Abstract
Context
Palliative services have historically been offered to terminal cancer patients, but much less so in other chronic illnesses like chronic obstructive pulmonary disease (COPD) because of difficulties in predicting the trajectory to death.
Objectives
The goal of this study was to determine if the change over time of key parameters (trajectory) in severe COPD patients can independently predict short-term mortality.
Methods
We analyzed data from 1218 patients with severe COPD. Multivariate models for trajectory change were used to forecast mortality at 12 months.
Results
Changes in several variables by defined cutpoints increase significantly and independently the odds of dying in 12 months. The earliest and strongest predictors were the decrease in gait speed by 0.14 m/sec or six-minute walk by 50m (OR 4.40, P<0.0001). Alternatively, if six-minute walk or gait speed were not used, change toward perceiving a very sedentary -state using single question- (OR 3.56, p=0.0007)and decrease in maximal inspiratory pressure >11 cm H2O (OR 2.19, p=0.0217). Then, and change toward feeling upset or downhearted (OR 2.44, p=0.0250), decrease in room air resting PaO2 >5 mmHg (OR 2.46, p=0.0156), increase in room air resting PaCO2 >3 mmHg (OR 2.8, p=0.0039). Change over time models were more discriminative (lower c-statistics) than change form baseline models.
Conclusion
The changes in defined variables and patient-reported outcomes by defined cutpoints were independently associated with increased 12-month mortality in patients with severe COPD. These results may inform clinicians when to initiate end-of-life communications and palliative care.
Keywords: Chronic obstructive pulmonary disease, severe COPD, end-stage COPD, palliative care, end-of-life care, gait speed, mortality, prediction tools
Introduction
Palliative care and end-of-life care are becoming increasingly important in the face of an aging population afflicted with multiple chronic conditions. Estimates show that about 27% of Medicare’s annual $327 billion budget goes to care for patients in their final year of life.1
Palliative services have most commonly been offered to terminal cancer patients where the prediction of mortality is much more precise.2 In cancer, compared with other chronic illnesses, the juncture at which to introduce a palliative focus is clearer for both patient and physician.
In the arena of chronic illnesses, changing the cynosure of care from therapeutic to palliative is often subtle. The current model for palliative care proposes an intertwined relationship between palliative and life-prolonging care.3–5 The efficacy of the current dichotomy is supported by a recent randomized study that reported that early referral to palliative care meaningfully improved quality of life in patients with lung cancer6 compared with usual care. That model, early referral to palliative care, may be applicable to other prevalent chronic and disabling conditions such as very severe chronic obstructive pulmonary disease (COPD).
In COPD, the fourth leading cause of death in the U.S., barriers to providing appropriate and timely end-of-life communications and palliative care are very much rooted in the inherent difficulties in predicting the trajectory to death. There is an existing knowledge gap about which changes in defined variables over time predict 12-month mortality, a window considered appropriate for referral to palliative care.7
FEV1 (forced expiratory volume in one second), the most used marker of disease in COPD, does not change significantly to predict survival once the patient is in the very severe stage (<35%). Although a decrease in the modified BODE (body mass index, airflow obstruction, dyspnea, and exercise capacity) score by more than one point has been reported as predictive of increased mortality in severe COPD, its value for daily clinical use and to initiate palliative care is limited. The BODE score is not routinely measured; further, the time frame for the increased mortality was not specified by the investigators. 8
The goal of this study was to demonstrate how the trajectory (change over time) of simple variables can predict 12-month mortality. We hypothesized that there are simple clinically measurable variables not previously defined whose change over time is meaningful and associated with short-term mortality in COPD. We envisioned that such results would be timely and critical for bridging the gap between patient and provider for initiating communications about end-of-life and palliative care interventions. From this, collaborative exchange and decisions can be made to redirect the focus of treatment.
Methods
The clinical data used for this analysis were collected as part of the National Emphysema Treatment Trial (NETT).9 Between January 1998 and July 2002, 1218 patients enrolled in the NETT. Clinical assessments and patient self-reports were collected at baseline, six months, 12 months and each subsequent year through 2003; mortality was ascertained as of September 30, 2008. This study was approved by the institutional review boards at all sites, and all patients signed informed consent forms before entering the study. In this study, patients with severe emphysema were randomized to medical therapy or lung reduction surgery. Characteristics, methods, inclusion and exclusion criteria have been previously published. 9
The outcome of interest was death in the 12 months following a clinical assessment. At each assessment time (6, 12, and 24 months), the patient was classified as either dying or not dying in the 12 months following the assessment time.
Predictors of interest were: decline from baseline to the assessment time or decline from the previous assessment to the current assessment time in clinical measures or patient self-reports. Candidate predictors were selected a priori from variables included in the NETT data set that were found to be prognostic of survival in prior studies and others that, because of simplicity, could be easily translated and applied to daily clinical practice. 8,10
A patient was considered to have had a clinically significant decline in a categorical variable if the response had changed from no to yes or had declined by at least one level. A patient was considered to have had a clinically significant decline in a continuous variable if the patient’s score had declined by more than half of a standard deviation. A half standard deviation change can be considered a clinically significant change in the absence of an established significant difference.11 Two predictors were generated for each variable at each assessment time (6, 12, and 24 months): decline from baseline and decline from the previous assessment; Table 1 lists the variables used to generate predictors.
Table 1.
Variables Studied and Included in All Models (in addition to sex, gender, marital status and arm in the randomized study)
| Variable | Mean Value or Percent at Baseline | Change Considered Clinically Significant (1/2 SD for continuous variables and otherwise stated for categorical ones ) |
|---|---|---|
| PaO2(mmHg) at rest on room air | 64.4 | ↓5.14 |
| PaCO2(mmHg) at rest on room air | 43.1 | ↑2.91 |
| Maximal inspiratory pressure(cmH2O) | 62.0 | ↓11.24 |
| FVC % predicted, pre BD (bronchodilator) | 59.1 | ↓7.43 |
| FEV1 % predicted, post BD | 26.8 | ↓3.59 |
| RV % predicted, post BD | 221.9 | ↑24.69 |
| TLC % predicted, post BD | 128.2 | ↓7.57 |
| DLCO % predicted | 27.9 | ↓4.82 |
| Maximum work (watts) | 39.0 | ↓10.81 |
| Gait speed (meters/second) | 1.00 | ↓0.137 |
| Six-minute walk | 371.1 | ↓49.3 |
| Weight (kg) | 70.4 | ↓6.72 |
| MRC dyspnea scale (scale 1–5) | 3.2 | ↑One level |
| Modified BODE index 8 | 5.3 | ↑One level |
| Feelings of being lonely or socially isolated?a | 7.9% | No to Yes |
| Trouble falling asleep or staying asleep?a | 31.0% Yes | No to Yes |
| Spells of feeling upset, downhearted, or blue?a | 16.1% Yes | No to Yes |
| Because of any impairment or health problem, did you need help with your personal care needs, such as eating, dressing, bathing, or getting around your home?a | 4.8% Yes | No to Yes |
| Avoid walking, have trouble walking, or walk more slowly than other people your age?a | 66.9% Yes | No to Yes |
| Spent most or all of day in chair, recliner of bed due to a health condition?a | 11.8% Yes | No to Yes |
For all items of the Quality of Well-Being questionnaire,30 patients were asked to respond to the following prompt “Please check which days (if any) over the past 3, not including today: a. no days; b. yesterday; c. 2 days ago; or d. 3 days ago.” Responses of “a. no days” were considered as “No “and any other response (“b. yesterday; c. 2 days ago; or d. 3 days ago”) was considered as a response of “Yes.” The latter method of characterization and the modified BODE score have been reported previously.8,14
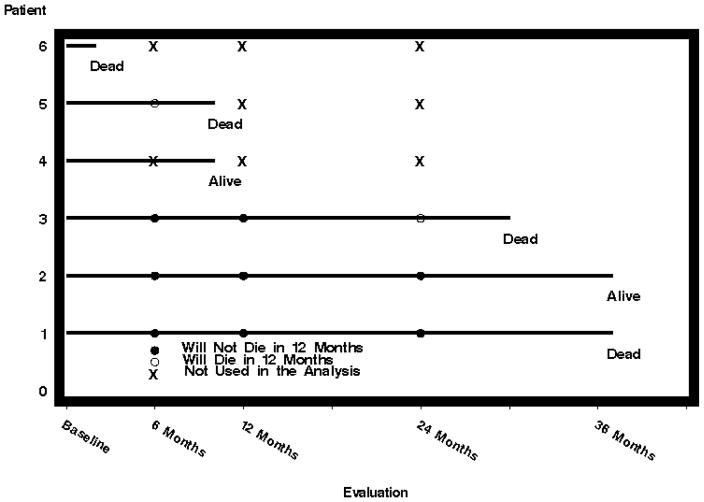
Figure 1 diagrams outcome and predictor availability over time for six example patients. Eighty-six patients who died before the first evaluation at six months were excluded from all analyses. These patients had only baseline scores, so they did not provide information on the prognostic power of changes in variables. Table 2 shows the available patient at each time point.
Fig. 1.
Diagram of outcome and predictor availability over time for 6 example patients.
Table 2.
Univariate Variables Prognostic for Dying Within 12 Months
| Variable With Significant Worsening from Baseline | Number of Patients Dying in the Next 12 Months | Number of Patients NOT Dying in the Next 12 Months | % of Patients Dying in the Next 12 Months Who Had Significant Worsening | % of Patients NOT Dying in the Next 12 Months Who Had Significant Worsening | P-value |
|---|---|---|---|---|---|
| Gait Speed at 6 months | 40 | 817 | 48% | 20% | <0.0001 |
| RV % predicted, post BD at 6 months | 46 | 835 | 20% | 10% | 0.0447 |
| PaCO2 at 6 Months | 45 | 830 | 33% | 20% | 0.0498 |
| Disability * at 6 Months | 54 | 893 | 13% | 3% | 0.0004 |
| Spent most or all of day in chair, recliner or bed due to a health condition? * at 6 months | 54 | 893 | 30% | 10% | <0.0001 |
| Maximal inspiratory pressure(cmH2O) at 12 Months | 35 | 690 | 37% | 17% | 0.0022 |
| Maximum work(watts) at 12 months | 29 | 649 | 41% | 23% | 0.0212 |
| Feeling upset or downhearted at 12 months | 45 | 756 | 33% | 16% | 0.0019 |
| PaO2(mmHg) at rest on room air at 24 months | 35 | 397 | 43% | 25% | 0.0211 |
| Weight (kg) at 24 months | 37 | 404 | 22% | 6% | 0.0009 |
| Feeling lonely or socially isolated at 24 months | 50 | 477 | 20% | 9% | 0.0095 |
Statistical Analysis
Separate univariate and multivariate analyses were run at 6, 12, and 24 months. No covariates other than the predictors described above were included in the multivariate modeling. Although baseline factors such as treatment arm and age would influence when the patient started to decline, those variables were not predictive of death after the patient had already started to decline.
At each time point and for each measure of clinically significant worsening (i.e., each predictor), Fisher’s Exact Test was used to compare the percentage of patients with clinically significant worsening who died in the next 12 months to the percentage of patients without clinically significant worsening who died in the next 12 months. A stepwise logistic model for predicting death in the next 12 months was fit for each time point, with covariates selected from the set of all clinical change measures available at the time point. These models were verified using bootstrapping,11,12 which is a recommended method for validating the predictive power of logistic models. Finally, a repeated measure logistic regression model for predicting death in the next 12 months and including clinical measures from multiple time points was run using SAS Proc GENMOD (SAS Institute Inc., Cary, NC). This repeated measures analysis adjusts for the correlation between measurements caused by the same patients having information at multiple time points. For completeness, this model also adjusted for treatment arm (NETT study arm: lung reduction surgery or medical treatment), age, gender, marital status, and disability.
Results
Mean age at baseline was 66.1 ± 6.1 years; 61% were male. Table 1 shows the variables studied (included in the models in addition to age, gender, marital status) and the corresponding change considered clinically significant worsening (half standard deviation or one category change). Table 3 summarizes variables prognostic for dying within 12 months in the univariate analyses. Changes from baseline in several variables were predictive of death within the next 12-month period.
Table 3.
Variables Significant in Stepwise Multivariate Logistic Modeling
| Variable With Significant Worsening from Baseline | Odds Ratio for Death Within 12 Months | 95% Confidence Interval | P-value | C-Statistic Using Only Baseline Variables | C-Statistic Using Change from Baseline |
|---|---|---|---|---|---|
| Gait speed decline by 0.137 at 6 months or six-minute walk decline by 50 meters | 4.40 | 2.23 to 8.67 | <0.0001 | 0.599 | 0.638 |
| New incidence of feeling upset or downhearted at 12 months | 2.44 | 1.12 to 5.32 | 0.0250 | 0.560 | 0.622 |
| PaO2(mmHg) at rest on room air decline by 5.14 at 12 months | 2.46 | 1.19 to 5.09 | 0.0156 | ||
| PaCO2(mmHg) at rest on room air increase by 2.91 at 24 months | 2.80 | 1.39 to 5.63 | 0.0039 | 0.587 | 0.620 |
Changes in gait speed or six-minute walk, becoming severely sedentary, RV% predicted, maximal inspiratory pressure, feeling downhearted, and maximum work were predictive for death. In later evaluations, changes in PaO2, PaCO2, weight, isolation, and disability were prognostic for death.
Stepwise multivariate logistic modeling (using gait speed/six-minute walk [Table 4] or alternatively using severe sedentarism instead of gait speed [Table 5]) shows factors significant during at least one evaluation. Repeated measures analysis using all of the endpoints and adjusting for treatment arm, age, gender, marital status, and disability confirmed the variables picked by the logistic models that were strongly associated with dying in the next 12 months (Table 6).
Table 4.
Variables Significant in Stepwise Multivariate Logistic Modeling (Alternative Model Not Using Gait Speed)
| Variable With Significant Worsening from Baseline | Odds Ratio for Death Within 12 Months | 95% Confidence Interval | P-value | C-Statistic Using Only Baseline Variables | C-Statistic Using Change from Baseline |
|---|---|---|---|---|---|
| Maximal inspiratory pressure(cmH2O) at 6 months | 2.19 | 1.12 to 4.29 | 0.0217 | 0.554 | 0.646 |
| New incidence of spending most or all of day in chair, recliner or bed due to a health condition at 6 months | 3.56 | 1.71 to 7.40 | 0.0007 | ||
| New incidence of feeling upset or downhearted at 12 months | 2.44 | 1.12 to 5.32 | 0.0250 | 0.560 | 0.622 |
| PaO2(mmHg) at rest on room air decline by 5.14 at 12 months | 2.46 | 1.19 to 5.09 | 0.0156 |
Table 5.
Repeated Measures Multivariate Logistic Modela
| Variable | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Maximal inspiratory pressure(cmH2O) | 2.29 | 1.45 to 3.62 | 0.0004 |
| Feeling upset or downhearted | 1.97 | 1.16 to 3.33 | 0.0117 |
| Gait Speed decline by 0.137 at 6 months or six-minute walk decline by 50 meters | 1.70 | 1.09 to 2.66 | 0.0202 |
| PaO2(mmHg) at rest on room air decline by 5.14 | 1.69 | 1.07 to 2.67 | 0.0240 |
This model takes into account all of the time points and results in four easy-to-interpret variables.
Table 6.
Distribution of Analyzed Patients Over Time
| Evaluation | Number (%) of Patients for Death Analysis | Number (%) of Patients Dying Within 12 Months of This Evaluation |
|---|---|---|
| Baseline | 1218 (100%) | -- |
| 6 | 1072 (88%) | 90 (8%) |
| 12 | 925 (76%) | 87 (9%) |
| 24 | 649 (53%) | 97 (15%) |
| 36 | 347 (28%) | 100 (29%) |
| 48 | 112 (9%) | 56 (50%) |
| 60 | 26 (2%) | 24 (92%) |
Discussion
Our results identified changes in defined variables that independently predict 12-month mortality in patients with severe COPD. In this analysis, we found that the very first indicator that predicts end of life in this population is the decline over time in gait speed or six-minute walk (Table 4) or alternatively (however, less strongly), the patient reporting a change to a very low physical activity (Table 5) –“spending most of the day in chair bed or recliner due to health condition.” These findings have very practical implications because patients who declined in gait speed by 0.14 meters/second or the six-minute walk by 50 meters or self-report severe sedentarism increased their odds of dying in the subsequent year about four times. Our results support and encourage the use of six-minute walk/gait speed as a routine measure in severe COPD for forecasting end of life. The robustness and predictive power of gait speed/distance walked was confirmed by bootstrapping models, a recommended method for validating the predictive power of logistic models (instead of having a derivative cohort and a validating one).12
We also found in the model that included sedentarism but not gait speed (Table 5) that a decline of maximal inspiratory pressure (MIP) by 14 mmHg is also a predictor of 12-month mortality. Most likely MIP is a surrogate marker for muscle mass. The fact that MIP did not enter the multivariate model that included gait speed/six-minute walk may suggest the comprehensiveness of the gait speed/six-minute walk as a global measure of physical function, including muscle function. Very likely, both gait speed/six-minute walk and extreme sedentarism inform about the same construct related to frailty and perhaps very low physical activity. These findings are particularly relevant and timely as very low physical activity is an event that has been recently highlighted as an independent and most important predictor of mortality in COPD. 13,14
Although the six-minute walking test change with time has been reported to predict survival in patients with COPD,15 there are critical differences that make our findings unique. First, we based our analysis on predicting the odds of dying at one year (a time frame that is considered appropriate for initiation of palliative care and end-of-life communications) whereas previous work was based on the survival models with a follow-up that was variable (one to two years). The latter may account for the reported overlap in walking tests that was seen by their authors as a limitation. Our analysis carries the robustness of a sample size five times larger in a population of well-characterized COPD patients. Second, we used predefined cutoffs to facilitate clinicians’ interpretation (of our results) and decision making in the identification of a patient with a high risk of dying in one year. Finally, we demonstrated that the change in the predictive parameters (trajectory) is more discriminative of death in one year compared with the prediction from a baseline value (higher c statistics from models based on trajectory compared with models from baseline variables).
Our intention in reporting the decline of gait speed (in addition to reporting six-minute walk) is because gait speed is gaining attention as a simple functional capacity indicator of very high significance in the elderly and one can be easily measured in the clinic or in the home setting. 16
The models at 24 months found that change in oxygenation and the development of depressive symptoms become independent predictors, and finally, one of the multivariate models included a change in PCO2 at 36 months.
Our findings on the changes in gas exchange, in a well-defined and reproducible clinical condition (room air and exacerbation free status), are in concert of previous reports that have shown them as important risk factors for mortality.17,18 We have shown that an increase of PCO2 by 3mmHg or deterioration of PO2 by >5mmHg are important changes as both are predictive of 12-month mortality. Although the changes in PO2 and PCO2 may seem trivial and not clinically relevant, the fact that they were taken in very defined conditions and their independent association with the end of life provide them value. The amount of decline observed in PO2 and PCO2 may represent a measure of a minimal clinically important difference. Even though we wanted to define a meaningful change in a generalized and simple way for all continuous variables (half standard deviation), we also analyzed the decline by 10 mmHg in both PO2 and PCO2 (a more common and intuitive meaningful decline) and as expected, that change much increased the odds of death in 12 months (analysis not shown). From a practical standpoint, the authors believe that a room air arterial blood gas is a measure that is feasible in the office care of severely ill patients (as it was done in the multicenter, large cohort that participated in this study).
Whereas the decline in functional capacity determinants seem to be the earlier signs of end of life in COPD patients, we consider patient perceptions of health and symptoms also to be of the utmost importance. The perception of depressive symptoms have been consistently significant in our results and in previous multivariate models.19 Our results add to the existing literature that the development of depressive symptoms, in the context of severe and very severe disease, independently indicate higher odds of dying in 12 months. Depression has been largely identified as a predictor of mortality in COPD.20–22 Our results not only confirm the observation that a patient’s perceptions of well-being and mental health are critically important to physical health, but also that the development of depressive symptoms in a patient with severe lung disease has a morbid meaning. This concept has been noted previously.23,24 Exploring the emotional needs of a patient and discussing care goals are a challenging task for every clinician. 25 To open the lines of communication, our results indicate that simple questions during a clinical encounter are informative and add to the overall assessment of the need of palliative care.
Although nothing can replace the overall gestalt of a patient’s worsening status that heralds the end of life, we believe that our results may be informative for the initiation of end-of- life communications and timely palliative care. Our results serve to fill a knowledge gap about the trajectory towards end of life in patients with very severe COPD.26 Whereas predictors of one-year survival exist, they are not informative to the clinician as they have no cutpoint or specific values that guide decisions and, therefore, are not commonly used to refer COPD patients for palliative care. Importantly, our results indicated that the change in parameters over time is more discriminative of death in one year (higher c-statistics) than the static values at baseline. Other studies that have looked at the relationships between different variables and mortality only predict which patients will live longer.10,27 They do not give any indication of whether or not a specific patient is close to death. The goal of this research was to see if these variables were indicators that patients had started their decline towards death. Indicators of this decline could be used to signal that a patient and their physician should consider a discussion of palliative care.
Understanding which changes in the trajectory of a very severely ill COPD patient are independently associated with approaching end of life may be of significant value in decision making for the early referral to palliative care in this prevalent disease. Early referral to palliative care in advanced lung cancer meaningfully improved quality of life compared with usual care.6 That model can possibly be applied to patients with very severe COPD and lead to an improved quality of life so much desired by patients.
Our study has several important limitations. First, our cohort was derived from a cohort of COPD patients with predominant emphysematous characteristics and may not completely represent the total spectrum of severe COPD. However, the quality of the data and the results (not just survival prediction based on a single evaluation) encouraged us to report these findings as they may represent the first available data to inform about the trajectory to death. Second, gait speed as it was calculated in this study represents identical information to the six-minute walk. Gait speed calculated from the six-minute walking test has been previously reported28 but it may provide a different result compared with gait speed that is obtained from a resting start. The value of the method presented lies in the independent association with mortality in 12 months. Third, inspiratory muscle strength is a measure that depends heavily on patient’s effort and technician coaching (something that may be different across laboratories), and can introduce significant variability and, therefore, requires confirmation. The strength of this broad analysis lies in the volume and diversity of patients (17 centers) with available data and the breadth of clinical and patient self-assessment questions available for analysis.
In summary, we found that in severe COPD patients, the trajectory to end of life is initially signaled by a decline in a physical function measure (six-minute walk/gait speed) or the development of a very severe sedentary life, followed by the onset of depressive symptoms, and a decline in oxygenation and eventually a decline in PCO2. Our results suggest very practical implications for the care of patients with severe COPD: the yearly measure of the six-minute walk or gait speed, the screening for severe physical inactivity or extreme sedentarism (one item question), depression (one item question), and the room-air assessment of oxygenation (first) and hypercapnia.
We believe that the novelty of this report is rooted in the identification of specific changes that are predictive of short-term survival and are more informative than predictions made from baseline values. Our findings may help fill the knowledge gap about forecasting end of life in COPD, what has been described as “prognostic paralysis,”29 when clinicians faced with uncertain illness trajectories prevaricate when considering end-of-life issues, and most importantly, from that awareness that would stimulate communication between patients, families and health care providers. The latter will lead to better symptomatic treatment and early referral for palliative care services, which can ameliorate both the functional and emotional burden in facing a terminal illness, promote better quality of life at the end of life, and decrease unnecessary care.
Acknowledgments
Dr. Benzo is supported by grant 1R01CA163293-01 from the National Heart Lung and Blood Institute, National Institutes of Health. The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS; formerly the Health Care Financing Administration); and the Agency for Healthcare Research and Quality (AHRQ).
Information regarding the National Emphysema Treatment Trial (NETT) Research Group is available in the Appendix at jpsmjournal.com.
Footnotes
Disclosures
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nicholas LH, Langa KM, Iwashyna TJ, Weir DR. Regional variation in the association between advance directives and end-of-life Medicare expenditures. JAMA. 2011;306:1447–1453. doi: 10.1001/jama.2011.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrahm JL, Hansen-Flaschen J. Hospice care for patients with advanced lung disease. Chest. 2002;121:220–229. doi: 10.1378/chest.121.1.220. [DOI] [PubMed] [Google Scholar]
- 3.Elkington H, White P, Addington-Hall J, Higgs R, Pettinari C. The last year of life of COPD: a qualitative study of symptoms and services. Respir Med. 2004;98:439–445. doi: 10.1016/j.rmed.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Lanken PN, Terry PB, Delisser HM, et al. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177:912–927. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 5.Thorns A, Cawley D. Palliative care in people with chronic obstructive pulmonary disease. BMJ. 2011;342:d106. doi: 10.1136/bmj.d106. [DOI] [PubMed] [Google Scholar]
- 6.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 7.Goodridge DM, Marciniuk DD, Brooks D, et al. End-of-life care for persons with advanced chronic obstructive pulmonary disease: report of a national interdisciplinary consensus meeting. Can Respir J. 2009;16:e51–53. doi: 10.1155/2009/987616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez FJ, Han MK, Andrei AC, et al. Longitudinal change in the BODE index predicts mortality in severe emphysema. Am J Respir Crit Care Med. 2008;178:491–499. doi: 10.1164/rccm.200709-1383OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 10.Martinez FJ, Foster G, Curtis JL, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173:1326–1334. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, et al. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 12.Efron B. The bootstrap and markov-chain monte carlo. J Biopharm Stat. 2011;21:1052–1062. doi: 10.1080/10543406.2011.607736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140:331–342. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 14.Benzo RP, Chang CC, Farrell MH, et al. Physical activity, health status and risk of hospitalization in patients with severe chronic obstructive pulmonary disease. Respiration. 2010;80:10–18. doi: 10.1159/000296504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto-Plata VM, Cote C, Cabral H, Taylor J, Celli BR. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J. 2004;23:28–33. doi: 10.1183/09031936.03.00034603. [DOI] [PubMed] [Google Scholar]
- 16.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124:459–467. doi: 10.1378/chest.124.2.459. [DOI] [PubMed] [Google Scholar]
- 18.Almagro P, Calbo E, Ochoa de Echaguen A, et al. Mortality after hospitalization for COPD. Chest. 2002;121:1441–1448. doi: 10.1378/chest.121.5.1441. [DOI] [PubMed] [Google Scholar]
- 19.Laurin C, Moullec G, Bacon SL, Lavoie KL. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med. 2012;185:918–923. doi: 10.1164/rccm.201105-0939PP. [DOI] [PubMed] [Google Scholar]
- 20.Gudmundsson G, Gislason T, Janson C, et al. Depression, anxiety and health status after hospitalisation for COPD: a multicentre study in the Nordic countries. Respir Med. 2006;100:87–93. doi: 10.1016/j.rmed.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Gardiner C, Gott M, Payne S, et al. Exploring the care needs of patients with advanced COPD: an overview of the literature. Respir Med. 2010;104:159–165. doi: 10.1016/j.rmed.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Fan VS, Ramsey SD, Giardino ND, et al. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007;167:2345–2353. doi: 10.1001/archinte.167.21.2345. [DOI] [PubMed] [Google Scholar]
- 23.Reinke LF, Engelberg RA, Shannon SE, et al. Transitions regarding palliative and end-of-life care in severe chronic obstructive pulmonary disease or advanced cancer: themes identified by patients, families, and clinicians. J Palliat Med. 2008;11:601–609. doi: 10.1089/jpm.2007.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocker GM, Dodek PM, Heyland DK. Toward optimal end-of-life care for patients with advanced chronic obstructive pulmonary disease: insights from a multicentre study. Can Respir J. 2008;15:249–254. doi: 10.1155/2008/369162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis JR, Engelberg RA, Nielsen EL, Au DH, Patrick DL. Patient-physician communication about end-of-life care for patients with severe COPD. Eur Respir J. 2004;24:200–205. doi: 10.1183/09031936.04.00010104. [DOI] [PubMed] [Google Scholar]
- 26.Curtis JR. Palliative and end-of-life care for patients with severe COPD. Eur Respir J. 2008;32:796–803. doi: 10.1183/09031936.00126107. [DOI] [PubMed] [Google Scholar]
- 27.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 28.Ilgin D, Ozalevli S, Kilinc O, et al. Gait speed as a functional capacity indicator in patients with chronic obstructive pulmonary disease. Ann Thorac Med. 2011;6:141–146. doi: 10.4103/1817-1737.82448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart S, McMurray JJ. Palliative care for heart failure. BMJ. 2002;325:915–916. doi: 10.1136/bmj.325.7370.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Revicki DA, Kaplan RM. Relationship between psychometric and utility-based approaches to the measurement of health-related quality of life. Qual Life Res. 1993;2:477–487. doi: 10.1007/BF00422222. [DOI] [PubMed] [Google Scholar]