Abstract
Extramammary Paget disease (empd) is a rare, slow-growing neoplasm, considered to be an adenocarcinoma of the apocrine glands. In men, the penoscrotal region is the most commonly affected area. The disease can present as carcinoma in situ or as invasive disease that can subsequently metastasize to lymph nodes and distant sites. Because of the rarity of empd, the medical literature available to guide management of the disease is limited, particularly in patients with metastases. In addition, metastatic disease may pose a diagnostic challenge, because invasive cancer of the genitourinary or gastrointestinal tract can occur in association with empd. In the present case series, we describe our experience in treating penoscrotal empd with multimodality therapy, and we review the existing literature concerning its diagnosis and management.
Keywords: Extramammary Paget disease, apocrine carcinoma, case series, treatment, pathology
1. INTRODUCTION
Extramammary Paget disease (empd) is a rare, slow-growing neoplasm considered to be an adenocarcinoma of the apocrine glands. Paget disease most commonly occurs in the nipple; the first case of penoscrotal empd was described by Crocker in 18881. The true incidence of empd is not known, but in older reports, it represented 6.5% of all cases of Paget disease2. A recent European study revealed an age-standardized incidence of invasive empd of 6 per million person–years3.
Invasive empd can present as carcinoma in situ or invasive disease (dermal invasion) that can subsequently metastasize to lymph nodes and distant sites4. The most common site of involvement in men for the invasive or carcinoma in situ variants is the penoscrotal area (50%)5,6.
The medical literature available to guide the management of empd is limited, particularly for metastatic disease. Another challenge is to differentiate metastatic empd from a concurrent invasive cancer. Here, we present our case series of 6 patients with empd of the penoscrotal region, our experience with systemic therapy, and a literature review for this rare malignancy.
2. METHODS
We identified 6 cases of penoscrotal empd treated at The Ottawa Hospital from 2007 to 2012. All cases underwent pathology review. After research ethics board approval, clinical and demographic data, including age at presentation, presence of a second malignancy, management, and follow-up were collected from patient records. The literature was reviewed for clinical and pathologic features, treatment options, and outcomes.
3. RESULTS
3.1. Case Series
3.1.1. Case Description 1
In March 2007, a 74-year-old white man was diagnosed with high-grade Ta urothelial cancer, for which he underwent transurethral resection of a bladder tumour. By April, he had developed right inguinal adenopathy, which fine-needle aspiration demonstrated to be metastatic urothelial carcinoma. He received radical intensity-modulated radiotherapy, 60 Gy in 30 fractions on tomotherapy to the bladder and right inguinal lymph nodes (40 Gy to whole pelvis, 20 Gy boost to gross tumour), with concurrent cisplatin chemotherapy (6 mg/m2 daily).
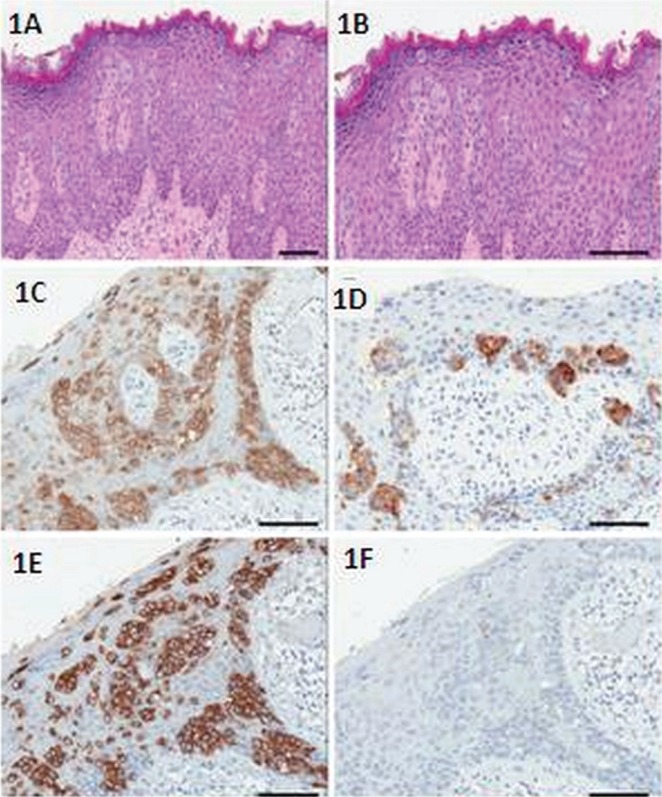
The patient was followed with interval cystoscopies. In early 2010, a lesion was noted on the right hemi-scrotum, and the initial pathology was described as metastatic urothelial carcinoma. In August 2010, the lesion progressed to resemble an extensive fungating cancer of the right groin, extending to the scrotum and upper thigh. The patient underwent a partial scrotectomy and excision of the overlying skin of the right medial thigh. Pathology revealed empd with poorly differentiated adenocarcinoma and invasion into the dermis. Margins were clear. The neoplastic cells had abundant clear cytoplasm, with round vesicular nuclei and prominent nucleoli (Figure 1). They were arranged as single cells and confluent aggregates. Colonic origin was excluded because of negative cytokeratin 20 (CK20) and cdx-2 and positive cytokeratin 7 (CK7) (see Table i). There was evidence of lymphovascular invasion.
FIGURE 1.
Histopathology of scrotal extramammary Paget disease in case 1. (1A) The neoplastic cells are arranged as single cells and confluent aggregates. (1B) They have abundant clear cytoplasm with round vesicular nuclei. The tumour cells express (1C) gross cystic disease fluid protein and (1D) carcinoembryonic antigen. The Paget cells are (1E) cytokeratin 7–positive and (1F) cytokeratin 20–negative.
TABLE I.
Immunohistochemistry of the patients in the case series
| Case |
Markers
|
|
|---|---|---|
| Positive | Negative | |
| 1 | gcdfp-15, CK7, cea, alcian blue | CDX2, CK20, S100 |
| 2 | CK7, cea, muc1, Ki67, androgen receptor, alcian blue | CDX2, CK20, S100, ttf1, muc2, muc5a |
| 3 | na | na |
| 4 | Cytokeratin AE1/AE3, gcdfp-15, androgen receptor | Prostate-specific antigen, estrogen receptor, progesterone receptor, her2 (fish) |
| 5 | CK7, cea, alcian blue | CK20, S100, prostate-specific antigen, androgen receptor, estrogen receptor, progesterone receptor, her2 |
| 6 | na | na |
gcdfp-15 = gross cystic disease fluid protein; CK = cytokeratin; cea = carcinoembryonic antigen; ttf1 = thyroid transcription factor 1; na = not available; her2 = human epidermal growth factor receptor 2; fish = fluorescence in situ hybridization.
The disease recurred in the suprapubic area, and a repeat excisional biopsy revealed a poorly differentiated carcinoma with positive margins. Follow-up computed tomography (ct) imaging confirmed periaortic adenopathy and a lytic lesion in T11.
It was the medical opinion of the treating physician that the distant sites of disease corresponded to metastatic urothelial carcinoma, and palliative chemotherapy [carboplatin area under the curve (auc) 4 day 1 and gemcitabine 1000 mg/m2 days 1 and 8 every 3 weeks] was started in July 2011. After 3 cycles, the disease progressed in the liver and bones. Docetaxel (100 mg/m2 every 3 weeks) was given for 2 cycles, but the hepatic lesions progressed. The patient received 1 cycle of doxorubicin (30 mg/m2 every 3 weeks) in December 2011, but because of deterioration in his performance status, no further chemotherapy was offered. He died in April 2012.
3.1.2. Case Description 2
A 77-year-old white man had a transanal excision in 2001 for T2N0 (0 of 3 nodes) well-differentiated adenocarcinoma of the lower rectum and anal canal, with positive lymphatic and perineural invasion and close margins. Abdominoperineal resection was offered, but the patient declined. He received concomitant chemoradiation (infusional 5-fluorouracil) to the pelvis, completed in January 2002. Follow-up endoscopies were normal.
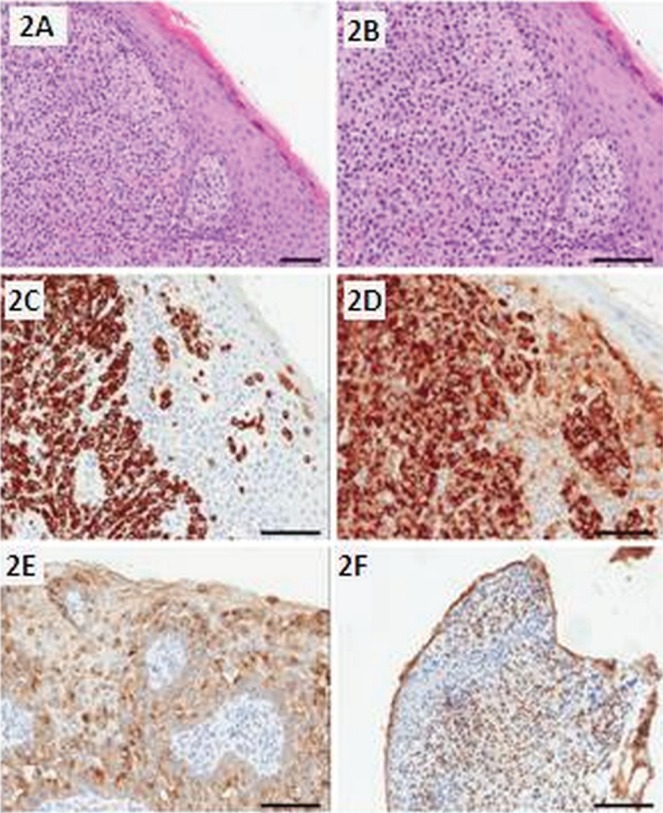
By June 2011, a nonpainful, firm, occasionally hemorrhagic subcutaneous nodule was noted in the patient’s left upper scrotum. In September 2011, excision of the scrotal lesion revealed invasive empd. This poorly differentiated tumour was arranged in small nests and fascicles within the dermis, accompanied by the typical epidermal component (Figure 2). The cells had pale eosinophilic cytoplasm and bland nuclei with few mitoses [immunohistochemistry (ihc) results are presented in Table i]. Repeat endoscopy, digital rectal examination of the prostate, and prostate-specific antigen (psa) were normal. Two months later, a 12-cm erythematous plaque was found extending onto the medial thigh and left scrotum. Some tenderness, pruritus, bleeding, and oozing in the area was evident.
FIGURE 2.
Histopathology of scrotal extramammary Paget disease in case 2. (2A) The tumour cells are arranged in sheets and nests within the dermis, with extension into the epidermis. (2B) The cells have a pale eosinophilic cytoplasm and bland nuclei. The tumour cells express (2C) cytokeratin 7, (2D) muc1, and (2E) carcinoembryonic antigen. (2F) The Paget cells from primary extramammary Paget disease can express the androgen receptor.
The patient was treated in January 2012 with a radical course of radiotherapy: 50 Gy in 20 fractions (250 cGy per fraction) over 4 weeks, using orthovoltage 150 kVp. Treatment was well tolerated. At December 2012, the patient was alive, with no recurrence.
3.1.3. Case Description 3
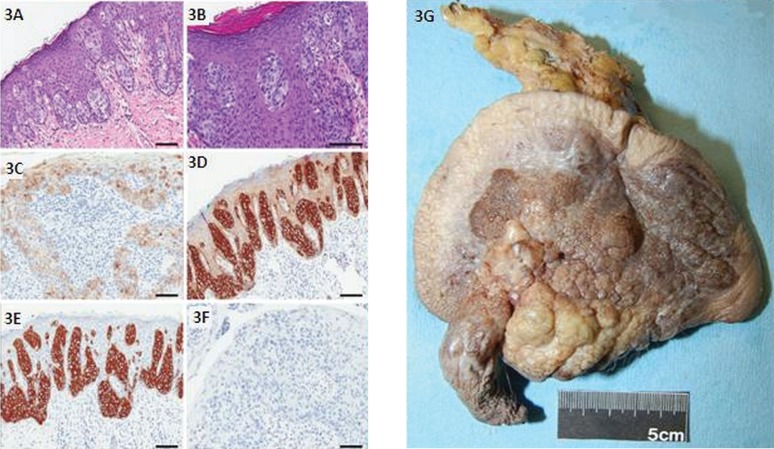
A 78-year-old white man presented with a 2-year history of a large erythematous lesion in the right groin. Excisional biopsy in December 2010 revealed an invasive apocrine carcinoma with close margins. Staging ct of chest, abdomen, and pelvis were negative. In July 2011, the patient underwent wide reexcision of primary site, with sentinel node biopsy (slnb) (Figure 3). The pathology report revealed extensive residual empd in the skin, but a negative lymph node. As of December 2012, this patient is doing well, with no evidence of disease.
FIGURE 3.
Histopathology and gross resection specimen of scrotal extramammary Paget disease in case 3. (3A) The tumour cells spread into the epidermis in single cells or small nest patterns. (3B) The cytoplasm is characteristically large and pale. The tumour cells express (3C) carcinoembryonic antigen and (3D) low molecular weight cytokeratins. The Paget cells from primary empd are (3E) cytokeratin 7–positive and (3F) cytokeratin 20–negative. Scale bar at 100 μm. (3G) The surgical resection specimen shows penile shaft, scrotal skin, and left inguinal lymph nodes. The skin surface is grey-tan brown-and-yellow, bosselated and firm, with nodular and granular lesions. The cut surface of the scrotal skin (not shown) demonstrated the lesion to be white, tan to red-brown and firm. Greatest depth was 1.0 cm at the base of the penile shaft, coming to within 0.1 cm of the deep margin. Sectioning through the underlying fibroadipose tissue revealed the presence of lymph nodes.
3.1.4. Case Description 4
A 67-year-old white man presented with a long story of scrotal rash. In 1993, the condition was treated as eczema. Scrotal biopsy in 1998 diagnosed Bowen disease, treated with topical fluorouracil. Repeat biopsy in 1999 revealed empd.
The patient received radiation, 60 Gy in 30 fractions, to the scrotal area. In December 2010, abnormal left inguinal lymph nodes were noted, which were positive for metastatic adenocarcinoma on biopsy. Bone scan revealed multiple areas of metastasis. Imaging of chest, abdomen, and pelvis by ct demonstrated retroperitoneal and left inguinal lymphadenopathy. In February 2011, magnetic resonance imaging of the spine showed diffuse metastatic or infiltrative disease involving the spine, and mild compression fracture with soft tissue in the anterior epidural space at T3.
A biopsy of the left inguinal lymph node confirmed carcinoma (ihc results in Table i). Repeat scrotal biopsy in March 2011 confirmed empd with dermal invasion. Given the positive inguinal lymph nodes, and because empd can accompany other tumours, urine cytopathology was done, revealing degenerated atypical urothelial cells. The patient’s carcinoembryonic antigen (cea) was elevated at 63.4 μg/L (normal level: <3 μg/L).
In that same month, the patient underwent hemiarthroplasty because of an impending left femoral head and neck fracture. The surgical specimen revealed an extensive metastatic carcinoma, suggestive of breast origin. A skeletal survey revealed sclerotic metastases in the skull and the cervical, thoracic, and lumbar spine.
The patient received radiation, 20 Gy in 5 fractions to the thoracic spine and left hip. Because of hypercalcemia [2.99 mmol/L (normal level: <2.52 mmol/L)], pamidronate 60 mg monthly was prescribed. In March 2011, weekly paclitaxel (80 mg/m2) was started. By April, pain in the lumbar spine and right hip led to the patient receiving palliative radiotherapy.
After 2 cycles of chemotherapy, ct imaging showed a partial response (pr), but the patient was admitted with sepsis. Because his tumour was androgen receptor–positive, total androgen blockade (goserelin acetate and bicalutamide) was started in May 2011, but the patient died shortly thereafter.
3.1.5. Case Description 5
A 73-year-old Asian man presented with a 2-year history of a skin lesion in the glans and penoscrotal area. Because of progressive pruritus, erythema, and thickening of the affected area, he sought medical attention. Initial treatment was topical therapy, with an empiric diagnosis of infection. Because this patient’s psa was elevated at 13 μg/L (normal level: <3 μg/L), he underwent prostatic biopsy in January 2011, which was positive only for prostatic intraepithelial neoplasia. Biopsy of the penile lesion demonstrated empd.
In April 2011, this patient underwent excision of penile shaft skin, foreskin, scrotal skin, and skin overlying the left inguinal area; bilateral inguinal lymph node dissection; and left sartorius transposition. Pathology revealed extensive empd involving the base of the penis, with extension to the penis shaft and the scrotum. Margins were negative.
The lesion consisted of an intra-epidermal, poorly differentiated large-cell carcinoma extending from the basal layer to the upper layer of the epidermis. Mitotic figures were present. Occasional cells containing droplets of mucin were identified by alcian blue stain. The diagnosis was further supported by ihc (Table i). The left inguinal lymph nodes were positive for metastatic adenocarcinoma (6 of 11). Restaging by magnetic resonance imaging in May 2011 revealed an enlarged left internal iliac node. Imaging of abdomen and pelvis by ct in October 2011 revealed retroperitoneal lymphadenopathy.
The patient declined referral to the cancer centre. Restaging ct imaging at March 2012 showed progressive disease (pd) in the retroperitoneal and pelvic lymph nodes. In May 2012, the patient’s psa was stable at 16 μg/L, and his cea was mildly elevated at 4.4 g/L (normal level: <3 g/L).
In July 2012, his psa rose to 59 μg/L, and ct imaging revealed lung metastasis, increasing subcutaneous edema, and adenopathy in the right groin and pelvis, with stable retroperitoneal nodes. In August, his psa dropped to 8.86 vg/L without treatment, and his cea rose to 17.3 g/L. He declined further biopsies, but agreed to receive chemotherapy (carboplatin auc 4 and paclitaxel 175 mg/m2 every 3 weeks). After 3 cycles, ct imaging showed pd, with new liver and left iliacus muscular metastases. At the time this report was written, the patient was receiving docetaxel (75 mg/m2 every 3 weeks), with pr after 2 cycles.
3.1.6. Case Description 6
A 53-year-old white man noted a small, slow-growing pruriginous papule on his left hemi-scrotum for approximately 2 years before seeking medical attention. He underwent excision in August 2004. Pathology revealed apocrine adenocarcinoma with evidence of pagetoid involvement of the surface epidermis. Margins were positive. The tumour was characterized by small cords and nests of epithelial cells that showed abundant foamy pink cytoplasm. Formation of numerous small duct-like structures was evident. Some of the ducts contained amorphous eosinophilic material. The epithelial cells also formed more solid areas in some foci. The epithelial cells showed moderate nuclear pleomorphism and atypia. Mitotic activity was not prominent in this tumour.
Imaging of chest, abdomen, and pelvis by ct; scrotal ultrasonography; cystoscopy; and prostate biopsy were negative for malignancy. The patient underwent partial scrotectomy in October 2004. In April 2009, he re-presented with dyspepsia and some change in his bowel habits. Imaging by ct showed retroperitoneal, pelvic, and inguinal lymphadenopathy. Fine-needle aspiration of the left inguinal lymphadenopathy revealed empd. By September 2009, he had developed cervical lymphadenopathy and lung and bone metastases.
The patient was treated with carboplatin (auc 6) and paclitaxel (175 mg/m2) every 3 weeks for 6 cycles. The best response was stable disease. By June 2011, he had developed lymphangitic carcinomatosis and hepatic and adrenal metastasis. The her2 (human epidermal growth factor receptor 2) amplification on biopsy material was negative. Gemcitabine (1000 mg/m2 days 1 and 8 every 3 weeks) was started in July 2011.
In September 2011, the patient was experiencing vomiting, dysphasia to solids, and retrosternal pressure. Restaging ct imaging revealed a node compressing the esophagus. He received palliative radiation to the area of esophageal compression to a dose of 20 Gy in 5 fractions. Because of painful osteoblastic metastases in the proximal left hip, the patient received radiation, 8 Gy in 1 fraction. With pd in October 2011, he was re-challenged with carboplatin (auc 4) and paclitaxel (140 mg/m2), with dose reduction because of worsening performance status. He received further palliative radiotherapy to the left hip, 15 Gy in 5 fractions.
Because of pd, doxorubicin (50 mg/m2) was started. After 2 cycles, his performance status had deteriorated, complicated by febrile neutropenia. Restaging investigations revealed pd in liver and lymph nodes. This patient died in March 2012.
3.1.7. Summary of Cases
In our case-series, mean age at diagnosis was 70 years (range: 53–78 years). Skin change followed by pruritus was the most common form of presentation. Three patients had an associated cancer (bladder, anorectal, and presumptively, prostate cancer). Follow-up ranged from 18 months to 12 years. After a mean follow up of 21 months, 2 patients were alive without disease. One patient was alive with his disease at 20 months although the disease had metastasized to lymph nodes 5 months after initial diagnosis. One patient died 13 months after the empd diagnosis, presumably because of an associated malignancy. Two patients died with metastatic empd. The patient in case 4 died after a follow-up of 12 years, but only 5 months after the diagnosis of invasive disease. The patient in case 6 died after 78 months of follow-up, 23 months after diagnosis of metastatic disease. The most common sites of metastases were lymph nodes, followed by lung and bone.
Baseline ct imaging and colonoscopies were not commonly performed, and cea was measured in only 2 cases and was elevated in both. The most common treatment was surgery, followed by reresection in cases of recurrent localized disease or positive margins. In only 1 case was a slnb done. For metastatic disease, the most common proposed treatment was taxane-based chemotherapy, followed by gemcitabine and doxorubicin. One patient received hormonal therapy on the basis of androgen receptor positivity in the tumour; however, his performance status deteriorated before response could be assessed.
4. DISCUSSION
The typical clinical presentation in empd consists of single or multifocal lesions that are raised, dry, erythematous, and slow-growing. Over time, they may evolve into eczematoid, crusted, ulcerated, or papillary lesions. Pruritus may precede the appearance of any skin abnormality7. Because of their benign appearance, these lesions are often neglected for several years before being presented for medical attention. The diagnosis can be challenging, and initial medical management commonly includes antifungal creams or topical steroids before a biopsy is done8,9. The differential diagnosis includes superficial spreading melanoma, Bowen disease, neuroendocrine carcinoma, mycosis fungoides, psoriasis, leukoplakia, eczema, fungal infection10, or intra-epidermal spread of visceral carcinoma (pagetoid effect)11. Extramammary Paget disease can also occur in association with invasive carcinomas of the genitourinary or gastrointestinal tract10. The frequency of that association varies from 9% to 32%12–19. Additionally, a familial association has been described11.
Information on the management of this rare entity is derived from small case series and case reports. A Surveillance, Epidemiology, and End Results (seer) analysis performed in 2011 found 328 cases of penis and anorectal empd from 1973 to 20075. The median age for a diagnosis of scrotal disease was 70.4 years; for penile disease, it was 73.6 years. After exclusion of vulvar disease, empd was most commonly seen in the scrotum and penis, followed by the anorectal area. Another seer study performed by Karam et al. for the same period of time, but looking at invasive empd of all sites, reported 1439 cases with a similar median age, predominance of Caucasian ethnicity (81%), and localized disease (80%)6. In that study, the 5-year disease-specific survival was 95% for localized disease, 85% for regional disease, and 52% for disease with distant metastasis. Poor prognosis was associated with primary anorectal empd, presence of distant metastasis, radiotherapy as monotherapy5, and older age6.
The incidence of empd in the Asian population is not clear. Before the seer analysis, the largest series in empd originated from Asia. In retrospective cohorts, the number of cases accumulating over a period of time seemed to be higher than was seen in Western studies. It is unclear if there is a racial or environmental reason behind the difference20. The correlation of cea levels with tumour progression in advanced empd21 is commonly described in Asian series. In Western series, however, the use of this test is seldom described.
Invasive genitourinary tract cancers (prostate, bladder, kidney)22–28 or gastrointestinal sites (colon, rectum, liver)29,30 can be associated, synchronously or metachronously, with penoscrotal empd, but the frequency of this association is not consistently reported. In a recent M.D. Anderson Cancer Centre report, the incidence of a second malignancy was 45%31, and in a European study, the overall standardized incidence ratio was 1.393.
4.1. Pathology
The presence of neoplastic cells with signs of glandular differentiation in the epidermis (Paget cells) characterizes empd. The pathologic classification makes a distinction between “primary empd,” in which the disease can be confined to the epidermis or may be associated with an underlying sweat gland adenocarcinoma, and “secondary empd,” which represents an extension from an underlying malignancy (that is, urethral or bladder carcinoma)32. The clinician should evaluate patients for an underlying second malignancy.
The pathogenesis of empd has been debated. One theory postulates that empd results from an underlying sweat gland adenocarcinoma that spreads into the epidermis33. Another theory suggests malignant transformation of pluripotent dermocytes that are capable of glandular differentiation24.
Histologically, Paget cells appear as large, pale, vacuolated cells with vesicular nuclei and prominent nucleoli. A cleft-like separation is often seen between the Paget cells and the adjacent non-neoplastic keratinocytes. The Paget cells may be single or arranged in rows or small nests, concentrated above the basal layer of the epidermis. They may also extend to the epithelium of adnexal structures. Dermal invasion is seen in small proportion of cases and implies a poor prognosis10. Lack of E-cadherin expression and lymphovascular invasion have been shown to be important pathologic predictors of metastasis34,35. Immunohistochemically, the Paget cells frequently show positive reactivity to epithelial membrane antigen, cea, gross cystic disease fluid protein, and low molecular weight cytokeratins36. Primary empd is usually CK7-positive and CK20-negative; secondary empd associated with urothelial carcinoma is usually CK7- and CK20-positive36,37. The tumour cells often stain positive to periodic acid–Schiff, mucicarmine, and alcian blue because of intracytoplasmic mucopolysaccharides. Although not well documented, her2 gene amplification and expression of hormone receptors (androgen receptor rather than estrogen receptor and progesterone receptor) have been described as poor prognostic markers. Their presence should prompt the use of targeted therapy38,39.
The main pathologic differential diagnoses of penoscrotal empd are malignant melanoma (similar pattern of intra-epidermal spread, but cea-negative, mucicarmine-negative, and S-100–positive) and pagetoid Bowen disease (similar pattern of spread, but cea-negative, mucicarmine-negative, CK7-negative, and p63-positive)40. Lesions combining the features of Paget disease and Bowen disease are occasionally reported41. Clear-cell papulosis, pagetoid dyskeratosis, and mucinous metaplasia should also be ruled out42.
4.2. Treatment
4.2.1. Surgery
Extramammary Paget disease imposes a surgical challenge, because the disease can be multifocal and because metastatic disease is sometimes clinically unapparent24,43. The standard treatment for local empd is surgical resection. The M.D. Anderson group recommends at least 1-cm to 1.5-cm margins of grossly visible tumour, with re-resection according to frozen section results31. Wide local excision may be followed by reconstructive surgical procedures to obtain better cosmesis. Another option is Mohs micrographic surgery. That technique consists of removal of tumour, review of margins under the microscope by the surgeon, tumour mapping, and resection of positive margins, potentially sparing normal tissue while achieving complete tumour resection44. Additionally, the reported recurrence rates are lower after Mohs micrographic surgery than after conventional excision (12.5%–28% vs. 33%–60%)20,43,45,46. When surgical margins are negative, patients experience durable relapse-free survival.
Dermal or lymph node invasion confers a worse prognosis31. In fact, in a multivariate analysis, a significant improvement in outcomes was observed for patients who had surgery compared with those who had no intervention (hazard ratio: 0.44), and the addition of radiotherapy to local surgery did not improve outcomes6. In the case of positive margins, re-excision with clear margins should be attempted. In one of our cases, that approach provided a good outcome.
Clinically positive local lymph nodes are resected, but the role for slnb in the search for occult metastases in empd is unclear, and the procedure is not routinely done. Nakamura et al.47 described 27 cases in which the incidence of sentinel lymph node metastasis was 37%. Lymph node metastasis occurred in 60% of tumours with microinvasion and in 44% with dermal invasion. Additionally, 67% of patients with a positive slnb who underwent lymphadenectomy (with <3 positive lymph nodes) did not experience recurrence.
4.2.2. Radiotherapy
A review of the literature suggests that radiation therapy is an effective and well-tolerated treatment that can provide good local control. Radiotherapy is an alternative when there are contraindications to excision such as medical inoperability or difficult location, or when patients decline surgery. Additionally, in recurrent empd, radiation therapy is an alternative to re-excision for post-surgery recurrences or when topical treatments fail. Because of widely divergent effectiveness, the place of radiation as monotherapy in the management of empd remains uncertain6. For example, a recent seer analysis did not show an extra survival benefit with the addition of adjuvant radiotherapy after surgery5; however, patients who received radiotherapy might have been high-risk patients who were not candidates for surgery or who were at high risk of local recurrence. However, to reduce local recurrences, adjuvant radiotherapy has been used in patients who are at high risk for recurrence postoperatively or who had positive margins at surgery48–54.
In our series, the first reported case had lymphovascular invasion and developed a local relapse. Some publications report complete regression of carcinoma in situempd after definitive radiotherapy55. Traditionally, radiation doses of 45 Gy–70 Gy have been prescribed in both the definitive and adjuvant settings48–54,56. Various radiation techniques have been used, including megavoltage and orthovoltage photon therapy, electron-beam therapy, and high-dose-rate mould brachytherapy50.
4.2.3. Other Local Therapies
Other nonsurgical modalities described in the literature are topical agents (imiquimod, 5-fluorouracil, bleomycin, retinoids), and CO2 laser ablation57.
4.2.4. Chemotherapy
Because of the rarity of metastatic empd, the optimal chemotherapy regimen and timing (neoadjuvant or adjuvant, monotherapy or combined therapy) is not established. Tumour responses have been reported with combination chemotherapy such as mitomycin C 3.5 mg/m2 and epirubicin 50 mg/m2 (day 1), vincristine 6 mg/m2 (days 1 and 7), cisplatin 30 mg/m2 (days 1–3), and 5-fluorouracil 350 mg/m2 (days 3–7), with pr and complete response58–60. The literature also describes prs with 5-fluorouracil plus cisplatin61 in a low-dose schedule (5-fluorouracil 500 mg daily, 7 days per week, and cisplatin 5 mg daily, 5 days per week), followed by oral tegafur uracil (600 mg daily)62. Complete radiologic response to docetaxel and carboplatin, with a disease-free survival of 13 months31, was reported for penoscrotal empd. Responses to docetaxel, weekly or every 3 weeks, alone or in combination, have also been reported in Japanese cases63, with complete response lasting 2 years64 and pr for up to 12 months despite prior use of other agents (mitomycin C, epirubicin, vincristine, cisplatin, and 5-fluorouracil)65.
4.2.5. Targeted Therapy
HER2:
One case of a patient with metastatic scrotal empd and her2 overexpression has been reported66. The patient was treated with paclitaxel and trastuzumab. A pr in the skin lesions was seen, but after 6 months, the patient developed brain metastasis67. Reponses with this combination68 or with trastuzumab alone69 in patients with vulvar empd have also been described.
Estrogen Receptor:
A patient with advanced penoscrotal empd and prostate cancer, in which the empd strongly expressed estrogen receptor alpha, received tamoxifen (20 mg daily) and bicalutamide (80 mg daily) and experienced stable disease for 6 months70. No description of the androgen receptor was noted.
5. CONCLUSIONS
Penoscrotal empd is a rare disease, and as such, clinical trials to better guide its management are lacking. In metastatic disease, the clinical course and response to treatments are widely variable. Local control is indispensable: it prevents further invasion and, therefore, metastatic disease. Surgery is the mainstay treatment for local control. Radiotherapy can be used in patients who are not candidates for, or who refuse, surgery. As described earlier, the duration to local or even metastatic recurrence can vary from months to years, and empd can be associated with other cancers in a metachronous or synchronous fashion. Long-term follow-up is therefore required. However, the appropriate investigations and follow-up schedule are not known. In the literature, further investigations to rule out associated malignancies were performed inconsistently, but include ct imaging of chest, abdomen, and pelvis; cystoscopy; and colonoscopy. Carcinoembryonic antigen is a tumour marker associated with invasive empd and may be helpful in assessing treatment response.
Once metastatic disease develops, outcomes are poor, despite case reports of complete response with chemotherapy. No standard systemic therapy has been established. Some centres use chemotherapy regimens developed for gastrointestinal adenocarcinomas or breast cancer; others base their choice on ihc findings, with the intent of using targeted therapy.
It is challenging, and not always possible, to differentiate metastatic empd from concurrent invasive cancer, especially in poorly differentiated adenocarcinomas. The enrolment of such patients in a rare disease consortium and in multicentre clinical trials designed for rare diseases, and the further advancement of knowledge concerning specific markers for the planning of targeted therapy, would provide much-needed information.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
7. REFERENCES
- 1.Darier J, Coulillaud P. A case of Paget’s disease of the anal and perineal scrotal region [French] Ann Dermatol Syphiligr. 1893;4:25–31. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 2.Fardal RW, Kierland RR, Clagett OT, Woolner LB. Prognosis on cutaneous Paget’s disease. Postgrad Med. 1964;36:584–93. doi: 10.1016/S0360-3016(00)00497-1. [DOI] [PubMed] [Google Scholar]
- 3.van der Zwan JM, Siesling S, Blokx WA, Pierie JP, Capocaccia R. Invasive extramammary Paget’s disease and the risk for secondary tumours in Europe. Eur J Surg Oncol. 2012;38:214–21. doi: 10.1016/j.ejso.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Evans AT, Neven P. Invasive adenocarcinoma arising in extramammary Paget’s disease of the vulva. Histopathology. 1991;18:355–60. doi: 10.1111/j.1365-2559.1991.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 5.Master VA, Herrel L, Johnson TV, Delman KA. Extramammary Paget’s disease of the penis and anogenital area: seer analysis [abstract 220] J Clin Oncol. 2011;29 doi: 10.1097/ACO.0b013e32830413cb. [Available online at: http://meetinglibrary.asco.org/content/72169-104; cited May 29, 2013] [DOI] [PubMed] [Google Scholar]
- 6.Karam A, Dorigo O. Treatment outcomes in a large cohort of patients with invasive extramammary Paget’s disease. Gynecol Oncol. 2012;125:346–51. doi: 10.1016/j.ygyno.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Rao VR, Henry DH. Extramammary Paget’s disease. Commun Oncol. 2004;1:109–15. doi: 10.1016/S1548-5315(11)70793-8. [DOI] [Google Scholar]
- 8.Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease. J Clin Pathol. 2000;53:742–9. doi: 10.1136/jcp.53.10.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JY, Li GK, Chung JH, Chow VL. Extramammary Paget’s disease: 20 years of experience in Chinese population. Int J Surg Oncol. 2012;2012:416–418. doi: 10.1016/S1542-3565(03)00226-X. [Available online at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3303748; cited December 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones RE, Jr, Austin C, Ackerman AB. Extramammary Paget’s disease. A critical reexamination. Am J Dermatopathol. 1979;1:101–32. doi: 10.1097/00000372-197900120-00002. [DOI] [PubMed] [Google Scholar]
- 11.Minicozzi A, Borzellino G, Momo R, Steccanella F, Pitoni F, de Manzoni G. Perianal Paget’s disease: presentation of six cases and literature review. Int J Colorectal Dis. 2010;25:1–7. doi: 10.1007/s00384-009-0797-9. [DOI] [PubMed] [Google Scholar]
- 12.Curtin JP, Rubin SC, Jones WB, Hoskins WJ, Lewis JL., Jr Paget’s disease of the vulva. Gynecol Oncol. 1990;39:374–7. doi: 10.1016/0090-8258(90)90269-Q. [DOI] [PubMed] [Google Scholar]
- 13.Kodama S, Kaneko T, Saito M, Yoshiya N, Honma S, Tanaka K. A clinicopathologic study of 30 patients with Paget’s disease of the vulva. Gynecol Oncol. 1995;56:63–70. doi: 10.1006/gyno.1995.1010. [DOI] [PubMed] [Google Scholar]
- 14.Fishman DA, Chambers SK, Schwartz PE, Kohorn EI, Chambers JT. Extramammary Paget’s disease of the vulva. Gynecol Oncol. 1995;2:266–70. doi: 10.1006/gyno.1995.1044. [DOI] [PubMed] [Google Scholar]
- 15.Lee SC, Roth LM, Ehrlich C, Hall JA. Extramammary Paget’s disease of the vulva. A clinicopathologic study of 13 cases. Cancer. 1977;39:2540–9. doi: 10.1002/1097-0142(197706)39:6<2540::AID-CNCR2820390635>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Tsukada Y, Lopez RG, Pickren JW, Piver MS, Barlow JJ. Paget’s disease of the vulva: a clinicopathologic study of eight cases. Obstet Gynecol. 1975;45:73–8. doi: 10.1016/j.lungcan.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Molinie V, Paniel BJ, Lessana–Leibowitch M, Moyal–Barracco M, Pelisse M, Escande JP. Paget’s disease of the vulva. 36 cases [French] Ann Dermatol Venereol. 1993;120:522–7. doi: 10.1080/07357900701560612. [DOI] [PubMed] [Google Scholar]
- 18.Goldblum JR, Hart WR. Perianal Paget’s disease—a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170–9. doi: 10.1097/00000478-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Olson DJ, Fujimura M, Swanson P, Okagaki T. Immunohistochemical features of Paget’s disease of the vulva with and without adenocarcinoma. Int J Gynecol Pathol. 1991;10:285–95. doi: 10.1097/00004347-199107000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ, Choe YS, Jung HD, et al. A multicenter study on extramammary Paget’s disease in Korea. Int J Dermatol. 2011;50:508–15. doi: 10.1111/j.1365-4632.2010.04661.x. [DOI] [PubMed] [Google Scholar]
- 21.Saida M, Takigawa M, Yamamoto A, Tsuchida T, editors. General Rules for Clinical and Pathological Studies on Malignant Neoplasms of the Skin [Japanese] Tokyo, Japan: Kanehara Shuppan Publications; 2002. [DOI] [Google Scholar]
- 22.Voigt H, Bassermann R, Nathrath W. Cytoreductive combination chemotherapy for regionally advanced unresectable extramammary Paget carcinoma. Cancer. 1992;70:704–8. doi: 10.1002/1097-0142(19920801)70:3<704::AID-CNCR2820700327>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Becker–Wegerich PM, Fritsch C, Schulte KW, et al. Carbon dioxide laser treatment of extramammary Paget’s disease guided by photodynamic diagnosis. Br J Dermatol. 1998;138:169–72. doi: 10.1046/j.1365-2133.1998.02046.x. [DOI] [PubMed] [Google Scholar]
- 24.Zollo JD, Zeitouni NC. The Roswell Park Cancer Institute experience with extramammary Paget’s disease. Br J Dermatol. 2000;142:59–65. doi: 10.1046/j.1365-2133.2000.03242.x. [DOI] [PubMed] [Google Scholar]
- 25.Powell FC, Bjornsson J, Doyle JA, Cooper AJ. Genital Paget’s disease and urinary tract malignancy. J Am Acad Dermatol. 1985;13:84–90. doi: 10.1016/S0190-9622(85)70148-X. [DOI] [PubMed] [Google Scholar]
- 26.Chanda JJ. Extramammary Paget’s disease: prognosis and relationship to internal malignancy. J Am Acad Dermatol. 1985;13:1009–14. doi: 10.1016/S0190-9622(85)70254-X. [DOI] [PubMed] [Google Scholar]
- 27.Ojeda VJ, Heenan PJ, Watson SH. Paget’s disease of the groin associated with adenocarcinoma of the urinary bladder. J Cutan Pathol. 1987;14:227–3. doi: 10.1111/j.1600-0560.1987.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 28.Koh KB, Nazarina AR. Paget’s disease of the scrotum: report of a case with underlying carcinoma of the prostate. Br J Dermatol. 1995;133:306–7. doi: 10.1111/j.1365-2133.1995.tb02635.x. [DOI] [PubMed] [Google Scholar]
- 29.Lai YL, Yang WG, Tsay PK, Swei H, Chuang SS, Wen CJ. Penoscrotal extramammary Paget’s disease: a review of 33 cases in a 20-year experience. Plast Reconstr Surg. 2003;112:1017–23. doi: 10.1097/01.PRS.0000076193.67701.6A. [DOI] [PubMed] [Google Scholar]
- 30.Li YC, Lu LY, Yang YT, Chang CC, Chen LM. Extramammary Paget’s disease of the scrotum associated with hepatocellular carcinoma. J Chin Med Assoc. 2009;72:542–6. doi: 10.1016/S1726-4901(09)70425-3. [DOI] [PubMed] [Google Scholar]
- 31.Hegarty PK, Suh J, Fisher MB, et al. Penoscrotal extramammary Paget’s disease: the University of Texas M.D. Anderson Cancer Center contemporary experience. J Urol. 2011;186:97–102. doi: 10.1016/j.juro.2011.02.2685. [DOI] [PubMed] [Google Scholar]
- 32.Harris DW, Kist DA, Bloom K, Zachary CB. Rapid staining with carcinoembryonic antigen aids limited excision of extramammary Paget’s disease treated by Mohs surgery. J Dermatol Surg Oncol. 1994;20:260–4. doi: 10.1111/j.1524-4725.1994.tb01622.x. [DOI] [PubMed] [Google Scholar]
- 33.Mehta NJ, Torno R, Sorra T. Extramammary Paget’s disease. South Med J. 2000;93:713–15. doi: 10.1097/JTO.0b013e318209edb9. [DOI] [PubMed] [Google Scholar]
- 34.Yang WJ, Kim DS, Im YJ, et al. Extramammary Paget’s disease of the penis and scrotum. Urology. 2005;65:972–5. doi: 10.1016/j.urology.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 35.van Randenborgh H, Paul R, Nahrig J, Egelhof P, Hartung R. Extramammary Paget’s disease of penis and scrotum. J Urol. 2002;168:2540–1. doi: 10.1016/S0022-5347(05)64192-4. [DOI] [PubMed] [Google Scholar]
- 36.Helm KF, Goellner JR, Peters MS. Immunohistochemical stains in extramammary Paget’s disease. Am J Dermatopathol. 1992;14:402–7. doi: 10.1097/00000372-199210000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Ordóñez NG, Awalt H, Mackay B. Mammary and extramammary Paget’s disease. An immunohistochemical and ultrastructural study. Cancer. 1987;59:1173–83. doi: 10.1002/1097-0142(19870315)59:6<1173::AID-CNCR2820590624>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 38.Diaz de Leon E, Carcangiu ML, Prieto VG, et al. Extramammary Paget disease is characterized by the consistent lack of estrogen and progesterone receptors but frequently expresses androgen receptor. Am J Clin Pathol. 2000;113:572–5. doi: 10.1309/P756-XXCB-TV71-U4XV. [DOI] [PubMed] [Google Scholar]
- 39.Plaza JA, Torres–Cabala C, Ivan D, Prieto VG. her-2/neu expression in extramammary Paget disease: a clinicopathologic and immunohistochemistry study of 47 cases with and without underlying malignancy. J Cutan Pathol. 2009;36:729–33. doi: 10.1111/j.1600-0560.2008.01148.x. [DOI] [PubMed] [Google Scholar]
- 40.Kohler S, Rouse RV, Smoller BR. The differential diagnosis of pagetoid cells in the epidermis. Mod Pathol. 1998;11:79–92. [PubMed] [Google Scholar]
- 41.Quinn AM, Sienko A, Basrawala Z, Campbell SC. Extramammary Paget disease of the scrotum with features of Bowen disease. Arch Pathol Lab Med. 2004;128:84–6. doi: 10.5858/2004-128-84-EPDOTS. [DOI] [PubMed] [Google Scholar]
- 42.Kuo TT, Chan HL, Hsueh S. Clear cell papulosis of the skin. A new entity with histogenetic implications for cutaneous Paget’s disease. Am J Surg Pathol. 2004;128:84–6. doi: 10.1097/00000478-198711000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd V, Davidson EJ, Davies–Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112:273–9. doi: 10.1111/j.1471-0528.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 44.Drake LA, Dinehart SM, Goltz RW, et al. Guidelines of care for Mohs micrographic surgery. American Academy of Dermatology. J Am Acad Dermatol. 1995;33:271–8. doi: 10.1016/0190-9622(95)90261-9. [DOI] [PubMed] [Google Scholar]
- 45.Hendi A, Brodland DG, Zitelli JA. Extramammary Paget’s disease: surgical treatment with Mohs micrographic surgery. J Am Acad Dermatol. 2004;51:767–73. doi: 10.1016/j.jaad.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Zampogna JC, Flowers FP, Roth WI, Hassenein AM. Treatment of primary limited cutaneous extramammary Paget’s disease with topical imiquimod monotherapy: two case reports. J Am Acad Dermatol. 2002;47(suppl 4):S229–35. doi: 10.1067/mjd.2002.126584. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura Y, Fujisawa Y, Ishikawa M, et al. Usefulness of sentinel lymph node biopsy for extramammary Paget’s disease. Br J Dermatol. 2012;167:954–6. doi: 10.1111/j.1365-2133.2012.11017.x. [DOI] [PubMed] [Google Scholar]
- 48.Besa P, Rich TA, Delclos L, Edwards CL, Ota DM, Wharton JT. Extramammary Paget’s disease of the perineal skin: role of radiotherapy. Int J Radiat Oncol Biol Phys. 1992;24:73–8. doi: 10.1016/0360-3016(92)91024-H. [DOI] [PubMed] [Google Scholar]
- 49.Hata M, Omura M, Koike I, et al. Role of radiotherapy as curative treatment of extramammary Paget’s disease. Int J Radiat Oncol Biol Phys. 2011;80:47–54. doi: 10.1016/j.ijrobp.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 50.Luk NM, Yu KH, Yeung WK, Choi CL, Teo ML. Extramammary Paget’s disease: outcome of radiotherapy with curative intent. Clin Exp Dermatol. 2003;28:360–3. doi: 10.1046/j.1365-2230.2003.01301.x. [DOI] [PubMed] [Google Scholar]
- 51.Yanagi T, Kato N, Yamane N, Osawa R. Radiotherapy for extramammary Paget’s disease: histopathological findings after radiotherapy. Clin Exp Dermatol. 2007;32:506–8. doi: 10.1111/j.1365-2230.2007.02425.x. [DOI] [PubMed] [Google Scholar]
- 52.Kim TH, Chang IH, Kim TH, Lee SY, Myung SC. Extramammary Paget’s disease of scrotum treated with radiotherapy. Urology. 2009;74:474.e1–3. doi: 10.1016/j.urology.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 53.Velenik V, Segedin B, Anderluh F, Oblak I, Strojan P. Radiotherapy for perianal Paget’s disease: a case report. Tumori. 2008;94:750–3. doi: 10.1177/030089160809400520. [DOI] [PubMed] [Google Scholar]
- 54.Guerrieri M, Back MF. Extramammary Paget’s disease: role of radiation therapy. Australas Radiol. 2002;46:204–8. doi: 10.1046/j.1440-1673.2001.01039.x. [DOI] [PubMed] [Google Scholar]
- 55.Moreno-Arias GA, Conill C, Castells-Mas A, Arenas M, Grimalt R. Radiotherapy for genital extramammary Paget’s disease in situ. Dermatol Surg. 2001;27:587–90. doi: 10.1046/j.1524-4725.2001.00304.x. [DOI] [PubMed] [Google Scholar]
- 56.Burrows NP, Jones DH, Hudson PM, Pye RJ. Treatment of extramammary Paget’s disease by radiotherapy. Br J Dermatol. 1995;132:970–2. doi: 10.1111/j.1365-2133.1995.tb16957.x. [DOI] [PubMed] [Google Scholar]
- 57.Hartman R, Chu J, Patel R, Meehan S, Stein JA. Extramammary Paget disease. Dermatol Online J. 2011;17:4. [PubMed] [Google Scholar]
- 58.Mochitomi Y, Sakamoto R, Gushi A, et al. Extramammary Paget’s disease/carcinoma successfully treated with a combination chemotherapy: report of two cases. J Dermatol. 2005;32:632–7. doi: 10.1111/j.1346-8138.2005.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 59.Yamazaki N, Yamamoto A, Wada T, Ishikawa M, Moriya Y, Nakanishi Y. A case of metastatic extramammary Paget’s disease that responded to combination chemotherapy. J Dermatol. 1999;26:311–16. doi: 10.1111/j.1346-8138.1999.tb03477.x. [DOI] [PubMed] [Google Scholar]
- 60.Balducci L, Athar M, Smith GF, Khansur T, McKenzie D, Crawford ED. Metastatic extramammary Paget’s disease: dramatic response to combined modality treatment. J Surg Oncol. 1998;38:38–44. doi: 10.1002/jso.2930380111. [DOI] [PubMed] [Google Scholar]
- 61.Kao SCH, Yap ML, Berry M, Santos L, Lin M, Goldrick A. Metastatic extramammary Paget’s disease responding to cisplatin, 5-fluorouracil and radiotherapy—a case report. Asia Pac J Oncol Hematol. 2009;1:1–4. [Google Scholar]
- 62.Kariya K, Tsuji T, Schwartz RA. Trial of low-dose 5-fluorouracil/cisplatin therapy for advanced extramammary Paget’s disease. Dermatol Surg. 2004;30:341–4. doi: 10.1111/j.1524-4725.2004.30093.x. [DOI] [PubMed] [Google Scholar]
- 63.Fujisawa Y, Umebayashi Y, Otsuka F. Metastatic extramammary Paget’s disease successfully controlled with tumour dormancy therapy using docetaxel. Br J Dermatol. 2006;154:375–6. doi: 10.1111/j.1365-2133.2005.07046.x. [DOI] [PubMed] [Google Scholar]
- 64.Nakamori R, Omoto Y, Yamanaka K, Habe K, Kurokawa I, Mizutani H. Complete remission of advanced extramammary Paget’s disease treated with docetaxel: a case report. Clin Exp Dermatol. 2012;37:194–5. doi: 10.1111/j.1365-2230.2011.04204.x. [DOI] [PubMed] [Google Scholar]
- 65.Oguchi S, Kaneko M, Uhara H, Saida T. Docetaxel induced durable response in advanced extramammary Paget’s disease: a case report. J Dermatol. 2002;29:33–7. doi: 10.1111/j.1346-8138.2002.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 66.Ogawa T, Nagashima Y, Wada H, et al. Extramammary Paget’s disease: analysis of growth signal pathway from the human epidermal growth factor receptor 2 protein. Hum Pathol. 2005;36:1273–80. doi: 10.1016/j.humpath.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 67.Takahagi S, Noda H, Kamegashira A, et al. Metastatic extramammary Paget’s disease treated with paclitaxel and trastuzumab combination chemotherapy. J Dermatol. 2009;36:457–61. doi: 10.1111/j.1346-8138.2009.00676.x. [DOI] [PubMed] [Google Scholar]
- 68.Hanawa F, Inozume T, Harada K, Kawamura T, Shibagaki N, Shimada S. A case of metastatic extramammary Paget’s disease responding to trastuzumab plus paclitaxel combination therapy. Case Rep Dermatol. 2011;3:223–7. doi: 10.1159/000333002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karam A, Berek JS, Stenson A, Rao J, Dorigo O. her-2/neu targeting for recurrent vulvar Paget’s disease: a case report and literature review. Gynecol Oncol. 2008;111:568–71. doi: 10.1016/j.ygyno.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 70.Iijima M, Uhara H, Ide Y, et al. Estrogen-receptor-alpha–positive extramammary Paget’s disease treated with hormonal therapy. Dermatology. 2006;213:144–6. doi: 10.1159/000093854. [DOI] [PubMed] [Google Scholar]