Abstract
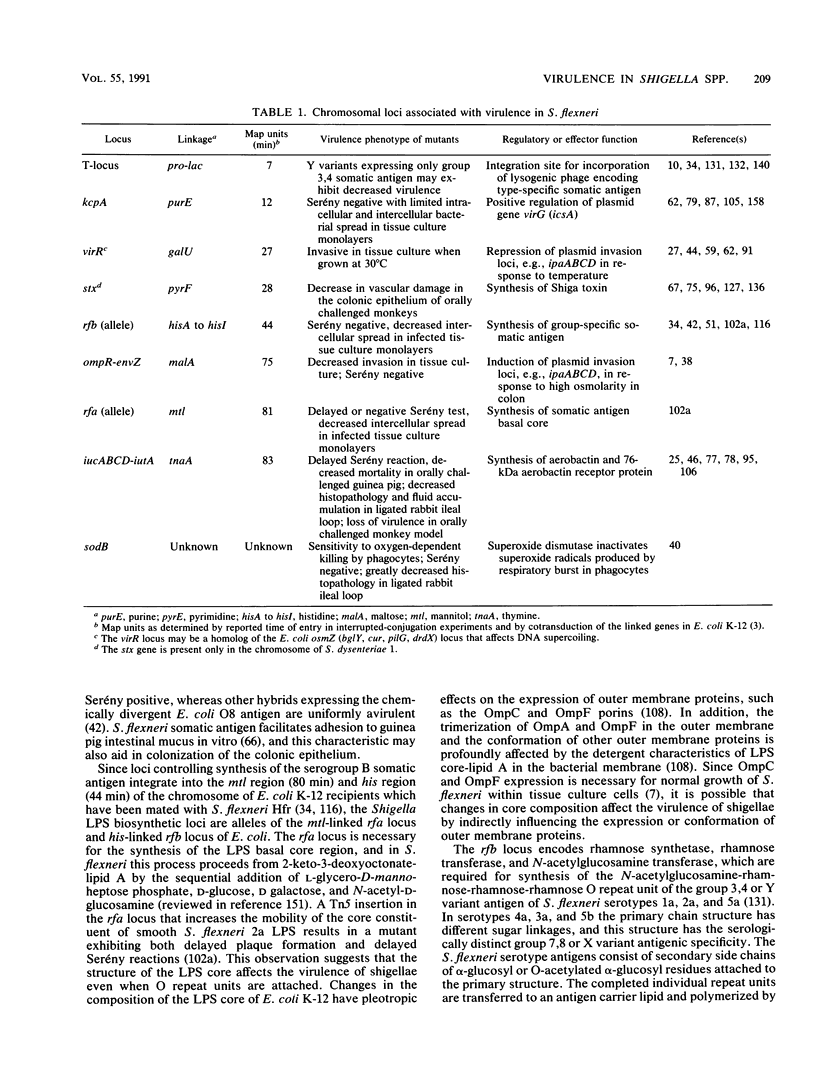
Shigella species and enteroinvasive strains of Escherichia coli cause disease by invasion of the colonic epithelium, and this invasive phenotype is mediated by genes carried on 180- to 240-kb plasmids. In addition, at least eight loci on the Shigella chromosome are necessary for full expression of virulence. The products of these genes can be classified as (i) virulence determinants that directly affect the ability of shigellae to survive in the intestinal tissues, e.g., the aerobactin siderophore (iucABCD and iutA), superoxide dismutase (sodB), and somatic antigen expression (rfa and rfb); (ii) cytotoxins that contribute to the severity of disease, e.g., the Shiga toxin (stx) and a putative analog of this toxin (flu); and (iii) regulatory loci that affect the expression of plasmid genes, e.g., ompR-envZ, which mediates response to changes in osmolarity, virR (osmZ), which mediates response to changes in temperature, and kcpA, which affects the translation of the plasmid virG (icsA) gene which is associated with intracellular bacterial mobility and intracellular bacterial spread. A single plasmid regulatory gene (virF) controls a virulence-associated plasmid regulon including virG (icsA) and two invasion-related loci, i.e., (i) ipaABCD, encoding invasion plasmid antigens that may be structural components of the Shigella invasion determinant; and (ii) invAKJH (mxi), which is necessary for insertion of invasion plasmid antigens into the outer membrane.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler B., Sasakawa C., Tobe T., Makino S., Komatsu K., Yoshikawa M. A dual transcriptional activation system for the 230 kb plasmid genes coding for virulence-associated antigens of Shigella flexneri. Mol Microbiol. 1989 May;3(5):627–635. doi: 10.1111/j.1365-2958.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Ashkenazi S., Cleary K. R., Pickering L. K., Murray B. E., Cleary T. G. The association of Shiga toxin and other cytotoxins with the neurologic manifestations of shigellosis. J Infect Dis. 1990 May;161(5):961–965. doi: 10.1093/infdis/161.5.961. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry B., Kaczorek M., Sansonetti P. J. Nucleotide sequence of the invasion plasmid antigen B and C genes (ipaB and ipaC) of Shigella flexneri. Microb Pathog. 1988 May;4(5):345–357. doi: 10.1016/0882-4010(88)90062-9. [DOI] [PubMed] [Google Scholar]
- Baudry B., Maurelli A. T., Clerc P., Sadoff J. C., Sansonetti P. J. Localization of plasmid loci necessary for the entry of Shigella flexneri into HeLa cells, and characterization of one locus encoding four immunogenic polypeptides. J Gen Microbiol. 1987 Dec;133(12):3403–3413. doi: 10.1099/00221287-133-12-3403. [DOI] [PubMed] [Google Scholar]
- Bennish M. L., Harris J. R., Wojtyniak B. J., Struelens M. Death in shigellosis: incidence and risk factors in hospitalized patients. J Infect Dis. 1990 Mar;161(3):500–506. doi: 10.1093/infdis/161.3.500. [DOI] [PubMed] [Google Scholar]
- Bernardini M. L., Fontaine A., Sansonetti P. J. The two-component regulatory system ompR-envZ controls the virulence of Shigella flexneri. J Bacteriol. 1990 Nov;172(11):6274–6281. doi: 10.1128/jb.172.11.6274-6281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. E., Craun G. F., Blake P. A. Epidemiology of common-source outbreaks of shigellosis in the United States, 1961-1975. Am J Epidemiol. 1978 Jul;108(1):47–52. [PubMed] [Google Scholar]
- Blaser M. J., Hale T. L., Formal S. B. Recurrent shigellosis complicating human immunodeficiency virus infection: failure of pre-existing antibodies to confer protection. Am J Med. 1989 Jan;86(1):105–107. doi: 10.1016/0002-9343(89)90239-8. [DOI] [PubMed] [Google Scholar]
- Bunning V. K., Raybourne R. B., Archer D. L. Foodborne enterobacterial pathogens and rheumatoid disease. Soc Appl Bacteriol Symp Ser. 1988;17:87S–107S. [PubMed] [Google Scholar]
- Butler T., Speelman P., Kabir I., Banwell J. Colonic dysfunction during shigellosis. J Infect Dis. 1986 Nov;154(5):817–824. doi: 10.1093/infdis/154.5.817. [DOI] [PubMed] [Google Scholar]
- Buysse J. M., Stover C. K., Oaks E. V., Venkatesan M., Kopecko D. J. Molecular cloning of invasion plasmid antigen (ipa) genes from Shigella flexneri: analysis of ipa gene products and genetic mapping. J Bacteriol. 1987 Jun;169(6):2561–2569. doi: 10.1128/jb.169.6.2561-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse J. M., Venkatesan M., Mills J. A., Oaks E. V. Molecular characterization of a trans-acting, positive effector (ipaR) of invasion plasmid antigen synthesis in Shigella flexneri serotype 5. Microb Pathog. 1990 Mar;8(3):197–211. doi: 10.1016/0882-4010(90)90047-t. [DOI] [PubMed] [Google Scholar]
- Clerc P. L., Berthon B., Claret M., Sansonetti P. J. Internalization of Shigella flexneri into HeLa cells occurs without an increase in cytosolic Ca2+ concentration. Infect Immun. 1989 Sep;57(9):2919–2922. doi: 10.1128/iai.57.9.2919-2922.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc P. L., Ryter A., Mounier J., Sansonetti P. J. Plasmid-mediated early killing of eucaryotic cells by Shigella flexneri as studied by infection of J774 macrophages. Infect Immun. 1987 Mar;55(3):521–527. doi: 10.1128/iai.55.3.521-527.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc P. L., Sansonetti P. J. Evidence for clathrin mobilization during directed phagocytosis of Shigella flexneri by HEp2 cells. Microb Pathog. 1989 Nov;7(5):329–336. doi: 10.1016/0882-4010(89)90036-3. [DOI] [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskaleros P. A., Payne S. M. Characterization of Shigella flexneri sequences encoding congo red binding (crb): conservation of multiple crb sequences and role of IS1 in loss of the Crb+ phenotype. Infect Immun. 1986 Nov;54(2):435–443. doi: 10.1128/iai.54.2.435-443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskaleros P. A., Payne S. M. Cloning the gene for Congo red binding in Shigella flexneri. Infect Immun. 1985 Apr;48(1):165–168. doi: 10.1128/iai.48.1.165-168.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskaleros P. A., Payne S. M. Congo red binding phenotype is associated with hemin binding and increased infectivity of Shigella flexneri in the HeLa cell model. Infect Immun. 1987 Jun;55(6):1393–1398. doi: 10.1128/iai.55.6.1393-1398.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire P., Baldwin T., Stevenson P., Griffiths E., Roberts M., Williams P., Hale T. L., Formal S. B. Expression in Escherichia coli K-12 of the 76,000-dalton iron-regulated outer membrane protein of Shigella flexneri confers sensitivity to cloacin DF13 in the absence of Shigella O antigen. Infect Immun. 1989 Sep;57(9):2794–2798. doi: 10.1128/iai.57.9.2794-2798.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman C. J., Ni Bhriain N., Higgins C. F. DNA supercoiling and environmental regulation of virulence gene expression in Shigella flexneri. Nature. 1990 Apr 19;344(6268):789–792. doi: 10.1038/344789a0. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Levine M. M., Hornick R. B., Formal S. B. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis. 1989 Jun;159(6):1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]
- Eiklid K., Olsnes S. Animal toxicity of Shigella dysenteriae cytotoxin: evidence that the neurotoxic, enterotoxic, and cytotoxic activities are due to one toxin. J Immunol. 1983 Jan;130(1):380–384. [PubMed] [Google Scholar]
- Endo Y., Tsurugi K., Yutsudo T., Takeda Y., Ogasawara T., Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem. 1988 Jan 15;171(1-2):45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- FALKOW S., SCHNEIDER H., BARON L. S., FORMAL S. B. VIRULENCE OF ESCHERICHIA-SHIGELLA GENETIC HYBRIDS FOR THE GUINEA PIG. J Bacteriol. 1963 Dec;86:1251–1258. doi: 10.1128/jb.86.6.1251-1258.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORMAL S. B., LABREC E. H., FALKOW S. RESTORATION OF VIRULENCE TO A STRAIN OF SHIGELLA FLEXNERI BY MATING WITH ESCHERICHIA COLI. J Bacteriol. 1965 Mar;89:835–838. doi: 10.1128/jb.89.3.835-838.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORMAL S. B., LABREC E. H., KENT T. H., FALKOW S. ABORTIVE INTESTINAL INFECTION WITH AN ESCHERICHIA COLI-SHIGELLA FLEXNERI HYBRID STRAIN. J Bacteriol. 1965 May;89:1374–1382. doi: 10.1128/jb.89.5.1374-1382.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A., Kay B. A., Russell R. G., Maneval D. R., Jr, Levin M. M. Enterotoxin and cytotoxin production by enteroinvasive Escherichia coli. Infect Immun. 1990 Nov;58(11):3717–3723. doi: 10.1128/iai.58.11.3717-3723.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine A., Arondel J., Sansonetti P. J. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a Tox- mutant of Shigella dysenteriae 1. Infect Immun. 1988 Dec;56(12):3099–3109. doi: 10.1128/iai.56.12.3099-3109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Gemski P., Baron L. S., Labrec E. H. A Chromosomal Locus Which Controls the Ability of Shigella flexneri to Evoke Keratoconjunctivitis. Infect Immun. 1971 Jan;3(1):73–79. doi: 10.1128/iai.3.1.73-79.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Gemski P., Baron L. S., Labrec E. H. Genetic Transfer of Shigella flexneri Antigens to Escherichia coli K-12. Infect Immun. 1970 Mar;1(3):279–287. doi: 10.1128/iai.1.3.279-287.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Labrec E. H., Palmer A., Falkow S. Protection of Monkeys Against Experimental Shigellosis with Attenuated Vaccines. J Bacteriol. 1965 Jul;90(1):63–68. doi: 10.1128/jb.90.1.63-68.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzon V. L., Arondel J., Sansonetti P. J. Contribution of superoxide dismutase and catalase activities to Shigella flexneri pathogenesis. Infect Immun. 1990 Feb;58(2):529–535. doi: 10.1128/iai.58.2.529-535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERBER D. F., WATKINS H. M. Growth of shigellae in monolayer tissue cultures. J Bacteriol. 1961 Dec;82:815–822. doi: 10.1128/jb.82.6.815-822.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Jr, Koeltzow D. E., Formal S. B. Phage conversion of Shigella flexneri group antigens. Infect Immun. 1975 Apr;11(4):685–691. doi: 10.1128/iai.11.4.685-691.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Jr, Sheahan D. G., Washington O., Formal S. B. Virulence of Shigella flexneri hybrids expressing Escherichia coli somatic antigens. Infect Immun. 1972 Aug;6(2):104–111. doi: 10.1128/iai.6.2.104-111.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots R. E., Formal S. B., Giannella R. A. Indomethacin inhibition of Salmonella typhimurium, Shigella flexneri, and cholera-mediated rabbit ileal secretion. J Infect Dis. 1974 Sep;130(3):280–284. doi: 10.1093/infdis/130.3.280. [DOI] [PubMed] [Google Scholar]
- Griffiths E., Stevenson P., Hale T. L., Formal S. B. Synthesis of aerobactin and a 76,000-dalton iron-regulated outer membrane protein by Escherichia coli K-12-Shigella flexneri hybrids and by enteroinvasive strains of Escherichia coli. Infect Immun. 1985 Jul;49(1):67–71. doi: 10.1128/iai.49.1.67-71.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göransson M., Sondén B., Nilsson P., Dagberg B., Forsman K., Emanuelsson K., Uhlin B. E. Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature. 1990 Apr 12;344(6267):682–685. doi: 10.1038/344682a0. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Formal S. B. Protein synthesis in HeLa or Henle 407 cells infected with Shigella dysenteriae 1, Shigella flexneri 2a, or Salmonella typhimurium W118. Infect Immun. 1981 Apr;32(1):137–144. doi: 10.1128/iai.32.1.137-144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Guerry P., Seid R. C., Jr, Kapfer C., Wingfield M. E., Reaves C. B., Baron L. S., Formal S. B. Expression of lipopolysaccharide O antigen in Escherichia coli K-12 hybrids containing plasmid and chromosomal genes from Shigella dysenteriae 1. Infect Immun. 1984 Nov;46(2):470–475. doi: 10.1128/iai.46.2.470-475.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Oaks E. V., Formal S. B. Identification and antigenic characterization of virulence-associated, plasmid-coded proteins of Shigella spp. and enteroinvasive Escherichia coli. Infect Immun. 1985 Dec;50(3):620–629. doi: 10.1128/iai.50.3.620-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Sansonetti P. J., Schad P. A., Austin S., Formal S. B. Characterization of virulence plasmids and plasmid-associated outer membrane proteins in Shigella flexneri, Shigella sonnei, and Escherichia coli. Infect Immun. 1983 Apr;40(1):340–350. doi: 10.1128/iai.40.1.340-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman A. B., Venkatesan M., Oaks E. V., Buysse J. M. Sequence and molecular characterization of a multicopy invasion plasmid antigen gene, ipaH, of Shigella flexneri. J Bacteriol. 1990 Apr;172(4):1905–1915. doi: 10.1128/jb.172.4.1905-1915.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley V. L., Payne S. M. Differential protein expression by Shigella flexneri in intracellular and extracellular environments. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4179–4183. doi: 10.1073/pnas.87.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Hromockyj A. E., Maurelli A. T. Identification of Shigella invasion genes by isolation of temperature-regulated inv::lacZ operon fusions. Infect Immun. 1989 Oct;57(10):2963–2970. doi: 10.1128/iai.57.10.2963-2970.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromockyj A. E., Maurelli A. T. Identification of an Escherichia coli gene homologous to virR, a regulator of Shigella virulence. J Bacteriol. 1989 May;171(5):2879–2881. doi: 10.1128/jb.171.5.2879-2881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulton C. S., Seirafi A., Hinton J. C., Sidebotham J. M., Waddell L., Pavitt G. D., Owen-Hughes T., Spassky A., Buc H., Higgins C. F. Histone-like protein H1 (H-NS), DNA supercoiling, and gene expression in bacteria. Cell. 1990 Nov 2;63(3):631–642. doi: 10.1016/0092-8674(90)90458-q. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Cultured mammalian cells attach to the invasin protein of Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6682–6686. doi: 10.1073/pnas.85.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990 Mar 9;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Izhar M., Nuchamowitz Y., Mirelman D. Adherence of Shigella flexneri to guinea pig intestinal cells is mediated by a mucosal adhesion. Infect Immun. 1982 Mar;35(3):1110–1118. doi: 10.1128/iai.35.3.1110-1118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. P. Structure-function analyses of Shiga toxin and the Shiga-like toxins. Microb Pathog. 1990 Apr;8(4):235–242. doi: 10.1016/0882-4010(90)90050-z. [DOI] [PubMed] [Google Scholar]
- Katakura S., Reinholt F. P., Kärnell A., Huan P. T., Trach D. D., Lindberg A. A. The pathology of Shigella flexneri infection in rhesus monkeys: an endoscopic and histopathological study of colonic lesions. APMIS. 1990 Apr;98(4):313–319. doi: 10.1111/j.1699-0463.1990.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Kato J., Ito K., Nakamura A., Watanabe H. Cloning of regions required for contact hemolysis and entry into LLC-MK2 cells from Shigella sonnei form I plasmid: virF is a positive regulator gene for these phenotypes. Infect Immun. 1989 May;57(5):1391–1398. doi: 10.1128/iai.57.5.1391-1398.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Grady G. F., Mata L. J., McIver J. The pathogenesis of Shigella diarrhea. I. Enterotoxin production by Shigella dysenteriae I. J Clin Invest. 1972 May;51(5):1212–1218. doi: 10.1172/JCI106915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R., Corwin L. M. Mutation in Shigella flexneri resulting in loss of ability to penetrate HeLa cells and loss of glycerol kinase activity. Infect Immun. 1974 May;9(5):916–923. doi: 10.1128/iai.9.5.916-923.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Holcombe J., Formal S. B. Molecular characterization of plasmids from virulent and spontaneously occurring avirulent colonial variants of Shigella flexneri. Infect Immun. 1979 May;24(2):580–582. doi: 10.1128/iai.24.2.580-582.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Washington O., Formal S. B. Genetic and physical evidence for plasmid control of Shigella sonnei form I cell surface antigen. Infect Immun. 1980 Jul;29(1):207–214. doi: 10.1128/iai.29.1.207-214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster F., Levin J., Walker L., Tung K. S., Gilman R. H., Rahaman M. M., Majid M. A., Islam S., Williams R. C., Jr Hemolytic-uremic syndrome after shigellosis. Relation to endotoxemia and circulating immune complexes. N Engl J Med. 1978 Apr 27;298(17):927–933. doi: 10.1056/NEJM197804272981702. [DOI] [PubMed] [Google Scholar]
- Kozlov YuV, Kabishev A. A., Lukyanov E. V., Bayev A. A. The primary structure of the operons coding for Shigella dysenteriae toxin and temperature phage H30 shiga-like toxin. Gene. 1988 Jul 30;67(2):213–221. doi: 10.1016/0378-1119(88)90398-8. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrec E. H., Schneider H., Magnani T. J., Formal S. B. EPITHELIAL CELL PENETRATION AS AN ESSENTIAL STEP IN THE PATHOGENESIS OF BACILLARY DYSENTERY. J Bacteriol. 1964 Nov;88(5):1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor K. M., Daskaleros P. A., Robinson R. E., Payne S. M. Virulence of iron transport mutants of Shigella flexneri and utilization of host iron compounds. Infect Immun. 1987 Mar;55(3):594–599. doi: 10.1128/iai.55.3.594-599.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor K. M., Payne S. M. Aerobactin genes in Shigella spp. J Bacteriol. 1984 Oct;160(1):266–272. doi: 10.1128/jb.160.1.266-272.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. M., Fournier R. S., Isberg R. R. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 1990 Jun;9(6):1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett M. C., Sasakawa C., Okada N., Sakai T., Makino S., Yamada M., Komatsu K., Yoshikawa M. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J Bacteriol. 1989 Jan;171(1):353–359. doi: 10.1128/jb.171.1.353-359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. Y., Reisner B. S., Straley S. C. YopM inhibits platelet aggregation and is necessary for virulence of Yersinia pestis in mice. Infect Immun. 1990 Oct;58(10):3262–3271. doi: 10.1128/iai.58.10.3262-3271.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. Y., Straley S. C. The yopM gene of Yersinia pestis encodes a released protein having homology with the human platelet surface protein GPIb alpha. J Bacteriol. 1989 Sep;171(9):4623–4632. doi: 10.1128/jb.171.9.4623-4632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., DuPont H. L., Formal S. B., Hornick R. B., Takeuchi A., Gangarosa E. J., Snyder M. J., Libonati J. P. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis. 1973 Mar;127(3):261–270. doi: 10.1093/infdis/127.3.261. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Brown J. E., Strömberg N., Westling-Ryd M., Schultz J. E., Karlsson K. A. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987 Feb 5;262(4):1779–1785. [PubMed] [Google Scholar]
- Lopez E. L., Diaz M., Grinstein S., Devoto S., Mendilaharzu F., Murray B. E., Ashkenazi S., Rubeglio E., Woloj M., Vasquez M. Hemolytic uremic syndrome and diarrhea in Argentine children: the role of Shiga-like toxins. J Infect Dis. 1989 Sep;160(3):469–475. doi: 10.1093/infdis/160.3.469. [DOI] [PubMed] [Google Scholar]
- Makino S., Sasakawa C., Kamata K., Kurata T., Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986 Aug 15;46(4):551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- Makino S., Sasakawa C., Yoshikawa M. Genetic relatedness of the basic replicon of the virulence plasmid in shigellae and enteroinvasive Escherichia coli. Microb Pathog. 1988 Oct;5(4):267–274. doi: 10.1016/0882-4010(88)90099-x. [DOI] [PubMed] [Google Scholar]
- Maurelli A. T., Baudry B., d'Hauteville H., Hale T. L., Sansonetti P. J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985 Jul;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T. Regulation of virulence genes in Shigella. Mol Biol Med. 1989 Oct;6(5):425–432. [PubMed] [Google Scholar]
- Maurelli A. T., Sansonetti P. J. Identification of a chromosomal gene controlling temperature-regulated expression of Shigella virulence. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2820–2824. doi: 10.1073/pnas.85.8.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. A., Buysse J. M., Oaks E. V. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect Immun. 1988 Nov;56(11):2933–2941. doi: 10.1128/iai.56.11.2933-2941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Intracellular parasitism: life in an extreme environment. J Infect Dis. 1974 Sep;130(3):300–306. doi: 10.1093/infdis/130.3.300. [DOI] [PubMed] [Google Scholar]
- Mounier J., Ryter A., Coquis-Rondon M., Sansonetti P. J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif X., Mazert M. C., Mounier J., Sansonetti P. J. Evaluation with an iuc::Tn10 mutant of the role of aerobactin production in the virulence of Shigella flexneri. Infect Immun. 1987 Sep;55(9):1963–1969. doi: 10.1128/iai.55.9.1963-1969.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill R. J., Gemski P., Formal S. B., Newland J. W. Deletion of the Shiga toxin gene in a chlorate-resistant derivative of Shigella dysenteriae type 1 that retains virulence. J Infect Dis. 1988 Oct;158(4):737–741. doi: 10.1093/infdis/158.4.737. [DOI] [PubMed] [Google Scholar]
- Newland J. W., Neill R. J. DNA probes for Shiga-like toxins I and II and for toxin-converting bacteriophages. J Clin Microbiol. 1988 Jul;26(7):1292–1297. doi: 10.1128/jcm.26.7.1292-1297.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., LaVeck G. D., Thompson M. R., Formal S. B. Production of Shigella dysenteriae type 1-like cytotoxin by Escherichia coli. J Infect Dis. 1982 Dec;146(6):763–769. doi: 10.1093/infdis/146.6.763. [DOI] [PubMed] [Google Scholar]
- Oaks E. V., Hale T. L., Formal S. B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986 Jul;53(1):57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks E. V., Wingfield M. E., Formal S. B. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985 Apr;48(1):124–129. doi: 10.1128/iai.48.1.124-129.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig T. G., Del Vecchio P. J., Brown J. E., Moran T. P., Rowland B. M., Judge T. K., Rothman S. W. Direct cytotoxic action of Shiga toxin on human vascular endothelial cells. Infect Immun. 1988 Sep;56(9):2373–2378. doi: 10.1128/iai.56.9.2373-2378.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Nakamura A., Nakaya R. Cinemicrographic study of tissue cell cultures infected with Shigella flexneri. Jpn J Med Sci Biol. 1968 Aug;21(4):259–273. doi: 10.7883/yoken1952.21.259. [DOI] [PubMed] [Google Scholar]
- Okada N., Sasakawa C., Tobe T., Yamada M., Nagai S., Talukder K. A., Komatsu K., Kanegasaki S., Yoshikawa M. Virulence-associated chromosomal loci of Shigella flexneri identified by random Tn5 insertion mutagenesis. Mol Microbiol. 1991 Jan;5(1):187–195. doi: 10.1111/j.1365-2958.1991.tb01839.x. [DOI] [PubMed] [Google Scholar]
- Okamura N., Nagai T., Nakaya R., Kondo S., Murakami M., Hisatsune K. HeLa cell invasiveness and O antigen of Shigella flexneri as separate and prerequisite attributes of virulence to evoke keratoconjunctivitis in guinea pigs. Infect Immun. 1983 Feb;39(2):505–513. doi: 10.1128/iai.39.2.505-513.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M. Iron and virulence in Shigella. Mol Microbiol. 1989 Sep;3(9):1301–1306. doi: 10.1111/j.1365-2958.1989.tb00281.x. [DOI] [PubMed] [Google Scholar]
- Prado D., Cleary T. G., Pickering L. K., Ericsson C. D., Bartlett A. V., 3rd, DuPont H. L., Johnson P. C. The relation between production of cytotoxin and clinical features in shigellosis. J Infect Dis. 1986 Jul;154(1):149–155. doi: 10.1093/infdis/154.1.149. [DOI] [PubMed] [Google Scholar]
- Pál T., Hale T. L. Plasmid-associated adherence of Shigella flexneri in a HeLa cell model. Infect Immun. 1989 Aug;57(8):2580–2582. doi: 10.1128/iai.57.8.2580-2582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pál T., Newland J. W., Tall B. D., Formal S. B., Hale T. L. Intracellular spread of Shigella flexneri associated with the kcpA locus and a 140-kilodalton protein. Infect Immun. 1989 Feb;57(2):477–486. doi: 10.1128/iai.57.2.477-486.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried G., Hindennach I., Henning U. Role of lipopolysaccharide in assembly of Escherichia coli outer membrane proteins OmpA, OmpC, and OmpF. J Bacteriol. 1990 Oct;172(10):6048–6053. doi: 10.1128/jb.172.10.6048-6053.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde J. E. Selective primary health care: strategies for control of disease in the developing world. XV. Acute diarrhea. Rev Infect Dis. 1984 Nov-Dec;6(6):840–854. doi: 10.1093/clinids/6.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout W. R., Formal S. B., Giannella R. A., Dammin G. J. Pathophysiology of Shigella diarrhea in the rhesus monkey: intestinal transport, morphological, and bacteriological studies. Gastroenterology. 1975 Feb;68(2):270–278. [PubMed] [Google Scholar]
- Sakai T., Sasakawa C., Makino S., Kamata K., Yoshikawa M. Molecular cloning of a genetic determinant for Congo red binding ability which is essential for the virulence of Shigella flexneri. Infect Immun. 1986 Feb;51(2):476–482. doi: 10.1128/iai.51.2.476-482.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Sasakawa C., Makino S., Yoshikawa M. DNA sequence and product analysis of the virF locus responsible for congo red binding and cell invasion in Shigella flexneri 2a. Infect Immun. 1986 Nov;54(2):395–402. doi: 10.1128/iai.54.2.395-402.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Sasakawa C., Yoshikawa M. Expression of four virulence antigens of Shigella flexneri is positively regulated at the transcriptional level by the 30 kiloDalton virF protein. Mol Microbiol. 1988 Sep;2(5):589–597. doi: 10.1111/j.1365-2958.1988.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Sankaran K., Ramachandran V., Subrahmanyam Y. V., Rajarathnam S., Elango S., Roy R. K. Congo red-mediated regulation of levels of Shigella flexneri 2a membrane proteins. Infect Immun. 1989 Aug;57(8):2364–2371. doi: 10.1128/iai.57.8.2364-2371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Hale T. L., Dammin G. J., Kapfer C., Collins H. H., Jr, Formal S. B. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun. 1983 Mar;39(3):1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun. 1981 Oct;34(1):75–83. doi: 10.1128/iai.34.1.75-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Mounier J. Metabolic events mediating early killing of host cells infected by Shigella flexneri. Microb Pathog. 1987 Jul;3(1):53–61. doi: 10.1016/0882-4010(87)90037-4. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., d'Hauteville H., Ecobichon C., Pourcel C. Molecular comparison of virulence plasmids in Shigella and enteroinvasive Escherichia coli. Ann Microbiol (Paris) 1983 May-Jun;134A(3):295–318. [PubMed] [Google Scholar]
- Sasakawa C., Adler B., Tobe T., Okada N., Nagai S., Komatsu K., Yoshikawa M. Functional organization and nucleotide sequence of virulence Region-2 on the large virulence plasmid in Shigella flexneri 2a. Mol Microbiol. 1989 Sep;3(9):1191–1201. doi: 10.1111/j.1365-2958.1989.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Makino S., Yamada M., Okada N., Yoshikawa M. Virulence-associated genetic regions comprising 31 kilobases of the 230-kilobase plasmid in Shigella flexneri 2a. J Bacteriol. 1988 Jun;170(6):2480–2484. doi: 10.1128/jb.170.6.2480-2484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Murayama S. Y., Makino S., Yoshikawa M. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect Immun. 1986 Feb;51(2):470–475. doi: 10.1128/iai.51.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Makino S., Kamata K., Yoshikawa M. Isolation, characterization, and mapping of Tn5 insertions into the 140-megadalton invasion plasmid defective in the mouse Sereny test in Shigella flexneri 2a. Infect Immun. 1986 Oct;54(1):32–36. doi: 10.1128/iai.54.1.32-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P., Payne S. M. Genetics and regulation of enterobactin genes in Shigella flexneri. J Bacteriol. 1988 Dec;170(12):5579–5587. doi: 10.1128/jb.170.12.5579-5587.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekizaki T., Harayama S., Brazil G. M., Timmis K. N. Localization of stx, a determinant essential for high-level production of shiga toxin by Shigella dysenteriae serotype 1, near pyrF and generation of stx transposon mutants. Infect Immun. 1987 Sep;55(9):2208–2214. doi: 10.1128/iai.55.9.2208-2214.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltmann G., Pál T., Tschäpe H. Surface hydrophobicity of plasmid-carrying virulent Shigella flexneri and their avirulent variants. J Basic Microbiol. 1986;26(5):283–287. doi: 10.1002/jobm.3620260508. [DOI] [PubMed] [Google Scholar]
- Sharon P., Ligumsky M., Rachmilewitz D., Zor U. Role of prostaglandins in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine. Gastroenterology. 1978 Oct;75(4):638–640. [PubMed] [Google Scholar]
- Silva R. M., Saadi S., Maas W. K. A basic replicon of virulence-associated plasmids of Shigella spp. and enteroinvasive Escherichia coli is homologous with a basic replicon in plasmids of IncF groups. Infect Immun. 1988 Apr;56(4):836–842. doi: 10.1128/iai.56.4.836-842.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. A. Immunochemistry of Shigella flexneri O-antigens: a study of structural and genetic aspects of the biosynthesis of cell-surface antigens. Bacteriol Rev. 1971 Jun;35(2):117–148. doi: 10.1128/br.35.2.117-148.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. A., Romanowska E. Structure and biology of Shigella flexneri O antigens. J Med Microbiol. 1987 Jun;23(4):289–302. doi: 10.1099/00222615-23-4-289. [DOI] [PubMed] [Google Scholar]
- Speelman P., Kabir I., Islam M. Distribution and spread of colonic lesions in shigellosis: a colonoscopic study. J Infect Dis. 1984 Dec;150(6):899–903. doi: 10.1093/infdis/150.6.899. [DOI] [PubMed] [Google Scholar]
- Stieglitz H., Fosmire S., Lipsky P. Identification of a 2-Md plasmid from Shigella flexneri associated with reactive arthritis. Arthritis Rheum. 1989 Aug;32(8):937–946. doi: 10.1002/anr.1780320802. [DOI] [PubMed] [Google Scholar]
- Stoll B. J., Glass R. I., Huq M. I., Khan M. U., Banu H., Holt J. Epidemiologic and clinical features of patients infected with Shigella who attended a diarrheal disease hospital in Bangladesh. J Infect Dis. 1982 Aug;146(2):177–183. doi: 10.1093/infdis/146.2.177. [DOI] [PubMed] [Google Scholar]
- Strockbine N. A., Jackson M. P., Sung L. M., Holmes R. K., O'Brien A. D. Cloning and sequencing of the genes for Shiga toxin from Shigella dysenteriae type 1. J Bacteriol. 1988 Mar;170(3):1116–1122. doi: 10.1128/jb.170.3.1116-1122.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stugard C. E., Daskaleros P. A., Payne S. M. A 101-kilodalton heme-binding protein associated with congo red binding and virulence of Shigella flexneri and enteroinvasive Escherichia coli strains. Infect Immun. 1989 Nov;57(11):3534–3539. doi: 10.1128/iai.57.11.3534-3539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Formal S. B., Sprinz H. Exerimental acute colitis in the Rhesus monkey following peroral infection with Shigella flexneri. An electron microscope study. Am J Pathol. 1968 Mar;52(3):503–529. [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timakov V. D., Petrovskaya V. G., Bondarenko V. M. Studies on the genetic control of shigella sub-group B type specific antigens. I. Behaviour of Shigella flexneri type specific antigens in sexual recombination of Shigella x E. coli. Ann Inst Pasteur (Paris) 1970 Jan;118(1):3–9. [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem. 1989 Jun 25;264(18):10867–10871. [PubMed] [Google Scholar]
- VOINO-IASENETSKII M. V., KHAVKIN T. N. IZUCHENIE VNUTRI'EPITELIAL'NO I LOKALIZATSII VOZBUDITELE I DIZENTERII PRI POMOSHCHI FLUORESTSIRUIUSHCHIKH ANTITEL. Zh Mikrobiol Epidemiol Immunobiol. 1964 Apr;41:98–100. [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Kopecko D. J. Characterization of invasion plasmid antigen genes (ipaBCD) from Shigella flexneri. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9317–9321. doi: 10.1073/pnas.85.23.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Kopecko D. J. Use of Shigella flexneri ipaC and ipaH gene sequences for the general identification of Shigella spp. and enteroinvasive Escherichia coli. J Clin Microbiol. 1989 Dec;27(12):2687–2691. doi: 10.1128/jcm.27.12.2687-2691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M. Nucleotide sequence of invasion plasmid antigen gene ipaA from Shigella flexneri 5. Nucleic Acids Res. 1990 Mar 25;18(6):1648–1648. doi: 10.1093/nar/18.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M., Buysse J. M., Vandendries E., Kopecko D. J. Development and testing of invasion-associated DNA probes for detection of Shigella spp. and enteroinvasive Escherichia coli. J Clin Microbiol. 1988 Feb;26(2):261–266. doi: 10.1128/jcm.26.2.261-266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS H. M. Some attributes of virulence in Shigella. Ann N Y Acad Sci. 1960 Nov 21;88:1167–1186. doi: 10.1111/j.1749-6632.1960.tb20107.x. [DOI] [PubMed] [Google Scholar]
- Wassef J. S., Keren D. F., Mailloux J. L. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989 Mar;57(3):858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Arakawa E., Ito K., Kato J., Nakamura A. Genetic analysis of an invasion region by use of a Tn3-lac transposon and identification of a second positive regulator gene, invE, for cell invasion of Shigella sonnei: significant homology of invE with ParB of plasmid P1. J Bacteriol. 1990 Feb;172(2):619–629. doi: 10.1128/jb.172.2.619-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H. Genetics of virulence of Shigella species. Microbiol Sci. 1988 Oct;5(10):307–310. [PubMed] [Google Scholar]
- Watanabe H., Nakamura A. Identification of Shigella sonnei form I plasmid genes necessary for cell invasion and their conservation among Shigella species and enteroinvasive Escherichia coli. Infect Immun. 1986 Aug;53(2):352–358. doi: 10.1128/iai.53.2.352-358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Nakamura A. Large plasmids associated with virulence in Shigella species have a common function necessary for epithelial cell penetration. Infect Immun. 1985 Apr;48(1):260–262. doi: 10.1128/iai.48.1.260-262.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Nakamura A., Timmis K. N. Small virulence plasmid of Shigella dysenteriae 1 strain W30864 encodes a 41,000-dalton protein involved in formation of specific lipopolysaccharide side chains of serotype 1 isolates. Infect Immun. 1984 Oct;46(1):55–63. doi: 10.1128/iai.46.1.55-63.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Timmis K. N. A small plasmid in Shigella dysenteriae 1 specifies one or more functions essential for O antigen production and bacterial virulence. Infect Immun. 1984 Jan;43(1):391–396. doi: 10.1128/iai.43.1.391-396.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton M., Spiegel R. A., Horan J. M., Tauxe R. V., Wells J. G., Barg N., Herndon J., Meriwether R. A., MacCormack J. N., Levine R. H. A large outbreak of antibiotic-resistant shigellosis at a mass gathering. J Infect Dis. 1990 Dec;162(6):1324–1328. doi: 10.1093/infdis/162.6.1324. [DOI] [PubMed] [Google Scholar]
- Williams K. M., Raybourne R. B. Demonstration of cross-reactivity between bacterial antigens and class I human leukocyte antigens by using monoclonal antibodies to Shigella flexneri. Infect Immun. 1990 Jun;58(6):1774–1781. doi: 10.1128/iai.58.6.1774-1781.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Sasakawa C., Okada N., Makino S. I., Yoshikawa M. Molecular cloning and characterization of chromosomal virulence region kcpA of Shigella flexneri. Mol Microbiol. 1989 Feb;3(2):207–213. doi: 10.1111/j.1365-2958.1989.tb01809.x. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Sasakawa C., Makino S., Okada N., Lett M. C., Sakai T., Yamada M., Komatsu K., Kamata K., Kurata T. Molecular genetic approaches to the pathogenesis of bacillary dysentery. Microbiol Sci. 1988 Nov;5(11):333-4, 339. [PubMed] [Google Scholar]
- de Lorenzo V., Herrero M., Neilands J. B. IS1-mediated mobility of the aerobactin system of pColV-K30 in Escherichia coli. Mol Gen Genet. 1988 Aug;213(2-3):487–490. doi: 10.1007/BF00339620. [DOI] [PubMed] [Google Scholar]




