Abstract
BACKGROUND
Blood transfusion is associated with an increased risk of organ damage, infection and alloimmunity. Neutrophil extracellular traps (NETs) are extracellular chromatin fibers decorated with neutrophil granular proteins that have been linked to cytotoxicity, thrombosis and autoimmunity. We questioned whether neutrophils in blood products release NETs during storage and thus could contribute to adverse reactions from blood transfusions.
STUDY DESIGN AND METHODS
We analyzed supernatants and blood smears of human red blood cell (RBC) units that either were or were not leukoreduced before storage for markers of NETs.
RESULTS
We identified extracellular DNA, which was associated with histones and myeloperoxidase, a marker of neutrophil granules, in supernatants and blood smears of non-leukoreduced RBC units. These markers of NETs were absent in leukoreduced RBC units. Importantly, NETs passed through blood transfusion filters and could therefore potentially be infused into patients.
CONCLUSIONS
Our studies indicate that NETs are liberated during storage of non-leukoreduced RBC units. Future studies should address whether NETs in RBC units could potentially contribute to transfusion-associated complications.
Keywords: Neutrophil extracellular traps, NETs, DNA, histones, red blood cell units, blood transfusion, storage
INTRODUCTION
Allogeneic transfusions of red blood cell (RBC) units have been associated with adverse reactions including organ injury, infection and alloimmunization.1 An increased risk for complications has also been associated with the age of RBC units.2 Currently, however, it remains unclear whether these associations are truly causal. Prospective clinical trials in this area are ongoing.3,4 RBCs generate bioactive lipids during storage that stimulate neutrophils in vitro and induce lung injury when infused into animals.5,6 Residual leukocytes in RBC units have also been linked to an increased risk of infection and organ dysfunction.7,8 Pre-storage leukocyte reduction of RBC units reduces the risks of both febrile nonhemolytic transfusion reactions and cytomegalovirus transmission.7,8 Other purported benefits of leukoreduction (e.g., reducing immunomodulatory effects of transfusion) remain controversial.7–9
In recent years, it has been shown that stimulated neutrophils and other leukocytes can release chromatin fibers decorated with neutrophil enzymes to form extracellular traps (NETs).10 NETs protect against infection,10,11 but also stimulate thrombosis,12 organ damage13 and autoimmunity.14,15 NET formation (NETosis) requires unwinding chromatin, which is induced by reactive oxygen species (ROS),16 neutrophil elastase and myeloperoxidase (MPO) 17 and importantly histone hypercitrullination by peptidylargininedeiminase 4.18 NETs may be liberated from neutrophils after lysis of the plasma membrane 16 or by an active mechanism which does not require cell death.13,19 Extracellular DNA in association with neutrophil enzymes can be found in the plasma of patients with deep vein thrombosis 20 and systemic thrombotic microangiopathies,12 autoimmunity 21 and transfusion-related acute lung injury (TRALI). 22,23
We hypothesized that storage of RBC units stimulates residual neutrophils to release NETs. Our study included cell-free supernatants of 9 non-leukoreduced and 14 leukoreduced outdated RBC units. We analyzed samples for the presence of DNA, histones and chromatin-MPO complexes. We also investigated samples of 5 non-leukoreduced RBC units stored for 14–16 days and 3 fresh blood samples and questioned whether neutrophils display morphological signs of NETosis by immunocytochemistry. Our data indicate that NETs are released during storage of RBC units.
MATERIALS AND METHODS
Preparation of RBC units and leukoreduction
Red blood cells units were prepared from whole blood units collected into 450 mL blood bags containing citrate-phosphate-dextrose (CPD) as an anticoagulant by centrifugation and stored in AS-5 solution as a preservative. Leukoreduced RBCs were obtained from whole blood units collected in 500 mL blood bags containing CPD as an anticoagulant and AS-3 as a preservative, then leukoreduced within 24h after blood collection using a Leukotrap SCRC leukocyte filtration system in conjunction with a high efficiency Pall BPF4 filter. Leukoreduced RBC units routinely contained less than 106 leukocytes per mL whereas non-reduced units contained approximately 5×109 leukocytes per mL. Untreated and leukoreduced RBC units were stored at 4°C for 42 days. The RBC units were anonymous, outdated units from the Blood Bank at Brigham and Women’s Hospital, Boston, MA. Samples from non-outdated RBC units (different from outdated units), stored for 14–16 days, were collected from segments. Segments are pieces of tubing containing approximately 1 ml of blood product. The tubing and blood bag are connected and thus filled with the same blood product. Segments are prepared by a heat-sealing device, which creates a closed band along the tubing. The band allows a segment to be cut off without opening the blood bag. Segments were used to analyze samples of units before they became outdated.
Supernatant preparation
An aliquot of RBCs was centrifuged for 10 min at 3000 g. The supernatant was collected and spun for 5 min at 10000 g. The resulting pellet was discarded and the supernatant was stored at −80°C.
Quantification of DNA
Supernatants were diluted 1:50 in phosphate buffered saline (PBS, Gibco, Grand Island, NY) containing 0.1% bovine serum albumin (BSA, Sigma, St. Louis, MO). One hundred μl of diluted supernatant were mixed with 100 μl of PBS containing SytoxGreen (final concentration 1 μM, Invitrogen, Grand Isle, NY) to label DNA. Fluorescence was recorded in a fluorometer (Fluoroskan, Thermo Fisher Scientific, Waltham, MA). Auto-fluorescence was considered as background and determined in samples mixed with PBS without SytoxGreen. DNA concentrations were calculated based on a standard curve of known concentrations of DNA (Invitrogen).
Passage through blood administration set
RBCs were passed through a y-type blood set containing a 170 μm filter (Hospira, Lake Forest, IL, product number 11994–48). We injected 50 ml of RBCs into the tubing of the device right above the filter unit. Blood passing through the filter was collected. Supernatants were prepared from three samples before and after the passage and DNA was quantified.
Quantification of nucleosomes
Supernatants were diluted 1:100 in PBS with 0.1% BSA and nucleosomes were quantified by ELISA (Cell Death Detection kit, Roche, Indianapolis, IN) according to manufacturer’s instructions.
Isolation and visualization of DNA
DNA was isolated from supernatants using a DNA isolation kit according to manufacturer’s instructions (Omega bio-tek, Norcross, GA), subjected to 2% agarose gel electrophoresis in the presence of ethidium bromide and visualized using a gel documentation system (Bio-Rad, Hercules, CA).
Western blot analysis for histone H3
Supernatants were analyzed by Western blotting for histone H3. Two μl of supernatants were mixed with Laemmli-Buffer supplemented with 5% beta-mercaptoethanol (Bio-Rad). After 3 min at 95°C, samples were subjected to 15% (w/v) SDS-polyacrylamide gel electrophoresis, followed by immunoblotting with a polyclonal rabbit anti-histone H3 (Abcam, Cambridge, MA, product number ab1791) as primary antibody and goat-anti-rabbit-IgG conjugated to horseradish peroxidase (Bio-Rad) as secondary antibody. Detection was carried out with a Pierce ECL Western Blotting Substrate (Thermo Scientific). Human recombinant histone H3 (New England Biolabs, Ipswich, MA) served as a positive control.
Quantification of MPO
Supernatants were diluted 1:100 in PBS with 0.1% BSA and MPO was quantified by a commercially available kit according to manufacturer’s instructions (Zen MPO ELISA, Invitrogen).
Detection of histone-MPO-complexes
Histone-MPO complexes were detected by an ELISA which employs components of the nucleosomes ELISA (Cell Death Detection Kit, Roche) and the MPO ELISA (Zen MPO ELISA). In brief, supernatants were diluted 1:100 in PBS with 0.1% BSA. Diluted cell-free fractions were mixed with biotinylated mouse-anti-histone antibodies and added to streptavidin-coated wells according to the instructions of the Cell Death Detection Kit. No anti-histone antibodies were added to control samples. After incubation and washing, rabbit-anti-myeloperoxidase antibodies were added to the wells (Component K, Zen MPO ELISA). Control samples did not receive anti-MPO antibodies. After an additional incubation and washing step, immobilized anti-myeloperoxidase antibodies were detected and quantified according the protocol of the Zen MPO ELISA.
Collection of fresh human blood
The investigation received approval from the Boston Children’s Hospital Institutional Review Board. After explaining the nature of the study, we obtained written informed consent from all donors. Ten ml of blood was collected into tubes containing EDTA (BD Vacutainer EDTA tubes, Franklin Lakes, NJ). Blood was centrifuged at 10 min at 3000 g within 30 min of blood collection. The plasma was collected and spun for 5 min at 10000 g. The resulting pellet was discarded and the plasma was stored at −80°C. Plasma was prepared from 3 donors.
Immunostaining of blood smears
Blood smears were fixed for 5 min with 100% methanol followed by 2% paraformaldehyde for 20 minute room temperature. Slides were rinsed with PBS, permeabilized with 0.1% Triton X-100 for 10 min at 4°C, blocked with 3% BSA for 1 h at 37°C, and stained with rabbit-anti-human-myeloperoxidase antibodies (1 μg/ml, Dako, Carpinteria, CA) and Alexa-488-conjugated goat-anti-rabbit-IgG (1.5 μg/ml, Invitrogen) in 0.3% BSA. DNA was labeled with Hoechst 33342 (1 μg/ml, Invitrogen) and images were acquired with a Zeiss Axiovert Microscope. Controls were prepared from freshly collected blood which was anticoagulated by EDTA. DNA stainings were quantified using ImageJ software (IMAGEJ, National Institutes of Health, Bethesda, MD).
Statistical evaluation
Statistical analysis was performed using Prism Software (GraphPad, LaJolla, CA) and included mean and Mann-Whitney test and ANOVA. Results were considered significant at P < 0.05.
RESULTS
Leukocytes release DNA during storage of RBC units
Current U.S. regulations permit storage of RBC units for up to 42 days. We examined whether neutrophils generate extracellular DNA in RBC units during storage. To test our hypothesis, we prepared supernatants of non-leukoreduced RBC units stored for 14–16 days and for 42 days and compared it to freshly prepared plasma from healthy donors. Quantification of DNA using a fluorescent DNA probe (Fig. 1A), revealed increased DNA levels at day 14–16 and in supernatants of RBC units stored for 42 days. We measured approximately 100-fold more DNA than in fresh controls (dotted line in Fig. 1A). We anticipated that leukocytes would be the source of extracellular DNA. Indeed leukoreduced RBC units, in which the leukocyte count is reduced from 5×109 to 106 cells before storage, contained only 88 ng/ml DNA after 42 days (Fig. 1A).
Figure 1. Leukocytes release high-molecular weight chromatin during storage of RBC units.
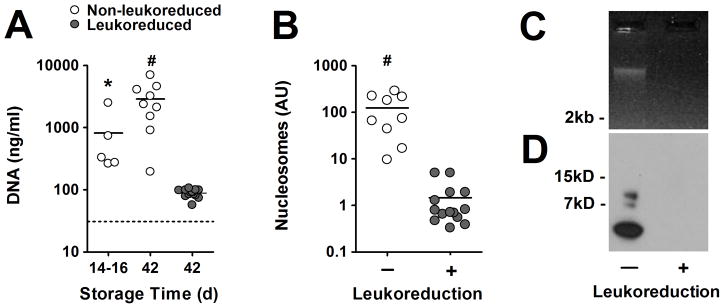
(A) DNA is elevated in non-leukoreduced RBC units at days14–16 (n=5) and day 42 (n=9), but not in leukoreduced RBC units at day 42 (n=14) indicating that leukocytes release DNA during storage of RBC units. Dotted line indicates plasma DNA levels of fresh blood. (B) High levels of nucleosomes are detected in supernatants from non-leukoreduced (n=9), but not in leukoreduced RBC units (n=14) stored for 42 days indicating that extracellular DNA is associated with histones. (C) Supernatants from non-leukoreduced but not from leukoreduced RBC units contained high-molecular weight DNA (> 2kB). (D) Bands of histone H3, smaller than the native 15kB H3 (not shown), were detected in supernatants indicating that histone H3 in RBC units is fragmented. Supernatants from leukoreduced RBC units did not contain histone H3. Data are presented as mean in panels A and B. P-value is calculated using the (A) ANOVA and (B) Mann-Whitney test (* P < 0.05, # P < 0.001 vs. leukoreduced RBC units stored for 42 days). DNA and histone H3 were analyzed from three RBC units and three leukoreduced RBC units stored for 42 days and panels B and C show representative results.
We next questioned whether the extracellular DNA would pass through a standard y-type blood set, which was comprised of a 170 μm filter. We found similar amounts of extracellular DNA in non-leukoreduced RBC samples before and after the passage through the administration set (before vs. after, p = 0.88, n=3) indicating that extracellular DNA can be infused into patients.
Stored RBC units contain high-molecular weight DNA-histone complexes
Leukocytes contain nuclear and mitochondrial DNA and both can be released to form NETs. 10,24 Nuclear and mitochondrial DNA can be distinguished by the presence of histones. We characterized DNA in supernatants from non-leukoreduced and leukoreduced RBC units stored for 42 days and measured extracellular nucleosomes (DNA-histone complexes) by an ELISA which employs antibodies against histones and DNA. We detected strongly elevated nucleosome levels in RBC units which contained leukocytes, and leukoreduction reduced nucleosome levels approximately 100-fold (Fig. 1B). These data suggest that leukocytes release nuclear DNA during RBC storage. Nuclear DNA can be liberated from leukocytes undergoing apoptosis, necrosis or NETosis. Apoptosis is characterized by fragmentation of chromosomal DNA, whereas DNA cleavage is largely absent in necrotic leukocytes or leukocytes undergoing NETosis. We isolated and visualized DNA in supernatants from RBC units stored for 42 days and detected a single DNA band of high-molecular weight (Fig. 1C). In agreement with our DNA and nucleosomes quantification, no DNA was observed in samples from leukoreduced RBC units. Our data show that the majority of extracellular DNA is not fragmented and thus does not derive from apoptotic cells. It furthermore shows that extracellular DNA does not degrade during blood storage. This is likely because the storage medium is deprived of free-calcium which is required for efficient nuclease activity. We next characterized extracellular histone H3 in supernatants from RBC units. Western blot analysis revealed three fragments of histone H3 smaller than the native 15kd H3 protein (Fig. 1D). Histones are sensitive to proteolytic cleavage and fragmentation of histones by active protein C or neutrophil serine proteases has recently been described.17,25
Neutrophils release extracellular traps in stored RBC units
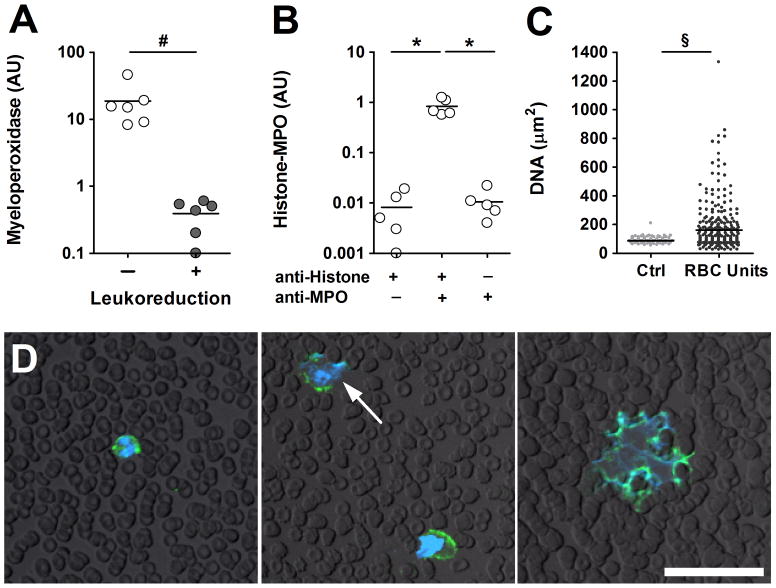
Histone fragmentation in neutrophils has been recently reported in response to cellular activation and NETosis.17 Activation and NETosis also discharges MPO from neutrophil granules. We quantified MPO in supernatants from RBC units and detected high levels of extracellular MPO in units containing leukocytes (Fig. 2A), whereas leukoreduction also reduced the amount of MPO in supernatants (Fig. 2A). We now questioned whether MPO in supernatants from the non-leukoreduced units was associated with extracellular nucleosomes as it occurs in NETs. 10,21 Using an ELISA, which employs anti-histone antibodies and anti-MPO antibodies,21 we were able to detect histone-MPO complexes (Fig. 2B) suggesting that neutrophils in RBC units undergo NETosis. During NETosis, the nucleus of neutrophils goes through characteristic morphological changes: the lobulated nucleus turns into rounded swollen shape before NETs are released as indicated by irregularly shaped DNA staining larger than a neutrophil.16 We analyzed the nuclear size of leukocytes from fresh blood and RBC units stored for 14–16 days. Staining and quantification of DNA revealed significantly larger DNA stainings in smears from stored blood compared to fresh controls (Fig. 2C) indicating that chromatin decondensation occurred during blood storage. Using immunocytochemistry, we identified neutrophils which displayed delobulated, diffuse nuclei or NETs indicated by extracellular DNA webs which contain MPO (Fig. 2D) in blood smears of 14–16 day old RBC units thus indicating that neutrophils undergo NETosis during blood storage.
Figure 2. Neutrophils release extracellular DNA traps in RBC units.
(A) MPO in supernatants was detected in RBC units stored for 42 days (n=6). Leukoreduction of RBCs units (n=6) greatly reduced the amount of extracellular MPO. (B) Histone-MPO complexes were detected in supernatants of non-leukoreduced RBC units (n=5). Controls lacked either anti-histone or anti-MPO antibodies. The presence of histone-MPO complexes suggests that neutrophils undergo NETosis during blood storage. (C) Analysis of nuclear size of blood smears from fresh blood or RBC units stored for 14–16 days. Blood smears showed larger DNA staining in stored (n = 304) compared to fresh blood (n = 61). (D) Identification of NETs by immunocytochemistry. Blood smears of 14–16 day old RBC units were stained for myeloperoxidase (green) and DNA (blue). Neutrophils were identified as MPO positive cells with small nuclei (left panel). Neutrophils undergoing NETosis were characterized as MPO-positive cells with disintegrated nuclei (arrows, middle panel) and NETs were identified as an extracellular DNA web containing MPO (right panel). Bar represents 50 μm. Data are presented as mean. P-values are calculated using (A, C) Mann-Whitney test and (B) ANOVA (* P < 0.05, # P< 0.01, § P < 0.001).
DISCUSSION
Our data suggest that NET formation occurs during RBC storage. In vitro, several mechanisms for NET formation exist. Viable neutrophils may release NETs by ejecting mitochondrial or nuclear DNA.13,19 Neutrophils may undergo a cell death program which leads to the liberation of chromatin decorated with granular proteins.16,17 Even though the underlying signaling events of these different mechanisms are not fully understood, NET formation is generally considered an active process which requires neutrophil stimulation. Storage of RBCs liberates proinflammatory lipids into the plasma and in vitro, these lipids prime the NAPDH oxidase system of neutrophils and potentiate ROS production.5 ROS can induce NET formation and thus proinflammatory lipids may facilitate NET formation during storage. Interestingly, plasma and lipids from stored RBCs induced transfusion-related acute lung injury (TRALI) in an isolated, perfused rat lung model.6 We and others have recently shown that NETs are implicated in TRALI.22,23 NETs are abundant in the microcirculation and alveolar space of lungs after induction of TRALI by autoantibodies. Importantly, treatment of mice with DNase1 or anti-histone antibodies ameliorated the disease suggesting that NETs contribute to TRALI in mice. 22,23 Moreover, patients with TRALI showed increased levels of MPO-DNA complexes in plasma indicating that NET formation occurs during TRALI in humans.22,23 Here we show that NET formation occurs during storage of RBCs and it is conceivable that NETs in blood products exert their toxic,13,26 prothrombotic12 or immunomodulatory functions14,15 after infusion into patients. Indeed, infusion of purified histones into mice causes platelet aggregation and lung injury.25,27
As of 2008, approximately 20% of RBC units and nearly half of whole blood-derived platelets collected in the U.S. were non-leukoreduced.28 Leukoreduction increases the cost per transfusion substantially; costs have been estimated to be up to $600 million per year in the U.S.8 Given the ever-increasing pressures to reduce health care costs, it is conceivable that the proportion of non-leukoreduced blood products transfused in the U.S. may increase over time. Several studies suggest that leukoreduction reduces the length of hospital stay after allogenic transfusion,29–31 however the clinical benefits of leukoreduction could not be confirmed in other studies and remains controversial.7,8,32,33 It would be interesting to investigate in future studies whether the presence of NET-biomarkers in RBC units can be linked to transfusion-related adverse effects.
Acknowledgments
Sources of Support: This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health grant R01 HL102101 (DDW).
We thank Lesley Cowan for help preparing the manuscript. This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health grant R01 HL102101 (DDW).
Footnotes
Conflict of Interest: The authors have no conflict of interest.
References
- 1.Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. First of two parts--blood transfusion. N Engl J Med. 1999;340:438–47. doi: 10.1056/NEJM199902113400606. [DOI] [PubMed] [Google Scholar]
- 2.Offner PJ. Age of blood: does it make a difference? Crit Care. 2004;8 (Suppl 2):S24–6. doi: 10.1186/cc2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glynn S. The red blood cell storage lesion: a method to the madness. Transfusion. 2010;50:1164–9. doi: 10.1111/j.1537-2995.2010.02674.x. [DOI] [PubMed] [Google Scholar]
- 4.Middelburg RA, van de Watering LM, van der Bom JG. Blood transfusions: good or bad? Confounding by indication, an underestimated problem in clinical transfusion research. Transfusion. 2010;50:1181–3. doi: 10.1111/j.1537-2995.2010.02675.x. [DOI] [PubMed] [Google Scholar]
- 5.Silliman CC, Moore EE, Kelher MR, Khan SY, Gellar L, Elzi DJ. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–54. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silliman CC, Voelkel NF, Allard JD, Elzi DJ, Tuder RM, Johnson JL, Ambruso DR. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–67. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blajchman MA. The clinical benefits of the leukoreduction of blood products. J Trauma. 2006;60:S83–90. doi: 10.1097/01.ta.0000199537.09201.7b. [DOI] [PubMed] [Google Scholar]
- 8.Silliman CC, Moore EE, Johnson JL, Gonzalez RJ, Biffl WL. Transfusion of the injured patient: proceed with caution. Shock. 2004;21:291–9. doi: 10.1097/00024382-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Aiboshi J, Moore EE, Ciesla DJ, Silliman CC. Blood transfusion and the two-insult model of post-injury multiple organ failure. Shock. 2001;15:302–6. doi: 10.1097/00024382-200115040-00009. [DOI] [PubMed] [Google Scholar]
- 10.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs TA, Kremer Hovinga JA, Schatzberg D, Wagner DD, Lammle B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012;120:1157–64. doi: 10.1182/blood-2012-02-412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–9. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–91. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–13. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–44. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 20.van Montfoort ML, Stephan F, Lauw MN, Hutten BA, Van Mierlo GJ, Solati S, Middeldorp S, Meijers JC, Zeerleder S. Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2013;33:147–51. doi: 10.1161/ATVBAHA.112.300498. [DOI] [PubMed] [Google Scholar]
- 21.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, Grone HJ, Brinkmann V, Jenne DE. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–5. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, Cifuni SM, Fuchs TA, von Andrian UH, Hartwig JH, Aster RH, Wagner DD. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–43. doi: 10.1182/blood-2012-01-405183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–71. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–53. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–14. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitaker BI, Schlumpf K, Schulman J, Green J. The 2009 National Blood Collection and Utilization Survey (NBCUS) Report. Washington, DC: US Department of Health and Human Services, Office of the Assistant Secretary for Health; 2011. Report of the US Department of Health and Human Services. [Google Scholar]
- 29.Hebert PC, Fergusson D, Blajchman MA, Wells GA, Kmetic A, Coyle D, Heddle N, Germain M, Goldman M, Toye B, Schweitzer I, vanWalraven C, Devine D, Sher GD Leukoreduction Study I. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. JAMA. 2003;289:1941–9. doi: 10.1001/jama.289.15.1941. [DOI] [PubMed] [Google Scholar]
- 30.Tartter PI, Mohandas K, Azar P, Endres J, Kaplan J, Spivack M. Randomized trial comparing packed red cell blood transfusion with and without leukocyte depletion for gastrointestinal surgery. Am J Surg. 1998;176:462–6. doi: 10.1016/s0002-9610(98)00245-1. [DOI] [PubMed] [Google Scholar]
- 31.van de Watering LM, Hermans J, Houbiers JG, van den Broek PJ, Bouter H, Boer F, Harvey MS, Huysmans HA, Brand A. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation. 1998;97:562–8. doi: 10.1161/01.cir.97.6.562. [DOI] [PubMed] [Google Scholar]
- 32.Phelan HA, Sperry JL, Friese RS. Leukoreduction before red blood cell transfusion has no impact on mortality in trauma patients. J Surg Res. 2007;138:32–6. doi: 10.1016/j.jss.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 33.Nathens AB, Nester TA, Rubenfeld GD, Nirula R, Gernsheimer TB. The effects of leukoreduced blood transfusion on infection risk following injury: a randomized controlled trial. Shock. 2006;26:342–7. doi: 10.1097/01.shk.0000228171.32587.a1. [DOI] [PubMed] [Google Scholar]