Abstract
Background & Aims
Cholesteryl Ester Storage Disease, an inherited deficiency of lysosomal acid lipase, is an underappreciated cause of progressive liver disease with no approved therapy. Presenting features include dyslipidemia, elevated transaminases, and hepatomegaly.
Methods
To assess the clinical effects and safety of the recombinant human lysosomal acid lipase, sebelipase alfa, 9 patients received 4 once-weekly infusions (0.35, 1, or 3 mg·kg−1) in LAL-CL01 which is the first human study of this investigational agent. Patients completing LAL-CL01 were eligible to enroll in the extension study (LAL-CL04) in which they again received 4 once-weekly infusions of sebelipase alfa (0.35, 1, or 3 mg·kg−1) before transitioning to long term every other week infusions (1 or 3 mg·kg−1).
Results
Sebelipase alfa was well-tolerated with mostly mild adverse events unrelated sebelipase alfa. No anti-drug antibodies were detected. Transaminases decreased in patients in LAL-CL01 and increased between studies. In 7 patients receiving ongoing sebelipase alfa treatment in LAL-CL04, mean±SD decreases for alanine transaminase and aspartate aminotransferase at week 12 compared to the baseline values in LAL-CL01 were 46±21U/L (-52%) and 21±14U/L (-36%), respectively (p<0.05). Through week 12 of LAL-CL04, these 7 patients also showed mean decreases from baseline in total cholesterol of 44±41mg/dL (-22%; p=0.047), low density lipoprotein-cholesterol of 29±31mg/dL (-27%; p=0.078), and triglycerides of 50±38mg/dL (-28%, p=0.016) and increases in high density lipoprotein-cholesterol of 5mg/dL (15%; p=0.016).
Conclusions
These data establish that sebelipase alfa, an investigational enzyme replacement, in patients with Cholesteryl Ester Storage Disease is well tolerated, rapidly decreases serum transaminases and that these improvements are sustained with long term dosing and are accompanied by improvements in serum lipid profile.
Keywords: lysosomal storage, enzyme replacement, fatty liver, hepatomegaly, dyslipidemia
Lysosomal Acid Lipase (LAL) Deficiency [(LAL Deficiency) OMIMD 278000] is a multisystem autosomal recessive disease caused by mutations in the LIPA gene, which encodes the enzyme, lysosomal acid lipase. LAL Deficiency leads to the accumulation of cholesteryl esters and triglycerides in the lysosomes of many tissues, including the liver, spleen, and cardiovascular system (1). LAL Deficiency presents as a clinical continuum with two major phenotypes: a rapidly progressive form, frequently called Wolman Disease, which manifests in infants, and a form that manifests post-infancy, also called Cholesteryl Ester Storage Disease (CESD). CESD is an under-appreciated cause of fatty liver, with prominent microvesicular steatosis, hepatic fibrosis and progression to cirrhosis and early death. Although the natural history of the disease has not been well studied, serious liver complications are frequently described. Splenomegaly and cardiovascular involvement are also commonly seen. Cardiovascular involvement includes accelerated (2) and premature (3) atherosclerosis associated with dyslipidemia (high total and low density lipoprotein-cholesterol [LDL], high triglyceride, and low high density lipoprotein-cholesterol [HDL]). The management of patients with CESD has mainly focused on control of the dyslipidemia through diet and the use of lipid lowering therapies including statins (4-10). Although laboratory improvements may be seen in some cases (4, 6-10), the underlying disease persists and disease progression still occurs (5, 11).
While the potential for enzyme replacement therapy as a treatment for patients with LAL Deficiency has been recognized for more than 25 years (12, 13), earlier attempts to produce recombinant LAL using different manufacturing approaches (Chinese Hamster Ovary (14), yeast (14), and plant-based production systems (15)) did not yield a therapeutic enzyme that progressed into clinical development.
Sebelipase alfa (SBC-102; Synageva BioPharma Corporation, Lexington, Massachusetts, USA) is a recombinant human LAL produced using methodologies that allow targeted expression of a gene sequence (16) in hen oviduct cells (17). The expressed gene sequence encodes for the same amino acid sequence as the native human LAL enzyme with secretion of the recombinant protein into egg white. Sebelipase alfa is the International Nonproprietary Name given to SBC-102 in 2012. In a rat model of LAL Deficiency that replicates a number of the abnormalities seen in patients with the disease (18, 19), sebelipase alfa produced a dose-dependent decrease in transaminases, improvement in liver pathology, and correction of impaired weight gain (20).
This is the first clinical report of the use of sebelipase alfa in patients with liver abnormalities due to CESD. The initial clinical trial and the long term treatment study were designed to characterize the safety, pharmacokinetics, and pharmacodynamic activity of repeat dosing with sebelipase alfa. The pharmacokinetic profile will be reported separately.
Patients and Methods
Sebelipase alfa
Sebelipase alfa is a glycoprotein with six potential N-linked glycosylation sites, of which five are occupied. Structural and compositional analyses demonstrate that sebelipase alfa glycans consist of predominantly N-acetylglucosamine and mannose terminated N-linked structures, which target proteins to the mannose receptors. N-glycans containing terminal mannose-6-phosphate moieties are also consistently expressed in sebelipase alfa, which allow for targeting to a wide variety of cells expressing the mannose-6-phosphate receptor. Cell culture studies (17) show that sebelipase alfa demonstrates mannose receptor dependent uptake and traffics to the lysosomes in a rat macrophage cell line. Additionally sebelipase alfa demonstrates mannose-6-phosphate receptor-dependent uptake and corrects the intracellular enzyme deficiency in fibroblasts from a LAL deficient patient. The specific activity of sebelipase alfa is approximately 260 U/mg protein [one unit is defined as the amount of activity that results in the hydrolysis of 1 μmole of a synthetic substrate, 4-methylumbelliferyl oleate, per minute under the assay conditions] (data on file, Synageva BioPharma Corporation).
Study design
A Phase 1/2 open-label, multicenter, dose-escalation study (LAL-CL01) was conducted between April 2011 and January 2012 across 6 sites in 4 countries. Patients completing LAL-CL01 were eligible to enroll in the LAL-CL04 extension study to further evaluate the clinical effects of sebelipase alfa. The research was conducted in accordance with the Declaration of Helsinki and Good Clinical practice guidelines. The protocols were approved by the appropriate ethics committees or institutional review boards at participating institutions and conducted in compliance with country specific regulatory requirements. The trials were registered as NCT01307098: CL01 (http://www.clinicaltrials.gov/ct2/show/NCT01307098) and NCT01488097: CL04 (http://www.clinicaltrials.gov/ct2/show/NCT01488097). All patients provided informed written consent before undergoing study-specific assessments or procedures.
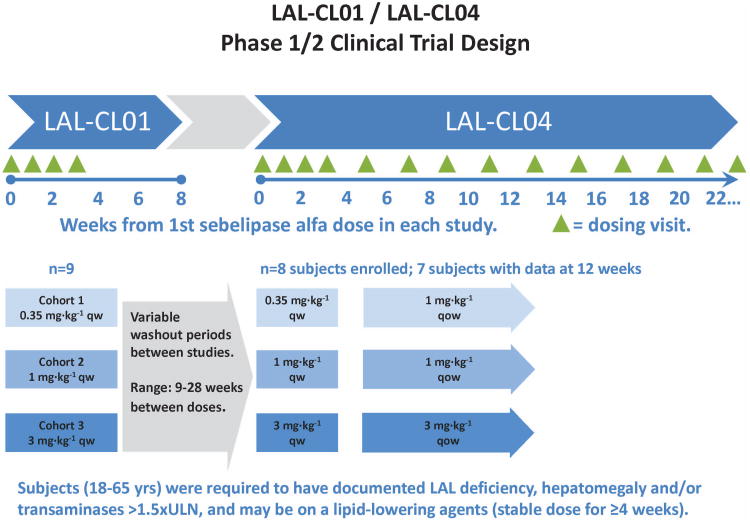
In LAL-CL01, screening assessments were conducted 7 to 28 days prior to the start of dosing. Eligible patients were treated in 3 sequential dose cohorts (Figure 1): 0.35 mg·kg−1 (low dose, Cohort 1: patients 1-3), 1 mg·kg−1 (intermediate dose, Cohort 2: patients 4-6), or 3 mg·kg−1 (high dose, Cohort 3: patients 7-9). In each cohort, patients were administered once-weekly infusions of sebelipase alfa on Day 0, Day 7, Day 14, and Day 21 with monitoring for safety. Dose escalation to 1 mg·kg−1 and 3 mg·kg−1 dose levels was based on review of safety data by an independent Safety Committee. Following completion of the last infusion on Day 21, patients returned on Day 28 and Day 52 for assessment of safety and the magnitude and reversibility of effects on serum transaminases, lipids, and other markers of disease activity. Throughout the study, patients on lipid lowering medications continued on their medications at a stable dose. Sebelipase alfa was provided as a liquid solution in single use glass vials and diluted for infusion in normal saline. Diluted sebelipase alfa was administered intravenously over a period of approximately 120 minutes.
Figure 1.
Schema of the LAL-CL01 and LAL-CL04 trial designs.
After completion of LAL-CL01, patients were eligible to enroll in the LAL-CL04 extension trial to evaluate the long-term effects of sebelipase alfa (Figure 1). In this study, patients resumed treatment with 4 once-weekly infusions of sebelipase alfa at the same dose as in LAL-CL01 study (0.35, 1.0 or 3.0 mg·kg−1) before transitioning to every other week infusions (1.0 or 3.0 mg·kg−1). Patients in the once-weekly 0.35 mg·kg−1 dosing regimen moved to 1 mg·kg−1 every other week. Patients in the 1 and 3 mg·kg−1 once-weekly dosing cohorts remained on the same dose but received it every other week.
Patients
Eligible patients were 18 to 65 years old. Deficient enzyme activity was confirmed upon entry into LAL-CL01 at our central laboratory (Willink Biochemical Genetics Unit Regional Genetics Laboratory, Manchester, England). Additionally, patients were required to have either hepatomegaly on physical exam or elevated transaminases (between 1.5 and 3 times the upper limit of normal of the clinical lab). Patients receiving statins or ezetimibe were only eligible if on a stable dose for at least 4 weeks prior to screening. Female patients of child-bearing potential and male partners of potentially fertile women were required to use a medically acceptable form of contraception. Patients were excluded from this study if they had severe hepatic dysfunction (Child-Pugh Class C); aspartate aminotransferase (AST) and/or alanine transaminase (ALT) persistently elevated to greater than three times the upper limit of normal; chronic liver disease attributed to a cause other than CESD; serological evidence of hepatitis B virus or hepatitis C virus; a score of 8 or more on a screening Alcohol Use Disorders Identification Test; previous hematopoietic bone marrow or liver transplant; known hypersensitivity to eggs; had received an investigational therapy for another indication within 30 days of screening; or they could not comply with the protocol for any other reasons.
Safety Assessments
Patients were monitored for safety and tolerability of sebelipase alfa at regular intervals from the time of signing the informed consent. In LAL-CL01, these assessments continued until the end of study assessment on Day 52. In the ongoing LAL-CL04 extension study, these assessments are analyzed to week 12. Safety evaluations included monitoring of adverse events, concomitant medications, physical examinations, vital signs, and clinical laboratory tests.
Pharmacodynamic assessments
Biological activity of sebelipase alfa was assessed by analysis of hepatic transaminases (ALT, AST), lipid parameters (total cholesterol, triglycerides, HDL, LDL), and serum ferritin. These were selected based on insights from preclinical studies of sebelipase alfa (20, 21) and disease biology (1). Ferritin was analyzed because it is increased in the presence of macrophage activation (22) and has been reported to be elevated in patients with LAL Deficiency (23).
Anti-drug Antibody assays
The presence of serum anti-sebelipase alfa antibodies was examined by use of a validated (24, 25) “bridging ELISA.” In these assays, sebelipase alfa was immobilized onto 96-well microtiter plates. Serum samples were subsequently added to the plates followed in series by biotinylated sebelipase alfa, streptavidin-peroxidase, and TMB substrate. This was used as the “screening assay” with a positivity cut-point predetermined by analysis of normal human serum samples. Putative positive samples were subjected to confirmatory immunodepletion assays in which sebelipase alfa was spiked into the serum samples, before the samples were evaluated using the same bridging assay. This assay has a sensitivity of ≥312 ng/mL in serum and has been used previously to successfully detect anti-sebelipase alfa antibodies in pre-clinical studies in rats and cynomolgus monkeys (data on file, Synageva Biopharma).
Statistical analysis
All patients who received at least 1 dose of sebelipase alfa were included in the safety analysis. Adverse events, vital signs, and laboratory tests including pharmacodynamic assessments and pharmacokinetics, were summarized. The study was not powered to detect differences between cohorts and therefore no statistical comparisons of cohorts were performed. Exploratory statistical analyses were performed to examine the effects of sebelipase alfa on key activity parameters in both LAL-CL01 and LAL-CL04. Wilcoxon's sign-rank test was used for statistical tests of change from baseline, without adjustment for multiplicity.
Results
Nine patients met study eligibility criteria and were sequentially enrolled to one of the three dose cohorts; all 9 patients received four weekly infusions in the LAL-CL01 study. At the time of this analysis, seven of these patients have received sebelipase alfa through Week 12 in the LAL-CL04 study. In these 7 patients, the median (range) wash-out period off between the last dose of sebelipase alfa in LAL-CL01 and the first dose of sebelipase alfa in LAL-CL04 was 15.1 weeks (range 9.0 to 28.3 weeks).
Demographics and baseline disease characteristics
Baseline characteristics of the study population are described in Table 1. All patients were Caucasian and two-thirds were male, with a mean age of 31.6 ± 10.7 years (range 19 to 45 years) at the time of enrollment. Dose cohorts were similar with respect to the patients' age and gender. Mean body mass index was 26.8 (SD ± 6.3; range 20.5 to 42.4). Medical history findings were consistent with those expected in this patient population. Seven patients had a history of hepatomegaly and/or splenomegaly, and 2 patients had evidence of more advanced liver disease; one subject in Cohort 2, had cirrhosis and portal hypertension and one subject in Cohort 3 had periportal fibrosis. Eight of the 9 patients had clinical evidence of hepatomegaly on physical exam. Seven (77.8%) patients also had a history of other cardiovascular conditions. Seven (77.8%) patients were on one or more medications at enrollment, and all 7 were receiving treatment with lipid-modifying therapies, including ezetimibe, statins, and other medications. No change in lipid modifying therapies was made during the studies. One patient (#3) discontinued lipid lowering medication between completion of LAL-CL01 and beginning LAL-CL04.
Table 1. Baseline Demographic and Disease Characteristics of All Patients in the LAL-CL- 01 Study.
ALT alanine transaminase
AST aspartate aminotransferase
GGT gamma-glutamyl transpeptidase
HDL high density lipoprotein-cholesterol
LDL low density lipoprotein-cholesterol
| Cohort | Cohort 1 (0.35 mg·kg−1) | Cohort 2 (1.0 mg·kg−1) | Cohort 3 (3.0 mg·kg−1) | Mean ± SD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Demographics | ||||||||||
| Baseline Age (years) | 41 | 29 | 27 | 42 | 45 | 19 | 21 | 19 | 41 | 31.6 ± 10.7 |
| Gender | M | M | F | F | M | M | M | F | M | 67% male |
| Baseline BMI (kg/m2) | 27.8 | 22.6 | 20.5 | 42.4 | 25.1 | 23.5 | 25.2 | 26.6 | 27.4 | 26.8 ± 6.3 |
| Baseline Laboratory Assessments | ||||||||||
| ALT (U/L) | 60 | 76 ↑ | 85 ↑ | 22 | 70 ↑ | 119 ↑ | 86 ↑ | 57 | 110 ↑ | 76±29 |
| AST (U/L) | 56 ↑ | 48 ↑ | 69 ↑ | 67 ↑ | 52 ↑ | 69 ↑ | 41 | 37 | 65 ↑ | 56 ± 12 |
| GGT (U/L) | 40 | 34 | 12 | 84 ↑ | 42 | 26 | 55 | 23 | 203 ↑ | 58 ± 58 |
| Total Cholesterol (mg/dL) | 130 | 391 ↑ | 256 ↑ | 171 | 116 | 178 | 188 | 182 | 220 | 204 ± 82 |
| HDL-Cholesterol (mg/dL) | 28 ↓ | 41 | 39 | 23 ↓ | 22 ↓ | 45 | 43 | 49 | 26 ↓ | 35 ± 10 |
| LDL-Cholesterol (mg/dL) | 74 | 300 ↑ | 208 ↑ | 137 | 70 | 118 | 112 | 135 | 143 | 144 ± 71 |
| Triglycerides (mg/dL) | 218 ↑ | 108 | 106 | 115 | 102 | 92 | 266 ↑ | 80 | 277 ↑ | 152 ± 79 |
| Alkaline Phosphatase (U/L) | 93 | 97 | 76 | 80 | 86 | 135 ↑ | 100 | 76 | 61 | 89 ± 21 |
| Serum Ferritin (ng/mL) | ||||||||||
| Male Patients | 221 | 300 | 283 | 182 | 283 | 342 | 269 ± 58 | |||
| Female Patients | 118 | 339 ↑ | 89 | 182 ± 137 | ||||||
| Baseline LAL Activity in leucocytes (umol/g/h) | 42 ↓ | 42 ↓ | 33 ↓ | 46 ↓ | 57 ↓ | 10 ↓ | 42 ↓ | 24 ↓ | 19 ↓ | 35± 15 |
| Baseline Hepatomegaly (PE) | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes: 8 of 9 |
| Lipid Lowering Medications | Yes:7 of 9 | |||||||||
| Statin | Yes | No | Yes* | Yes | Yes | No | No | Yes | Yes | Yes: 6 of 9 |
| Ezetimibe | No | No | Yes* | Yes | No | Yes | No | No | No | Yes: 3 of 9 |
| Other (niacin, cholestyramine) | Yes | No | No | No | No | Yes | No | No | No | Yes: 2 of 9 |
Baseline is defined as date of first dose. BMI = Body Mass Index. LDL = Low Density Lipoprotein. HDL = High Density Lipoprotein.↑ = Above central lab upper limit of normal (ALT: 67 U/L; AST: 50 U/L; GGT: 73 U/L for male subjects, 73 U/L for female subjects; alkaline phosphatase: 129 U/L; Total-C: 232 mg/dL; LDL-C: 162 mg/dL; Triglycerides: 199 mg/dL; Ferritin: 400 ng/mL for male subjects, 150 ng/mL for female subjects). ↓ = Below central lab lower limit of normal (HDL-C: 35 mg/dL; LAL activity in leucocytes 350 umol/g/h).
Subject discontinued medications in between study LAL-CL01 and LAL-CL04.
Safety
LAL-CL01
All 9 patients completed LAL-CL01 with no serious adverse events, treatment-related discontinuations, withdrawals or dose reductions. A total of 36 infusions of sebelipase alfa were administered to the 9 patients with no infusion-related reactions, no requirement for premedication with antipyretics or antihistamines, or modification of infusion rate due to infusion related side effects. No anti-sebelipase alfa antibodies were detected in any subject.
Seven of the nine patients experienced adverse events. Nausea, headache and diarrhea were reported in 2 or more patients (Table 2). The overall frequency of adverse events was comparable for the two highest dose cohorts and less frequent in Cohort 1. The majority of events were mild (grade 1) and only six events in 2 patients were considered possibly related to study drug. One subject in Cohort 3 developed asymptomatic elevation in cholesterol (total cholesterol increased from 220 mg/dL at baseline to 772 mg/dL at Day 28), which was classified as a grade 4 event based on the National Cancer Institute Common Terminology Criteria adverse event grading scale used in this study (ctep.cancer.gov/reporting/ctc.html). One additional subject in Cohort 1 had an asymptomatic increase in cholesterol to over 400 mg/dL, from 391 mg/dL at baseline to 509 mg/dL at Day 28, which was not considered an adverse event by the investigator. There were no clinically relevant trends in systolic or diastolic blood pressure, heart rate, respiratory rate, or body temperature during this study.
Table 2. Overview of the Number (Percentage) of Patients Reporting Adverse Events After Treatment with Sebelipase alfa in Study LAL-CL01.
| System Organ Class Preferred Term | Cohort 1: 0.35 mg·kg−1 N=3 | Cohort 2: 1 mg·kg−1 N=3 | Cohort 3:3 mg·kg−1 N=3 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Events n | Patients n (%) | Events n | Patients n (%) | Events n | Patients n (%) | |
|
| ||||||
| Any treatment emergent adverse event | 4 | 1 (33.3) | 23 | 3 (100) | 17 | 3 (100) |
|
| ||||||
| Gastrointestinal disorders | ||||||
| Diarrhea | 1 | 1 (33.3) | 3 | 2 (66.7) | 0 | 0 |
| Nausea | 3 | 1 (33.3) | 0 | 0 | 1 | 1 (33.3) |
|
| ||||||
| Nervous system disorders | ||||||
| Headache | 0 | 0 | 3 | 1 (33.3) | 4 | 2 (66.7) |
LAL-CL04
At the time of this report, 7 of the 9 patients have transitioned into the ongoing extension study and have been administered a total of 56 infusions over the 12 week period. With the exception of occasional episodes of mild diarrhea temporally associated with some infusions in 2 patients, the overall safety profile through 12 weeks in LAL-CL04 remains consistent with that in LAL-CL01 with no findings of clinical concern. A single patient during LAL-CL04 experienced acute cholecystitis classified as a serious adverse event but deemed by the investigator unlikely to be related to sebelipase alfa. All 7 patients remained negative for anti-sebelipase alfa antibodies through the 12 week visit.
Pharmacodynamic Activity
Liver transaminases
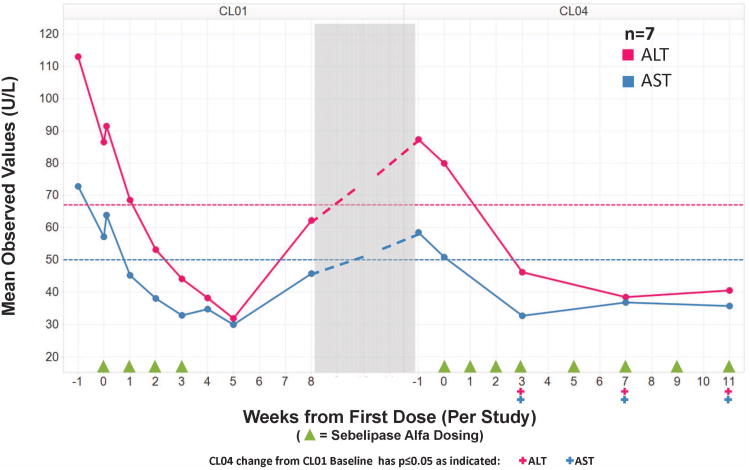
Following initiation of treatment with sebelipase alfa, levels of ALT and AST decreased rapidly in 8 of the 9 patients, regardless of whether their baseline levels were within or above the normal range. No obvious difference in response was seen between cohorts, and therefore data were pooled for all patients. This reduction in AST and ALT was apparent within 2 weeks of the first infusion and levels continued to decrease in most patients through Day 28. By Day 28, approximately 1 week after the fourth infusion, transaminases had normalized in all 6 patients with abnormal baseline ALT and in 4 (66.7%) of 6 patients with abnormal baseline AST. For the set of all 9 patients, treatment was associated with statistically significant decreases (p<0.05) in AST and ALT from baseline to Day 28. The mean decreases from baseline to Day 28 were 39 ± 26 U/L (41% decrease) for ALT and 18 ± 15 U/L (32% decrease) for AST (Figure 2). As shown in Figure 2, the mean improvements in ALT and AST were sustained for two weeks after the last infusion but had partially reversed 3 weeks later. There was no evidence of a dose-related effect in the time to onset or magnitude of the reduction in AST and ALT, or in the maintenance of that effect after discontinuation of treatment.
Figure 2. Mean hepatic transaminases (U/L) in 7 patients in the LAL-CL01 and LAL-CL04 study.
ALT alanine transaminase
AST aspartate aminotransferase
Transaminases, which decreased with therapy in LAL-CL01, increased in patients off treatment. All 7 patients who re-initiated sebelipase alfa in LAL-CL04 had rapid reductions in transaminases similar to those seen in LAL-CL01, which were sustained with the transition to every other week dosing. In the 7 patients receiving ongoing treatment with sebelipase alfa in LAL-CL04, the statistically significant (p<0.05) decreases at week 12 for ALT and AST (shown as mean decrease ± SD compared to the baseline values in LAL-CL01) were 46 ± 21 U/L (52% decrease) and 21 ± 14 U/L (36% decrease), respectively (Figure 2).
In the one patient with a markedly elevated gamma-glutamyl transpeptidase of 203 U/L at baseline in LAL-CL01, gamma-glutamyl transpeptidase declined to 96 U/L at Day 28 before increasing to 187 U/L off treatment between studies. Upon reinitiating treatment in LAL-CL04, gamma-glutamyl transpeptidase decreased to 64 U/L at week 12.
Serum lipids
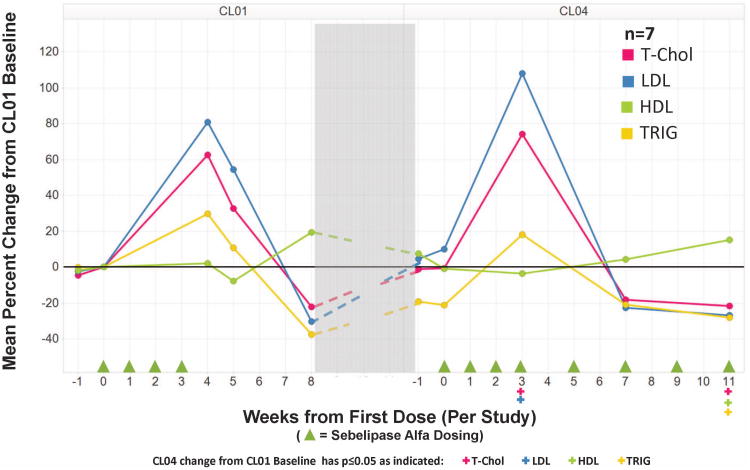
Total cholesterol, triglycerides, and LDL increased in most patients between baseline and Day 28. Increases in total cholesterol and triglycerides were observed for 8 patients with 7 of these patients also had increases in LDL. On average, total cholesterol increased by 140 ± 168 mg/dL (70%), triglycerides increased by 82 ± 91 mg/dL (69%), and LDL increased by 113 ± 169 mg/dL (87%) between baseline and Day 28. The magnitudes of the increases in these serum lipids were comparable in the two lowest dose cohorts, and more pronounced in Cohort 3. While one subject in Cohort 3 showed changes comparable to those seen in the 6 patients in Cohorts 1 and 2, the largest increase in total cholesterol, LDL, or triglycerides were seen in the other two patients in the high dose cohort. Post treatment lipid monitoring was not performed for patients in Cohort 1. By Day 52, thirty-one days after the last dose of sebelipase alfa in LAL-CL01, 5 of the 6 patients in Cohorts 2 and 3 had levels for each lipid parameter that were below their baseline values. One patient had normal total cholesterol and triglycerides, and a borderline abnormal LDL that was approaching baseline levels. HDL levels were generally stable during treatment with sebelipase alfa in LAL-CL01, with no consistent changes between baseline and Day 28 (range -8 to +9 mg/dL).
In the 7 patients receiving ongoing treatment with sebelipase alfa in LAL-CL04 through Week 12, all 7 patients showed decreases from their original LAL-CL01 baseline values in triglycerides (p=0.016) and increases in HDL (p=0.016); 6 of 7 patients had decreases in total cholesterol (p=0.047) and LDL (p=0.078). The mean ±SD decreases for total cholesterol, LDL, and triglycerides were 44±41 mg/dL (-22%), 29 ± 31 mg/dL (-27%), and 50 ± 38 mg/dL (-28%), respectively. Mean HDL increased from 35 ± 9 mg/dL to 40 ± 9 mg/dL (15%)(Figure 3). The one patient who did not have a decrease in total cholesterol and LDL had discontinued lipid lowering therapy between completing LAL-CL01 and beginning LAL-CL04.
Figure 3. Mean percent change from CL01 baseline for serum lipids in 7 patients in the LAL-CL01 and LAL-CL04 study.
HDL high density lipoprotein-cholesterol
LDL low density lipoprotein-cholesterol
T-Chol Total cholesterol
Trig Triglycerides
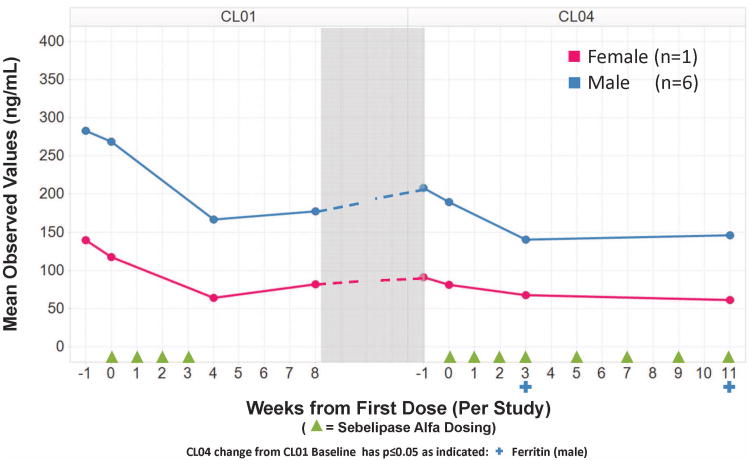
In addition to effects on liver transaminases and serum lipids, serum ferritin decreased in all 9 patients between baseline and Day 28. Mean decreases in serum ferritin from baseline to Day 28 were comparable for Cohort 2 (112 ± 34 ng/mL) and Cohort 3 (119 ± 69 ng/mL), and appeared to be slightly greater than those for Cohort 1 (54± 11 ng/mL). Ferritins measured at the beginning of LAL-CL04 were higher than the last follow-up measurements at the conclusion of LAL-CL01 in 5 of 7 patients. Nonetheless, by week 12 of treatment in LAL-CL04, there was a decrease in serum ferritin in all 7 patients compared to both the baseline values in LAL-CL01 and the baseline values in LAL-CL04. Mean changes by gender for these 7 patients are shown in Figure 4.
Figure 4. Mean serum ferritin by gender in 7 patients in the LAL-CL01 and LAL-CL04 study.
Discussion
Herein we report on the first–in-human experience of enzyme replacement therapy with the recombinant human lysosomal acid lipase enzyme, sebelipase alfa, in CESD patients. Diagnosis of CESD requires a high index of clinical suspicion as the combination of fatty liver, elevated transaminases and dyslipidemia is also seen in patients with the much more common diagnosis of metabolic syndrome. As is the case for some other lysosomal storage diseases (26), substrate accumulation in LAL Deficiency is also associated with macrophage activation, elevation of ferritin (23), and chitotriosidase (27). The results of these studies demonstrate that sebelipase alfa is well tolerated with infrequent, mild reactions. The majority of adverse events were mild and unrelated to study drug. Taken together, this patient population has now received more than 90 infusions with no infusion-related allergic or hypersensitivity-type reactions. Our initial clinical experience suggests that sebelipase alfa may be less immunogenic than many other enzyme replacement therapies. This decreased immunogenicity may be due to (A) the rapid clearance of sebelipase alfa from the circulation following administration (data not shown), (B) the ubiquitous expression of homologous lipases that may decrease the likelihood that administered sebelipase alfa will represent a “foreign” antigen likely to induce an immune response even in patients where the enzyme is completely absent. (28), and (C) the finding that these patients, as expected in patients with CESD, do have some residual LAL enzyme activity (Table 1).
While the main objective of these studies was to evaluate the safety of sebelipase alfa, they provide evidence that enzyme replacement therapy with sebelipase alfa in patients with CESD is mobilizing accumulated lysosomal lipid, and that this is accompanied by normalization of serum transaminases and improvements in the serum lipid profile. Additionally, there was evidence that substrate mobilization is associated with rapid reductions in ferritin which suggests that correction of enzyme deficiency also is accompanied by a reduction in macrophage activation. The significant reductions in ALT, AST and ferritin after the commencement of enzyme replacement therapy with sebelipase alfa were rapid and sustained. The time course of the reversibility of the effects of sebelipase alfa on these pharmacodynamic biomarkers after cessation of dosing following LAL-CL01 supports consideration of either weekly or every other week dosing.
Characteristically, some patients demonstrated elevated serum total cholesterol, LDL, and/or triglycerides, despite having been on various regimens of lipid lowering agents (5). Increases in total cholesterol, triglyceride and LDL were seen in all cohorts after 4 weekly infusions of sebelipase alfa in LAL-CL01, which rapidly reversed following discontinuation of therapy. In most patients, serum cholesterol, triglyceride and/or LDL were below their baseline values at Day 52. The transient rise in serum total cholesterol, LDL, and triglyceride following initiation of treatment with sebelipase alfa may be the result of increased free cholesterol and fatty acids in the cytoplasm leading to increased secretion of VLDL and down-regulation of the LDL receptor. The finding that there was not a concomitant rise in either alkaline phosphatase or gamma-glutamyl transpeptidase suggests that increases in cytoplasmic free fatty acids and cholesterol are not accompanied by transient intrahepatic cholestasis (supplemental Figure 1). More importantly, with continued treatment with sebelipase alfa in LAL-CL04, the mean serum total cholesterol, LDL, and triglycerides all decreased to below original baseline levels at week 12. This trend strongly suggests that continued sebelipase alfa treatment is correcting the dyslipidemia associated with enzyme deficiency in addition to its effects on mobilizing accumulated lipid from the liver, spleen, and other tissues.
Decreased HDL levels have also been described in patients with CESD and a recent study has established a link between LAL activity, cholesteryl ester, and triglyceride breakdown products and expression of the ABCA1 transporter which is important for reverse cholesterol transport (29). With the more sustained dosing in LAL-CL04, HDL levels significantly increased by week 12 compared to baseline in LAL-CL01. With enzyme replacement, the decreases seen in LDL and triglycerides, together with the increases seen in HDL, are important given the recognized clinical sequelae of premature atherosclerosis reported in CESD (1, 2).
In summary, these initial human clinical studies demonstrate that sebelipase alfa is well tolerated with an acceptable safety profile over a broad range of doses in this CESD patient population. ,. We have established that enzyme replacement produces a rapid and sustained reduction in transaminases in patients with CESD. Consistent with the hypothesis that sebelipase alfa can rapidly mobilize abnormal lysosomal lipid from affected tissues in these patients, treatment was associated with rapid but transient increases in serum total cholesterol, LDL, and triglycerides. As anticipated from the recognized association between CESD and dyslipidemia, continued treatment with enzyme replacement led to statistically significant decreases to below baseline levels in not only serum total cholesterol and triglycerides, downward trends in LDL, and but also modest but significant improvements in HDL. Taken together, these findings provide evidence that sebelipase alfa corrects a broad range of abnormalities associated with this inherited enzyme deficiency and has the potential to improve the clinical course for patients afflicted with CESD.
Supplementary Material
Supplementary Figure 1. Mean hepatic transaminases, alkaline phosphatase, and gamma-glutamyl transpeptidase (U/L) in 7 patients in the LAL-CL01 and LAL-CL04 study
Acknowledgments
We would like to thank the patients with CESD and their physicians and health care personnel. We thank Andrea Gwosdow, Ph.D. and Cherie Dewar for assistance in editing and preparing the manuscript.
Financial support: These studies were supported by Synageva BioPharma Corporation. This work was also supported in part by grant UL1TR000067 from the National Center for Advancing Translational Sciences, National Institutes of Health to the Mount Sinai School of Medicine and by research project PRVOUK-P24/LF1/3 to TH. Synageva Biopharma Corporation is the manufacturer of sebelipase alfa and conducted the statistical analysis. All authors had access to the study data, reviewed early and final drafts of the manuscript and were fully responsible for the content and editorial decisions related to this manuscript.
List of abbreviations in order of appearance
- LAL
lysosomal acid lipase
- CESD
cholesteryl ester storage disease
- LDL
low density lipoprotein-cholesterol
- HDL
high density lipoprotein-cholesterol
- AST
aspartate aminotransferase
- ALT
alanine transaminase
Contributor Information
Manisha Balwani, Email: manisha.balwani@mssm.edu.
Catherine Breen, Email: catherine.breen@cmft.nhs.uk.
Gregory M Enns, Email: greg.enns@stanford.edu.
Patrick B Deegan, Email: patrick.deegan@addenbrookes.nhs.uk.
Tomas Honzík, Email: HonzikT@seznam.cz.
Simon Jones, Email: Simon.Jones@cmft.nhs.uk.
John P Kane, Email: john.kane@ucsf.edu.
Vera Malinova, Email: MalinovaV@seznam.cz.
Reena Sharma, Email: reena.sharma@srft.nhs.uk.
Eveline O Stock, Email: eoestreicher@medicine.ucsf.edu.
Vassili Valayannopoulos, Email: vassili.valaya@nck.aphp.fr.
J Edmond Wraith, Email: Ed.Wraith@cmft.nhs.uk.
Jennifer Burg, Email: Jennifer.Burg@synageva.com.
Stephen Eckert, Email: Stephen.Eckert@synageva.com.
Eugene Schneider, Email: Eugene.Schneider@synageva.com.
Anthony G Quinn, Email: Anthony.Quinn@synageva.com.
References
- 1.Grabowski G, Charnas L, Du H. Acid lipase deficiency: Wolman disease and cholesteryl ester storage disease. In: Beaudet A, Vogelstein B, Kinzler K, Antonarakis S, Ballabio A, editors. The Metabolic and Molecular Basis of Inherited Metabolic Disease (Online) 8th. New York: McGraw-Hill, Inc; 2012. [accessed June 14, 2012]. [Google Scholar]
- 2.Beaudet AL, Ferry GD, Nichols BL, Jr, Rosenberg HS. Cholesterol ester storage disease: clinical, biochemical, and pathological studies. J Pediatr. 1977;90:910–914. doi: 10.1016/s0022-3476(77)80557-x. [DOI] [PubMed] [Google Scholar]
- 3.Elleder M, Chlumska A, Ledvinova J, Poupetova H. Testis - a novel storage site in human cholesteryl ester storage disease. Autopsy report of an adult case with a long-standing subclinical course complicated by accelerated atherosclerosis and liver carcinoma. Virchows Arch. 2000;436:82–87. doi: 10.1007/pl00008203. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg HN, Le NA, Short MP, Ramakrishnan R, Desnick RJ. Suppression of apolipoprotein B production during treatment of cholesteryl ester storage disease with lovastatin. Implications for regulation of apolipoprotein B synthesis. J Clin Invest. 1987;80:1692–1697. doi: 10.1172/JCI113259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Bisceglie AM, Ishak KG, Rabin L, Hoeg JM. Cholesteryl ester storage disease: hepatopathology and effects of therapy with lovastatin. Hepatology. 1990;11:764–772. doi: 10.1002/hep.1840110509. [DOI] [PubMed] [Google Scholar]
- 6.Tarantino MD, McNamara DJ, Granstrom P, Ellefson RD, Unger EC, Udall JN., Jr Lovastatin therapy for cholesterol ester storage disease in two sisters. J Pediatr. 1991;118:131–135. doi: 10.1016/s0022-3476(05)81866-9. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama S, McCoy E. Long-term treatment of a homozygous cholesteryl ester storage disease with combined cholestyramine and lovastatin. J Inherit Metab Dis. 1992;15:291–292. doi: 10.1007/BF01799650. [DOI] [PubMed] [Google Scholar]
- 8.Glueck CJ, Lichtenstein P, Tracy T, Speirs J. Safety and efficacy of treatment of pediatric cholesteryl ester storage disease with lovastatin. Pediatr Res. 1992;32:559–565. doi: 10.1203/00006450-199211000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Gasche C, Aslanidis C, Kain R, Exner M, Helbich T, Dejaco C, Schmitz G, et al. A novel variant of lysosomal acid lipase in cholesteryl ester storage disease associated with mild phenotype and improvement on lovastatin. J Hepatol. 1997;27:744–750. doi: 10.1016/s0168-8278(97)80092-x. [DOI] [PubMed] [Google Scholar]
- 10.Tadiboyina VT, Liu DM, Miskie BA, Wang J, Hegele RA. Treatment of dyslipidemia with lovastatin and ezetimibe in an adolescent with cholesterol ester storage disease. Lipids Health Dis. 2005;4:26. doi: 10.1186/1476-511X-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen K, Horslen S. Metabolic liver disease in children. Liver Transpl. 2008;14:713–733. doi: 10.1002/lt.21520. [DOI] [PubMed] [Google Scholar]
- 12.Besley GT, Broadhead DM, Lawlor E, McCann SR, Dempsey JD, Drury MI, Crowe J. Cholesterol ester storage disease in an adult presenting with sea-blue histiocytosis. Clin Genet. 1984;26:195–203. doi: 10.1111/j.1399-0004.1984.tb04367.x. [DOI] [PubMed] [Google Scholar]
- 13.Meyers WF, Hoeg JH, Demosky BS, Herbst JJ, Brewer HB. The use of parenteral hyperalimentation and elemental formula feeding in the treatment of Wolman disease. Nutrition Res. 1985;5:423–429. [Google Scholar]
- 14.Du H, Levine M, Ganesa C, Witte DP, Cole ES, Grabowski GA. The role of mannosylated enzyme and the mannose receptor in enzyme replacement therapy. Am J Hum Genet. 2005;77:1061–1074. doi: 10.1086/498652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du H, Cameron TL, Garger SJ, Pogue GP, Hamm LA, White E, Hanley KM, et al. Wolman disease/cholesteryl ester storage disease: efficacy of plant-produced human lysosomal acid lipase in mice. J Lipid Res. 2008;49:1646–1657. doi: 10.1194/jlr.M700482-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson RA, Sando GN. Cloning and expression of cDNA encoding human lysosomal acid lipase/cholesteryl ester hydrolase. Similarities to gastric and lingual lipases. J Biol Chem. 1991;266:22479–22484. [PubMed] [Google Scholar]
- 17.Quinn AG, Harvey A, Chen M, Christmann L, Leavitt M, Hu W, Nagy T, et al. ASHG, 2010. Washington, DC: 2010. SBC-102, a recombinant enzyme replacement therapy, corrects key abnormalities due to lysosomal acid lipase deficiency. [Google Scholar]
- 18.Yoshida H, Kuriyama M. Genetic lipid storage disease with lysosomal acid lipase deficiency in rats. Lab Anim Sci. 1990;40:486–489. [PubMed] [Google Scholar]
- 19.Kuriwaki K, Yoshida H. Morphological characteristics of lipid accumulation in liver-constituting cells of acid lipase deficiency rats (Wolman's disease model rats) Pathol Int. 1999;49:291–297. doi: 10.1046/j.1440-1827.1999.00862.x. [DOI] [PubMed] [Google Scholar]
- 20.Leavitt M, Burt AD, Hu W, Canty D, Gray M, Bray A, Harvey C, et al. Recombinant lysosomal acid lipase normalized liver weight, transaminases and histopathological abnormalities in an in vivo model of cholesterol storage ester disease. J Hepatol. 2011;54(1):S358. [Google Scholar]
- 21.Leavitt M, Hu W, Canty D, Gray M, Bray A, Rutkowski J, Harvey A, et al. Efficacy of SBC-102, a recombinant enzyme replacement therapy, across a broad range of doses in an in vivo model of lysosomal acid lipase deficiency. J Ped Gastroenterol Nutr. 2011;52(1):E20. [Google Scholar]
- 22.Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61:933–952. doi: 10.1136/gut.2010.214312. [DOI] [PubMed] [Google Scholar]
- 23.Al Essa M, Nounou R, Sakati N, Le Quesne G, Joshi S, Archibald A, Ozand PT. Wolman's disease: The King Faisal Specialist Hospital and Research Centre experience. Ann Saudi Med. 1998;18:120–124. doi: 10.5144/0256-4947.1998.120. [DOI] [PubMed] [Google Scholar]
- 24.Mire-Sluis AR, Barrett YC, Devanarayan V, Koren E, Liu H, Maia M, Parish T, et al. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods. 2004;289:1–16. doi: 10.1016/j.jim.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco-Kent D, Fiscella M, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48:1267–1281. doi: 10.1016/j.jpba.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Vitner EB, Platt FM, Futerman AH. Common and uncommon pathogenic cascades in lysosomal storage diseases. J Biol Chem. 2010;285:20423–20427. doi: 10.1074/jbc.R110.134452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.vom Dahl S, Harzer K, Rolfs A, Albrecht B, Niederau C, Vogt C, van Weely S, et al. Hepatosplenomegalic lipidosis: what unless Gaucher? Adult cholesteryl ester storage disease (CESD) with anemia, mesenteric lipodystrophy, increased plasma chitotriosidase activity and a homozygous lysosomal acid lipase -1 exon 8 splice junction mutation. J Hepatol. 1999;31:741–746. doi: 10.1016/s0168-8278(99)80356-0. [DOI] [PubMed] [Google Scholar]
- 28.Holmes RS, Cox LA, VandeBerg JL. Comparative studies of mammalian acid lipases: Evidence for a new gene family in mouse and rat (Lipo) Comp Biochem Physiol Part D Genomics Proteomics. 2010;5:217–226. doi: 10.1016/j.cbd.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowden KL, Bilbey NJ, Bilawchuk LM, Boadu E, Sidhu R, Ory DS, Du H, et al. Lysosomal acid lipase deficiency impairs regulation of ABCA1 gene and formation of high density lipoproteins in cholesteryl ester storage disease. J Biol Chem. 2011;286:30624–30635. doi: 10.1074/jbc.M111.274381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Mean hepatic transaminases, alkaline phosphatase, and gamma-glutamyl transpeptidase (U/L) in 7 patients in the LAL-CL01 and LAL-CL04 study