Abstract
Objectives
Administrative data have been used to compare carotid endarterectomy (CEA) and carotid artery stenting (CAS). However, there are limitations in defining symptom status, CMS high-risk status, as well as complications. Therefore, we did a direct comparison between administrative data and physician chart review as well as between data collected for the National Surgical Quality Improvement Program (NSQIP) and physician chart review for CEA and CAS.
Methods
We performed an outcomes analysis on all CEA and CAS procedures from 2005–2011. We obtained ICD-9 diagnosis codes from hospital discharge records regarding symptom status, high-risk status, and perioperative stroke. We also obtained data on all CEA patients submitted to NSQIP over the same time period. A physician then performed a chart review of the same patients to determine symptom status, high-risk status, and perioperative strokes and the results were compared.
Results
We identified 1342 patients who underwent CEA or CAS between 2005–2011 and 392 patients who underwent CEA that were submitted to NSQIP. Administrative data identified fewer symptomatic patients (17.0% vs. 34.0%), fewer physiologic high-risk patients (9.3% vs. 23.0%), fewer anatomic high-risk patients (0% vs. 15.2%), and a similar proportion of perioperative strokes (1.9% vs. 2.0%). However, administrative data identified 8 false positive and 9 false negative perioperative strokes. NSQIP data identified more symptomatic patients compared to chart review (44.1% vs. 30.3%), fewer physiologic high-risk patients (13.0% vs. 18.6%), fewer anatomic high-risk patients (0% vs. 6.6%), and a similar proportion of perioperative strokes (1.5% vs. 1.8%, only 1 false negative stroke and no false positives).
Conclusions
Administrative data are unreliable for determining symptom status, high-risk status, and perioperative stroke and should not be used to analyze CEA and CAS. NSQIP data do not adequately identify high-risk patients, but do accurately identify perioperative strokes and to a lesser degree, symptom status.
Introduction
Administrative data have been utilized to compare carotid endarterectomy (CEA) and stenting (CAS) 1–3. Large databases populated with administrative data are valuable research tools. However, the accuracy of administrative data in determining pre-existing disease, symptom status, high-risk status, and perioperative complications has been questioned 4. Studies utilizing the Nationwide Inpatient Sample report that 90%–97% of the patients undergoing carotid revascularization are asymptomatic 1–3 while multicenter studies and retrospective reviews report that only 56%–72% of patients are asymptomatic 5–8. ICD-9 codes (International Classification of Diseases) are nonspecific and imprecise. They do not specify the extent of disease within a diagnosis (e.g. class III–IV congestive heart failure [CHF]), do not provide laterality of disease, and lack the temporal timing of onset. These limitations with ICD-9 codes limit the ability to distinguish preexisting disease from new disease and perioperative complications.
Other large datasets that utilize clinical data, such as the National Surgical Quality Improvement Program (NSQIP), may provide a more accurate measurement of symptom status, high-risk status, and perioperative complications. NSQIP is not reliant on hospital coders. Trained clinical nurse reviewers input data prospectively. However, the current iteration of NSQIP does not have procedure specific comorbidities or complications. For example, a history of transient ischemic attack (TIA) or stroke are obtained but may have occurred more than 6 months before surgery or may have been contralateral to the carotid undergoing treatment. Similarly, there is no measure of disease severity. The ability to accurately determine symptom status and high-risk status with NSQIP has not been studied. The purpose of this study is to determine the accuracy with which administrative data and NSQIP data capture symptom status, high-risk status, and perioperative complications as compared to chart review by a trained physician.
Methods
Patients
We obtained hospital administrative discharge data on all patients undergoing CEA or CAS from January 1, 2005 to December 31, 2011 at our institution. Patients were identified using ICD-9 procedure codes for CEA (38.12) or CAS (00.63). We also identified all patients undergoing CEA at our institution that were submitted to NSQIP. We used our institution’s administrative discharge data and prospectively collected clinical data submitted to NSQIP, to determine symptom status, high-risk status, and perioperative stroke and compared these results to physician chart review. This study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center and Harvard Medical School.
Hospital administrative discharge data
We identified patients undergoing CEA or CAS. Patients having coronary artery bypass (CABG) (36.11–36.16) or cardiac valve repair (35.X) during the same hospitalization were identified. All ICD-9 diagnosis codes associated with each patient’s discharge record were reviewed to determine symptom status and physiologic and anatomic high-risk status. Physiologic high-risk variables include age >80 years, CHF Class III/IV, left ventricular ejection fraction (LVEF) <30%, unstable angina, myocardial infarction (MI) within 30 days, severe lung disease (forced expiratory volume in 1 second <30% of predicted or home oxygen), hemodialysis, and CABG or valve repair within 30 days. Anatomic high-risk variables include contralateral laryngeal nerve palsy, restenosis after prior CEA, radiation therapy to the neck, a high lesion, prior neck surgery, and contralateral internal carotid artery occlusion. Patients were considered symptomatic if they had ICD-9 codes for TIA (435.X, 781.4, V12.54), amaurosis fugax/retinal vascular occlusion (362.3X, 368.12), or stroke (433.11, 433.31, 433.91, 434.01, 434.11, 434.91). Patients without any of these diagnosis codes were considered asymptomatic. As has been previously reported, ICD-9 diagnosis codes were used to identify comorbid conditions that would qualify the patient for CMS physiologic high-risk status 3 (Table 1). Patients without these conditions were considered non-high risk. Perioperative strokes were identified using ICD-9 code 997.02 (iatrogenic cerebrovascular infarction or hemorrhage).
Table 1.
Physiologic and anatomic high-risk variables and how they are defined by the Centers for Medicare and Medicaid Services, administrative data, and the National Surgical Quality Improvement Program Database
| CMS Definitions | Administrative Data | NSQIP Definitions |
|---|---|---|
| Physiologic High Risk | ||
| Age > 80 years | Age > 80 years | Age > 80 years |
| Recent MI within 30 days | ** 412 | History of a non-Q wave or a Q wave MI within 6 months of surgery |
| Unstable angina | ** 411.1 | Angina within 30 days of surgery |
| CHF class III or IV | ** 428 | Newly diagnosed CHF or chronic CHF with new symptoms within 30 days of surgery |
| LVEF < 30% | Unavailable | Unavailable |
| Hemodialysis | ** 585.3 – 585.9, 586, V42.0, V45.1 – V45.12, V56.0 – V56.32, V56.8 | Acute or chronic renal failure requiring peritoneal dialysis, hemodialysis, hemofiltration, hemodiafiltration, or ultrafiltration within 2 weeks of surgery |
| Severe pulmonary disease * | ** 490 – 492.8, 493, 494 – 494.1, 495– 505, 506.4 | Severe COPD *** |
| CABG/valve repair within 30 days | ** 36.11 – 36.16, 35 | CABG/valve repair performed at the same time as CEA |
| Anatomic High Risk | ||
| Contralateral ICA occlusion | Unavailable | Unavailable |
| Restenosis after CEA | Unavailable | Unavailable |
| Prior neck surgery | Unavailable | Unavailable |
| Contralateral laryngeal nerve palsy | Unavailable | Unavailable |
| History of neck irradiation | Unavailable | Unavailable |
| High or Low lesion | Unavailable | Unavailable |
CMS, Centers for Medicare and Medicaid Services; MI, myocardial infarction; CHF, congestive heart failure; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting; ICA, internal carotid artery; CEA, carotid endarterectomy; COPD, chronic obstruction pulmonary disease
Forced expiratory volume in 1 second (FEV1) < 30% of predicted or home oxygen
ICD-9-CM codes
Functional disability from COPD, hospitalization for treatment of COPD, chronic bronchodilator therapy, or an FEV1 < 75% of predicted
Prospectively collected NSQIP data
NSQIP is a national multi-institutional, risk-adjusted, prospectively collected clinical database created to facilitate quality control review of outcomes. The registry collects information on demographics, comorbidities, intraoperative information, and 30-day perioperative outcomes. Specially trained clinical nurse reviewers, trained by the ACS, input data at each participating institution using standardized definitions. We obtained a list of all patients undergoing CEA at our institution whose data were submitted to NSQIP. We calculated the proportion of patients that were symptomatic, physiologic and anatomic high-risk, and those with perioperative strokes. Physiologic high-risk variables utilized in NSQIP include age >80 years, active CHF, MI within 6 months, angina within 1 month, and hemodialysis 9. Patients were considered symptomatic if they had a history of TIA or stroke.
Physician chart review
One of the study authors (R.B.) performed a thorough chart review of the same patients identified using hospital administrative data and the patients submitted to NSQIP. The senior author clarified all questions encountered during chart review. Symptom status, physiologic and anatomic high-risk status, and perioperative strokes were identified. Symptomatic was defined as ipsilateral carotid territory TIA or stroke within 6 months of their carotid procedure. Discrepancies in the identification of symptom status, high-risk status, and perioperative strokes were identified and recorded. The majority of patients were not examined by a neurologist unless there was a suspicion for a perioperative stroke or if they were enrolled in a clinical trial requiring neurologic evaluation.
Defining high-risk variables
CMS has specific criteria defining high-risk for CEA 10. When performing chart review, all CMS guidelines were adhered to when identifying both physiologic and anatomic high-risk patients. However, ICD-9 coding lacks the level of detail and specificity outlined by CMS. Furthermore, there are no ICD-9 codes for anatomic high-risk variables so these were unable to be assessed with administrative data. NSQIP has specific definitions of comorbidities that differ from CMS 11 and lacks anatomic details. Table 1 lists high-risk variables (physiologic and anatomic) and how they are identified and defined by CMS, administrative data, and NSQIP respectively.
Statistical analysis
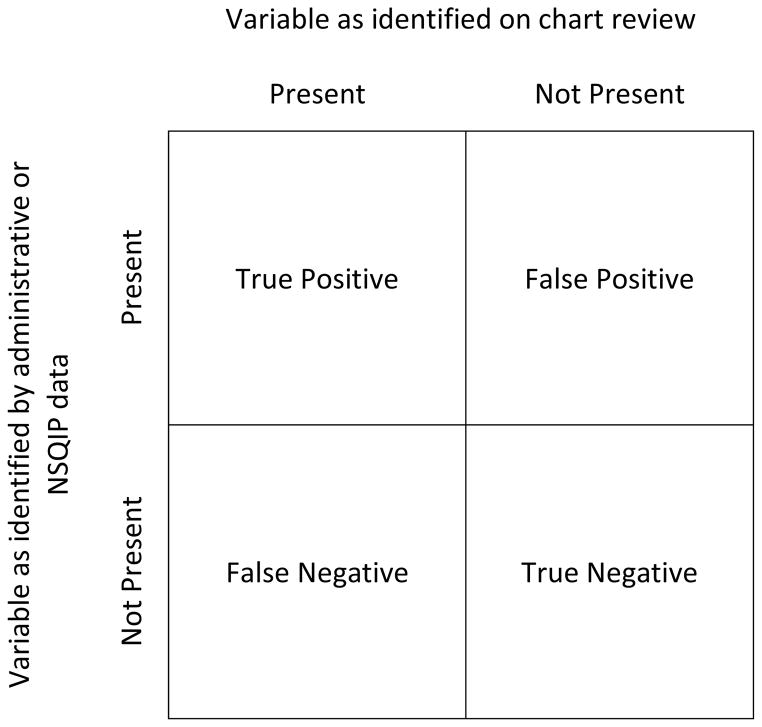
We calculated the proportion of patients who were considered symptomatic, physiologic high-risk, anatomic high-risk, and who experienced a perioperative stroke by administrative data and NSQIP data respectively and then compared them to physician chart review of the same patients. We then calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and kappa coefficient of the ability of administrative data and NSQIP data to properly identify symptom status, high-risk status, and perioperative strokes as compared to chart review (Figure 1 shows the 2×2 table used to calculate the sensitivity, specificity, PPV, and NPV). Sensitivity measures the accuracy of identifying a variable (i.e. symptomatic, high-risk) as being present in patients in whom it truly is present. Specificity measures the accuracy of identifying a variable as being absent in patients who do not have the variable. PPV measures the proportion of patients identified as having a variable that truly have the variable. NPV measures the proportion of patients identified as not having a variable that truly do not have that variable. Kappa is a measure of agreement beyond chance between two databases or methods of identifying data. Kappa values >0.75 indicate excellent agreement, 0.40–0.75 fair to good agreement, and values <0.4 poor agreement. All statistical tests were performed using STATA 12 software (StataCorp, College Station, TX).
Figure 1.
2×2 table used to calculate sensitivity, specificity, positive predictive value, and negative predictive value.
Results
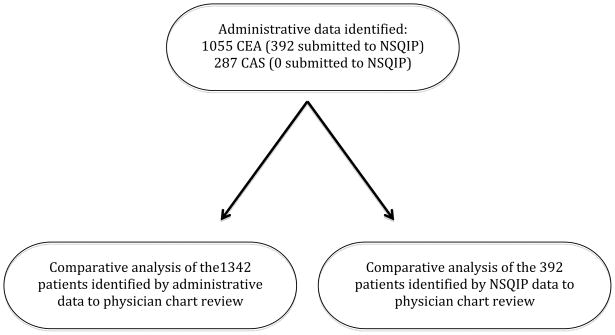
Administrative data identified 1342 patients who underwent carotid revascularization (1055 CEA and 287 CAS) at our institution from 2005–2011. We identified 392 CEA patients submitted to NSQIP, all of whom were included in the 1055 CEA patients identified by administrative data. A chart review was then performed of the 1342 patients identified with administrative data and the 392 patients submitted to NSQIP to make direct comparisons to each method (Figure 2).
Figure 2.
Flow diagram depicting all patients captured by administrative data, NSQIP data, and physician chart review.
Comparison of administrative data and physician chart review
Comparing administrative data to chart review, administrative data identified fewer symptomatic patients (17.0% vs. 34.0%), fewer physiologic high-risk patients (9.3% vs. 23.0%), fewer anatomic high-risk patients (0% vs. 15.2%), and a similar proportion of perioperative strokes (1.9% vs. 2.0%). When examining individual high-risk variables, administrative data overestimated recent MI, CHF, hemodialysis, and severe pulmonary disease while underestimating unstable angina, LVEF <30%, and CABG/valve repair (Table 2). Administrative data are unable to identify anatomic high-risk variables using ICD-9 codes.
Table 2.
Comparison of outcomes analysis using administrative data and physician chart review in 1342 patients who underwent CEA or CAS between 2005 and 2011
| Administrative Data | Physician chart review | |||
|---|---|---|---|---|
|
| ||||
| n | % | n | % | |
| Symptomatic | 228 | 17.0% | 456 | 34.0% |
| Physiologic High Risk | 125 | 9.3% | 308 | 23.0% |
| Age > 80 years | 265 | 19.7% | 265 | 19.7% |
| Recent MI | 174 | 13.0% | 9 | 0.7% |
| Unstable angina | 1 | 0.0% | 16 | 1.2% |
| CHF class III or IV | 125 | 9.3% | 10 | 0.7% |
| LVEF < 30% | . | . | 35 | 2.6% |
| Hemodialysis | 108 | 8.0% | 13 | 1.0% |
| Severe pulmonary disease* | 250 | 18.6% | 15 | 1.1% |
| CABG/valve repair within 30 days | 32 | 2.4% | 48 | 3.6% |
| Anatomic High Risk | . | . | 204 | 15.2% |
| Contralateral ICA occlusion | . | . | 82 | 6.1% |
| Restenosis after CEA | . | . | 108 | 8.0% |
| Prior neck surgery | . | 41 | 3.1% | |
| Contralateral laryngeal nerve palsy | . | . | 2 | 0.1% |
| History of neck irradiation | . | . | 29 | 2.2% |
| High or Low lesion | . | . | 29 | 2.2% |
| Perioperative stroke | 26 | 1.9% | 27 | 2.0% |
MI, myocardial infarction; CHF, congestive heart failure;
LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting
ICA, internal carotid artery; CEA, carotid endarterectomy
Forced expiratory volume in 1 second < 30% of predicted or home oxygen
Symptom status
Administrative data identified 228 symptomatic patients while chart review identified 456. However, there was poor overlap. Of 228 symptomatic patients identified by administrative data, only 167 were truly symptomatic. This means that 289 of the 456 symptomatic patients identified by chart review were misclassified as being asymptomatic by administrative data. The sensitivity of administrative data for determining symptom status is 36.6%, specificity 93.1%, PPV 73.2%, and NPV 74.1%. The kappa is 0.34 indicating poor agreement between administrative data and chart review (Table 3).
Table 3.
Sensitivity, specificity, positive predictive value, negative predictive value, and kappa of administrative data compared to chart review.
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Kappa | |
|---|---|---|---|---|---|
| Symptomatic | 36.6% | 93.1% | 73.2% | 74.1% | 0.34 |
| Physiologic High Risk | 19.5% | 93.7% | 48.0% | 79.6% | 0.17 |
| Age > 80 years | 100.0% | 100.0% | 100.0% | 100.0% | 1.00 |
| MI within 30 days | 33.3% | 55.4% | 1.7% | 97.2% | −0.01 |
| Unstable angina | 6.3% | 100.0% | 100.0% | 96.2% | 0.11 |
| CHF class III or IV | 10.0% | 0.4% | 0.8% | 96.6% | −0.03 |
| LVEF < 30% | . | . | . | . | . |
| Hemodialysis | 61.5% | 73.6% | 7.4% | 98.2% | 0.08 |
| Severe pulmonary disease* | 46.7% | 35.5% | 2.8% | 94.4% | −0.02 |
| CABG/valve repair within 30 days | 66.7% | 100.0% | 100.0% | 95.6% | 0.78 |
| Perioperative stroke | 66.7% | 99.4% | 69.2% | 99.3% | 0.67 |
High-risk status
Administrative data identified 125 physiologic high-risk patients while chart review identified 308. Only 60 of the 125 were truly physiologic high-risk leaving 248 of the 308 misclassified by administrative data as being non-high risk. The sensitivity of administrative data for determining high-risk status is 19.5%, specificity 93.7%, PPV 48.0%, and NPV 79.6%. The kappa is 0.17 indicating poor agreement between administrative data and chart review. There was also poor concordance with the individual high-risk variables with the exception of age >80 years and CABG/valve repair. (Table 3) The kappa for age >80 and CABG/valve repair is 1.00 and 0.78 respectively, indicating excellent agreement. The kappa for the other high-risk variables ranges from −0.03 to 0.11 indicating poor agreement.
Perioperative stroke
Administrative data identified 26 perioperative strokes while chart review identified 27. Of the 26 strokes identified by administrative data, 18 were true perioperative strokes and 8 were false positive identifications. Nine perioperative strokes were missed entirely by administrative data. Table 4 lists the explanations for the 8 false positive and 9 false negative perioperative strokes identified by administrative data. The sensitivity of administrative data for determining perioperative strokes is 66.7%, specificity 99.4%, PPV 69.2%, and NPV 99.3%. The kappa is 0.67 indicating fair to good agreement with chart review (Table 3).
Table 4.
Explanations for the 8 false positive strokes and 9 false negative strokes identified by administrative data
| 8 false positive perioperative strokes
|
| 4 patients were falsely coded as having a perioperative stroke |
| 1 preoperative stroke was miscoded as being a postoperative stroke |
| 3 strokes occurred after admission to the hospital for other reasons |
| One occurred after a laparoscopic cholecystectomy |
| One occurred after admission to the hospital for treatment of pneumonia |
| One occurred after admission to the hospital for lower extremity ischemia
|
| 9 false negative perioperative strokes
|
| 3 were post-discharge strokes |
| 2 were misclassified as preoperative strokes |
| 4 strokes were not coded at all in the discharge data |
Comparison of NSQIP data and physician chart review
When comparing NSQIP to chart review, NSQIP identified more symptomatic patients (44.1% vs. 30.3%), fewer physiologic high-risk patients (13.0% vs. 18.6%), fewer anatomic high-risk patients (0% vs. 6.6%), and a similar proportion of perioperative strokes (1.5% vs. 1.8%). When examining individual high-risk variables, NSQIP overestimated recent MI, unstable angina, CHF, and severe pulmonary disease while underestimating LVEF <30% and hemodialysis. (Table 5) There was complete agreement in identifying patients >80 years and those who underwent CABG/valve repair. NSQIP is unable to identify anatomic high-risk variables.
Table 5.
Comparison of outcomes analysis using NSQIP data and physician chart review in 392 patients who underwent CEA between 2005 and 2011
| NSQIP data | Physician chart review | |||
|---|---|---|---|---|
|
| ||||
| n | % | n | % | |
| Symptomatic | 173 | 44.1% | 119 | 30.3% |
| Physiologic High Risk | 51 | 13.0% | 73 | 18.6% |
| Age > 80 years | 76 | 19.4% | 76 | 19.4% |
| Recent MI | 7 | 1.8% | 2 | 0.5% |
| Unstable angina | 8 | 2.0% | 1 | 0.3% |
| CHF class III or IV | 3 | 0.8% | 0 | 0.0% |
| LVEF < 30% | . | . | 4 | 1.0% |
| Hemodialysis | 3 | 0.8% | 5 | 1.3% |
| Severe pulmonary disease* | 41 | 10.4% | 4 | 1.0% |
| CABG/valve repair within 30 days | 3 | 0.8% | 3 | 0.8% |
| Anatomic High Risk | . | . | 26 | 6.6% |
| Contralateral ICA occlusion | . | . | 20 | 5.1% |
| Restenosis after CEA | . | . | 15 | 3.8% |
| Prior neck surgery | . | . | 4 | 1.0% |
| Contralateral laryngeal nerve palsy | . | . | 0 | 0.0% |
| History of neck irradiation | . | . | 2 | 0.5% |
| High or Low lesion | . | . | 2 | 0.5% |
| Perioperative stroke | 6 | 1.5% | 7 | 1.8% |
MI, myocardial infarction; CHF, congestive heart failure;
LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting
ICA, internal carotid artery; CEA, carotid endarterectomy
Forced expiratory volume in 1 second < 30% of predicted or home oxygen
Symptom status
NSQIP identified 173 symptomatic patients while chart review identified 119. Of the 173 patients identified by NSQIP, 109 were truly symptomatic. This means that 10 of the 119 symptomatic patients identified by chart review were misclassified as being asymptomatic using NSQIP. The sensitivity of NSQIP for determining symptomatic status is 91.6%, specificity 76.6%, PPV 63.0%, and NPV 95.4%. The kappa of NSQIP is 0.60 indicating fair to good agreement between NSQIP and chart review (Table 6).
Table 6.
Sensitivity, specificity, positive predictive value, negative predictive value, and kappa of NSQIP data compared to chart review.
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Kappa | |
|---|---|---|---|---|---|
| Symptomatic | 91.6% | 76.6% | 63.0% | 95.4% | 0.60 |
| Physiologic High Risk | 13.7% | 87.1% | 19.6% | 81.5% | 0.01 |
| Age > 80 years | 100.0% | 100.0% | 100.0% | 100.0% | 1.00 |
| MI within 30 days | 50.0% | 98.5% | 14.3% | 99.7% | 0.21 |
| Unstable angina | 0.0% | 98.0% | 0.0% | 99.7% | −0.01 |
| CHF class III or IV | 0.0% | 99.2% | 0.0% | 100.0% | −0.01 |
| LVEF < 30% | . | . | . | . | . |
| Hemodialysis | 60.0% | 100.0% | 100.0% | 99.5% | 0.75 |
| Severe pulmonary disease* | 75.0% | 90.2% | 7.3% | 99.7% | 0.11 |
| CABG/valve repair within 30 days | 100.0% | 100.0% | 100.0% | 100.0% | 1.00 |
| Perioperative stroke | 85.7% | 100.0% | 100.0% | 99.7% | 0.92 |
High-risk status
NSQIP identified 51 physiologic high-risk patients while chart review identified 73. Only 10 of the 51 were truly physiologic high-risk patients leaving 63 of the 73 misclassified by NSQIP as being non-high risk. The sensitivity of NSQIP for determining high-risk status is 13.7%, specificity 98.4%, PPV 58.3%, and NPV 82.6%. The kappa of NSQIP is 0.01 indicating poor agreement between NSQIP and chart review. There was also poor concordance with the individual high-risk variables with the exception of age >80 years, hemodialysis, and CABG/valve repair (kappa of 1.00, 0.75, and 1.00 respectively indicating excellent agreement). (Table 6) The kappa for the other high-risk variables ranges from −0.01 to 0.21 indicating poor agreement.
Perioperative stroke
NSQIP identified 6 perioperative strokes while chart review identified 7. There was 1 false negative stroke and 0 false positives with NSQIP. This1 missed perioperative stroke was simply not captured by the person responsible for NSQIP data input. The sensitivity of NSQIP for determining perioperative stroke is 85.7%, specificity 100%, PPV 100%, and NPV 99.7%. The kappa of NSQIP is 0.92 indicating excellent agreement with chart review (Table 6).
Discussion
Many studies have used administrative data to study patients undergoing CEA or CAS 1–3. The benefit of these databases is their large sample size, while the downside is their reliance on ICD-9 hospital discharge coding. With this study we sought to examine the accuracy of ICD-9 coding as compared to chart review and we have found poor concordance between administrative and clinical data. Administrative data are poor at determining symptom status and high-risk status. While perioperative stroke rates were similar between administrative data and chart review, administrative data identified the wrong patients and had poor accuracy. NSQIP data were also poor at determining high-risk status, but were accurate for determination of perioperative strokes and to a lesser extent symptom status.
Our study is the first to examine the accuracy of identifying symptom status and physiologic high-risk status with administrative data. Recently, the validity of prior studies utilizing administrative data to compare outcomes after CEA and CAS has been questioned 4. Our results validate this question as we have found that administrative data cannot accurately determine symptom status. ICD-9 codes lack the specificity needed to accurately determine the extent and severity of a patient’s comorbid condition, lack laterality of disease, and lack the temporal timing of onset. Because of this lack of detail, the use of ICD-9 codes to determine high-risk status may overestimate certain individual high-risk variables such as class III/IV CHF, recent MI, severe pulmonary disease, and hemodialysis. For example, when examining the rate of recent MI, administrative data identified 174 patients while chart review identified 9. This discrepancy is due to the lack of specificity and timing of ICD-9 codes. ICD-9 diagnosis code 412 is used for myocardial infarction. However, it does not specify timing of the MI (within the past 30 days or remote). Chart review was able to determine that only 9 patients had a MI within 30 days of their carotid procedure. Furthermore, there are no ICD-9 codes available to estimate anatomic high-risk status.
Other studies have examined the accuracy with which acute ischemic strokes are identified with administrative data, but not in the setting of CEA. Goldstein 12 reported that only 79% of patients with a primary discharge diagnosis indicating cerebral infarction actually had an acute stroke and that 7% of patients with an ICD-9 code indicating the absence of cerebral infarction actually had an acute stroke. Reker et al. 13 also showed that there is incredible variability in which ICD-9 code is reported to indicate an acute stroke and to the accuracy with which these codes actually represent new strokes. The rates of perioperative stroke were similar in our study, but this is misleading. Administrative data falsely identified patients as having a perioperative stroke and failed to identify others that did. In our study, 3 of 27 patients experienced their perioperative stroke after discharge from the hospital. Post-discharge events are not captured with hospital discharge data and this highlights a very important limitation of most administrative datasets. Studies have shown that in-hospital data underestimates the true risk of perioperative stroke 5,6,14. NSQIP, however, provides data on outcomes out to 30 days. We feel that true perioperative outcomes include 30-day outcomes and our study highlights that administrative data underestimates the true 30-day perioperative risk. In the setting of CEA the analysis is further complicated by the distinction of pre-operative stroke (indicating CEA for symptomatic disease) versus a postoperative complication, as stroke is a difficult endpoint to measure accurately with administrative data. Mortality, however, is a hard endpoint and can be accurately determined with administrative data 15,16.
We found NSQIP to be poor at determining high-risk status most likely due to the different definitions NSQIP uses to define patient comorbid conditions. CMS has specific high-risk criteria and the NSQIP definition of these differs substantially. CMS is more specific and specifies a greater severity of disease and/or a shorter timeframe for an event to occur. Prior studies have used NSQIP to analyze CEA. One study by Kang et al. 9 did not attempt to determine symptom status because NSQIP does not provide laterality (of symptoms or procedure) and only states if the patient has a history of TIA or stroke and does not provide timing of the event. Gupta et al. 17 did stratify patients by symptom status. Similar to our analysis they specified that a patient was symptomatic if they had a history of a prior TIA or stroke and found a similar proportion of symptomatic patients (43.5% vs. 44.1%). This methodology overestimates the number of symptomatic patients as the sensitivity was 91.6% but the PPV was only 63.0%. This indicates that NSQIP is more accurate at identifying those patients who are truly symptomatic but also identifies patients in whom stroke or TIA was likely more than 6 months before their CEA procedure or contralateral, thus making them asymptomatic. One perioperative stroke was missed by NSQIP and no false positive strokes were identified. NSQIP is more accurate at determining perioperative strokes, but remains poor at identifying high-risk patients and therefore has limited value for comparative effectiveness of CEA and CAS. These limitations of NSQIP have prompted a new iteration of NSQIP to be created, which is currently in use and now captures procedure specific comorbidities and complications, which should make NSQIP a better research and quality improvement tool in the future 18
Other large clinical databases such as the Vascular Study Group of New England (VSGNE) and the Vascular Quality Initiative (VQI) may be better designed to accurately study CEA and CAS. The VSGNE is a regional quality improvement registry that prospectively collects over 140 data points 19 and specifically identifies preoperative symptom status, laterality of disease and procedure, anatomic details, high-risk status, as well as the laterality of perioperative strokes. This level of clinical detail makes the VSNGE and VQI valuable research tools.
Medicare data may provide a higher level of accuracy compared to purely hospital discharge data since the timing of prior procedures (e.g. CABG) could potentially be captured using longitudinal data. Additionally, Medicare may be useful in determining the date of onset of new neurologic symptoms or complications, thus potentially improving the accuracy of determining symptom status and post-procedure strokes. However, knowledge of this timing would not be expected to improve the inherent limitations in the ability to identify post-procedure strokes or the severity of comorbid conditions that we identified in this analysis.
Because of the limitations associated with using administrative data, a present on admission (POA) indicator has been developed to improve coding accuracy. The goal of the POA indicator is to help determine if a diagnosis is a pre-existing disease, a new diagnosis, or a complication that occurred during the hospitalization. Prior studies have shown that the addition of a POA indicator more accurately characterized preexisting conditions from complications 20,21. This should help in determining if a stroke is a perioperative complication or the reason for the procedure. However, the POA indicator will not aid in determining high-risk status, as the current ICD-9 codes are less specific than needed. Improved education of hospital coders may improve the accuracy and reliability of administrative data. ICD-10 codes should improve diagnostic accuracy with regards to symptom status, high-risk status, and perioperative strokes, but the extent of this improvement cannot be determined until ICD-10 codes are fully implemented.
As stated earlier, administrative data have well known limitations. NSQIP is a clinical database, however, it is expensive and voluntary. NSQIP only captures a random selection of all CEAs performed at an institution and does not collect data on CAS. The preoperative characteristics and postoperative complications collected are not procedure specific, NSQIP has definitions of comorbid conditions that differ from CMS, and it does not capture anatomic high-risk criteria. NSQIP does specify a history of TIA or stroke but provides no details on the timing or laterality.
Physician chart review is susceptible to limitations as well. Despite thorough review, information can be overlooked and missed entirely. The accuracy of chart review is reliant upon complete and accurate documentation within the chart. Minor periprocedural strokes may be missed by the clinician or not entered into the medical record. As this is a retrospective review, not all patients were evaluated by a neurologist or had neuroimaging. The true “gold standard” for accurately determining comorbidities and complications is uncertain. However, we feel that physician chart review is the most accurate method available for retrospective analysis. Clearly, further comparisons will be needed to monitor the progress of each of these systems and the relative value of each for answering various clinical questions.
Conclusions
Administrative data are poor at determining symptom status, high-risk status, and accurately detecting perioperative strokes after CEA and CAS. This is in large part due to the lack of specificity with ICD-9 diagnosis codes as they fail to provide information on the severity, laterality, and temporal onset of disease. However, while NSQIP does not adequately identify high-risk patients, it is more accurate at determining perioperative strokes and to a lesser degree, symptom status. The new iteration of NSQIP, as it captures procedure specific comorbid conditions and complications, will make this an even more robust database in the future.
Acknowledgments
This work was supported by the NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734
Footnotes
Author Disclosures: M.L. Schermerhorn is a consultant for Endologix, Medtronic, and Boston Scientific. For all other authors none were disclosed.
This was manuscript was presented at the plenary session of the Peripheral Vascular Surgical Society Meeting in National Harbor, MD on June 6, 2012.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eslami MH, McPhee JT, Simons JP, Schanzer A, Messina LM. National trends in utilization and postprocedure outcomes for carotid artery revascularization 2005 to 2007. J Vasc Surg. 2011;53:307–15. doi: 10.1016/j.jvs.2010.08.080. [DOI] [PubMed] [Google Scholar]
- 2.Vogel TR, Dombrovsky VY, Haser PB, Scheirer JC, Graham AM. Outcomes of carotid artery stenting and endarterectomy in the United States. J Vasc Surg. 2009;49:325–30. doi: 10.1016/j.jvs.2008.08.112. [DOI] [PubMed] [Google Scholar]
- 3.Giles KA, Hamdan AD, Pomposelli FB, Wyers MC, Schermerhorn ML. Stroke and death after carotid endarterectomy and carotid artery stenting with and without high risk criteria. J Vasc Surg. 2010;52:1497–504. doi: 10.1016/j.jvs.2010.06.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hertzer NR. The Nationwide Inpatient Sample may contain inaccurate data for carotid endarterectomy and carotid stenting. J Vasc Surg. 2012;55:263–6. doi: 10.1016/j.jvs.2011.08.059. [DOI] [PubMed] [Google Scholar]
- 5.Sidawy AN, Zwolak RM, White RA, Siami FS, Schermerhorn ML, Sicard GA, et al. Risk-adjusted 30-day outcomes of carotid stenting and endarterectomy: results from the SVS Vascular Registry. J Vasc Surg. 2009;49:71–9. doi: 10.1016/j.jvs.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 6.Goodney PP, Likosky DS, Cronenwett JL Vascular Study Group of Northern New England. Factors associated with stroke or death after carotid endarterectomy in Northern New England. J Vasc Surg. 2008;48:1139–45. doi: 10.1016/j.jvs.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Hobson RW, 2nd, Howard VJ, Roubin GS, Brott TG, Ferguson RD, Pompa JJ, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40:1106–11. doi: 10.1016/j.jvs.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Halm EA, Tuhrim S, Wang JJ, Rockman C, Riles TS, Chassin MR. Risk factors for perioperative death and stroke after carotid endarterectomy: results of the New York Carotid Artery Surgery Study. Stroke. 2009;40:221–9. doi: 10.1161/STROKEAHA.108.524785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang JL, Chung TK, Lancaster RT, LaMuraglia GM, Conrad MF, Cambria RP. Outcomes after carotid endarterectomy: Is there a high-risk population? A National Surgical Quality Improvement Program report. J Vasc Surg. 2009;49:331–9. doi: 10.1016/j.jvs.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Pub. 100–03 Medicare National Coverage Determinations. Department of Health and Human Services, Centers for Medicare and Medicaid Services; 2006. [Google Scholar]
- 11.American College of Surgeons. [Last accessed May 25, 2012];National Quality Improvement Program. Available at http://site.acsnsqip.org/wp-content/uploads/2012/03/ACS_NSQIP_Participant_User_Data_File_User_Guide.pdf.
- 12.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke. Stroke. 1998;29:1602–4. doi: 10.1161/01.str.29.8.1602. [DOI] [PubMed] [Google Scholar]
- 13.Reker DM, Hamilton BB, Duncan PW, Yeh SC, Rosen A. Stroke: who’s counting what? J Rehabil Res Dev. 2001;38:281–9. [PubMed] [Google Scholar]
- 14.Fokkema M, Bensley RP, Lo RC, Hamdan AD, Wyers MC, Moll FL, et al. In-hospital versus post discharge adverse events following carotid endarterectomy. J Vasc Surg. doi: 10.1016/j.jvs.2012.11.072. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holt PJ, Poloniecki JD, Thompson MM. Multicentre study of the quality of a large administrative data set and implications for comparing death rates. Br J Surg. 2012;99:58–65. doi: 10.1002/bjs.7680. [DOI] [PubMed] [Google Scholar]
- 16.Wennberg DE, Lucas FL, Birkmeyer JD, Bredenberg CE, Fisher ES. Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patients characteristics. JAMA. 1998;279:1278–81. doi: 10.1001/jama.279.16.1278. [DOI] [PubMed] [Google Scholar]
- 17.Gupta PK, Pipinos II, Miller WJ, Gupta H, Shetty S, Johanning JM, et al. A population-based study of risk factors for stroke after carotid endarterectomy using the ACS NSQIP database. J Surg Res. 2011;167:182–91. doi: 10.1016/j.jss.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 18.American College of Surgeons. [Last accessed June 21, 2012];National Quality Improvement Program. Available at http://site.acsnsqip.org/
- 19.Vascular Study Group of New England. [Last accessed June 22, 2012]; Available at http://www.vascularweb.org/regionalgroups/vsgne/Pages/home.aspx/index.html.
- 20.Pine M, Jordan HS, Elixhauser A, Fry DE, Hoaglin DC, Jones B, et al. Enhancement of claims data to improve risk adjustment of hospital mortality. JAMA. 2007;297:71–6. doi: 10.1001/jama.297.1.71. [DOI] [PubMed] [Google Scholar]
- 21.Fry DE, Pine M, Jordan HS, Elixhauser A, Hoaglin DC, Jones B, et al. Combining administrative and clinical data to stratify surgical risk. Ann Surg. 2007;246:875–8. doi: 10.1097/SLA.0b013e3180cc2e7a. [DOI] [PubMed] [Google Scholar]