Abstract
At present there is no cure for chronic obstructive pulmonary disease (COPD). However, some nonpharmacologic treatments, such as rehabilitation and lung volume reduction surgery, as well as pharmacologic intervention, can relieve some of the patient’s symptoms and improve quality of life, while also reducing the rate of exacerbations and hospitalizations. There needs to be a paradigm shift away from the unjustified nihilistic approach to COPD towards considering it a preventable and treatable disease. After patients quit smoking and start to lead healthier lifestyles, long-acting bronchodilators, such as long-acting beta-adrenergic agents (LABA) and long-acting antimuscarinic agents (LAMA), are recommended as the cornerstone of treatment for COPD, either as monotherapy or in combination. COPD is characterized by a reduced maximum expiratory flow and slow forced emptying of the lungs, which progress over time and are not completely reversible. In this condition, gas gets trapped in the lungs and pulmonary hyperinflation occurs. LABA and LAMA improve airway patency and deflate the lungs. Indacaterol is the first once-daily LABA approved for treatment of COPD, and is administered by inhalation through the Breezhaler® device. The speed of bronchodilation is similar to that with salbutamol (ie, about five minutes) and longer (ie, 24 hours) than that with traditional LABA, with the same 12-hour effect as salmeterol and formoterol, both of which require twice-daily administration. This is why indacaterol has been called the “ultra-LABA”. On the one hand, the fast onset of action provides immediate relief of symptoms, and on the other, its constant 24-hour bronchodilation provides “pharmacologic stenting” which facilitates lung emptying, thereby decreasing trapped gas and pulmonary hyperinflation. Once-daily administration of a fast and long-acting bronchodilator can improve patient adherence with therapy, which is known to be a major problem for many medical treatments. Dose-finding trials have shown that 75 μg is the minimum dose needed to achieve clinically important improvement. However, indacaterol 150 μg and 300 μg achieve an even greater improvement in lung function and patient-oriented outcomes. Further, these two doses of indacaterol significantly reduce pulmonary hyperinflation, thereby improving exercise tolerance and ability to perform day-to-day activities. It is more effective on lung volumes at the 300 μg dose than formoterol, and better than salmeterol and tiotropium at the 150 μg dose, at least in the acute setting. It is noteworthy that few studies document these results in patients with COPD and moderate airflow obstruction. These are exactly the kind of patients our research should be concentrating on, in view of the accelerated decay in forced expiratory volume in one second at this stage of the disease. Finally, all the relevant studies show that indacaterol is consistently well tolerated by patients with COPD at every stage, and that it has a high safety profile.
Keywords: indacaterol, chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a major medical problem as well as a burden on global public health services. It causes tremendous difficulties for individuals and societies due to it being the cause of severe lifelong disability and even premature death. COPD is one of the leading causes of morbidity and mortality worldwide.1 Cigarette smoking is the principal cause of COPD, and quitting is the most effective measure to combat progression of the disease.2,3 At present there is no cure for COPD. However, some nonpharmacologic treatments, such as rehabilitation4,5 and lung volume reduction surgery,6,7 as well as pharmacologic treatment, can relieve symptoms and improve quality of life while also reducing the rate and severity of exacerbations as well as the number of hospital visits and admissions.8,9 In other words, COPD cannot be cured, but substantial benefits can be obtained for patients and society by abandoning the conventional approach,10 and taking the view, once and for all, that COPD is a preventable and treatable disease.11
COPD has been defined as a “disorder”,12 a “disease state”,13 or a “pathologic condition”14 resulting from chronic bronchitis, pulmonary emphysema, and small airway disease.15–18 It has also been suggested that bronchial asthma might be considered among the phenotypes of COPD.19 Asthma can, in fact, cause poorly reversible airflow obstruction.20 However, we share the view that COPD and asthma are two different disease entities,21 each with their own pathway of pharmacologic treatment. In asthma, inhaled corticosteroids (ICS) are the cornerstone of pharmacotherapy for the disease, whereas monotherapy with bronchodilators is strongly discouraged.22 Long-acting beta-adrenergic agents (LABA) can be added to ICS to improve asthma control.22 In contrast, long-acting bronchodilators, such as LABA23,24 and/or long-acting antimuscarinic agents (LAMA),25 are recommended as the foundation of treatment for COPD, either as monotherapy or used in combination.26–28 It has been suggested that ICS can be added to LABA29 and LAMA30 in patients with severe airflow obstruction (forced expiratory volume in one second [FEV1] <50% or <60% of predicted values), who remain symptomatic despite regular treatment with long-acting bronchodilators and have frequent exacerbations.31,32 FEV1 < 50% predicted is the threshold suggested by the 2011 Global Initiative for Chronic Obstructive Lung Disease document,9 and FEV1 < 60% predicted is the threshold indicated by the European Medicines Agency.
Many clinical studies have demonstrated the efficacy of LABA and LAMA in reducing the symptoms of COPD and the incidence of exacerbations.33 Understanding of the mechanisms via which long-acting bronchodilators provide benefits for patients with COPD requires a brief description of the underlying pathophysiology.
Pathophysiologic background
COPD is characterized by reduced maximum expiratory flow and slow forced emptying of the lungs, which progresses over time and is not completely reversible. These pathophysiologic abnormalities are determined by a varying combination of airway disease and lung parenchymal destruction. The latter causes not only a reduction in lung elastic recoil pressure, but also loss of alveolar attachments supporting the small airways.34 Unsupported small airways are compressed during expiration by the positive intrathoracic pressure, such that expiratory flow limitation develops. Under these circumstances, gas gets trapped in the lungs and pulmonary hyperinflation occurs.35
After the seminal work by Fletcher and Peto,36 an accelerated decline in FEV1 has been universally accepted as the paradigm for the natural history of COPD. However, air trapping causes vital capacity and forced vital capacity to decrease, while residual volume increases due to both loss of elastic recoil and small airway obstruction/closure.37 As the disease progresses, the residual volume expands, vital capacity falls, along with a fall in FEV1, and functional residual capacity and total lung capacity increase. Hence, a new paradigm should be adopted to understand the natural history of COPD, ie, modification of lung volumes with evolution of the disease. This would emphasize the decline in FEV1 secondary to the modification in lung volume determined by air trapping, as shown in Figure 1. This concept is important because it brings the focus of therapy onto the escape of trapped gas rather than on the conventional issue of dilation of the large airways.
Figure 1.
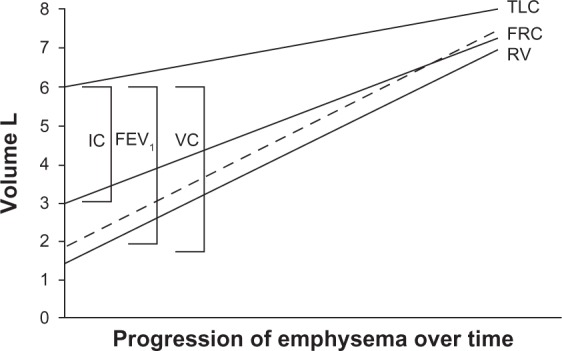
Schematic illustration showing that the natural history of chronic obstructive pulmonary disease is characterized by a progressive increase in gas trapping measured as a progressive increase in residual volume.
Note: A lesser increase in TLC leads to progressive decline in VC, imposing a reduction in FEV1.© 2010 European Respiratory Society. Reproduced with permission of the European Respiratory Society Eur Respir J March 2010 35:676-680; doi: 10.1183/09031936.00120609.37
Abbreviations: FEV1, forced expiratory volume in one second; FRC, functional residual capacity; IC, inspiratory capacity; TLC, total lung capacity; VC, vital capacity; RV, residual volume; L, liters.
The rise in residual volume, functional residual capacity, and total lung capacity above predicted values is termed pulmonary hyperinflation. With the act of breathing, abnormal airway resistance and expiratory flow limitation may prevent full expiration, such that the end-expiratory lung volume is set above the relaxed functional residual capacity. This condition is defined as “dynamic pulmonary hyperinflation”. A slight degree of dynamic pulmonary hyperinflation is present even at rest in patients with severe COPD.38,39 Dynamic pulmonary hyperinflation magnifies during acute exacerbation40,41 and exercise42 even in mild COPD.43 Dynamic pulmonary hyperinflation increases the elastic work of breathing while decreasing the pressure-generating capacity of the respiratory muscles due to its unfavorable geometric arrangement. This combination of events causes poor exercise tolerance and dyspnea,44 as well as cardiovascular complications.45
A simple measurement of dynamic hyperinflation is the change in inspiratory capacity, which mirrors the concomitant variation in functional residual capacity. Both resting inspiratory capacity and changes in inspiratory capacity are better correlated with dyspnea than FEV1.46
By relaxing smooth muscle in the airways, LABA and LAMA improve the patency of both the large and small airways, thereby decreasing air trapping.47,48 With sustained bronchodilation, less dynamic hyperinflation occurs during exercise or exacerbations, and breathing becomes less uncomfortable because of ventilation at a lower lung volume. It has been suggested that these complex effects of bronchodilation on pulmonary mechanics and the dynamics of breathing might be the major mechanism via which long-acting bronchodilators decrease the frequency and severity of exacerbations.49
Indacaterol: an “ultra-LABA”
Indacaterol is the first once-daily LABA50 approved for treatment of COPD, and is administered by inhalation through the Breezhaler device (Novartis Pharma AG, Basel, Switzerland). This paper reviews and discusses some of the recent data on the effects of indacaterol in patients with COPD.
Extensive preclinical studies on indacaterol have documented its rapid onset and long duration of action due to its biochemical structure.51–53 The speed of bronchodilation is similar to that of salbutamol (ie, both of five minutes) and longer than that of traditional LABA (ie, 24 hours) such as salmeterol and formoterol, which require twice-daily administration. This is why indacaterol has been called an “ultra-LABA”.54 This rapid onset of bronchodilation is not affected by time of administration, ie, in the morning or in the evening.55 However, due to the circadian rhythm of bronchomotor tone, with a peak of airflow resistance in the morning, administration in the morning might be preferable for rapid relief of the patient’s symptoms.56
The duration of bronchodilation is also important. On the one hand, it has been suggested that once-daily administration might improve patient adherence with treatment,57 and on the other, persistent 24-hour airway patency provides a type of “pharmacologic stenting” which facilitates lung emptying and thereby decreases trapped gas and pulmonary hyperinflation.58 As mentioned earlier, this action should be regarded as key to the effectiveness of bronchodilating therapy in COPD.
Dose-finding studies are an essential step in drug development. Studies using different doses have been performed with indacaterol.59–61 Indacaterol 150 μg was found to be the lowest effective dose for global targeted improvement in FEV1, but with the 300 μg dose being selected for the second step. No safety signal was observed with any dose of indacaterol.33,62 Some recent studies have focused on indacaterol 75 μg and documented that it improves lung function and symptoms, compared with placebo, in patients with COPD and moderate-to-severe airflow obstruction, suggesting that it can be used as a regular therapy for COPD.63,64 This dose has been selected in the US,65 whereas the 150 μg and 300 μg doses are marketed in other countries.
Analysis of the relationship between improvement in lung function, most commonly FEV1, and patient-related outcomes, such as dyspnea and quality of life, is important. Indeed, it is a common belief that spirometry may not fully capture the impact of COPD on patients’ health status. However, in a pooled analysis of three clinical studies on indacaterol, involving more than 3000 patients with COPD and moderate-to-severe airflow obstruction, Jones et al66 showed significant improvements in patient-related outcomes such as dyspnea (assessed by the transitional dyspnea index)67 and quality of life (assessed by the Saint George Respiratory Questionnaire),68 which were associated with greater improvements in FEV1.66 These authors concluded that interventions which significantly improve FEV1 are also likely to produce better clinical and patient-related outcomes. A recently reported study investigated the effectiveness of indacaterol in patients with COPD on regular treatment with ICS as well as in ICS-naive patients.69 Decramer et al report that the positive action of indacaterol on lung function and symptoms was not affected by ICS treatment.69 They also found that, whereas indacaterol 150 μg was effective in patients with COPD and moderate airflow obstruction, higher doses of 300 μg might be needed in patients with more severe functional impairment. However, a recent study of the acute effects of indacaterol by Cazzola et al concluded that a subgroup of patients with COPD can gain some benefits in lung function from an increase of the dose from 150 μg to 300 μg, although that improvement may be associated with a mild transient decrease in pulse-oximetry.70
Safety profile
The safety message by Chapman et al33,62 was reinforced by analysis of a data base containing data on more than 4000 patients with COPD and moderate-to-severe airflow obstruction, enrolled in studies lasting more than six months, by Worth et al.71 The overall cerebrocardiovascular profile of indacaterol was similar to that of placebo and comparable with that of other long-acting bronchodilators. Further, Donohue et al pooled data from 11 clinical studies investigating indacaterol 75 μg, 150 μg, 300 μg, and 600 μg, formoterol 12 μg twice daily, salmeterol 50 μg twice daily, tiotropium 18 μg, and placebo.72 Overall, almost 10,000 data/patients were analyzed to investigate whether any dose of indacaterol might be associated with adverse events compared to placebo; in particular, serious adverse events, such as plasma potassium, blood glucose, QTc intervals and vital signs. The risk of acute respiratory adverse events was not significantly increased with any of the active treatments compared with placebo. However, some patients reported mild cough, but with attenuation over time. This can occur within seconds of inhalation and with rapid resolution, and has not been associated with bronchospasm.33 In summary, indacaterol has a good safety and tolerability profile, and is appropriate for maintenance treatment of patients with COPD and moderate-to-severe airflow obstruction. This may be relevant in particular for elderly patients with COPD and multiple comorbidity.
Efficacy versus established therapies
A recent review addressed the comparative efficacy of indacaterol in COPD.33 In particular, indacaterol at different doses was compared with formoterol73–75 and salmeterol,76,77 which are well established LABA used as regular pharmacotherapy for patients with COPD. Indacaterol 150 μg and 300 μg achieved greater bronchodilation and better patient-related outcomes than formoterol 12 μg or salmeterol 50 μg twice daily in patients with moderate-to-severe airflow obstruction.
Compared with tiotropium, the published data indicated that indacaterol was at least as effective and safe as tiotropium in an “open-label” six-month study.78 Hence, two effective and safe long-acting bronchodilators provide patients and physicians with more flexibility for treating patients with COPD.78 However, to circumvent the limitations related to the open-label design, Vogelmeier et al performed a blinded noninferiority comparison of indacaterol 150 μg and 300 μg, tiotropium 18 μg, and placebo. Both indacaterol doses had a faster onset of action on day 1, providing clinically relevant treatment-placebo differences of 120–130 mL in FEV1 five minutes post-dosing.79 At this time point, treatment with both indacaterol doses resulted in a statistically superior FEV1 (about 80 mL) compared with tiotropium. In a blinded parallel-group comparison by Buhl et al, indacaterol 150 μg and tiotropium 18 μg had similar effects on trough FEV1.80 However, patients treated with indacaterol showed greater improvements in terms of dyspnea and quality of life. In this regard, it is worth mentioning the study by Chapman et al, which reported that patients with COPD may show a preference for one inhaler (Breezhaler) over another (HandiHaler®, Boeheringer-Ingelheim, Ingelheim, Germany).81 This may be an important factor for optimum dose delivery and successful management of COPD.
Regarding concerns about the scientific credibility of open-label protocols versus full blinded designs, Beeh et al, on analyzing the published studies, reported that there was no difference between the two types of design in terms of objective measurements such as lung function, whereas some slight bias might be introduced for subjective measurements, such as dyspnea and quality of life.82 The authors recommend transparency concerning study design and consideration of any potential source of bias, suggesting that, under such circumstances, data from open-label studies can provide valuable and credible evidence of the effects of therapy.
Rodrigo and Neffen undertook a systematic comparison between indacaterol, tiotropium, and twice-daily LABA from five trials representing almost 6000 patients.83 This systematic review suggests that patients with COPD and moderate-to-severe airflow obstruction treated with indacaterol had better clinical outcomes with regard to dyspnea and health status than those treated with tiotropium or twice-daily LABA. Cope et al investigated the efficacy of indacaterol relative to alternative bronchodilators by means of a patient-level mixed-treatment comparison, involving a combination of four randomized controlled trials.84 They found that indacaterol 150 μg provided better FEV1 than formoterol or salmeterol, and a greater improvement in quality of life than tiotropium, while the 300 μg dose demonstrated the greatest response overall. A multiple comparison study investigated indacaterol 75 μg, tiotropium 18 μg, salmeterol 50 μg twice daily, and formoterol 12 μg twice daily at 12 weeks in a network meta-analysis from 21 clinical trials.85 They concluded that indacaterol 75 μg provided levels of improvement in health-related quality of life and lung function comparable with those of tiotropium, salmeterol, and formoterol. However, although 75 μg can be accepted as the minimum effective dose of indacaterol, it should be noted that the 150 μg and 300 μg doses provide better bronchodilation without significant side effects. In fact, these two doses are marketed in almost all countries, while the US Food and Drug Administration allows only the minimum 75 μg dose.
Indacaterol versus LABA-ICS combinations
A number of studies have addressed the issue of indacaterol versus fixed-dose combination of salmeterol-fluticasone. Balint et al compared the onset of action of indacaterol 150 μg and 300 μg with that of salbutamol 200 μg and salmeterol-fluticasone 50/500 μg in a randomized, double-blind, crossover trial using FEV1 at five minutes post-dosing as the primary variable.86 Indacaterol was faster-acting than salmeterol-fluticasone, and as fast as salbutamol. Two other studies investigating indacaterol versus a LABA-ICS combination were performed as a network meta-analysis. Cope et al compared indacaterol 150 μg and 300 μg with two doses of formoterol-budesonide (9/320 μg and 9/160 μg) and salmeterol-fluticasone (50/250 μg and 50/500 μg).87 They found that indacaterol monotherapy at both doses was as good as both LABA-ICS combinations in terms of lung function, health status, and breathlessness. A similar study was performed with indacaterol 75 μg, which was shown to be an effective monotherapy in terms of lung function, although the study was inconclusive in terms of breathlessness and health status.88 At present, there are still no appropriately designed prospective studies comparing indacaterol directly with LABA-ICS combinations. This is an important issue, given that some publications have shown that the LABA-ICS combination is widely used in patients with COPD and moderate airflow obstruction,32,89 despite no guidelines recommending treatment with ICS in the absence of frequent exacerbations. In this regard, it should be remembered that, in the COPD population with moderate airflow obstruction, the prevalence of “frequent exacerbations” averages about 22%, ie, occurs in a minority of the overall “moderate” patient population.32
Maintenance-naive patients with moderate COPD
Decramer et al have addressed the issue of regular pharmacotherapy using indacaterol 150 μg and 300 μg in patients with COPD not receiving other maintenance treatment.90 The data were pooled from three randomized, placebo-controlled studies, and the results from an open-label tiotropium treatment arm in one study were also available for comparison. Both doses of indacaterol led to clinically relevant and statistically significant improvements over placebo at week 26. Tiotropium also improved trough FEV1, and there was no statistically significant difference between the treatments, as shown in Figure 2. However, indacaterol was slightly better than tiotropium in improving patient-related outcomes, such as dyspnea, bad days, and quality of life. All treatment regimens reduced the exacerbation rate compared with placebo, namely by about 0.70 in the treated groups versus 0.91 in the placebo group, with no difference between treatments. The overall incidence of adverse events was not significantly different between the groups, and none of these events were serious. A potential limitation of this study is the fact that the subgroup analyses were not preplanned and no statistical power calculation was done. In addition, the use of open-label tiotropium may have introduced bias. However, its value relies on the investigation of patients with COPD naive to regular pharmacotherapy.
Figure 2.
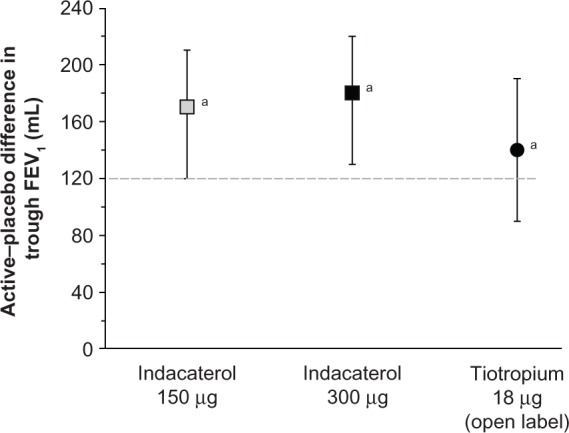
Effect of active treatments (differences compared with placebo) on trough FEV1 at week 26.
Notes: Data are least squares means with 95% confidence intervals. aP < 0.001 versus placebo. The broken line indicates the minimum clinically important difference.© 2012 Elsevier Limited. Reproduced with permission from Decramer et al.91
Abbreviation: FEV1, forced expiratory volume in one second.
The effect of tiotropium in patients naive to maintenance therapy was reported by Troosters et al in a subgroup of patients from the UPLIFT population.91–93 They found that regular treatment with tiotropium was associated with significant benefits and slower disease progression in patients with COPD who were not on maintenance therapy. The annual rate of decline of FEV1 was about 10 mL less, on average, in the tiotropium group compared with the placebo arm.91 This was not the case in the whole UPLIFT population, where a difference amounting to an average of 7 mL was observed in patients with moderate obstruction in the active arm compared with conventional treatment.92,93 Data from recent clinical investigations indicate that clinicians continue to be confronted with patients newly detected as having COPD.94,95 In the work by Decramer et al, the majority of patients naive to treatment (62%–70%) had mild-to-moderate airflow obstruction.90 Therefore, research on the impact of pharmacotherapy in patients naive to maintenance treatment is important. In particular, greater attention should be paid to patients with COPD and moderate airflow obstruction. Their symptoms might well be underestimated due both to a lower impact on daily activity and to the fact that the exacerbation rate is lower than in more severe COPD.32 Further, a recent study by Tantucci and Modina reviewed spirometric data for patients with COPD recruited in the placebo arms of 14 recent clinical trials, and analyzed the decline in FEV1 according to separation in the classic four-stage Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification.96,97 They found that the loss of lung function, assessed as reduction in expiratory airflow, was more rapid in the early stages of COPD than in the later stages, as shown in Figure 3.
Figure 3.
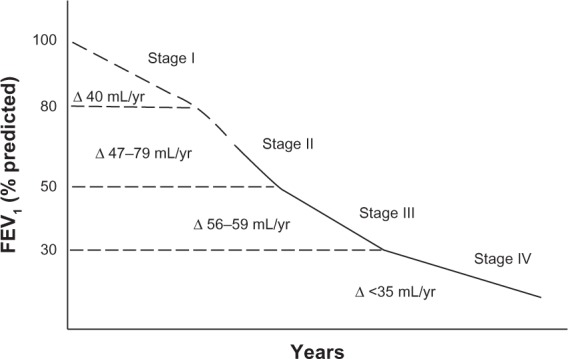
Range of average rate of decline of FEV1 in patients with chronic obstructive pulmonary disease according to severity of airflow obstruction.
Notes: The dashed segment of the line highlights any stage or part of it where consistent information is still lacking. Control data from the UPLIFT study were not considered in the analysis.© 2012 Dove Medical Press. Reproduced with permission from Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruc Pulmon Dis. 2012;7:95–99.96
Abbreviation: FEV1, forced expiratory volume in one second.
In conclusion, the studies by Decramer et al and Troosters et al show that long-acting bronchodilators, such as indacaterol at 150 μg and 300 μg and tiotropium 18 μg, are effective in gaining benefits for patients with COPD naive to other maintenance pharmacologic therapies.90,91 On the other hand, long-acting bronchodilation seems to slow the FEV1 decline in patients with moderate COPD, where the rate of loss of lung function is accelerated.93,96 These conclusions are meaningful in terms of planning studies specifically designed to address the effects of long-acting bronchodilators in treatment-naive patients in the early stages of the disease, for whom regular pharmacotherapy with long acting bronchodilators might reduce the faster progression of the disease.3
Combination of indacaterol and tiotropium
It has been well known since the 1980s that a combination of bronchodilators can be more effective than any single agent used alone. In the COMBIVENT study, a short-term adrenergic agonist (albuterol) was combined with a short-term anticholinergic agent (ipratropium) to obtain greater bronchodilation than possible with either drug used alone.98 LABA and LAMA are also powerful bronchodilators with different mechanisms of action. They can be associated either by separate administration, as was done for indacaterol with tiotropium, or in a single device, as was done for indacaterol with glycopyrronium.27,28 The latter is a new LAMA with a fast onset similar to indacaterol, and a long duration of action similar to that of tiotropium.99 Van Noord et al have shown that combination of indacaterol and glycopyrronium in the same device allows rapid and sustained bronchodilation with significant improvements when compared with indacaterol 150 μg and 300 μg in patients with COPD.28 No significant cardiovascular adverse event was observed.100 Recently, Mahler et al reported on data from two studies comparing indacaterol 150 μg and placebo in patients already taking tiotropium 18 μg.27 They found greater bronchodilation and lung deflation (increase in inspiratory capacity) with indacaterol and tiotropium compared with tiotropium monotherapy. Adverse effects were similar in the two groups and mild. Therefore, combination of long-acting bronchodilators with different mechanisms of action may be useful in the management of patients with COPD for whom a greater degree of bronchodilation and lung deflation is needed. In this regard, a recent clinical trial reported by Vogelmeier et al compared a once-daily indacaterol-glycopyrronium combination with a twice-daily salmeterol-fluticasone combination.101 They found better data in terms of bronchodilation and patient-related outcomes with the former combination than with the latter in patients with COPD and moderate-to-severe airflow obstruction but without exacerbations in the previous year.
Indacaterol, hyperinflation, and exercise tolerance
It is known that pulmonary hyperinflation causes poor exercise tolerance in patients with COPD, as discussed earlier. It has been shown that bronchodilators, both short-acting47,48,102 and long-acting,103,104 can improve exercise tolerance by decreasing lung hyperinflation. Some recent studies have found that indacaterol is also effective at doing this. Beier et al reported a greater increase in inspiratory capacity after indacaterol 300 μg than after formoterol twice daily, although the effect on FEV1 was essentially equivalent, as shown in Figure 4.74
Figure 4.
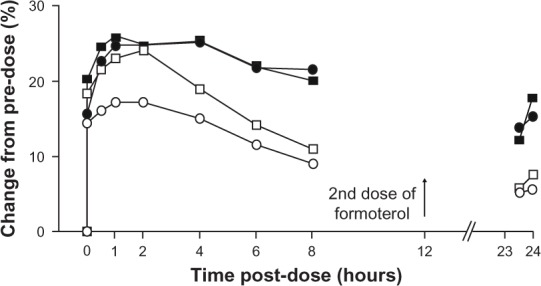
Comparison of effects of indacaterol and formoterol on FEV1 and inspiratory capacity as percent change in unadjusted mean values from predose.
Notes: Error bars omitted for clarity. n = 30 from each treatment. Black squares, indacaterol FEV1; black circles, indacaterol inspiratory capacity; empty squares, formoterol FEV1; empty circles, formoterol inspiratory capacity.From © 2009 Elsevier Limited. Reproduced with permission from Beier et al.74
Abbreviation: FEV1, forced expiratory volume in one second.
Rossi et al noted a significant improvement in peak inspiratory capacity in a crossover protocol comparing the acute effect of indacaterol 150 μg versus open-label tiotropium 18 μg and placebo in patients with COPD and moderate airflow obstruction.105 Spirometry and lung volumes were both measured. The data show that indacaterol 150 μg has a significant bronchodilating action, similar to the effect of the recommended dose of tiotropium on spirometric variables (FEV1 and forced vital capacity), but slightly superior at improving inspiratory capacity and reducing lung hyperinflation, as seen in Figure 5.
Figure 5.
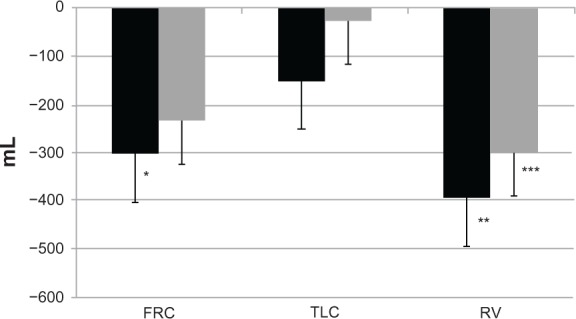
Changes in FRC, TLC, and RV at hour 4 after administration.
Notes: FRC was decreased compared with predose by 305+100mL with indacaterol (p=0.01*), and by 236+88 with tiotropium (p=0.053); TLC by 152+114mL with indacaterol (p=0.208), and by 28+85mL with tiotropium (p=0.806); and RV by 395+125mL with indacaterol (p=0.004**), and by 301+106mL with tiotropium (p=0.029***). Data are expressed as the mean+standard error of the mean. Black and gray bars are indacaterol and tiotropim, respectively.© 2012 Elsevier Limited. Reproduced with permission from Rossi et al.105
Abbreviations: FRC, functional residual capacity; TLC, total lung capacity; RV, residual volume.
Hence, these data and those of Beier et al show that indacaterol, at two different doses, is very effective at reducing lung hyperinflation, which remains a key target in the management of patients with COPD and moderate to severe airflow obstruction.74 Beeh et al investigated the effect of indacaterol 300 μg on peak inspiratory capacity in patients with COPD and a significant degree of resting pulmonary hyperinflation (functional residual capacity > 120% predicted) during exercise in a crossover study with evaluation after two weeks of treatment.106 They found that peak inspiratory capacity was significantly greater after indacaterol than after placebo. Resting inspiratory capacity, trough FEV1, dyspnea indices, and endurance time were also improved by indacaterol 300 μg. Similar results were obtained by O’Donnell et al, who studied the impact of the lower dose of indacaterol, ie, 150 μg, on exercise tolerance in patients with COPD and moderate to severe airflow obstruction.107 They found that indacaterol 150 μg was effective in improving endurance time from the first day of treatment onwards. Taken together, the data from these studies show that indacaterol, at both doses, ie, 300 μg and 150 μg, is successful in reducing pulmonary hyperinflation, which in turn decreases dyspnea and increases exercise tolerance.74,105–107 In this regard, the studies by Ofir et al and O’Donnell et al in patients with COPD and mild airflow obstruction should also be borne in mind.43,102
One could speculate that greater exercise tolerance would encourage patients with COPD to pursue a more active lifestyle with significant implications for their quality of life and perhaps even survival. In fact, a recent study by Hataji et al documents that indacaterol can improve daily activity in patients with COPD.108 It is noteworthy that such a great body of literature suggests that daily exercise, even mild and pleasant, can be a powerful tool to reducing mortality from all causes.109
Conclusion
COPD is no longer considered an irreversible disease and can now be treated effectively.110 Long-acting bronchodilators are not simply symptomatic drugs. Their complex effects on the underlying pathophysiology of COPD and benefits in terms of patient-related outcomes could encourage a paradigm shift from symptomatic drugs to “disease modifiers”. Indacaterol is a new long-acting bronchodilator approved as regular therapy for patients with COPD and moderate to severe airflow obstruction. The available literature shows that significant bronchodilation can be obtained following inhalation of indacaterol from the Breezhaler device at the three marketed doses, ie, 75 μg in the US, and 150 μg and 300 μg in other countries worldwide.
Indacaterol has a rapid onset of action, as fast as that of salbutamol and formoterol, and faster than salmeterol, tiotropium, and the fluticasone-salmeterol combination. Indacaterol also has a longer-lasting effect, ie, 24 hours, which is longer than for formoterol and salmeterol and similar to that of tiotropium. At present, indacaterol is the only bronchodilator available for COPD therapy which combines rapid onset with a 24-hour effect. Hence its definition as an “ultra-LABA”.
The data show that the significant bronchodilation achieved by indacaterol translates into perceivable benefits for the patient, including relief of symptoms, better quality of life, and reduction of exacerbations. Indacaterol decreases pulmonary hyperinflation, thereby improving exercise tolerance and everyday activities. Its key effect on lung volumes appears superior to that of formoterol for the 300 μg dose, and better than salmeterol and tiotropium for the 150 μg dose, at least in acute settings. It is noteworthy that some studies document these results in patients with COPD and moderate airflow obstruction. These are exactly the type of patients our research should be concentrating on, in view of the accelerated decrease in FEV1 seen at this stage of the disease. Further, all the studies have shown that indacaterol is consistently well tolerated by patients with COPD at every stage and that it has a good safety profile.
Therefore, we can conclude that the “ultra-LABA” indacaterol is effective and safe in the treatment of COPD and it will eventually replace the traditional LABA. Just as importantly, indacaterol can be successfully and safely combined with a LAMA, to maximize bronchodilation and deflate the lungs efficiently and continuously in patients with stable COPD.
Acknowledgments
The authors thank Chris Botterill for copy editing the manuscript and Pamela Micheletti for technical and editing assistance.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hanania NA, Marciniuk DD. A unified front against COPD: clinical practice guidelines from the American College of Physicians, the American College of Chest Physicians, the American Thoracic Society, and the European Society. Chest. 2011;140(3):565–566. doi: 10.1378/chest.11-1152. [DOI] [PubMed] [Google Scholar]
- 2.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166(5):675–679. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 3.Maltais F, Dennis N, Chan CK. Rationale for earlier treatment in COPD: a systematic review of published literature in mild-to-moderate COPD. COPD. 2013;10(1):79–103. doi: 10.3109/15412555.2012.719048. [DOI] [PubMed] [Google Scholar]
- 4.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;4:CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Egan C, Deering BM, Blake C, et al. Short term and long term effects of pulmonary rehabilitation on physical activity in COPD. Respir Med. 2012;106(12):1671–1679. doi: 10.1016/j.rmed.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 6.National Emphysema Treatment Trial Research Group Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med. 2001;345(15):1075–1083. doi: 10.1056/NEJMoa11798. [DOI] [PubMed] [Google Scholar]
- 7.Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363(13):1233–1244. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 8.National Clinical Guideline Centre Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care London, UK: National Clinical Guideline Centre; Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/EnglishAccessed April 12, 2013 [Google Scholar]
- 9.Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease Revised 2011. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2011_Feb21.pdfAccessed April 12, 2013
- 10.Van der Palen J, Monninkhof E, van der Valk P, Visser A. Managing COPD: no more nihilism! Patient Educ Couns. 2004;52(3):221–223. doi: 10.1016/S0738-3991(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 11.Celli BR, MacNee W, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 12.Siafakas NM, Vermeire P, Pride NB, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD) Eur Respir J. 1995;8(8):1398–1420. doi: 10.1183/09031936.95.08081398. [DOI] [PubMed] [Google Scholar]
- 13.ATS Statement Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;152(5):S78–S121. [PubMed] [Google Scholar]
- 14.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9(5):377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 15.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278(25):1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 16.Cosio M, Ghezzo H, Hogg JC, et al. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med. 1978;298(23):1277–1281. doi: 10.1056/NEJM197806082982303. [DOI] [PubMed] [Google Scholar]
- 17.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 18.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraft M. Asthma and chronic obstructive pulmonary disease exhibit common origins in any country! Am J Respir Crit Care Med. 2006;174(3):238–244. doi: 10.1164/rccm.2604007. [DOI] [PubMed] [Google Scholar]
- 20.Fabbri LM, Romagnoli M, Corbetta L, et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(3):418–424. doi: 10.1164/rccm.200203-183OC. [DOI] [PubMed] [Google Scholar]
- 21.Barnes PJ. Against the Dutch hypothesis: asthma and chronic obstructive pulmonary disease are distinct diseases. Am J Respir Crit Care Med. 2006;174(3):240–243. doi: 10.1164/rccm.2604008. [DOI] [PubMed] [Google Scholar]
- 22.Pocket Guide for Asthma Management and Prevention A pocket guide for physician and nurses Updated 2011Global Initiative for Asthma; 2011Available from: http://www.ginasthma.org/Accessed April 12, 2013 [Google Scholar]
- 23.Rossi A, Khirani S, Cazzola M. Long-acting β2-agonists (LABA) in chronic obstructive pulmonary disease: efficacy and safety. Int J Chron Obstruct Pulmon Dis. 2008;3(4):1–9. doi: 10.2147/copd.s1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res. 2010;11(1):149. doi: 10.1186/1465-9921-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigo GJ, Nannini LJ. Tiotropium for the treatment of stable chronic obstructive pulmonary disease: a systematic review with meta-analysis. Pulm Pharmacol Ther. 2007;20(5):495–502. doi: 10.1016/j.pupt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Sethi S, Cote C. Bronchodilator combination therapy for the treatment of chronic obstructive pulmonary disease. Curr Clin Pharmacol. 2011;6(1):48–61. doi: 10.2174/157488411794941331. [DOI] [PubMed] [Google Scholar]
- 27.Mahler DA, D’Urzo A, Bateman ED, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomized, double-blind comparison. Thorax. 2012;67(9):781–788. doi: 10.1136/thoraxjnl-2011-201140. [DOI] [PubMed] [Google Scholar]
- 28.van Noord JA, Buhl R, LaForce C, et al. QVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary disease. Thorax. 2010;65(12):1086–1091. doi: 10.1136/thx.2010.139113. [DOI] [PubMed] [Google Scholar]
- 29.Calverley PM, Anderson JA, Celli B, et al. TORCH Investigators Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 30.Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease. A randomized trial. Ann Intern Med. 2007;146(8):545–555. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 31.Qaseem A, Wilt TJ, Weiberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the ACP, ACCP, ATS and ERS. Ann Intern Med. 2011;155(3):179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 32.Hurst JR, Vestbo J, Anzueto A, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro M, Chapman KR. Comparative efficacy of indacaterol in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2012;7(3):145–152. doi: 10.2147/COPD.S19805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saetta M, Ghezzo H, Kim WD, et al. Loss of alveolar attachments in smokers. A morphometric correlate of lung function impairment. Am Rev Respir Dis. 1985;132(4):894–900. doi: 10.1164/arrd.1985.132.4.894. [DOI] [PubMed] [Google Scholar]
- 35.Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med. 2006;119(10):S1, 21–31. doi: 10.1016/j.amjmed.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Fletcher C, Peto R, Tinker C, et al. The Natural History of Chronic Bronchitis and Emphysema An Eight-Year Study of Early Chronic Obstructive Lung Disease in Working Men in London. Oxford, UK: Oxford University Press; 1976. [Google Scholar]
- 37.Macklem PT. Therapeutic implications of the pathophysiology of COPD. Eur Respir J. 2010;35(3):676–680. doi: 10.1183/09031936.00120609. [DOI] [PubMed] [Google Scholar]
- 38.Dal Vecchio L, Polese G, Poggi R, Rossi A. “Intrinsic” positive end-expiratory pressure in stable patients with chronic obstructive pulmonary disease. Eur Respir J. 1990;3(1):74–80. [PubMed] [Google Scholar]
- 39.Khirani S, Polese G, Aliverti A, et al. On-line monitoring of lung mechanics during spontaneous breathing: a physiologic study. Respir Med. 2010;104(3):463–471. doi: 10.1016/j.rmed.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 40.O’ Donnell DE, Parker CM. COPD exacerbations – 3: pathophysiology. Thorax. 2006;61(4):354–361. doi: 10.1136/thx.2005.041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossi A, Khirani S. PEEPi and the air-bag effect. Respiration. 2009;77(3):256–258. doi: 10.1159/000203732. [DOI] [PubMed] [Google Scholar]
- 42.O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770–777. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 43.Ofir D, Laveneziana P, Webb KA, Lam YM, O’Donnell DE. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(6):622–629. doi: 10.1164/rccm.200707-1064OC. [DOI] [PubMed] [Google Scholar]
- 44.Calverley PM, Koulouris NG. Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology. Eur Respir J. 2005;25(1):186–199. doi: 10.1183/09031936.04.00113204. [DOI] [PubMed] [Google Scholar]
- 45.Tzani P, Aiello M, Elia D, et al. Dynamic hyperinflation is associated with a poor cardiovascular response to exercise in COPD patients. Respir Res. 2011;12:150. doi: 10.1186/1465-9921-12-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diaz O, Villafranca C, Ghezzo H, et al. Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at rest. Eur Respir J. 2000;16(2):269–275. doi: 10.1034/j.1399-3003.2000.16b14.x. [DOI] [PubMed] [Google Scholar]
- 47.Tantucci C, Duguet A, Similowski T, Zelter M, Derenne JP, Milic-Emili J. Effect of salbutamol on dynamic hyperinflation in chronic obstructive pulmonary disease. Eur Respir J. 1998;12(4):799–804. doi: 10.1183/09031936.98.12040799. [DOI] [PubMed] [Google Scholar]
- 48.Dellacà RL, Pompilio PP, Walker PP, Duffy N, Pedotti A, Calverley PM. Effect of bronchodilation on expiratory flow limitation and resting lung mechanics in COPD. Eur Respir J. 2009;33(6):1329–1337. doi: 10.1183/09031936.00139608. [DOI] [PubMed] [Google Scholar]
- 49.Wedzicha JA, Decramer M, Seemugal TA. The role of bronchodilator treatment in the prevention of exacerbations of COPD. Eur Respir J. 2012;40(6):1545–1554. doi: 10.1183/09031936.00048912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slaton RM, Cruthirds DL. Indacaterol (Arcapta Neohaler) for chronic obstructive pulmonary disease. P T. 2012;37(2):86–98. [PMC free article] [PubMed] [Google Scholar]
- 51.Cazzola M, Page CP, Calzetta L, Matera MG. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64(3):450–504. doi: 10.1124/pr.111.004580. [DOI] [PubMed] [Google Scholar]
- 52.Yorgancioglu A. Indacaterol in chronic obstructive pulmonary disease: an update for clinicians. Ther Adv Chronic Dis. 2012;3(1):25–36. doi: 10.1177/2040622311426204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones PW, Barnes N, Vogelmeier C, Lawrence D, Kramer B. Efficacy of indacaterol in the treatment of patients with COPD. Prim Care Respir J. 2011;20(4):380–388. doi: 10.4104/pcrj.2011.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cazzola M, Matera MG, Lötvall J. Ultra-long acting β2 agonists in development for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2005;14(7):775–783. doi: 10.1517/13543784.14.7.775. [DOI] [PubMed] [Google Scholar]
- 55.Magnussen H, Verkindre C, Jack D, et al. Indacaterol once-daily is equally effective dosed in the evening or morning in COPD. Respir Med. 2010;104(12):1869–1876. doi: 10.1016/j.rmed.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Calverley PM, Lee A, Towse L, van Noord J, Witek TJ, Kelsen S. Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax. 2003;58(10):855–860. doi: 10.1136/thorax.58.10.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toy EL, Baulieu NU, McHale JL, et al. Treatment of COPD: relationship between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105(3):435–441. doi: 10.1016/j.rmed.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Beeh KM, Beier J. The short, the long, and the “ultra-long”: why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Adv Ther. 2010;27(3):150–159. doi: 10.1007/s12325-010-0017-6. [DOI] [PubMed] [Google Scholar]
- 59.Rennard S, Bantje T, Centanni S, et al. A dose-ranging study of indacaterol in obstructive airways disease, with a tiotropium comparison. Respir Med. 2008;102(7):1033–1044. doi: 10.1016/j.rmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Barnes PJ, Pocock SJ, Magnussen H, et al. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pulm Pharmacol Ther. 2010;23(3):165–171. doi: 10.1016/j.pupt.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Renard D, Looby M, Kramer B, Lawrence D, Morris D, Stanski DR. Characterization of the bronchodilatory dose response to indacaterol in patients with chronic obstructive pulmonary disease using model-based approaches. Respir Res. 2011;12:54. doi: 10.1186/1465-9921-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B. Long-term safety and efficacy of indacaterol, a novel long-acting β2 agonist in subjects with COPD: a randomized, placebo-controlled study. Chest. 2011;140(1):68–75. doi: 10.1378/chest.10-1830. [DOI] [PubMed] [Google Scholar]
- 63.Kerwin EM, Gotfried MH, Lawrence D, Lassen C, Kramer B. Efficacy and tolerability of indacaterol 75 μg once daily in patients aged ≥ 40 years with chronic obstructive pulmonary disease: results from 2 double-blind, placebo-controlled 12-week study. Clin Ther. 2011;33(12):1974–1984. doi: 10.1016/j.clinthera.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 64.Gotfried MH, Kerwin EM, Lawrence D, Lassen C, Kramer B. Efficacy of indacaterol 75 μg once daily on dyspnea and health status: results of two double-blind, placebo-controlled 12 week studies. COPD. 2012;9(6):629–636. doi: 10.3109/15412555.2012.729623. [DOI] [PubMed] [Google Scholar]
- 65.Chowdhury BA, Seymour SM, Michele TM, Durmowicz AG, Liu D, Rosebraugh CJ. The risks and benefits of indacaterol – the FDA’s review. N Engl J Med. 2011;365(24):2247–2249. doi: 10.1056/NEJMp1109621. [DOI] [PubMed] [Google Scholar]
- 66.Jones PW, Donohue JF, Nedelman J, Pascoe S, Pinault G, Lassen C. Correlating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysis. Respir Res. 2011;12:161. doi: 10.1186/1465-9921-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurements of dyspnea* Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 68.Jones PW. Quality of life measurements for patients with diseases of the airways. Thorax. 1992;46:676–682. doi: 10.1136/thx.46.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Decramer M, Dahl R, Kornmann O, Korn S, Lawrence D, McBryan D. Effects of long-acting bronchodilators in COPD patients according to COPD severity and ICS use. Respir Med. 2013;107(2):223–232. doi: 10.1016/j.rmed.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 70.Cazzola M, Segreti A, Stirpe E, et al. Effect of an additional dose of indacaterol in COPD patients under regular treatment with indacaterol. Respir Med. 2012;107(1):107–111. doi: 10.1016/j.rmed.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 71.Worth H, Chung KF, Felser JM, Hu H, Rueegg P. Cardio- and cerebrovascular safety of indacaterol vs formoterol, salmeterol, tiotropium and placebo in COPD. Respir Med. 2011;105(4):571–579. doi: 10.1016/j.rmed.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 72.Donohue JF, Singh D, Kornmann O, Lawrence D, Lassen C, Kramer B. Safety of indacaterol in the treatment of patients with COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:477–492. doi: 10.2147/COPD.S23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bauwens O, Ninane V, Van de Maele B, et al. 24-hour bronchodilator efficacy of single doses of indacaterol in subjects with COPD: comparison with placebo and formoterol. Curr Med Res Opin. 2009;25(2):463–470. doi: 10.1185/03007990802675096. [DOI] [PubMed] [Google Scholar]
- 74.Beier J, Beeh KM, Brookman L, Peachey G, Hmissi A, Pascoe S. Bronchodilator effects of indacaterol and formoterol in patients with COPD. Pulm Pharmacol Ther. 2009;22(6):492–496. doi: 10.1016/j.pupt.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Dahl R, Chung KF, Buhl R, et al. Efficacy of a new once-daily long-acting inhaled β2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65(6):473–479. doi: 10.1136/thx.2009.125435. [DOI] [PubMed] [Google Scholar]
- 76.Korn S, Kerwin E, Atis S, et al. Indacaterol once-daily provides superior efficacy to salmeterol twice-daily in COPD: a 12-week study. Respir Med. 2011;5105:719–726. doi: 10.1016/j.rmed.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 77.Kornmann O, Dahl R, Centanni S, et al. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J. 2011;37(2):273–279. doi: 10.1183/09031936.00045810. [DOI] [PubMed] [Google Scholar]
- 78.Donohue JF, Fogarty C, Lötvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease. Indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182(2):155–162. doi: 10.1164/rccm.200910-1500OC. [DOI] [PubMed] [Google Scholar]
- 79.Vogelmeier C, Ramos-Barbon D, Jack D, et al. Indacaterol provides 24-hour bronchodilation in COPD: a placebo-controlled blinded comparison with tiotropium. Respir Res. 2010;11:135. doi: 10.1186/1465-9921-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buhl R, Dunn LJ, Disdier C, et al. Blinded 12-week comparison of once-daily indacaterol and tiotropium in COPD. Eur Respir J. 2011;38(4):797–803. doi: 10.1183/09031936.00191810. [DOI] [PubMed] [Google Scholar]
- 81.Chapman KR, Fogarty CM, Peckitt C, et al. Delivery characteristics and patients’ handling of two single-dose dry-powder inhalers used in COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:353–363. doi: 10.2147/COPD.S18529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beeh KM, Beier J, Donohue JF. Clinical trial design in chronic obstructive pulmonary disease: current perspectives and considerations with regard to blinding of tiotropium. Respir Res. 2012;13:52. doi: 10.1186/1465-9921-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rodrigo GJ, Neffen H. Comparison of indacaterol with tiotropium or twice-daily long-acting beta-agonists for stable COPD: a systematic review. Chest. 2012;142(5):1104–1110. doi: 10.1378/chest.11-2252. [DOI] [PubMed] [Google Scholar]
- 84.Cope S, Capkun-Niggli G, Gale R, et al. Efficacy of once-daily indacaterol relative to alternative bronchodilators value in COPD patients: a patient-level mixed treatment comparison. Value Health. 2012;15(3):524–533. doi: 10.1016/j.jval.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 85.Cope S, Zhang J, Williams J, Jansen JP. Efficacy of once-daily indacaterol 75 μg relative to alternative bronchodilators in COPD: a study level and a patient level network meta-analysis. BMC Pulm Med. 2012;12:29. doi: 10.1186/1471-2466-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balint B, Watz H, Amos C, Owen R, Higgins M, Kramer B. Onset of action of indacaterol in patients with COPD: comparison with salbutamol and salmeterol-fluticasone. Int J Chron Obstruct Pulmon Dis. 2010;5:311–318. doi: 10.2147/copd.s12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cope S, Capkun-Niggli G, Gale R, Jardim JR, Jansen JP. Comparative efficacy of indacaterol 150 μg and 300 μg versus fixed-dose combinations of formoterol + budesonide or salmeterol + fluticasone for the treatment of chronic obstructive pulmonary disease – a network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2011;6:329–344. doi: 10.2147/COPD.S18759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cope S, Kraemer M, Zhang J, Capkun-Niggli G, Jansen JP. Efficacy of indacaterol 75 μg versus fixed-dose combinations of formoterol-budesonide or salmeterol-fluticasone for COPD: a network meta-analysis. Int J Chronic Obstruct Pulmon Dis. 2012;7:415–420. doi: 10.2147/COPD.S31526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corrado A, Rossi A. How far is real life from COPD therapy guidelines. An Italian Observational Study. Respir Med. 2012;106(7):989–997. doi: 10.1016/j.rmed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 90.Decramer M, Rossi A, Lawrence D, McBryan D. Indacaterol therapy in patients with COPD not receiving other maintenance treatment. Respir Med. 2012;106(12):1706–1714. doi: 10.1016/j.rmed.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 91.Troosters T, Celli B, Lystig T, et al. Tiotropium as a first maintenance drug in COPD: secondary analysis of the UPLIFT trial. Eur Respir J. 2010;36(1):65–73. doi: 10.1183/09031936.00127809. [DOI] [PubMed] [Google Scholar]
- 92.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 93.Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374(9696):1171–1178. doi: 10.1016/S0140-6736(09)61298-8. [DOI] [PubMed] [Google Scholar]
- 94.Pelkonen M, Notkola IL, Nissinen A, Tukiainen H, Kostela H. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest. 2006;130(4):1129–1137. doi: 10.1378/chest.130.4.1129. [DOI] [PubMed] [Google Scholar]
- 95.van Durme YM, Verhamme KM, Stijnen T, et al. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam study. Chest. 2009;135(2):368–377. doi: 10.1378/chest.08-0684. [DOI] [PubMed] [Google Scholar]
- 96.Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruc Pulmon Dis. 2012;7:95–99. doi: 10.2147/COPD.S27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Update 2010. Available from: http://www.goldcopd.com.
- 98.In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. COMBIVENT Inhalation Aerosol Study Group. Chest. 1994;105(5):1411–1419. doi: 10.1378/chest.105.5.1411. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 99.Buhl R, Banerji D. Profile of glycopyrronium for once-daily treatment of moderate-to-severe COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:729–741. doi: 10.2147/COPD.S36001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Van de Maele B, Fabbri LM, Martin C, Horton R, Dolker M, Overend T. Cardiovascular safety of QVA149, a combination of indacaterol and NVA237, in COPD patients. COPD. 2010;7(6):418–427. doi: 10.3109/15412555.2010.528812. [DOI] [PubMed] [Google Scholar]
- 101.Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60. doi: 10.1016/S2213-2600(12)70052-8. [DOI] [PubMed] [Google Scholar]
- 102.O’Donnell DE, Laveneziana P, Ora J, Webb KA, Lam YA, Ofir D. Evaluation of acute bronchodilator reversibility in patients with symptoms of GOLD stage I COPD. Thorax. 2009;64(3):216–223. doi: 10.1136/thx.2008.103598. [DOI] [PubMed] [Google Scholar]
- 103.O’Donnell DE, Voduc N, Fitzpatrick M, Webb KA. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J. 2004;24(1):86–94. doi: 10.1183/09031936.04.00072703. [DOI] [PubMed] [Google Scholar]
- 104.O’Donnell DE, Flϋge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832–840. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 105.Rossi A, Centanni S, Cerveri I, et al. Acute effects of indacaterol on lung hyperinflation in moderate COPD: a comparison with tiotropium. Respir Med. 2012;106(1):84–90. doi: 10.1016/j.rmed.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 106.Beeh KM, Wagner F, Khindri S, Drollmann AF. Effect of indacaterol on dynamic lung hyperinflation and breathlessness in hyperinflated patients with COPD. COPD. 2011;8(5):340. doi: 10.3109/15412555.2011.594464. [DOI] [PubMed] [Google Scholar]
- 107.O’Donnell DE, Casaburi R, Vincken W, et al. INABLE 1 study group Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir Med. 2011;105(7):1030–1036. doi: 10.1016/j.rmed.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 108.Hataji O, Naito M, Ito K, Watanabe F, Gabazza EC, Taguchi O. Indacaterol improves daily physical activity in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:1–5. doi: 10.2147/COPD.S38548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chang YH, Chen RC, Wahlqvist ML, Lee MS. Frequent shopping by men and women increases survival in the older Taiwanese population. J Epidemiol Commun Health. 2012;66(7):e20. doi: 10.1136/jech.2010.126698. [DOI] [PubMed] [Google Scholar]
- 110.Russell R, Anzueto A, Weisman I. Optimizing management of chronic obstructive pulmonary disease in the upcoming decade. Int J Chron Obstruct Pulmon Dis. 2011;6:47–61. doi: 10.2147/COPD.S13758. [DOI] [PMC free article] [PubMed] [Google Scholar]


