Abstract
Background
The prognosis of patients with brain metastases from non-small-cell lung cancer (nsclc) is poor. However, some reports suggest that patients with brain metastases at the time of initial diagnosis have a more favourable survival than do patients with advanced nsclc without brain metastases.
Methods
In a retrospective cohort of all new lung cancer patients seen at a Canadian tertiary centre between July 2005 and June 2007, we examined survival after a diagnosis of brain metastases for patients with brain metastases at initial diagnosis and patients who developed brain metastases later in their illness.
Results
During the 2-year period, 91 of 878 patients (10.4%) developed brain metastases. Median age in this cohort was 64 years. In 45, brain metastases were present at initial diagnosis, and in 46, brain metastases developed later in the course of the illness. Median survival in the entire cohort was 7.8 months. Survival after the diagnosis of brain metastases was similar for patients with brain metastases at diagnosis and later in the illness (4.8 months vs. 3.7 months, p = 0.53). As a result, patients who developed brain metastases later in their illness had a longer overall survival than did patients with brain metastases at diagnosis (9.8 months vs. 4.8 months). Among patients who received chemotherapy, the survival of patients with brain metastases at diagnosis was still poor (6.2 months).
Conclusions
Our data show limited survival in patients with brain metastases from nsclc. Careful patient selection for more aggressive treatment approaches is necessary.
Keywords: Early prognosis, late prognosis, whole-brain radiation, chemotherapy
1. INTRODUCTION
Worldwide, lung cancer is the leading cause of cancer death in both men and women, causing more than 1.18 million deaths per year1. Approximately 85% of lung cancer cases are non-small-cell lung cancer (nsclc). Most patients either present with advanced disease or develop recurrence at some point during their illness, and the 5-year survival rate for all lung cancer patients is only about 15%2. The prognosis of patients with advanced nsclc is generally considered poor, with a median survival of 8–10 months and a 2-year survival of no more than 10%–20%3,4.
Brain metastases are a common problem in patients with metastatic nsclc. About 7%–10% of nsclc patients present with brain metastases at the time of initial diagnosis, and as many as 20%–40% of patients develop brain metastases at some point during their illness5–7. The incidence of brain metastases appears to be increasing, which may reflect improvements in diagnostic imaging8,9. Alternatively, patients may be more at risk for the development of brain metastases as a result of the modest improvements in survival associated with more effective systemic therapies9.
Historically, the survival of patients with brain metastases has been considered very poor6,10, with the risk of death and significant impairments in quality of life being increased by a factor of 411–13. The median survival of patients with untreated brain metastases is reported to be 1–3 months7,10,11. The combination of whole-brain radiation (wbrt) and corticosteroids, which has been the standard treatment to palliate symptoms, may result in modest improvements in survival for patients well enough to receive this treatment14–16.
Because of limited survival, nsclc patients with brain metastases have not generally been considered candidates for systemic therapy, and they have often been excluded from clinical trials of systemic therapy9,17. In recent times, there has been a shift toward more aggressive local management of selected patients with solitary or oligo-metastatic brain metastasis from nsclc. Management approaches include surgical resection and advanced radiation techniques such as stereotactic radiosurgery8,18. In addition, patients with treated brain metastases who maintain a good performance status have been included in many trials of systemic therapy. A retrospective review of patients with stable treated brain metastases from nsclc included in chemotherapy trials reported survival similar to that of patients with advanced disease without brain metastases17. However, those observations are all potentially subject to selection bias. We sought to determine the generalizability of those findings to patients with brain metastases from nsclc followed at a Canadian tertiary care centre.
We hypothesized that patients presenting with brain metastases from nsclc at diagnosis experience longer survival after the diagnosis of brain metastases than do those presenting with brain metastases from nsclc later in the course of illness. Identifying subgroups of patients with brain metastases who have favorable prognostic factors and who may benefit from more aggressive measures is important to limit the number of patients in whom systemic treatment may be deferred and even withheld because of the perception of poor outcome.
The current study examined survival in a cohort of nsclc patients with brain metastasis treated at a tertiary centre. The primary outcome of interest was survival after a diagnosis of brain metastases for patients presenting with brain metastases at the time of initial diagnosis and for patients developing brain metastases at a point later in their illness.
2. METHODS
We retrospectively reviewed all new lung cancer patients seen at a Canadian tertiary cancer centre between July 2005 and June 2007. Patients with nsclc were identified through a search of the institutional electronic database. Among the identified patients, those with brain metastases who were scheduled to receive wbrt were selected. Patients with brain metastases who never received radiation were not identified in the search. Patients were eligible if they had histologically or cytologically confirmed nsclc. Patients whose treatment was managed primarily at another institution and who were referred only for radiation treatment were not included because information concerning those patients was incomplete. Patients with a diagnosis of small-cell lung cancer were not included. The sample size for this descriptive study was not calculated a priori, and the study population reflects patients with brain metastases from nsclc scheduled to receive wbrt and followed at our centre over a 2-year period.
Data were abstracted from the medical records of all eligible patients, including age, sex, date of diagnosis, diagnostic and staging information, Eastern Cooperative Oncology Group performance status, weight loss, smoking status, intercurrent medical problems, and descriptive information about diagnosis and treatment of brain metastases. The latter information included whether patients were symptomatic or asymptomatic at diagnosis of brain metastases, systemic therapy used, and the patient’s last known vital status, with date. Family physicians were contacted to determine vital status if the patient had not been seen within 3 months of the present review and was not known to be deceased. Patients not known to be deceased at the time of the analysis were censored at the date of last follow-up. If Eastern Cooperative Oncology Group performance status was not explicitly stated, it was derived, where possible, from the description of the patient’s functional activities.
Our primary aim in the present study was to compare survival from the time of diagnosis of brain metastases for patients presenting with brain metastases at the time of initial diagnosis (hereafter called the “early” group) and patients who developed brain metastases later in their illness (hereafter called the “late” group). Brain metastases were considered to be present at diagnosis if detected within 1 month of the diagnosis of lung cancer. For patients with relapsed early-stage disease, survival was counted from the diagnosis of metastatic disease. A secondary aim was to compare overall survival from the time of diagnosis of advanced or metastatic disease to death in patients with early and with late brain metastases. Additional analyses compared baseline characteristics, descriptive information about brain metastases, and patterns of treatment after diagnosis of brain metastases, including referral to medical oncology, for the groups of nsclc patients with early and late brain metastases.
Data were analyzed using the SPSS software application (version 16: SPSS, Chicago, IL, U.S.A.). Frequency tables for all variables were generated. Overall survival was calculated for the whole group using the Kaplan–Meier method. Survival between patients with early and with late brain metastases was compared using a log-rank test (two-sided). Chi-square tests (for categorical variables) and t-tests (for continuous variables) were used to explore differences between the groups for other variables.
The study was reviewed and approved by the Hamilton Health Sciences Research Ethics Board.
3. RESULTS
3.1. Patient Population
Between July 2005 and June 2007, 878 new lung cancer patients were seen at our institution. Of those 878 patients, 91 (10.4%) had developed brain metastases from nsclc. Table i summarizes the characteristics of those 91 patients [43 men (47%), 48 women (53%); median age: 64 years (standard deviation: 10.6 years)]. Adenocarcinoma was the most frequent histology in this group. Eastern Cooperative Oncology Group performance status at the time of diagnosis of brain metastases was not recorded in 50 patients (55%) and was derived from the description of functional status in 18 patients (20%).
TABLE I.
Baseline characteristics for all patients with brain metastases
| Characteristic | Value |
|---|---|
| Patients (n) | 91 |
| Age (years) | |
| Median | 64 |
| sd | 10.6 |
| Sex [n (%)] | |
| Men | 43 (47) |
| Women | 48 (53) |
| Stage [n (%)] | |
| ia/b | 7 (7.5) |
| iia/b | 6 (7.5) |
| iiia | 11 (12) |
| iiib | 12 (13) |
| iv | 55 (60) |
| Histology [n (%)] | |
| Adenocarcinoma | 47 (52) |
| Squamous cell | 11 (12) |
| Large cell | 3 (3) |
| nos | 30 (33) |
| Smoking [n (%)] | |
| Never-smoker | 7 (8) |
| Ex-smoker | 31 (34) |
| Current smoker | 52 (57) |
| Unknown | 1 (1) |
| Weight loss [n (%)] | |
| <5 | 39 (43) |
| 5–10 | 10 (11) |
| >10 | 23 (25) |
| Unknown | 19 (21) |
| ecog score at Dx | |
| 0 | 1 (1) |
| 1 | 20 (22) |
| 2 | 9 (10) |
| 3 | 11 (12) |
| Unknown | 50 (55) |
sd = standard deviation; nos = n ot o therwise s pecified; ecog = Eastern Cooperative Oncology Group; Dx = diagnosis.
Half the cohort (n = 45) presented with early brain metastases; the remaining half (n = 46) developed late brain metastases (Table ii). Most patients (n = 79, 87%) were symptomatic when diagnosed with brain metastases. A greater proportion of patients with early brain metastases had a solitary lesion (44% vs. 30% with late metastases) and underwent surgical resection of the metastases (27% vs. 13%). The radiation treatment schedules were similar for both groups (Table ii). Table iii summarizes the systemic therapy given to the patients. Patients with early brain metastases were significantly less likely to be seen by a medical oncologist (51% vs. 74% with late metastases, p = 0.02) and less likely to receive chemotherapy (18% vs. 41%, p = 0.01). In neither group were the patients likely to receive chemotherapy after the diagnosis of brain metastases (18% vs. 15%).
TABLE II.
Descriptive statistics concerning brain metastases
| Variable |
Brain metastases Dx group [n (%)]
|
|
|---|---|---|
| Early | Late | |
| Metastases | 45 (49.5) | 46 (50.5) |
| Symptomatic metastases | 38 (84) | 41 (89) |
| Solitary metastasis | 20 (44) | 14 (30) |
| Surgery for metastases | 12 (27) | 6 (13) |
| Radiation for metastases | 44 (98) | 46 (100) |
| Radiation dose | ||
| 20 Gy in 5 fractions | 28 (64) | 33 (72) |
| 30 Gy in 10 fractions | 16 (36) | 13 (28) |
Dx = diagnosis.
TABLE III.
Summary of systemic therapy
| Variable |
Brain metastases Dx group [n (%)]
|
|
|---|---|---|
| Early | Late | |
| Referral to medical oncology | 23 (51) | 34 (74) |
| Systemic therapy at any time | ||
| First line | 8 (18) | 19 (41) |
| Second line | 2 (4) | 5 (11) |
| Third line | 0 | 2 (4) |
| Systemic therapy after metastases Dx | 8 (18) | 7 (15) |
Dx = diagnosis.
3.2. Survival Data
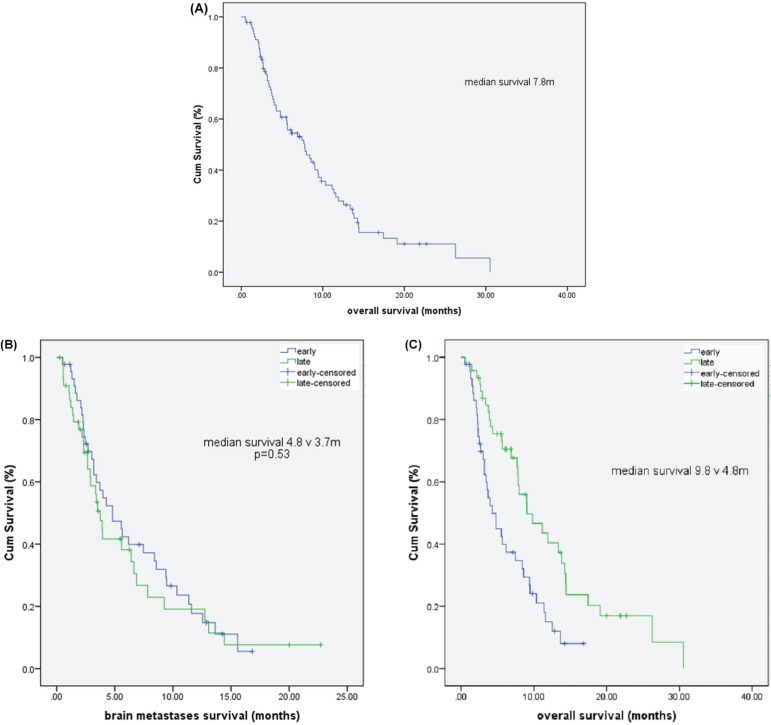
At the time of analysis, 23 patients (25.3%) were living, and 68 (74.7%) were known to have died. In this retrospective review, we could not reliably determine whether patients died primarily from progressive brain metastases, disease in other sites, or a combination. Median overall survival for the entire patient population was 7.8 months [Figure 1(A)]. We observed no differences in survival after diagnosis for patients in the early and late brain metastases groups [3.7 months vs. 4.8 months, p = 0.53, Figure 1(B)]. Compared with patients having early brain metastases, patients with late brain metastases experienced significantly longer overall survival from diagnosis of advanced disease [9.8 months vs. 4.8 months, Figure 1(C)]. In an exploratory analysis limited to patients who received chemotherapy, the survival was still worse in patients with early brain metastases than in patients with late brain metastases (6.2 months vs. 14.3 months, p = 0.08).
FIGURE 1.
(A) Cumulative overall survival for all patients. (B) Cumulative survival after a diagnosis of brain metastases (early vs. later). (C) Cumulative overall survival with advanced disease, by time of diagnosis of brain metastases (early vs. later).
4. DISCUSSION
Historically, brain metastases from nsclc were felt to represent a poor outlook6,10. More recently, there has been a shift toward more aggressive local management in selected patients to maximize survival and neurologic function8. Approximately 25% of patients with solitary or oligo-metastatic brain metastasis from nsclc will be eligible for surgical resection of their metastatic brain lesion or lesions19. A number of retrospective series showed promising survival of 11.6–14 months in selected patients who, with good performance and controlled extracranial disease, underwent complete resection of solitary or oligo-metastatic brain metastasis from nsclc8,20–22. It is difficult, however, to assess the effect of selection bias in those studies.
In addition to more aggressive local management of the brain, there has been renewed interest in integrating systemic treatment agents in the management of brain metastases from nsclc. Some evidence suggests that brain metastases respond to chemotherapy at a rate similar to that seen with the primary tumour and systemic disease9. One randomized trial of nsclc patients with brain metastases demonstrated similar survival for patients treated with initial chemotherapy and delayed wbrt and for patients treated with immediate wbrt23. Additionally, a retrospective review of patients included in systemic therapy clinical trials reported similar survival for those with neurologically stable brain metastases from nsclc at diagnosis and for those with no brain metastases17. However, those reports were based mostly on selected groups of younger patients with good neurologic function and performance status, a small number of lesions, and controlled primary and extracranial disease. Those reports were therefore subject to bias, making it hard to conclude whether the observed favourable survival data were a result of the treatment itself or the selection of patients with favourable prognostic factors. Targeted agents in patients with brain metastases from nsclc remains an area of ongoing clinical trials research24,25.
In view of the uncertainty of the available published data, and the improved outcomes with aggressive local and systemic approaches in selected patients with brain metastasis from nsclc, the present study examined the generalizability of those findings in a cohort of consecutive nsclc patients with brain metastasis seen at our institution. To maximize the sample size and broaden the generalizability of the findings, we included patients diagnosed with stage iv nsclc and patients with relapsed early-stage disease. Patients with early-stage disease have inherently longer survival. To account for potential confounding of stage and overall survival, we counted survival from the diagnosis of metastatic disease in the early-stage patients. Although that approach has limitations, the survival of patients in the late brain metastases group was similar to that which would be expected for patients with stage iv nsclc. However, patients with early brain metastases experienced survival inferior to that which would be expected.
Our main aim was to examine the survival of nsclc patients after early or late diagnosis of brain metastases. The incidence of brain metastases in our series was lower than expected from the literature, and we have no clear explanation for that departure. In contrast to other reports in the literature, our data suggest that a diagnosis of brain metastases from nsclc conveys a relatively poor prognosis regardless of whether patients have early or late brain metastases. This lack of a difference appears to be the case despite the fact that patients with early brain metastases were more likely than patients with late brain metastases to have a solitary brain lesion and to undergo surgical resection.
The poor outcomes observed in our series might potentially be explained by the low rate of referral to medical oncology and the lesser utilization of chemotherapy in patients in the early metastasis group. However, in a retrospective study such as this one, that hypothesis cannot be analyzed. However, in an exploratory analysis limited to patients who received chemotherapy, poorer survival in patients in the early group was still demonstrated. Our data highlight the need to implement strategies to improve the rate of referral for this group of patients.
Another potential explanation for poor outcomes is the predominant use of wbrt in these patients. However, stereotactic radiation was not available in our institution during the study period.
Lastly, information on performance status was not recorded for half the patients, thus limiting interpretation of the findings.
Despite promising advancements in neurosurgical techniques and systemic chemotherapy, our data show that many patients with brain metastasis from nsclc still experience limited survival. Our data do not support an aggressive approach to the treatment of all patients with brain metastasis from nsclc. Timing of the development of brain metastasis does not appear helpful in defining approaches to treatment. Identification of better prognostic groups remains an important challenge for selecting appropriate treatment options for this group of patients. Incorporation of a multidisciplinary approach for these patients appears desirable, with the goal of improving survival, preserving neurologic function, and maximizing quality of life.
Various prognostic models have been developed for patients with brain metastasis from a number of primary tumour types, including nsclc, to identify patients with favourable prognostic factors. The models are based on age, Karnofsky performance status, primary tumour status, extracranial system metastasis, lesion number, and largest brain lesion volume26–30. The absence of key information did not allow us to evaluate those models in our dataset.
Our study also has other limitations. Patients at our institution do not undergo routine brain imaging at the time of diagnosis if they have no neurologic symptoms. There is therefore some potential for misclassification between the early and late metastases groups. We were not able to distinguish whether patients died from progressive central nervous system disease or as a result of their systemic disease. Such data would help in understanding the appropriateness of the low rate of systemic therapy that we observed after a diagnosis of brain metastases in this series. As well, a small number of patients not considered well enough to receive brain radiation would not have been captured in the search of our electronic database. Nevertheless, our data demonstrate that many patients with brain metastasis from nsclc still experience poor outcomes, highlighting the need to individualize treatment decisions for such patients.
5. CONCLUSIONS
Our data suggest that approximately half of all patients with brain metastases from nsclc present with early metastases. These patients often present with multiple lesions, although, compared with patients who develop late brain metastases, those presenting with early metastases are more likely to have a solitary lesion and to undergo surgical resection. Survival after a diagnosis of brain metastases was not influenced by the time at which the patient developed brain metastases. However, patients with early brain metastases did appear less likely to be assessed for and to be offered systemic therapy.
Our data suggest that survival after a diagnosis of brain metastases is poor regardless of the time during the illness that brain metastases develop, and they caution against aggressive systemic measures in all patients. Further research is needed to better understand the competing risks of central nervous system and systemic metastatic disease from nsclc and to identify the patient populations with brain metastases who will benefit most from systemic treatments. In clinical trials, the determination of neurologic endpoints, such as freedom from neurologic progression, and the determination of global endpoints, such as time to disease progression and overall survival, pose methodologic challenges. In patients with brain metastases from nsclc, careful consideration should be given to the issue of patient selection for more aggressive treatment approaches.
6. CONFLICT OF INTEREST DISCLOSURES
All authors have no financial conflicts of interest to declare.
7. REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society’s Steering Committee . Canadian Cancer Statistics 2010. Toronto, ON: Canadian Cancer Society; 2010. [DOI] [Google Scholar]
- 3.Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase iii study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the tax 326 study group. J Clin Oncol. 2003;21:3016–24. doi: 10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 4.Schiller JH, Harrington D, Belani CP, et al. on behalf of the Eastern Cooperative Oncology Group Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 5.Robinet G, Barlesi F, Gervais R, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small cell lung cancer (nsclc) with measurable asymptomatic inoperable brain metastases (bm): a multicenter phase ii trial (gfpc 07-01) [abstract 7595] J Clin Oncol. 2010:28. doi: 10.1002/cncr.23659. [Available online at: http://meetinglibrary.asco.org/content/47370-74; cited May 31, 2013] [DOI] [PubMed] [Google Scholar]
- 6.Schuette W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer. 2004;45(suppl 2):S253–7. doi: 10.1016/j.lungcan.2004.07.967. [DOI] [PubMed] [Google Scholar]
- 7.Louie AV, Rodrigues G, Yaremko B, et al. Management and prognosis in synchronous solitary resected brain metastasis from non–small cell lung cancer. Clin Lung Cancer. 2009;10:174–9. doi: 10.3816/CLC.2009.n.024. [DOI] [PubMed] [Google Scholar]
- 8.Al-Shamy G, Sawaya R. Management of brain metastases: the indispensable role of surgery. J Neurooncol. 2009;92:275–82. doi: 10.1007/s11060-009-9839-y. [DOI] [PubMed] [Google Scholar]
- 9.Bernardo G, Cuzzoni Q, Strada MR, et al. First-line chemotherapy with vinorelbine, gemcitabine, and carboplatin in the treatment of brain metastases from non-small-cell lung cancer: a phase ii study. Cancer Invest. 2002;20:293–302. doi: 10.1081/CNV-120001173. [DOI] [PubMed] [Google Scholar]
- 10.Penel N, Brichet A, Prevost B, et al. Prognostic factors of synchronous brain metastases from lung cancer. Lung Cancer. 2001;33:143–54. doi: 10.1016/S0169-5002(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 11.Flannery TW, Suntharalingam M, Kwok Y, et al. Gamma knife stereotactic radiosurgery for synchronous versus metachronous solitary brain metastases from non-small cell lung cancer. Lung Cancer. 2003;42:327–33. doi: 10.1016/S0169-5002(03)00357-X. [DOI] [PubMed] [Google Scholar]
- 12.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 13.Greig NH. Chemotherapy of brain metastases: current status. Cancer Treat Rev. 1984;11:157–86. doi: 10.1016/0305-7372(84)90006-9. [DOI] [PubMed] [Google Scholar]
- 14.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase iii results of the rtog 9508 randomized trial. Lancet. 2004;363:1665–72. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 15.Vecht CJ, Haaxma–Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583–90. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez de Cos J, Sojo González MA, Montero MV, Pérez Calvo MC, Vicente MJ, Valle MH. Non-small cell lung cancer and silent brain metastasis. Survival and prognostic factors. Lung Cancer. 2009;63:140–5. doi: 10.1016/j.lungcan.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Bradbury PA, Twumasi–Ankrah P, Ding K, et al. The impact of brain metastases on overall survival (os) in National Cancer Institute of Canada Clinical Trials Group (ncic ctg) clinical trials (ct) in advanced non-small cell lung cancer (nsclc) J Clin Oncol. 2009:27. doi: 10.1080/07357900701560612. [Available online at: http://meetinglibrary.asco.org/content/32489-65; cited May 31, 2013] [DOI] [PubMed] [Google Scholar]
- 18.Bindal AK, Bindal RK, Hess KR, et al. Surgery versus radiosurgery in the treatment of brain metastases. J Neurosurg. 1996;84:748–54. doi: 10.3171/jns.1996.84.5.0748. [DOI] [PubMed] [Google Scholar]
- 19.Wroński M, Arbit E, Burt M, Galicich JH. Survival after surgical treatment of brain metastases from lung cancer: a follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg. 1995;83:605–16. doi: 10.3171/jns.1995.83.4.0605. [DOI] [PubMed] [Google Scholar]
- 20.Abrahams JM, Torchia M, Putt M, Kaiser LR, Judy KD. Risk factors affecting survival after brain metastases from non–small cell lung carcinoma: a follow-up study of 70 patients. J Neurosurg. 2001;95:595–600. doi: 10.3171/jns.2001.95.4.0595. [DOI] [PubMed] [Google Scholar]
- 21.Bindal RK, Sawaya R, Leavens ME, Lee JJ. Surgical treatment of multiple brain metastases. J Neurosurg. 1993;79:210–16. doi: 10.3171/jns.1993.79.2.0210. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa H, Miyawaki Y, Fujita T, et al. Surgical treatment of brain metastases of lung cancer: retrospective analysis of 89 cases. J Neurol Neurosurg Psychiatry. 1994;57:950–6. doi: 10.1136/jnnp.57.8.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinet G, Thomas P, Breton JL, et al. Results of a phase iii study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small cell lung cancer: Groupe Francais de Pneumo-Cancerologie (gfpc) protocol 95-1. Ann Oncol. 2001;12:59–67. doi: 10.1023/A:1008338312647. [DOI] [PubMed] [Google Scholar]
- 24.Olson JJ, Paleologos NA, Gaspar LE, et al. The role of emerging and investigational therapies for metastatic brain tumors: a systematic review and evidence-based clinical practice guideline of selected topics. J Neurooncol. 2010;96:115–42. doi: 10.1007/s11060-009-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Improta G, Zupa A, Fillmore H, et al. Protein pathway activation mapping of brain metastasis from lung and breast cancers reveals organ type specific drug target activation. J Proteome Res. 2011;10:3089–97. doi: 10.1021/pr200065t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (rpa) of prognostic factors in three Radiation Therapy Oncology Group (rtog) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–51. doi: 10.1016/S0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 27.Weltman E, Salvajoli JV, Brandt RA, et al. Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys. 2000;46:1155–61. doi: 10.1016/S0360-3016(99)00549-0. [DOI] [PubMed] [Google Scholar]
- 28.Lorenzoni J, Devriendt D, Massager N, et al. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys. 2004;60:218–24. doi: 10.1016/j.ijrobp.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Golden DW, Lamborn KR, McDermott MW, et al. Prognostic factors and grading systems for overall survival in patients treated with radiosurgery for brain metastases: variation by primary site. J Neurosurg. 2008;109(suppl):77–86. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 30.Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005;23:6207–19. doi: 10.1200/JCO.2005.03.145. [DOI] [PubMed] [Google Scholar]