 2.13 | 0.95 |
| Time interval | 2000–2011 vs. 1993–1999 | 0.41 | 0.23 to 0.73 | 0.002 | 0.78 | 0.37 to 1.64 | 0.50 |
By Cox proportional hazard analysis.
Wald test.
hr = hazard ratio; ci = confidence interval; py = pack–years; rt = radiotherapy; crt = chemoradiation.
It should be noted that the TNM staging system changed during the intervals studied. Imaging was not consistently available for all patients, and thus a retrospective restaging of patients from the earlier intervals was not possible. To determine if staging played a significant role in patient survival, we specifically analyzed the effect of T and N stage by hpv status for the entire cohort and each time period (Table iv). In all scenarios, T and N stage were not statistically significant predictive factors.
TABLE IV.
Prognostic significance of T and N stage by time frame and human papillomavirus (hpv) status
| Variable | Overall | 1993–1999 | 2000–2005 | 2006–2011 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| hr | 95% cia | p Valuea | hr | 95% cia | p Valuea | hr | 95% cia | p Valuea | hr | 95% cia | p Valuea | ||
| Recurrence-free survival | |||||||||||||
| Overall | |||||||||||||
| T Stage | 3+4 vs. 1+2 | 0.99 | 0.51 to 1.95 | 0.98 | 1.68 | 0.53 to 5.3 | 0.38 | 0.79 | 0.28 to 2.26 | 0.67 | 0.76 | 0.20 to 2.94 | 0.69 |
| N Stage | 2+3 vs. 0+1 | 0.69 | 0.37 to 1.29 | 0.25 | 2.04 | 0.64 to 6.45 | 0.23 | 0.56 | 0.21 to 1.47 | 0.24 | 0.60 | 0.17 to 2.13 | 0.43 |
| hpv-positive | |||||||||||||
| T Stage | 3+4 vs. 1+2 | 0.87 | 0.27 to 2.77 | 0.81 | —b | 1.08 | 0.27 to 4.34 | 0.91 | 0.53 | 0.06 to 4.75 | 0.57 | ||
| N Stage | 2+3 vs. 0+1 | 1.54 | 0.43 to 5.50 | 0.51 | —b | 0.71 | 0.15 to 3.42 | 0.67 | 1.17 | 0.13 to 10.50 | 0.89 | ||
| hpv-negative | |||||||||||||
| T Stage | 3+4 vs. 1+2 | 0.79 | 0.35 to 1.78 | 0.56 | 1.87 | 0.59 to 5.94 | 0.29 | 0.46 | 0.09 to 2.27 | 0.34 | 0.69 | 0.12 to 4.14 | 0.69 |
| N Stage | 2+3 vs. 0+1 | 0.87 | 0.39 to 1.92 | 0.72 | 1.66 | 0.53 to 5.27 | 0.39 | 1.16 | 0.27 to 4.96 | 0.84 | 0.43 | 0.08 to 2.60 | 0.36 |
| Overall survival | |||||||||||||
| Overall | |||||||||||||
| T Stage | 3+4 vs. 1+2 | 1.43 | 0.83 to 2.46 | 0.20 | 1.16 | 0.49 to 2.8 | 0.73 | 1.8 | 0.80 to 4.09 | 0.16 | 1.4 | 0.38 to 5.2 | 0.62 |
| N Stage | 2+3 vs. 0+1 | 0.69 | 0.41 to 1.17 | 0.17 | 1.65 | 0.693.94 | 0.26 | 0.76 | 0.32 to 1.80 | 0.53 | 0.35 | 0.10 to 1.32 | 0.12 |
| hpv-positive | |||||||||||||
| T Stage | 3+4 vs. 1+2 | 1.99 | 0.80 to 4.9 | 0.14 | 1.10 | 0.18 to 6.80 | 0.92 | 2.67 | 0.8 to 8.77 | 0.11 | 1.97 | 0.12 to 31.8 | 0.63 |
| N Stage | 2+3 vs. 0+1 | 0.59 | 0.23 to 1.51 | 0.27 | 2.59 | 0.36 to 18.5 | 0.34 | 0.33 | 0.10 to 1.11 | 0.07 | 0.30 | 0.02 to 4.76 | 0.39 |
| hpv-negative | |||||||||||||
| T Stage | 3+4 vs. 1+2 | 0.96 | 0.49 to 1.9 | 0.91 | 1.24 | 0.45 to 3.44 | 0.68 | 1.06 | 0.32 to 3.51 | 0.92 | 0.83 | 0.19 to 3.70 | 0.8 |
| N Stage | 2+3 vs. 0+1 | 1.49 | 0.77 to 2.92 | 0.24 | 1.27 | 0.47 to 3.41 | 0.64 | 3.64 | 1.00 to 12.40 | 0.05 | 0.63 | 0.14 to 2.84 | 0.55 |
Calculated using a Cox proportional hazards model.
Calculation not possible because of low patient numbers in this subset.
hr = hazard ratio; ci = confidence interval.
4. INTERPRETATION
In the United States and Europe, there is compelling evidence that opc is on the rise, and that the increase is primarily a result of increasing rates of hpv-related opc3,4. The strongest study was carried out by Chaturvedi and colleagues3, who used multiple methods to test for hpv in 271 specimens collected by three population-based cancer registries in the Surveillance, Epidemiology, and End Results Residual Tissue Repositories Program. They definitively found that the prevalence of hpv increased significantly from 1984 to 2004 and that survival was significantly improved for hpv-positive patients compared with hpv-negative patients. It has been assumed that this same phenomenon is occurring elsewhere in the world.
In Canada, population-based studies have provided indirect evidence of the phenomenon by demonstrating that the incidence of head-and-neck cancers at hpv-related anatomic sites (oropharynx) are rising and that those of smoking-related sites (for example, oral cavity) are declining2,11,12. However, no previous study has performed hpv testing of tumour samples, and thus we have attempted to provide the first direct evidence of this dramatic shift in disease prevalence and demographics.
The data we provide have several potential methodologic shortcomings:
Inclusion of patients in the pathology database may have varied over time.
Changes in the catchment area of our institution are possible.
The TMN staging system changed over the study period.
A small but increased potential for bias is present because, in the early time frame, patients were lost because of charts being unavailable.
Patients and samples were drawn from a single centre.
The samples obtained reflect a relatively small proportion of the patients actually treated at our centre.
The fact that hpv-negative cases remained stable during the three time intervals while the hpv-positive cases increased dramatically argues against the first two points playing a major role. Although the TNM staging system did change over time, our analysis stratified by time period and T and N stage (Table iv) argues against that factor being a major confounder. Regarding chart unavailability, 7 of 39 patients were excluded for the 1993–1999 period compared with 3 of 60 and 3 of 68 for the 2000–2005 and 2006–2011 periods respectively (p < 0.05). However, all 7 of the patients from the earliest period were hpv-negative, compared with only 1 of 3 patients from each of the two subsequent periods, which would result in a conservative bias (toward a null result), and thus would be unlikely to have affected the validity of the study.
Although a single-centre study raises questions about generalizability, the head-and-neck cancer team at London Health Sciences Centre manages a high-volume program that treats approximately 400 newly diagnosed head-and-neck cancers each year, approximately one quarter of which are opcs. Although it is just one centre, it may be even more representative of the Canadian experience than that studied by Chaturvedi et al.3, particularly when accounting for the disparity in country population size.
Of approximately 100 opcs handled at the London Health Sciences Centre annually, about 60% are tonsillar cancers, implying that roughly 1000 cases were treated during the entire study period. However, many patients are initially biopsied in the community (with the biopsy thus being unavailable) or are treated based solely on fine-needle aspiration of a cervical lymph node. Indeed, Chaturvedi et al. were able to retrieve samples from fewer than 5% of the patients identified in their three population-based registries, compared with approximately 16% of the patients located in our study.
Given that hpv status is being incorporated into clinical care pathways and clinical trials, accurate determination of hpv status has become extremely important in contemporary head-and-neck oncology practice. Immunohistochemical expression of p16 in oropharyngeal samples is highly correlated with hpv status14,15 and is used in many centres throughout Canada because it is inexpensive and easily implemented in any pathology lab. Although most opcs are clearly positive or negative by p16 immunohistochemistry, ambiguous staining patterns are not infrequent15. In addition, p16 appears to be a highly sensitive (96.8%), but only moderately specific (83.8%) marker of hpv status compared with the “gold standard” of hpv E6/E7 expression by reverse transcriptase real-time pcr15. Other methods include conventional pcr, which appears to yield a high rate of false positives15, in situ hybridization, and real-time pcr. The authors have had experience with in situ hybridization and have previously demonstrated a very high correlation with p16 status14; however, other investigators have reported difficulties optimizing the assay and a poorer correlation with p16 and E6/E7 expression15. We currently favour real-time pcr, with all samples tested in triplicate and repeated if any discrepancies arise. We reported a very high correlation with p16 status13, and our method has the advantage of working with dna, which is significantly more stable than rna. The pcr techniques also offer the ability to carry out hpv type-specific testing, which may be important, because different hpv-types may portend a different prognosis, as is observed in cervical cancer13,16.
The implications of what has been described as a “slow epidemic” of head-and-neck cancer are significant. Given the trends that have been observed and the approximately 40-year lag time between infection and manifestation of the disease, hpv-positive cancer will be a problem for the foreseeable future. Although hpv vaccination offers the potential to stem this trend, vaccination rates in Canada remain low17 and will not demonstrate an effect on head-and-neck squamous cell carcinoma rates for decades. In the interim, the hpv opc epidemic will have tremendous implications for health care resources: These typically younger, healthier patients have a high chance of surviving their disease9, and they will have to live with the toxicity of treatment for many decades.
In Canada, most centres favor concurrent chemotherapy and radiation for the primary treatment of these cancers. This treatment is effective, and most patients have an excellent post-treatment quality of life. However, a subset of patients suffer severe side effects, including hearing loss, neuropathy, renal failure, dysphagia requiring long-term gastrostomy tube dependence, and osteoradionecrosis18. Given the favourable prognosis in this patient cohort, tremendous interest has been expressed for de-escalating therapy to decrease toxicity. Potential de-escalation strategies include reduced doses of radiation or chemotherapy (or both), or avoidance of chemotherapy entirely for low-risk patients. Indeed, O’Sullivan and colleagues19 retrospectively demonstrated that hpv-positive patients with early-stage neck disease (N2B or less) may be adequately treated with radiotherapy alone. Future prospective trials focusing solely on hpv-positive disease—such as Radiation Therapy Oncology Group 1016, which is comparing chemo-radiation with radiation and cetuximab (http://www.clinicaltrials.gov/ct2/show/NCT01302834)—are essential to optimize treatment for this patient cohort.
5. CONCLUSIONS
The rates of hpv-related tonsillar cancer are on the rise in Canada. Although patients tend to experience improved survival after treatment, this rising burden of disease has tremendous implications for the demand on health care resources. Furthermore, these young patients (and the health care system) will also have to deal with the toxicity-related consequences of their cancer treatment for decades to come. Available prophylactic vaccinations hold the potential to eradicate hpv-related disease in the long term; however, further public health interventions are needed to increase vaccination uptake. In the interim, prospective trials are needed to delineate the optimal management of this patient cohort.
6. ACKNOWLEDGMENT
This study was supported by a research grant from Merck Canada Inc.
7. CONFLICT OF INTEREST DISCLOSURES
The authors declare that no financial conflict of interest exists.
8. REFERENCES
- 1.Reid JL, Hammond D, Burkhalter R, Ahmed R. Tobacco Use in Canada: Patterns and Trends. 2012 Edition. Waterloo, ON: Propel Centre for Population Health Impact, University of Waterloo; 2012. [DOI] [Google Scholar]
- 2.Auluck A, Hislop G, Bajdik C, Poh C, Zhang L, Rosin M. Trends in oropharyngeal and oral cavity cancer incidence of human papillomavirus (hpv)–related and hpv-unrelated sites in a multicultural population: the British Columbia experience. Cancer. 2010;116:2635–44. doi: 10.1002/cncr.21285. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Näsman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (hpv) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–6. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 5.Kliewer EV, Demers AA, Elliott L, Lotocki R, Butler JR, Brisson M. Twenty-year trends in the incidence and prevalence of diagnosed anogenital warts in Canada. Sex Transm Dis. 2009;36:380–6. doi: 10.1097/OLQ.0b013e318198de8c. [DOI] [PubMed] [Google Scholar]
- 6.D’Souza G, Kreimer AR, Viscidi R, et al. Case–control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 7.Sellors JW, Mahony JB, Kaczorowski J, et al. Prevalence and predictors of human papillomavirus infection in women in Ontario, Canada. Survey of hpv in Ontario Women (show) Group. CMAJ. 2000;163:503–8. doi: 10.1093/jnci/djs494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 9.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols AC, Faquin WC, Westra WH, et al. hpv-16 infection predicts treatment outcome in oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. 2009;140:228–34. doi: 10.1016/j.otohns.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 11.Nichols AC, Yoo J, Hammond JA, et al. Early-stage squamous cell carcinoma of the oropharynx: radiotherapy vs. trans-oral robotic surgery (orator)—study protocol for a randomized phase ii trial. BMC Cancer. 2013;13:133. doi: 10.1186/1471-2407-13-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson–Obaseki S, McDonald JT, Corsten M, Rourke R. Head and neck cancer in Canada: trends 1992 to 2007. Otolaryngol Head Neck Surg. 2012;147:74–8. doi: 10.1177/0194599812437332. [DOI] [PubMed] [Google Scholar]
- 13.Nichols AC, Dhaliwal SS, Palma DA, et al. Does hpv type affect outcome in oropharyngeal cancer? J Otolaryngol Head Neck Surg. 2013;42:9. doi: 10.1186/1916-0216-42-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols AC, Finkelstein DM, Faquin WC, et al. Bcl2 and human papilloma virus 16 as predictors of outcome following concurrent chemoradiation for advanced oropharyngeal cancer. Clin Cancer Res. 2010;16:2138–46. doi: 10.1158/1078-0432.CCR-09-3185. [DOI] [PubMed] [Google Scholar]
- 15.Jordan RC, Lingen MW, Perez–Ordonez B, et al. Validation of methods for oropharyngeal cancer hpv status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–54. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burger RA, Monk BJ, Kurosaki T, et al. Human papillomavirus type 18: association with poor prognosis in early stage cervical cancer. J Natl Cancer Inst. 1996;88:1361–8. doi: 10.1093/jnci/88.19.1361. [DOI] [PubMed] [Google Scholar]
- 17.Canadian Cancer Society and the National Cancer Institute of Canada . Canadian Cancer Statistics 2006. Toronto, ON: Canadian Cancer Society; 2006. [DOI] [Google Scholar]
- 18.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an rtog analysis. J Clin Oncol. 2008;26:3582–9. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–50. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]