Abstract
A series of four related phenol derivatives, with 2,2'-dipicolylamine substituents at the ortho positions, were prepared and their Zn2+ coordination complexes studied by spectroscopic methods. X-ray crystal diffraction analysis of a dinuclear zinc complex with two bridging acetate anions showed a ternary structure with highly charged interior and lipophilic exterior, which helps explain why this class of water-soluble complexes can effectively diffuse through cell membranes. The stability of the dinuclear zinc complexes in aqueous solution was found to be strongly anion dependent; that is, bridging oxyanions, such as acetate and pyrophosphate, lock the two Zn2+ cations to the surrounding ligand and greatly enhance ligand/zinc affinity. Overall, the results provide new insight into the structural and mechanistic factors that control the recognition and chemosensing performance of phenoxide bridged dipicolylamine molecular probes.
Keywords: Anion recognition, zinc coordination, nuclear magnetic resonance, X-ray crystallography, titration, spectroscopic analysis
Introduction
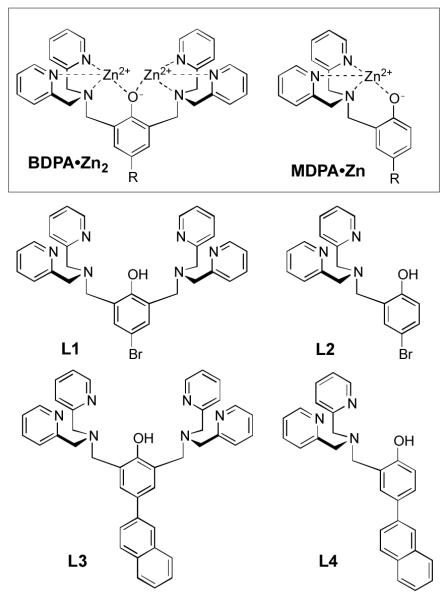
Phenol derivatives with 2,2'-dipicolylamine (DPA) substituents at the ortho positions are known to readily form coordination complexes with Zn2+, Cu2+, Ni2+, Co3+, Fe2+, and Fe3+ cations, and quite a few X-ray crystal structures of dinuclear complexes have been described.1 This report focuses on the Zn2+ complexes with generic molecular structure, BDPA•Zn2 (Scheme 1). These compounds are part of a larger family of Zn2+-DPA complexes that have been developed for various supramolecular applications such as sensing of anionic biomolecules in aqueous solution, protein labeling, DNA targeting, RNA hydrolysis, biomembrane targeting, and cell penetration.2,3,4 The long term goal of our research is to invent new classes of molecular probes for biological imaging, and we have discovered that Zn2+-DPA complexes have a remarkable ability to recognize anionic cell membrane surfaces selectively in complex biological environments such as cell culture and living animals.5 During these studies we observed an unusual phase transfer feature with water-soluble BDPA•Zn2 complexes, namely, an ability to penetrate cell membranes.6 We exploited this finding by developing oligomers of MDPA•Zn as effective cell permeating peptides.7 However, non-selective cell penetration is not a desirable attribute for most bioimaging applications. In order to rationally improve the performance of BDPA•Zn2 complexes as bioimaging probes and chemical sensors, a better understanding is needed of the fundamental coordination chemistry that controls the molecular recognition.
Scheme 1.
Here, we report a study of the solid-state structure and solution dynamics of some MDPA•Zn and BDPA•Zn2 complexes using a combination of spectrometric methods. Specifically, we have examined the Zn2+ complexation properties of four ligands, L1–L4 (Scheme 1). We find that Zn2+ affinity of these ligands in water is strongly anion dependent, that is, Zn2+ affinity is greatly enhanced by the presence of bridging oxyanions, such as acetate (OAc−) and pyrophosphate (PPi4−). Furthermore, oxyanion bridging of the two Zn2+ cations in BDPA•Zn2 produces a lipophilic ternary complex, which helps explain why water-soluble BDPA•Zn2 complexes can effectively diffuse through cell membranes. The article concludes with a discussion of how this study can be used to improve the performance of DPA coordination complexes as molecular imaging agents and optical chemosensors for Zn2+ and oxyanions.
Results and Discussion
Synthesis and Solid-State Structures
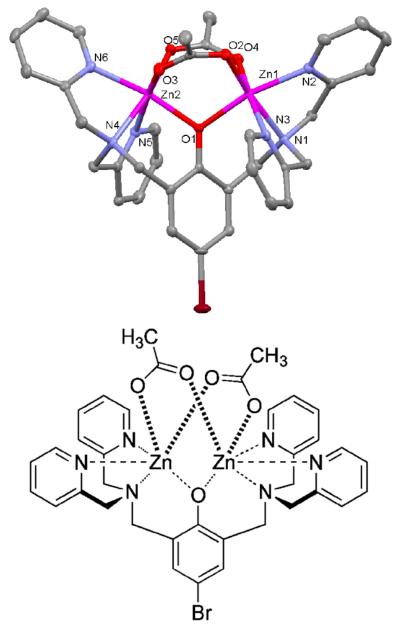
The ligands, L1–L4, were prepared in straightforward fashion using established procedures that produced a Mannich reaction of the parent phenol with one or two molar equivalents of paraformaldehyde and DPA.3c An X-ray crystal structure was obtained for the zinc acetate complex of L1. Single crystals were obtained by recrystallizing a methanol solution containing L1 mixed with two molar equivalents each of Zn(OAc)2, triethylamine, and NaBF4.1a X-ray diffraction analysis revealed a molecular formula of Zn2L1(OAc)2(BF4)•2MeOH. The C2 symmetric structure (Figure 1) contains two, six-coordinate Zn2+ centers with distorted octahedral geometries and an N3O3 donor set. Each Zn2+ is coordinated by a DPA tertiary amine and two pyridyl nitrogen atoms, as well as the central phenoxy anion and two bridging OAc− anions. The average Zn-N distances are 2.23 Å for the Zn-tertiary amine interactions and 2.17 Å for the Zn-pyridyl nitrogen interactions. The average Zn–O distances are 2.04 Å for the Zn-phenoxy bonds and 2.05 Å for the Zn-acetoxy bonds. The trans-axial angles through the distorted octahedral Zn atoms are all in the range of 162–171°, and the Zn1⋯Zn2 distance is 3.36 Å. These bond lengths and angles are consistent with similar Zn2+-DPA structures found in the literature.1 The molecular structure has a highly charged interior and lipophilic exterior, and suggests a structural process that enables partitioning of water-soluble BDPA•Zn2 complexes into vesicle and cell membranes.7 The process involves reversible association of BDPA•Zn2 complexes with one or two fatty acids or phospholipids at the membrane surface to produce membrane-soluble coordination complexes that are analogous to the structure in Figure 1.
Figure 1.
X-ray crystal structure of Zn2L1(OAc)2(BF4)•2MeOH. The structure omits the BF4− anion and two MeOH molecules for clarity. Thermal ellipsoids are indicated for all non-hydrogen atoms at the 50% probability level.
Solution-State UV Titrations
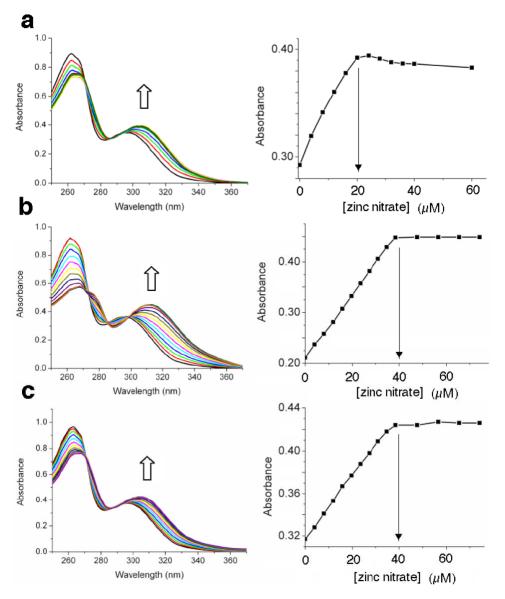
The two phenolic ligands, L3 and L4, have appended naphthalene chromophores and they are known to exhibit ratiometric spectral changes when they form zinc complexes.3c Thus, L3 and L4 were chosen as sensing ligands for a study of the effects of bridging oxyanions on relative zinc complex stoichiometry and stability. Shown in Figure 2 are the results of an absorbance titration experiment that added Zn(NO3)2 to control mono-DPA ligand L4 in methanol:water (4:1). There is a large bathochromic shift in absorption maxima as the ligand forms the expected L4:Zn2+ salt structure (see MDPA•Zn in Scheme 1) with 1:1 stoichiometry. The next set of absorbance titration experiments added Zn(NO3)2 to bis-DPA ligand L3 in methanol:water (4:1) and yielded intriguing results. As shown in Figure 3 the stoichiometry at signal saturation depended on the identity of the counter anion. Addition of Zn(NO3)2 to a solution of L3 reached saturation when the L3:Zn2+ stoichiometry was 1:1 (Figure 3a). In contrast, the saturated L3:Zn2+ stoichiometry was clearly 1:2 when the titration was conducted in the presence of Na4PPi (Figure 3b) or NaOAc (Figure 3c). The data is rationalized by considering the two stepwise, zinc association equilibria in Scheme 2. When the free L3 ligand (represented as generic ligand BDPA in Scheme 2) is titrated with Zn(NO3)2, the first apparent Zn2+ association constant, K1, is larger than the second stepwise constant, K2.8 Thus, the absorbance titration isotherm in Figure 3a reflects the first Zn2+ association step in Scheme 2; that is, the conversion of free BDPA ligand into mononuclear zinc complex BDPA•Zn.9 When high-affinity, bridging oxyanions, such as PPi4− or OAc−, are present in the solution, the second Zn2+ association constant, K2, is greatly enhanced such that it is larger than K1 (Scheme 2). Thus, the absorbance changes in Figure 3b and 3c reflect the conversion of generic ligand BDPA directly into dinuclear zinc complex BDPA•Zn2 with no measurable accumulation of the intermediate mononuclear zinc complex BDPA•Zn.
Figure 2.
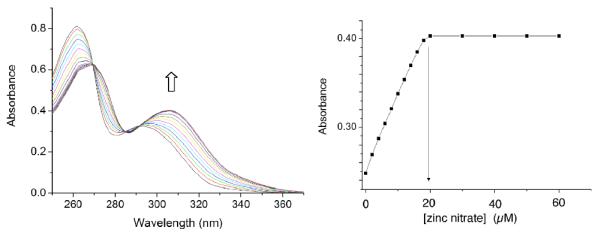
(left) Absorbance spectra of L4 (20 μM) upon titration of Zn(NO3)2 in methanol/water (4:1 volume ratio); (right) absorbance at 306 nm.
Figure 3.
(a) Absorbance spectra of L3 (20 μM) upon titration of Zn(NO3)2 in methanol/water (4:1 volume ratio); (b) same titration repeated in the presence of Na4PPi (40 μM); (c) same titration repeated in the presence of NaOAc (40 μM). In each case, the graph on the right shows absorbance intensity change for the spectral wavelength maxima band.
Scheme 2.
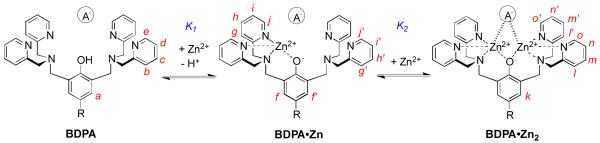
Stepwise association of BDPA with Zn2+ to form BDPA•Zn2 is pushed to the right by the presence of bridging oxyanions, A− = OAc− or PPi4−.
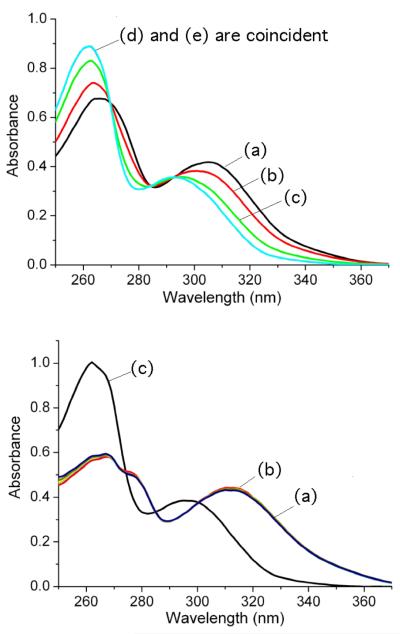
A meaningful quantitative analysis of the above absorption titration curves was not possible, primarily for two reasons — the aqueous methanol solvent was not buffered (K1 is pH dependent) and the ligand/Zn2+ association was too strong for the absorption titration method. However, semi-quantitative confirmation of the ligand/Zn2+ stabilization provided by the bridging oxyanions was gained by conducting competition experiments using ethylenediaminetetraacetic acid (EDTA) as a competitive Zn2+ binder. The top of Figure 4 shows the change in absorption spectra when a solution of L3:2•Zn(NO3)2 in water/methanol (4:1) was titrated with two molar equivalents of EDTA. The spectra clearly show that the EDTA stripped the two zinc cations and produced the free L3 ligand. In contrast, the set of spectra at the bottom of Figure 4 show that addition of two molar equivalents of Na4PPi to the solution of L3:2•Zn(NO3)2 greatly stabilized the dinuclear L3•Zn2 structure and prevented EDTA stripping of the zinc cations. Even the addition of one hundred molar equivalents of EDTA was unable to remove any measurable amount of Zn2+. The data show clearly that PPi4−, a strongly binding, bridging oxyanion, can stabilize the dinuclear L3•Zn2 complex by several orders of magnitude compared to a weakly binding anion such as NO3−.
Figure 4.
(top) Absorption spectra of L3:2•Zn(NO3)2 complex (20 μM) showing complete conversion to L3 upon addition of two molar equivalents of EDTA in water/methanol (4:1 volume ratio). (a) L3:2•Zn(NO3)2 with no EDTA, (b) L3:2•Zn(NO3)2 plus 0.5 equiv. EDTA, (c) L3:2•Zn(NO3)2 plus 1.0 equiv. EDTA, (d) L3:2•Zn(NO3)2 plus 2.0 equiv. EDTA, (e) L3. (bottom) Absorption spectra of L3:2•Zn(NO3)2 complex (20 μM) in the presence of Na4PPi (40 μM) is unchanged by the addition of one hundred molar equivalents of EDTA in water/methanol (4:1 volume ratio). (a) L3:2•Zn(NO3)2 plus Na4PPi, (b) L3:2•Zn(NO3)2 plus Na4PPi and 100 equiv. EDTA, (c) L3.
Solution-State NMR Titrations
Additional structural evidence for the bridging anion stabilization effect was obtained by monitoring analogous Zn(NO3)2 titration experiments using 1H NMR spectroscopy. Shown in Figure 5 are partial 1H NMR spectra from the titration of mono-DPA ligand L2 with Zn(NO3)2 in CD3OD:D2O (4:1). As expected, there was a smooth transition from free L2 to L2:Zn complex with very strong affinity and 1:1 ligand/zinc stoichiometry. The two pyridyl rings in the DPA unit exhibit an equivalent set of four proton chemical shifts. Figures 6–8 show 1H NMR spectra obtained during the titration of bis-DPA ligand L1 with Zn(NO3)2 in CD3OD:D2O (4:1) under three different conditions respectively: (i) no additional salt present, (ii) presence of NaOAc, and (iii) presence of Na4PPi. The peak assignments on the spectra refer to the atom labels in Scheme 2 as elucidated by analyzing COSY spectra. The partial NMR spectra in Figure 6 show the titration of L1 with Zn(NO3)2 with no other salt present. The first species to appear is the asymmetric L1:Zn complex with 1:1 stoichiometry. The most diagnostic peaks are the two aryl peaks on the central phenoxy ring which are non-equivalent at 6.98 and 7.25 ppm (assigned as f and f' on the generic mononuclear BDPA·Zn structure in Scheme 2). These peaks disappear as more Zn(NO3)2 is added, and they are replaced by the symmetric dinuclear L1:2•Zn complex. However, the pyridyl proton peaks are very broad broad, indicating a fluxional structure. The only sharp NMR peak is at 6.86 ppm and corresponds to the two equivalent protons on the central phenoxy ring (labeled as k on the generic BDPA·Zn2 structure in Scheme 2). The sodium salts of several anions were investigated to see if they influenced the zinc association equilibria. The presence of NaCl had no effect, with the titration spectra clearly showing stepwise production of the mononuclear species followed by the dinuclear complex (data not shown). In contrast, the presence of NaOAc promoted formation of the dinuclear zinc species with no evidence for any intermediate mononuclear structure. The spectra in Figure 7 show direct conversion of free L1 into the dinuclear L1:2•Zn complex with eight, broadened inequivalent pyridyl proton signals. This spectral feature is consistent with the X-ray structure in Figure 1, which is a C2 symmetric bis-acetoxy bridged complex with one zinc-coordinated pyridyl ring in pseudo axial position and the partner pyridyl ring in pseudo equatorial position. The broadness of the pyridyl peaks indicates that the pyridyl rings undergo exchange of pseudo axial/pseudo equatorial Zn2+-coordination positions. In Figure 8 is the titration of L1 with Zn(NO3)2 in the presence of Na4PPi. Again, the spectra show direct conversion of free L1 into the dinuclear L1:2•Zn complex. In this case, the eight inequivalent pyridyl peaks of the dinuclear L1:2•Zn complex are quite sharp indicating that the PPi strongly bridges the two zinc cations and produces a rigid, C2 symmetric structure. Indeed, it is extremely likely that the structure is analogous to that shown in Figure 1 with a single tetradentate PPi4− replacing the two bidentate OAc− anions.3b,3d The increased rigidity of the PPi4− bridged structure is due to the higher anionic charge and stronger tetradentate zinc-coordination by the PPi4− which prevents the zinc-oxygen bond dissociation events that allow the pyridyl rings to exchange between pseudo axial and pseudo equatorial coordination positions.
Figure 5.
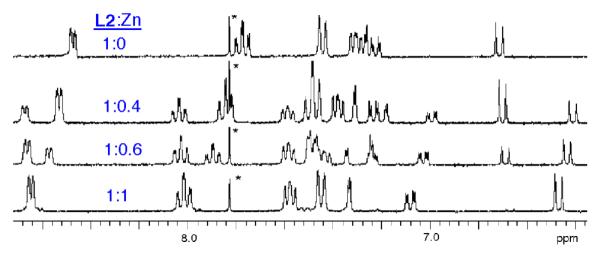
Partial 1H NMR spectra of L2 (10 mM) with increasing amounts of Zn(NO3)2 in CD3OD/D2O (4:1 volume ratio). The asterisk indicates a solvent peak.
Figure 6.
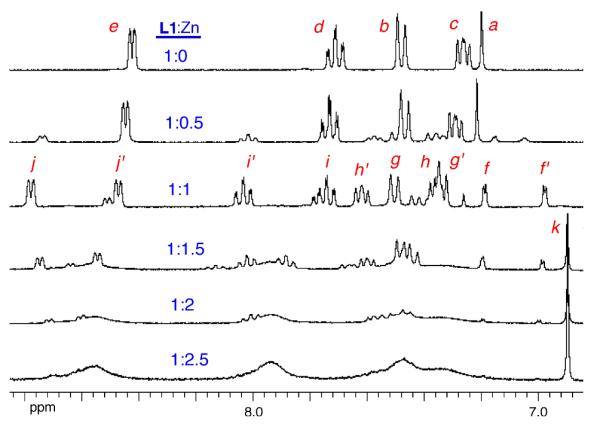
Partial 1H NMR spectra of L1 (10 mM) with increasing amounts Zn(NO3)2 in CD3OD/D2O (4:1 volume ratio).
Figure 8.
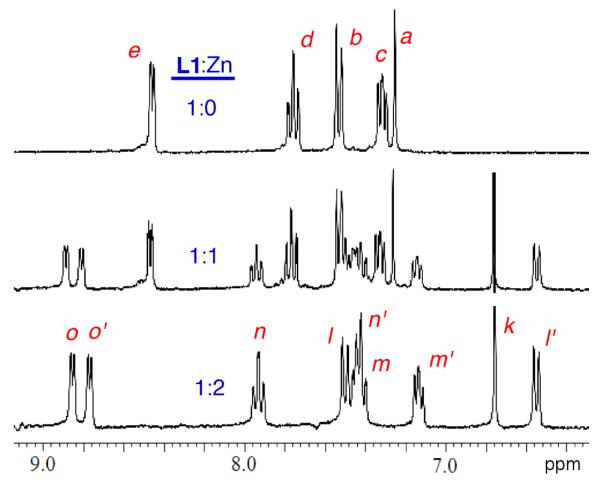
Partial 1H NMR spectra of L1 (10 mM) in the presence of Na4PPi (20 mM) with increasing amounts Zn(NO3)2 in CD3OD/D2O (4:1 volume ratio).
Figure 7.
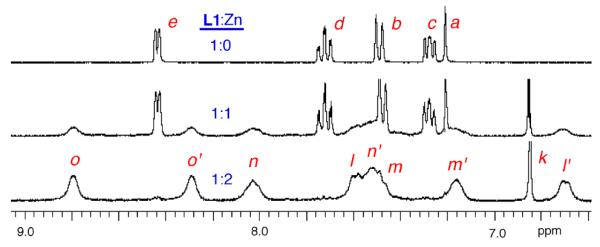
Partial 1H NMR spectra of L1 (10 mM) in the presence of NaOAc (20 mM) with increasing amounts Zn(NO3)2 in CD3OD/D2O (4:1 volume ratio).
Conclusions
The crystallographic and spectroscopic results demonstrate that strongly bridging oxyanions such as OAc− and PPi4− associate with water soluble, dinuclear BDPA·Zn2 complexes in aqueous solution and form relatively rigid, C2 symmetric coordination structures with octahedral geometries around each Zn2+ center (Figure 1).1,3b,3d It is reasonable to conclude that if the bridging anions were fatty acids or phospholipids then the resulting coordination complexes would be lipophilic enough to partition into a biomembrane. The relatively high lipophilicity of DPA ligands has been reported by nuclear imaging researchers to produce poor pharmacokinetics and they have mitigated this problem by developing DPA analogues with increased hydrophilicity.10 It should be possible to refine these hydrophilic molecular designs and produce next-generation, membrane impermeable dinuclear zinc bis-DPA complexes with improved cell recognition and bioimaging performance.
The solution-state absorption and NMR titration data show clearly that bridging oxyanions lock the two Zn2+ cations to the surrounding ligand and greatly enhance ligand/zinc affinity (Scheme 2). Thus, at sufficiently low zinc concentrations the molecular recognition process can be viewed as a three-component assembly of mononuclear zinc-ligand complex, second Zn2+ cation, and bridging oxyanion. This stepwise process can be employed to create optical anion sensor designs that use the anion-mediated association of the second zinc cation to induce a change in ligand optical properties.2c,5b At higher zinc concentrations the bis-DPA ligand is saturated with both Zn2+ cations (i.e., it exists entirely as a dinuclear BDPA•Zn2 complex) but the two sets of zinc-coordinated pyridyl rings are in a fluxional state. Association of a bridging oxyanion rigidifies the zinc-coordinated pyridyl rings, a process that may decrease non-radiative decay of excited state energy and be the basis of a “turn on” fluorescent sensor design.3c,11 In addition to anion sensing, many DPA-containing receptors have been investigated as Zn2+ cation sensors and while it has been reported that the response of a bis-DPA Zn2+ sensor can be anion dependent,12 the impact of this anion dependence on analytical performance for this class of zinc sensors has not been widely discussed. Finally, it is worth noting that the three-component molecular assembly describe here mimics a common biological strategy for targeting the surface of biomembranes.13 Metal cations often act as bridging cofactors that enable membrane association of peripheral membrane proteins (e.g., membrane targeting by annexin V mediated by Ca2+ ions).14
Experimental
Synthesis
The known ligands L3 and L4 were prepared by a literature method,3c (an improved procedure is described in reference 11) and ligands L1 and L2 were prepared by the following related procedure.
L1: A solution of 4-bromophenol (0.88 g), 1 M HCl (0.5 mL), paraformaldehyde (0.35 g) and 2,2'-dipicolylamine (2.1 g) in ethanol (20 mL) and water (40 mL) was refluxed for 36 hours, then cooled to room temperature and neutralized with Na2CO3. The neutral suspension was extracted into chloroform, dried with sodium sulfate, and the solvent was evaporated to yield a yellow oil that was purified on a silica gel column using an eluent of 97:3 CHCl3: MeOH give to give L1 as a pale yellow oil (2.5 g), 83% yield. 1H NMR (300 MHz, CDCl3, ppm): δ 8.53 (m, 4H), 7.61 (td, 4H, J1=7.8 Hz, J2=1.8 Hz), 7.44 (d, 4H, J= 8.1 Hz), 7.33 (s, 2H), 7.14 (m, 4H), 3.87 (s, 8H), 3.77 (s, 4H). 13C NMR (75 MHz, CDCl3, ppm): δ 158.9, 155.1, 148.8, 136.6, 131.4, 126.5, 122.8, 122.0, 110.2, 59.7, 54.2. MS (FAB+): HRMS C32H32BrN6O calcd 595.1821, found 595.1832.
L2: The above procedure was used with the following stoichiometry: 4-bromophenol (0.88 g), 1 M HCl (0.5 mL), paraformaldehyde (0.35 g), 2,2'-dipicolylamine (1.0 g). The same work up and purification gave L2 as pale yellow oil (1.7 g), 87 % yield. 1H NMR (300 MHz, CDCl3, ppm): δ 8.55 (m, 2H), 7.60 (td, 2H, J1=7.8 Hz, J2=1.8 Hz), 7.31 (d, 2H, J=7.8 Hz), 7.22 (dd, 1H, J1=8.7 Hz, J2=2.4 Hz), 7.14 (m, 3H), 6.80 (d, 1H, t=8.7 Hz), 3.87 (s, 4H), 3.74 (s, 2H). 13C NMR (75 MHz, CDCl3, ppm): δ 157.8, 156.7, 148.6, 136.7, 132.4, 131.5, 125.0, 122.9, 122.1, 118.3, 110.1, 58.6, 56.1. MS (FAB+): HRMS C19H19BrN3O calcd 384.0711, found 384.0699.
X-ray Crystallography
L1 Dinuclear Zinc Acetate Complex
A solution of L1 ligand and two molar equivalents of triethylamine, Zn(OAc)2, and NaBF4 in methanol was refluxed for 30 minutes and then allowed to cool to room temperature.4a Colorless crystals were formed after 24 h and they were found to be suitable for X-ray diffraction analysis. C38H44BBrF4N6O7Zn2, Mr=994.25, triclinic, unit cell dimensions a=10.2479(5), b=12.5046(6), c=17.2421(10) Å, α=69.363(3), ß=81.066(3), γ=86.542(3)°, V=2042.60(18) Å3, T=100(2) K, , Z=2. The structure was refined on F2 to wR2=0.0809, conventional R1=0.0310 [11493 reflections with I >2σ(I)], and a goodness of fit=1.055 for 10254 refined parameters. The asymmetric unit contains the Zn2L1 complex coordinated by two OAc anions, one BF4 anion, and two molecules of CH3OH. One of the CH3OH molecules is disordered over two sites, and site occupancy for the principal component is 0.872(70).
Absorption Titrations
Appropriate aliquots of Zn(NO3)2 solution (20 mM in 4:1 CH3OH:H2O volume ratio) were added to 3.0 mL solutions of ligand L3 or L4 in the presence or absence of NaCl, NaOAc, or Na4PPi in the same solvent and the absorption spectrum acquired at 25 °C.
1H NMR Titrations
Appropriate aliquots of Zn(NO3)2 solution (100 mM in 4:1 CH3OD:D2O volume ratio) were added to 0.75 mL solutions of ligand L1 or L2 in the presence or absence of NaCl, NaOAc, or Na4PPi in the same solvent and the 1H NMR spectrum acquired at 25 °C.
Acknowledgements
This work was supported by the University of Notre Dame and the NIH (USA). We are grateful to B. Noll and A. Oliver for acquiring and analyzing the X-ray data.
Footnotes
Supporting Information Available NMR assignments and CIF file for X-ray structure can be obtained via http://www.tandfonline.com. The crystallographic data is also provided in CCDC 901795, which can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB21EZ, UK; fax (+44) 1223-336-033; or deposit@ccdc.cam.uk).
Notes and References
- 1.(a) Adams H, Bradshaw D, Fenton DE. Inorg. Chim. Acta. 2002;332:195–200. [Google Scholar]; (b) Matsufuji K, Shiraishi H, Miyasato Y, Shiga T, Ohba M, Yokoyama T, Okawa H. Bull. Chem. Soc. Jpn. 2005;78:851–858. [Google Scholar]; (c) Dalgaard P, Hazell A, McKenzie CJ, Moubaraki B, Murray KS. Polyhedron. 2000;19:1909–1915. [Google Scholar]; (d) Torelli S, Belle C, Gautier-Luneau I, Pierre JL. Inorg. Chem. 2000;39:3526–3536. doi: 10.1021/ic991450z. [DOI] [PubMed] [Google Scholar]; (e) Belle C, Beguin C, Gautier-Luneau I, Hamman S, Philouze C, Pierre JL, Thomas F, Torelli S. Inorg. Chem. 2002;41:479–491. doi: 10.1021/ic010534g. [DOI] [PubMed] [Google Scholar]; (f) Suzuki M, Kanatomi H, Murase I. Chem. Lett. 1981:1745–1748. [Google Scholar]; (g) Seo JS, Sung N-D, Hynes RC, Chin J. Inorg. Chem. 1996;35:7472–7473. [Google Scholar]; (h) Maeda Y, Ishida A, Ohba M, Sugihara S, Hayami S. Bull. Chem. Soc. Jpn. 2002;75:2441–2448. [Google Scholar]; (i) Albedyhl S, Averbuch-Pouchot MT, Belle C, Krebs B, Pierre J-L, Saint-Aman E, Torelli S. Eur. J. Inorg. Chem. 2001:1457–1464. [Google Scholar]; (j) Torelli S, Belle C, Gautier-Luneau I, Hamman S, Pierre J-L. Inorg. Chim. Acta. 2002;333:144–147. [Google Scholar]; (k) Durot S, Hossain LH, Hamman S, Jamet H, Orio M, Gautier-Luneau I, Luneau D, Philouze C, Pierre J-L, Belle C. Inorg. Chem. 2010;49:7832–7840. doi: 10.1021/ic1006567. [DOI] [PubMed] [Google Scholar]
- 2.(a) Sakamoto T, Ojida A, Hamachi I. Chem. Comm. 2009;2:141–152. doi: 10.1039/b812374h. [DOI] [PubMed] [Google Scholar]; (b) Kruppa M, König B. Chem. Rev. 2006;106:3520–3560. doi: 10.1021/cr010206y. [DOI] [PubMed] [Google Scholar]; (c) O'Neil EJ, Smith BD. Coord. Chem. Rev. 2006;250:3068–3080. [Google Scholar]; (d) Kim SK, Lee DH, Hong J, Yoon J. Acc. Chem. Res. 2009;42:23–31. doi: 10.1021/ar800003f. [DOI] [PubMed] [Google Scholar]; (e) Zhou Y, Xu Z, Yoon J. Chem. Soc. Rev. 2011;40:2222–2234. doi: 10.1039/c0cs00169d. [DOI] [PubMed] [Google Scholar]; (f) Ngo HT, Liu X, Jolliffe KA. Chem. Soc. Rev. 2012;41:4928–4965. doi: 10.1039/c2cs35087d. [DOI] [PubMed] [Google Scholar]; For reviews on molecular recognition using Zn2+-DPA complexes, see:; references therein; references therein; references therein
- 3.(a) Han MS, Kim DH. Angew., Chem. Int. Ed. 2002;41:3809–3811. doi: 10.1002/1521-3773(20021018)41:20<3809::AID-ANIE3809>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]; (b) Lee DH, Im JH, Son SK, Chung YK, Hong J-I. J. Am. Chem. Soc. 2003;125:7752–7753. doi: 10.1021/ja034689u. [DOI] [PubMed] [Google Scholar]; (c) Lee DH, Kim SY, Son SK, Chung YK, Hong J-I. Angew. Chem. Int. Ed. 2004;43:4777–4780. doi: 10.1002/anie.200453914. [DOI] [PubMed] [Google Scholar]; (d) Lee JH, Park J, Lah MS, Chin J, Hong J-I. Org. Lett. 2007;9:3729–3731. doi: 10.1021/ol071306e. [DOI] [PubMed] [Google Scholar]; (e) Bae SW, Cho MS, Jeong AR, Choi BR, Kim D-E, Yeo W-S, Hong J-I. Small. 2010;6:1499–1503. doi: 10.1002/smll.201000564. [DOI] [PubMed] [Google Scholar]; (f) Oh DJ, Kim KM, Ahn KH. Chem. Asian J. 2011;6:2033–2038. doi: 10.1002/asia.201000621. [DOI] [PubMed] [Google Scholar]; (g) Liu G, Choi KY, Bhirde A, Swierczewska M, Yin J, Lee SW, Park JH, Hong J-I, Xie J, Niu G, Kiesewetter DO, Lee S, Chen X. Angew. Chem. Int. Ed. 2012;51:445–449. doi: 10.1002/anie.201105565. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Honda K, Fujishima S-H, Ojida A, Hamachi I. ChemBioChem. 2007;8:1370–1372. doi: 10.1002/cbic.200700146. [DOI] [PubMed] [Google Scholar]; (i) Ojida A, Honda K, Shimi D, Kiyonaka S, Moria Y, Hamachi I. J. Am. Chem. Soc. 2006;128:10452–10459. doi: 10.1021/ja0618604. [DOI] [PubMed] [Google Scholar]; (j) Honda K, Ojida A, Hamachi I. Chem. Comm. 2006:4024–4026. doi: 10.1039/b608684e. [DOI] [PubMed] [Google Scholar]; (k) Drewry JA, Burger S, Mazouchi A, Duodu E, Ayers P, Gradinaru CC, Gunning PT. Med. Chem. Commun. 2012;3:763–770. doi: 10.1021/ic3008393. [DOI] [PubMed] [Google Scholar]; For papers on molecular recognition using dinuclear BDPA•Zn2 complexes, see:
- 4.(a) Morrow JR, Iranzo O. Curr. Opin. Chem. Biol. 2004;8:192–200. doi: 10.1016/j.cbpa.2004.02.006. [DOI] [PubMed] [Google Scholar]; (b) Ganesh V, Bodewits K, Bartholdson SJ, Natale D, Campopiano DJ, Marque-Rivas JC. Angew. Chem. Int. Ed. 2009;48:356–370. doi: 10.1002/anie.200804168. [DOI] [PubMed] [Google Scholar]; (c) Kinoshita E, Takahashi M, Takeda H, Shiro M, Koike T. Dalton Trans. 2004:1189–1194. doi: 10.1039/b400269e. [DOI] [PubMed] [Google Scholar]; (d) Ciavatta L, Mareque JC, Natale D, Salvatore F. Ann. Chim-Rome. 2006;96:317–325. doi: 10.1002/adic.200690033. [DOI] [PubMed] [Google Scholar]; For papers on molecular recognition and catalysis using structurally related hydroxyl bridged dinuclear Zn2+-DPA complexes, see:
- 5.(a) Koulov AV, Stucker K, Lakshmi C, Robinson JP, Smith BD. Cell Death Diff. 2003;10:1357–1359. doi: 10.1038/sj.cdd.4401315. [DOI] [PubMed] [Google Scholar]; (b) Koulov AV, Hanshaw RG, Stucker KA, Lakshmi C, Smith BD. Isr. J. Chem. 2005;45:373–379. [Google Scholar]; (c) Hanshaw RG, Lakshmi C, Lambert TN, Johnson JR, Smith BD. Chem. Bio. Chem. 2005;6:2214–2220. doi: 10.1002/cbic.200500149. [DOI] [PubMed] [Google Scholar]; (d) Leevy WM, Gammon ST, Johnson JR, Lampkins AJ, Jiang H, Marquez M, Piwnica-Worms D, Smith BD. Bioconjugate Chem. 2008;19:686–692. doi: 10.1021/bc700376v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Smith BA, Akers WJ, Leevy WM, Lampkins AJ, Xiao S, Wolter W, Suckow MA, Achilefu S, Smith BD. J. Am. Chem. Soc. 2010;132:67–69. doi: 10.1021/ja908467y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Smith BA, Xiao S, Wolter W, Wheeler J, Suckow MA, Smith BD. Apoptosis. 2011;16:722–731. doi: 10.1007/s10495-011-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Smith BA, Xie B-W, van Beek ER, Que I, Blankevoort V, Xiao S, Cole EL, Hoehn. M.; Kaijzel EL, Löwik CWGM, Smith BD. ACS Chem. Neurosci. 2012;3:530–537. doi: 10.1021/cn3000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) DiVittorio KM, Leevy WM, O'Neil EJ, Johnson JR, Vakulenko S, Morris JD, Rosek KD, Serazin N, Hilkert S, Hurley S, Marquez M, Smith BD. ChemBioChem. 2008;9:286–293. doi: 10.1002/cbic.200700489. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jiang H, O'Neil EJ, DiVittorio KM, Smith BD. Org. Lett. 2005;7:3013–3016. doi: 10.1021/ol0510421. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JR, Jiang H, Smith BD. Bioconjugate Chem. 2008;19:1033–1039. doi: 10.1021/bc700466z. [DOI] [PubMed] [Google Scholar]
- 8.Reference 4d indicates that a structurally related hydroxyl bridged dinuclear Zn2+-DPA complex exhibits a similar set of stepwise equilibria for binding two Zn2+ cations.
- 9.There is no doubt that the second zinc association step subsequently occurs, however, it does not produce a detectable change in absorbance.
- 10.Maresca KP, Marquis JC, Hillier SM, Lu G, Femia FJ, Zimmerman CN, Eckelman WC, Joyal JL, Babich JW. Bioconjugate Chem. 2010;21:1032–1042. doi: 10.1021/bc900517x. [DOI] [PubMed] [Google Scholar]
- 11.Pathberiya LG, Barlow N, Nguyen T, Graham B, Tuck KL. Tetrahedron. 2012;68:9435–9439. [Google Scholar]; For a discussion of other mechanisms that may control the fluorescence signal, see:
- 12.Jang YJ, Jun EJ, Lee YJ, Kim YS, Kim JS, Yoon J. J. Org. Chem. 2005;70:9603–9606. doi: 10.1021/jo0509657. [DOI] [PubMed] [Google Scholar]
- 13.Hanshaw RG, Stahelin RV, Smith BD. Chem. – Eur. J. 2008;14:1690–1697. doi: 10.1002/chem.200701589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert TN, Smith BD. Coord. Chem. Rev. 2003;240:129–141. [Google Scholar]