Abstract
Objective
To characterize the size, location, conformation, and features of incident geographic atrophy (GA) as detected by annual stereoscopic color photographs and fluorescein angiograms (FAs).
Design
Retrospective cohort study within a larger clinical trial
Participants
Patients with bilateral large drusen who developed GA during the course of the Complications of Age-related Macular Degeneration Prevention Trial (CAPT).
Methods
Annual stereoscopic color photographs and FAs were reviewed from 114 CAPT patients who developed GA in the untreated eye during 5-6 years of follow-up. Geographic atrophy was defined according to the Revised GA Criteria for identifying early GA23. Color-optimized fundus photographs were viewed concurrently with the FAs during grading.
Main Outcome Measures
Size and distance from the fovea of individual GA lesions, number of areas of atrophy, and change in visual acuity (VA) when GA first developed in an eye.
Results
At presentation, the median total GA area was 0.26mm2 (0.1 Disc area). GA presented as a single lesion in 89 (78%) of eyes. The median distance from the fovea was 395μm. Twenty percent of incident GA lesions were subfoveal and an additional 18% were within 250μm of the foveal center. Development of GA was associated with a mean decrease of 7 letters from the baseline visual acuity level compared to 1 letter among matched early age-related macular degeneration (AMD) eyes without GA. GA that formed in areas previously occupied by drusenoid pigment epithelial detachments (DPED) were on average larger (0.53 vs. 0.20 mm2; p=0.0001), more central (50 vs. 500 microns from the center of the fovea; p<0.0001), and associated with significantly worse visual outcome (20/50 vs. 20/25; p=0.0003) than GA with other drusen types as precursors.
Conclusions
Incident geographic atrophy most often appears on color fundus photographs and fluorescein angiograms as a small, singular, parafoveal lesion, though a large minority of lesions are subfoveal or multifocal at initial detection. The characteristics of incident geographic atrophy vary with precursor drusen types. These data can facilitate design of future clinical trials of therapies for GA.
An estimated 7 to 10 million Americans have early signs of age-related macular degeneration (AMD) and are at significant risk for developing geographic atrophy (GA)1,2. Over a fifteen year time period, the estimated incidence of progression from early AMD to GA is nearly 14%.3 It is known that large areas of geographic atrophy significantly impair visual acuity and visual function, but the nature of geographic atrophy when it first develops and the visual impact that is associated with incident geographic atrophy has not been thoroughly investigated.
Geographic atrophy develops when the retinal pigment epithelial cell layer and overlying photoreceptors breakdown in a discrete area of the retina. Although the exact mechanism of degeneration is only partially understood, geographic atrophy is known to develop frequently in areas of the macula previously occupied by drusen.4 Large size and number of drusen are known risk factors for development of GA.5 In the Beaver Dam Eye Study (BDES), a large population-based study of adults aged 43 to 84 years, the overall five-year incidence of GA was 0.3%6. This rate more than tripled (1%) in patients with large drusen (>125um) and was as high as 8% in eyes with very large drusen (>250um). A positive correlation was also found between total drusen area and the development of GA.
Once geographic atrophy develops, the atrophic areas enlarge slowly over time and tend to spare the fovea until late in the course of disease.7 The risk of loss of central vision progresses steadily with time. In the BDES, 42% of patients with geographic atrophy were legally blind (VA<20/200).8 Furthermore, patients with GA and good central VA often have substantial visual dysfunction.9 Parafoveal or partial foveal scotomas, that are common in patients with GA, interfere with the ability to perform tasks that require large continuous areas of central vision for processing visual information, such as reading, driving and recognizing faces. In addition, patients with GA often have decreased visual function as well as decreased contrast sensitivity in dim illumination.10
Several investigators have described the progression of geographic atrophy in patients with large, well-established GA lesions.11-17 However, there is limited literature on the characteristics of GA at its initial detection4 and none on the natural history of GA early in the course of disease. Incident and early GA may not have been described previously because small areas of early GA are particularly difficult to delineate from color photographs alone. Furthermore, the standard criteria for delineating GA do not lend themselves well to early GA lesions.
The standard definitions of GA used to evaluate color fundus photographs in previous observational studies and clinical trials require an area of depigmentation with a diameter at least 175μm and at least two of the following three features must be present in addition: more or less circular shape, sharply demarcated edges, and visible choroidal vessels.4,6,16-22 However, as described previously23, when the retinal pigment epithelial (RPE) cell layer first begins to recede in a discrete area of retina, these features may not be present.
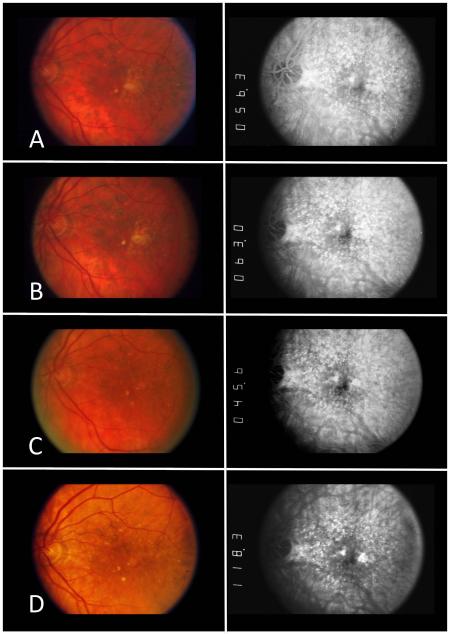
Recently, we developed a new method23 to more accurately detect areas of very early geographic atrophy using information from both stereoscopic fundus photographs and fluorescein angiograms. GA is defined in this revised grading system as “an area in which the RPE is absent, as evidenced by hyperfluorescence on fluorescein angiograms plus one additional feature indicative of RPE atrophy, specifically: visible choroidal vessels, sharp edges, or marked excavation on either color photography or FA” and was designed to be more sensitive and specific for identifying early geographic atrophy. In brief, using this revised method, areas of incident GA were detected reliably (97.8% intragrader, 93.3% intergrader agreement) a year earlier on average than using traditional methods.23 Of the individual incident GA lesions identified using this method, 36.4% did not initially meet the traditional color fundus photograph (CFP) criteria used in many previous studies,4,6,16-22 and would have been missed without this method. In later years, however, over 80% of those failing to meet traditional CFP criteria when first identified using the revised criteria, developed additional features eventually fulfilling standard color fundus photograph criteria for GA. These revised criteria for GA were utilized in the current study because this method is able to reliably detect areas of GA earlier than previously possible. (See Figure 1).
Figure 1.
Color fundus photographs (CFP) and corresponding fluorescein angiograms (FA) over time from a subject who developed geographic atrophy during the course of the Complications of Age-related macular degeneration Prevention Trial( CAPT). The subject’s visual acuity remained ≥20/32 at all visits. (A) Initial CAPT study visit (Year 0). On CFP, there were soft confluent drusen. Corresponding FA demonstrated a staining pattern consistent with drusen. (B) One year before geographic atrophy developed (Year 1), the appearance of the drusen on CFP has become more confluent and an area of depigmentation/incipient atrophy develops just temporal to the fovea. The FA shows a corresponding area of hyperfluorescence in a staining pattern consistent with drusen. (C) Incident geographic atrophy was detected at Year 2 using the revised grading criteria. The CFP again shows a focal area of depigmentation with well-defined borders just temporal to the fovea in same location occupied by confluent drusen the previous year. This lesion would not have met the standard grading criteria for geographic atrophy using CFP alone. In this case, the sharp borders of the window defect on fluorescein angiography confirm the presence of geographic atrophy. (Area=0.16 mm2). (D) One year after detection using the revised criteria (Year 3), the area of geographic atrophy becomes larger and more apparent on CFP alone, meeting the standard CFP criteria used in previous studies (i.e. depigmentation, circular, sharp borders). The fluorescein angiogram again confirms the presence of geographic atrophy. (Area=0.2mm2).
The purpose of this report is to describe the characteristics of incident geographic atrophy lesions with both precise quantitative measurements and qualitative assessments of the patterns of GA development. The photographic files of the Complications of Age-related Macular Degeneration Prevention Trial (CAPT) provided the opportunity to study early GA lesions because a high proportion (30%) of the approximately 1,000 untreated eyes developed GA of the macula during 5-6 years follow-up with annual imaging consisting of two modalities: standard 30° stereoscopic color fundus photographs and stereoscopic fluorescein angiograms.
METHODS
Eyes included in this Early GA substudy were from patients who participated in the CAPT study, a multicenter randomized clinical trial to evaluate low-intensity laser treatment in the prevention of vision loss from AMD. The CAPT study was compliant with the Heath Insurance Portability and Accountability Act (HIPAA), adhered to the tenets of the Declaration of Helsinki, and required written informed consent from all study participants. The CAPT methodology has been described in detail previously.20 In short, 1052 participants with bilateral large drusen (≥10 drusen at least 125μm in diameter) were enrolled from 22 clinical centers from 1999 to 2001 and followed annually for 5-6 years with stereoscopic fundus photographs and fluorescein angiograms. One eye of each patient was randomized to laser treatment and the contralateral eye to observation. Each eye was required to have visual acuity ≥20/40 at entry. Neither eye could have GA within 500 microns of the foveal center or >1 disc area (DA) or 1.5mm2 in total size or any choroidal neovascularization (CNV). For each annual visit, graders at the CAPT Photograph Reading Center recorded the presence or absence of GA as a secondary outcome measure. According to the original CAPT grading protocol, “GA was considered present when the color fundus photographs showed an area of atrophy >250μm in diameter accompanied by 2 of the following 3 features: visible choroidal vessels, sharp edges, and approximately circular shape.” The Early GA substudy, which investigated the natural history of early geographic atrophy, includes data only from untreated eyes.
Study Participants
Of the 1052 subjects enrolled in CAPT, GA was noted in 306 untreated eyes by the Reading Center graders at some point during the CAPT study and were considered for inclusion in the Early GA substudy. Because one of the objectives of the overall project was to characterize changes in incident lesions over time, eyes were excluded from the substudy if any GA was present at CAPT baseline (not new) or if GA was first identified at the last CAPT follow-up visit so that no photographic sets were available after the initial presentation of GA to monitor change over time. Eyes were also excluded if the photographic quality was insufficient to identify and measure atrophic areas accurately.
Image Grading
Definition of GA
We have described a new method for the identification of early geographic atrophy using both stereoscopic color fundus photographs and fluorescein angiograms in a previous publication23. This grading system was established to be more sensitive and specific for the identification of early geograhic atrophy than a traditional single-modality approach. Using this grading system, GA was defined as one or more areas in which the RPE was absent, as evidenced by hyperfluorescence on fluorescein angiograms plus one additional feature indicative of RPE atrophy, specifically: visible choroidal vessels, sharp edges, or marked excavation on either color photography or FA. Atrophic drusen ((i.e., degenerating drusen associated with RPE atrophy at its margins) were not considered GA unless the drusenoid material was completely encircled by a 360° rim of atrophy. This distinction was made to include regressing drusen located underneath a larger area of atrophy and exclude individual drusen or areas of confluent drusen that are associated with early atrophic changes. Similarly, drusen bordering areas of GA were not included in the measurement of GA areas unless the druse was contiguous with the area of GA and completely encircled by a rim of atrophy. Peripapillary atrophy was not included in GA measurements.
Grading protocol
Each GA lesion was reviewed on digitized images derived from the color films. The area of each GA lesion and distance from the center of the fovea were measured using ImageJ software (National Institutes of Health, Bethesda, Maryland. Available at: http://rsb.info.nih.gov/ij. Accessed December 27, 2012) after calibrating and color-optimizing each image. A standard template grid for age-related maculopathy (ARM) classification was centered over the fovea of each stereo image pair.18 The diameter of the inner circle on this grid corresponds to 3mm (2DD) in the average fundus. This diameter was used to calibrate the size of each fundus prior to measurement. All eyes except one had spherical equivalent < 6 diopters so that variability in magnification due to variability in axial length was minimal.24 Previous investigators have noted that the detection of AMD lesions on digital images is facilitated when contrast enhancement techniques are employed.25 Similarly, in this study, each digitized image was color-optimized to increase the contrast of the red spectrum by contrast stretching over the range of red pixel values from the original image. The maximum limit of the red color spectrum was adjusted down to the upper limit of the color balance histogram and the minimum was adjusted up to its lower limit. When the edges of a lesion were indistinct on the color image, the grader referred to the fluorescein angiogram, often by superimposing the color slide directly on top of the FA negative on a lightbox in order to determine landmarks at the borders of GA on the color photo, which could then be outlined on the digitized color images.
Sequence of grading
Photographic sets taken from annual visits, consisting of stereoscopic color photographs and fluorescein angiograms for each patient, were viewed and graded sequentially. Transit or early recirculation phases of the fluorescein angiogram were typically used to identify window defects. After each stereoscopic image set was reviewed for the presence of areas of gepgraphic atrophy, the digitized images were used to outline and measure each GA lesion. After grading the last patient follow-up visit, all color photographs, including those taken before the first appearance of GA, were reviewed in reverse-chronological order noting the type(s) of precursor drusen that were located in areas where GA later formed. Drusen categories consisted of: soft confluent drusen, calcified drusen, and drusenoid pigment epithelial detachment (PED), defined as an area of confluent drusen in which the RPE is significantly elevated and often associated with characteristic pigment deposits.26-29
Comparison Groups for Assessing the Impact of GA on Visual Acuity
Visual acuity measurements, performed by CAPT-certified examiners following a standard protocol for refraction and visual acuity testing30,31, before and after the onset of GA were compared to the acuity measurements performed on matched controls. Only eyes that developed GA at least two years after baseline and who never developed CNV were included as cases for this comparison. Control eyes did not develop GA, CNV, or any other retinal disease affecting visual acuity during follow-up. One control was selected to match each case on the basis of phakia (exact match on phakic/pseudophakic), baseline visual acuity score (within 2 letters), age at baseline (within 2 years), gender (exact match), and length of follow-up (within 1 year). Ninety-one patients met the criteria for a case and appropriate controls were identified for 87 cases.
Data Analysis
Standard descriptive analyses were performed for the patient and eye characteristics, visual acuity, and GA lesion measures. Continuous measures were summarized by using mean, standard deviation, median, quartiles and range. Categorical variables were analyzed by frequency tabulation. Paired t-tests and Wilcoxon signed-rank tests were used for comparing VA change between eyes that developed GA and matched controls that did not develop GA. Two group t-tests and Wilcoxon rank sum tests were used to compare GA lesion size in eyes with different GA precursors. Data analysis was performed in STATA 10 (StataCorp, College Station, TX) or SAS v9.1 (SAS Inc, Cary, NC).
RESULTS
Study Participants
114 eyes were included in these analyses of the 306 CAPT participants originally identified as having GA in the untreated eye by the CAPT Reading Center. Among the 192 excluded patients, 30 (15.6%) had GA at the initial CAPT visit and could not be considered incident lesions, 100 (52.1%) first developed GA at the final CAPT visit with no follow-up photographs for tracking these lesions over time, 43 (22.4%) had photographs missing or unsuitable for grading, and 19 (9.9%) did not have GA that met either the original CAPT grading criteria (error in original grading) or the new revised criteria.
Baseline Characteristics
The mean age at the time of enrollment in CAPT of the Early GA substudy patients was 70.6 years (range 52-85) (Table 1). All patients were Caucasian; 5.3% were current cigarette smokers; and 35.1% had hypertension. At enrollment into CAPT, the mean baseline best-corrected visual acuity was 20/25+1 letter. Substudy eyes were in the more severe stage of early AMD (previously classified as intermediate AMD) with 87.7% having very large drusen (≥250 μm in diameter) and 90.4% having focal hyperpigmentation. Areas of depigmentation were present in 7.0% of eyes.
Table 1.
Baseline Characteristics of Patients and Eyes in the Early Geographic Atrophy Substudy (N=114)
| Characteristic | n (%) |
|---|---|
| Age | |
| 50-59 | 8 ( 7.0) |
| 60-69 | 33 (29.0) |
| 70-79 | 66 (57.9) |
| >79 | 7 ( 6.1) |
| Mean (standard deviation) | 70.6 (6.3) |
| Gender | |
| Female | 70 (61.4) |
| Male | 44 (38.6) |
| Race | |
| White | 114 (100) |
| Cigarette smoking | |
| Never | 51 (44.7) |
| Quit | 57 (50.0) |
| Current | 6 ( 5.3) |
| Hypertension | |
| Normal | 55 (48.3) |
| Suspect | 16 (14.0) |
| Definite | 40 (35.1) |
| Unknown | 3 ( 2.6) |
| Visual Acuity | |
| ≥20/20 | 56 (49.1) |
| 20/25-20/40 | 54 (47.4) |
| ≤20/50 | 4 ( 5.5) |
| Mean | 20/25 +1 letter |
| Predominant drusen size (μm) | |
| 64-125 | 43 (37.7) |
| 125-249 | 64 (56.1) |
| ≥250 | 7 ( 6.1) |
| Largest drusen size (μm) | |
| 125-249 | 14 (12.3) |
| ≥250 | 100 (87.7) |
| Focal hyperpigmentation | |
| None/questionable | 10 ( 8.8) |
| < 250μ | 67 (58.8) |
| >= 250μ | 36 (31.6) |
| Cannot grade/determine/missing | 1 ( 0.9) |
| RPE depigmentation | |
| None | 106 (93.0) |
| Any | 8 ( 7.0) |
RPE = retinal pigment epithelium
Characteristics of Incident GA
Number of distinct GA areas at detection
There were 162 individual areas of GA identified in the 114 eyes at the time when any GA was first detected (Table 2). Most eyes initially had one (78.1%) or two (13.2%) areas of GA; however, up to 7 distinct areas were observed.
Table 2.
Lesion-specific and eye-specific characteristics of incident Geographic Atrophy
| Characteristic | Mean (standard deviation) |
Median (interquartile range) |
Minimum - Maximum |
|---|---|---|---|
| Lesion-specific (n=162) | |||
| Area of distinct lesion (mm2) † | 0.41 (0.57) | 0.20 (0.13, 0.39) | 0.01 – 3.82 |
| Distance to fovea center (μm)‡ | 560 (430) | 540 (200, 790) | 0 - 1810 |
| Eye-specific (n=114) | |||
| Total area (mm2)† | 0.57 (0.75) | 0.26 (0.14, 0.63) | 0.01 – 3.82 |
| Minimum distance to foveal center (μm) |
430 (400) | 395 (60, 620) | 0 - 1810 |
Area could not be determined in one eye and was excluded from analysis.
Distance to fovea center could not be determined for one lesion and was excluded from analysis.
Area of incident GA
The total area of incident GA ranged from 0.01 (approximately the area of one large druse) to 3.82 mm2 (approximately 1.5 DA) (Table 2). The median incident area was 0.26 mm2 (0.1DA) for the total area of GA in an eye (Figure 2, available at http://aaojournal.org). The median area for an individual GA lesion was 0.20 mm2 (approximately the area of 16 large drusen or the area of GA in Figure 1D).
Location of incident GA
The median distance of the most proximal edge of any geographic atrophy (most central lesion when GA was multifocal) in an eye was 395 microns (Table 2). There was a subfoveal GA lesion in 23 (20.2%) of the 114 eyes, GA was encroaching on the center of the fovea (≤250 μm) in 20 (17.5%) and parafoveal (250-1500 μm) in 70 (61.4%) of eyes. The most proximal edge was >1500 μm from the foveal center in only 1 eye (0.9%). The median distance from the foveal center of individual incident lesions was 540 microns.
Defining features of incident GA
When using a combination of both fundus photography and FA (i.e. when a feature was considered present if it was apparent on either medium), sharp edges were present for 82.1% of the 162 individual lesions, 60.5% appeared excavated, and choroidal vessels were visible in 54.3% (Table 3). Other features noted on color photographs that are characteristic of GA but not used in the definition of GA for the substudy, were: roughly circular shape, present in 70.4% of lesions, and hyperpigmented border, present in 33.3% of lesions.
Table 3.
Defining Features of Geographic Atrophy Lesions on Color Photographs and Fluorescein Angiograms
| Characteristic | Color Photograph n (%) |
Fluorescein Angiogram n (%) |
Multimodal (Color or Fluorescein) |
|---|---|---|---|
| Depigmented | |||
| Yes | 153 (94.4) | --- | --- |
| No | 9 ( 5.6) | --- | --- |
| Hyperfluorescent | |||
| Yes | --- | 162 (100) | --- |
| No | --- | 0 ( 0.0) | --- |
| Sharp edges | |||
| Yes | 85 (52.5) | 109 (67.3) | 133 (82.1) |
| No | 77 (47.5) | 53 (32.7) | 29 (17.9) |
| Excavated | |||
| Yes | 54 (33.3) | 77 (47.5) | 98 (60.5) |
| No | 108 (66.7) | 85 (52.5) | 64 (39.5) |
| Visible choroidal vessels | |||
| Yes | 85 (52.5) | 22 (13.6) | 88 (54.3) |
| No | 77 (47.5) | 140 (86.4) | 74 (45.7) |
| Circular | |||
| Yes | 114 (70.4) | --- | --- |
| No | 48 (29.6) | --- | --- |
| Hyperpigmented border | |||
| Yes | 54 (33.3) | --- | --- |
| No | 108 (66.7) | --- | --- |
Visual acuity loss associated with incident GA
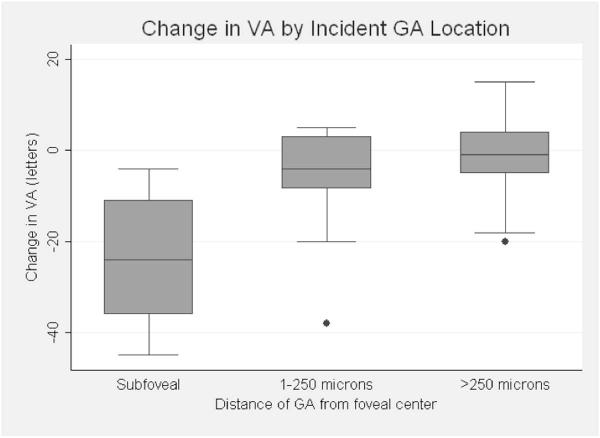
The mean baseline best-corrected visual acuity for both the 87 eyes that developed GA and their 87 matched controls was 20/25+2 letters (81 letters) at the year of enrollment into the CAPT study. One year before GA developed in an eye, there was a mean (± SD) loss of 5 (± 9) letters relative to baseline compared to a mean loss of 0.7 (± 6) letters among control eyes (p=0.0003). At first detection of GA, mean VA had decreased 7 (± 12) letters (an additional 2 letters from the prior year) compared to a 1 (± 7) letter total decline from baseline among controls (p<0.0001). The degree of visual acuity loss was directly related to the proximity to the center of the fovea (Figure 3). The mean number of letters lost was 24 for eyes with subfoveal GA, 5 when the proximal edge of atrophy was within the foveal avascular zone, and 2 when the proximal edge was ≥ 250 μm from the center (p<0.0001).
Figure 3.
Change in visual acuity (VA) from baseline by incident geographic atrophy (GA) location. Boxplots of the number of letters lost from the Complications of Age-related macular degeneration Prevention Trial (CAPT) baseline to first detection of geographic atrophy in eyes with GA that was subfoveal, within the foveal avascular zone but not subfoveal, or more than 250 microns from the foveal center. Top, middle and bottom horizontal lines of the boxes represent the 75th percentile, median, and 25th percentile; horizontal lines on the whiskers represent the most extreme values within 1.5 interquartile range of the box. The dots outside the 1.5 interquartile range of the box were outliers.
Precursors to GA
The majority of geographic atrophy (84%) formed in areas previously occupied by drusen. 35% were preceded by soft confluent drusen, 33% by a drusenoid PED, 12% by calcified drusen and 4% by a combination of 2 or more drusen types, when GA was multifocal. 8% were preceded by depigmentation only, without any clinical evidence of drusen in the area of the eye where GA later formed during the study period. In the remaining 8%, no precursor was identified.
The median size of an incident GA lesion preceded by a drusenoid pigment epithelial detachment was 0.5mm2 as opposed to 0.2mm2 for other drusen-types (p=0.0001). The median distance from the center of the fovea was 60μm for GA lesions preceded by drusenoid PED and 500μm for lesions preceded by other drusen types (p<0.0001). The mean visual acuity associated with incident GA preceded by drusenoid PED was 20/50 versus 20/25 for eyes with other types of precursor drusen (p=0.0003).
DISCUSSION
Although the presentation of incident GA is diverse, there were some specific patterns. The majority of incident GA consisted of small areas of parafoveal atrophy (Table 2). However, 20% of these eyes of patients with bilateral large drusen had clinically significant, large, central GA at the time of first detection. This is similar to the proportion of central GA in a report from the AREDS group (24%),4 but is in contrast to the notion that GA begins as small extrafoveal lesions that grow slowly and spare the fovea until late in the course of disease.
Incident geographic atrophy is associated with a decline in visual acuity (Figure 2). However, as expected, only eyes with GA lesions at or encroaching on the foveal center, experience significant VA loss. Interestingly, the decline of VA begins at least a year before GA develops. This may be due to stretching, thinning, or partial atrophy of the RPE over drusen. Other investigators have noted that very large drusen are associated with decreased retinal sensitivity.32-33 (Takiura K, et al. Multifocal Electoretinogram in Patients with Soft Drusen in Macula. IOVS 2001;42:ARVO Abstract S73.)
Reports from previous investigations have noted that drusenoid pigment epithelial detachments are associated with a high rate of progression to advanced AMD, most notably, central geographic atrophy.27,29 (Klein ML, et al. Clinical features and natural course of drusenoid pigment epithelial detachments in age-related macular degeneration. IOVS 2004;45:E-Abstract 3047.) Incidence rates of central GA following drusenoid PED are reported between 50-75% over 10 years. This is in contrast to patients with large soft indistinct drusen of any type, where the 10-year incidence of geographic atrophy in any location ranges between 4.4-11.7%.5
In the CAPT Early GA population, 70% of patients who initially presented with central subfoveal GA had a drusenoid PED in the area before GA developed. By directly comparing geographic atrophy in areas previously occupied by drusenoid PEDs and other drusen types, it is clear that patients with drusenoid PEDs have a distinct disease process which places them at substantially higher risk, not only for any GA, but in particular, for GA that has a substantial impact on vision. (Figure 4, available at http://aaojournal.org)
This study is the first to describe the characteristics of incident geographic atrophy in detail as seen on stereoscopic color fundus photographs and fluorescein angiograms. This information is important for prognostic purposes and for a complete understanding of the natural history of this disease process. Furthermore, clinical trials for several novel treatments to halt the progression of GA are currently underway. Because GA results in irreversible loss of vision, these treatments would be most effective if used when GA first develops. Even if GA is subfoveal upon initial presentation, patients would benefit enormously from keeping the area small to allow better vision with eccentric fixation.
There are several limitations of this study. As with any study that relies on fundus photographs for measuring lesions, there is the potential for error in uniform calibration of images. In this study, no patient had more than 6 diopters of refractive error, so magnification variance should not have been a significant factor.24 Another limitation of this study is that all included subjects had multiple (≥10) bilateral large drusen at baseline. The risk of developing GA increases with increasing drusen area.5,6,34,35 As stated earlier, we have found that different drusen patterns confer variable risk for GA presentation, with drusenoid PEDs being a significant risk factor for central GA. This CAPT population, with multiple bilateral large drusen, might not be representative of the AMD population in general. The last limitation of this study was the lack of microperimetry data or Optical Coherence Tomography (OCT) to determine whether the smallest of areas, identified as GA, were definitively atrophic with photoreceptor loss.
The CAPT study did not include data from newer imaging modalities such as fundus autofluorescence (FAF), OCT, or microperimetry, which have been used in many recent investigations of GA.11,36-47 A recent study noted that measurements of GA size obtained from color fundus photograhs and fundus autofluorescence images are comparable, with the median difference in total area measurements close to zero.46 When there were differences, these were attributable to either 1) discrepancies in the perceived presence/absence of foveal involvement or 2) in the number of lesions included in total area measurements. As has been noted previously23, areas of GA, especially early GA, are difficult to identify using color fundus photography alone, and are more easily identified on higher contrast media, such as FA, or FAF. In contrast, a disadvantage of FAF in identifying and delineating areas of GA is that the fluorescence of a normal fovea in a FAF image is blocked by foveal pigments and therefore appears decreased compared to the normal parafoveal and peripheral macula. Furthermore, precise foveal localization is difficult with FAF even in normal FAF images.47 In cases of parafoveal GA, it is often difficult to identify foveal sparing. These results are consistent with our own findings that, given the limits of any single imaging modality, multimodal imaging is likely most reliable for identifying and measuring GA, especially small early lesions.
There is also a potential role for en-face OCT imaging in the identification and measurement of GA lesions. In one recent study47, size of GA measured using en-face OCT images (defined as complete choroidal signal enhancement) correlated significantly with FAF measurements. In this study, delineations of the margins of choroidal signal enhancement on a series of B-scan images were projected onto the en-face image for area measurement. Using this method, GA size was consistently smaller when measured using OCT as compared with FAF, though the authors do not mention whether this difference was statistically significant.47 This was the first study to our knowledge to define OCT criteria for delineating GA. OCT is unique amongst the imaging modalities for delineating GA in that the precise determination of both structural integrity of individual retinal layers and foveal localization is possible. Further investigations should be aimed at determining the feasibility of identifying small areas of early GA with OCT. In the future, it would be of great interest to compare the presentation of early GA lesions on multiple imaging modalities, not only for descriptive purposes but also to determine which imaging modality is able to capture geographic atrophy most reliably at its earliest stages.
In summary, we identified 114 patients who developed GA in their untreated eye during the 5 year follow-up period in the CAPT study. For these eyes, the size of incident GA lesions was small, ranging from 0.01 to 3.82mm2 (median=0.20mm2). The majority of incident GA areas were parafoveal with a median distance of 395 microns from the foveal center, though 20% of incident GA lesions were directly subfoveal when GA was first detected. Incident GA tended to present as a singular atrophic locus, but 22% presented in a multifocal pattern with between 2 and 7 distinct atrophic areas. Incident GA was associated with a significant decline in visual acuity, with the greatest decline occurring a year before GA is clinically identified. However, this decline in VA was an average, which was heavily influenced by eyes that presented with central lesions. Most of eyes with central incident GA had a prior drusenoid PED. Type of precursor drusen has a major impact on prognosis, incidence, and pattern of GA that develops.
These data on number, area, and location of incident GA, as well as precursor lesions, increase our understanding of the natural history of geographic atrophy and will be useful in designing future clinical trials evaluating agents to prevent the development or halt the progression of GA.
Supplementary Material
Acknowledgments
Supported by grants EY012211, EY012261, and EY012279, from the National Eye Institute, National Institutes of Health, Department of Health and Human Services, an unrestricted grant from Research to Prevent Blindness and a grant from the Doris Duke Charitable Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: No conflicting relationship exists for any author.
Presented in part at the Association for Research in Vision and Ophthalmology Meeting. Ft. Lauderdale, Florida, May 2009.
REFERENCES
- 1.Eye Diseases Prevalence Research Group Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Chou CF, Klein BE, et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129:75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Knudtson MD, et al. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–62. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 4.Klein ML, Ferris FL, III, Armstrong J, et al. AREDS Research Group Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115:1026–31. doi: 10.1016/j.ophtha.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Tomany SC, et al. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2002;109:1767–79. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 7.Sunness JS. [Accessed December 27, 2012];The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol Vis [serial online] 1999 5:25. Available at: http://www.molvis.org/molvis/v5/a25/ [PubMed] [Google Scholar]
- 8.Klein R, Wang Q, Klein BE, et al. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995;36:182–91. [PubMed] [Google Scholar]
- 9.Sunness JS, Rubin GS, Zuckerbrod A, Applegate CA. Foveal-sparing scotomas in advanced dry age-related macular degeneration. J Vis Impair Blind. 2008;102:600–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Sunness JS, Rubin GS, Applegate CA, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104:1677–91. doi: 10.1016/s0161-6420(97)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreyhaupt J, Mansmann U, Pritsch M, et al. Modelling the natural history of geographic atrophy in patients with age-related macular degeneration. Ophthalmic Epidemiol. 2005;12:353–62. doi: 10.1080/09286580591005723. [DOI] [PubMed] [Google Scholar]
- 12.Maguire P, Vine AK. Geographic atrophy of the retinal pigment epithelium. Am J Ophthalmol. 1986;102:621–5. doi: 10.1016/0002-9394(86)90535-0. [DOI] [PubMed] [Google Scholar]
- 13.Schatz H, McDonald HR. Atrophic macular degeneration: rate of spread of geographic atrophy and visual loss. Ophthalmology. 1989;96:1541–51. doi: 10.1016/s0161-6420(89)32694-7. [DOI] [PubMed] [Google Scholar]
- 14.Holz FG, Bindewald-Wittich A, Fleckenstein M, et al. FAM-Study Group Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143:463–72. doi: 10.1016/j.ajo.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Meuer SM, Knudtson MD, Klein BE. The epidemiology of progression of pure geographic atrophy: the Beaver Dam Eye Study. Am J Ophthalmol. 2008;146:692–9. doi: 10.1016/j.ajo.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106:1768–79. doi: 10.1016/S0161-6420(99)90340-8. [DOI] [PubMed] [Google Scholar]
- 17.Sunness JS, Margalit E, Srikumaran D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114:271–7. doi: 10.1016/j.ophtha.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein R, Davis MD, Magli YL, et al. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98:1128–34. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 19.Age-Related Eye Disease Study Research Group The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study report number 6. Am J Ophthalmol. 2001;132:668–81. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 20.Complications of Age-Related Macular Degeneration Prevention Trial Research Group Laser treatment in patients with bilateral large drusen: the Complications of Age-Related Macular Degeneration Prevention trial. Ophthalmology. 2006;113:1974–86. doi: 10.1016/j.ophtha.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Klaver CC, Assink JJ, van Leeuwen R, et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2001;42:2237–41. [PubMed] [Google Scholar]
- 22.Mitchell P, Wang JJ, Foran S, Smith W. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology. 2002;109:1092–7. doi: 10.1016/s0161-6420(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 23.Brader HS, Ying GS, Martin ER, Maguire MG, Complications of Age-Related Macular Degeneration Prevention Trial (CAPT) Research Group New grading criteria allow for earlier detection of geographic atrophy in clinical trials. Invest Ophthalmol Vis Sci. 2011;52:9218–25. doi: 10.1167/iovs.11-7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pach J, Pennell DO, Romano PE. Optic disc photogrammetry: magnification factors for eye position, centration, and ametropias, refractive and axial; and their application in the diagnosis of optic nerve hypoplasia. Ann Ophthalmol. 1989;21:454–62. [PubMed] [Google Scholar]
- 25.Hubbard LD, Danis RP, Neider MW, et al. Age-Related Eye Disease 2 Research Group Brightness, contrast, and color balance of digital versus film retinal images in the Age-Related Eye Disease Study 2. Invest Ophthalmol Vis Sci. 2008;49:3269–82. doi: 10.1167/iovs.07-1267. [DOI] [PubMed] [Google Scholar]
- 26.Roquet W, Roudot-Thoraval F, Coscas G, Soubrane G. Clinical features of drusenoid pigment epithelial detachment in age related macular degeneration. Br J Ophthalmol. 2004;88:638–42. doi: 10.1136/bjo.2003.017632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casswell AG, Kohen D, Bird AC. Retinal pigment epithelial detachments in the elderly: classification and outcome. Br J Ophthalmol. 1985;69:397–403. doi: 10.1136/bjo.69.6.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AREDS Summary Grading Protocol [Accessed December 27, 2012];Fundus Photograph Reading Center. Appendix 15B. Wisconsin Age-related Maculopathy Grading System. Section 7.3 Drusenoid PED. Available at: http://eyephoto.ophth.wisc.edu/ResearchAreas/AREDS/CHAPTER1 5B.html#DrusenoidPED.
- 29.Cukras C, Agrón E, Klein ML, et al. Age-Related Eye Disease Study Research Group. Natural history of drusenoid pigment epithelial detachment in age-related macular degeneration: Age-Related Eye Disease Study report no. 28. Ophthalmology. 2010;117:489–99. doi: 10.1016/j.ophtha.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology. 1991;98(suppl):741–56. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 31.Examination Procedures. EMMES Corp.; Rockville, MD: [Accessed December 27, 2012]. 2000. Age-Related Eye Disease Study Manual of Procedures. Available at: https://web.emmes.com/study/areds/mopfiles/chp7_mop.pdf. [Google Scholar]
- 32.Midena E, Vujosevic S, Convento E, et al. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007;91:1499–503. doi: 10.1136/bjo.2007.119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerth C, Hauser D, Delahunt PB, et al. Assessment of multifocal electroretinogram abnormalities and their relation to morphologic characteristics in patients with large drusen. Arch Ophthalmol. 2003;121:1404–14. doi: 10.1001/archopht.121.10.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Age-Related Eye Disease Study Research Group The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS report no. 17. Arch Ophthalmol. 2005;123:1484–98. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Complications of Age-related Macular Degeneration Prevention Trial (CAPT) Research Group Risk factors for choroidal neovascularization and geographic atrophy in the Complications of Age-Related Macular Degeneration Prevention trial. Ophthalmology. 2008;115:1474–9. doi: 10.1016/j.ophtha.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Fleckenstein M, Schmitz-Valckenberg S, Adrion C, et al. Tracking progression with spectral-domain optical coherence tomography in geographic atrophy caused by age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:3846–52. doi: 10.1167/iovs.09-4533. [DOI] [PubMed] [Google Scholar]
- 37.Holz FG, Bellman C, Staudt S, et al. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:1051–6. [PubMed] [Google Scholar]
- 38.Hartmann KI, Bartsch DU, Cheng L, et al. Scanning laser ophthalmoscope imaging stabilized microperimetry in dry age-related macular degeneration. Retina. 2011;31:1323–31. doi: 10.1097/IAE.0b013e31820a6850. [DOI] [PubMed] [Google Scholar]
- 39.Meleth AD, Mettu P, Agron E, et al. Changes in retinal sensitivity in geographic atrophy progression as measured by microperimetry. Invest Ophthalmol Vis Sci. 2011;52:1119–26. doi: 10.1167/iovs.10-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Ruckmann A, Fitzke FW, Bird AC. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci. 1997;38:478–86. [PubMed] [Google Scholar]
- 41.Bearelly S, Cousins SW. Fundus autofluorescence imaging in age-related macular degeneration and geographic atrophy. Adv Exp Med Biol. 2010;664:395–402. doi: 10.1007/978-1-4419-1399-9_45. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz-Valckenberg S, Jorzik J, Unnebrink K, Holz FG, FAM Study Group Analysis of digital scanning laser ophthalmoscopy fundus autofluorescence images of geographic atrophy in advanced age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2002;240:73–8. doi: 10.1007/s00417-001-0413-3. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz-Valckenberg S, Bindewald-Wittich A, Dolar-Szczasny J, et al. Fundus Autofluorescence in Age-Related Macular Degeneration Study Group Correlation between the area of increased autofluorescence surrounding geographic atrophy and disease progression in patients with AMD. Invest Ophthalmol Vis Sci. 2006;47:2648–54. doi: 10.1167/iovs.05-0892. [DOI] [PubMed] [Google Scholar]
- 44.Brar M, Kozak I, Cheng L, et al. Correlation between spectral-domain optical coherence tomography and fundus autofluorescence at the margins of geographic atrophy. Am J Ophthalmol. 2009;148:439–44. doi: 10.1016/j.ajo.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz-Valckenberg S, Fleckenstein M, Gobel AP, et al. Optical coherence tomography and autofluorescence findings in areas with geographic atrophy due to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1–6. doi: 10.1167/iovs.10-5619. [DOI] [PubMed] [Google Scholar]
- 46.Khanifar AA, Lederer DE, Ghodasra JH, et al. Comparison of color fundus photographs and fundus autofluorescence images in measuring geographic atrophy area. Retina. 2012;32:1884–91. doi: 10.1097/IAE.0b013e3182509778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sayegh RG, Simader C, Scheschy U, et al. A systematic comparison of spectral-domain optical coherence tomography and fundus autofluorescence in patients with geographic atrophy. Ophthalmology. 2011;118:1844–51. doi: 10.1016/j.ophtha.2011.01.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.