Abstract
To explore stem cell therapy for Parkinson’s disease (PD), three adult rhesus monkeys were first rendered hemiparkinsonian by unilateral intracarotid 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) infusion. Five months postinfusion, they were given MRI-guided stereotaxic intrastriatal and intranigral injections of green fluorescent protein (GFP)-labeled cultures of dopaminergic neurons derived from human embryonic stem cells (DA-hES cells). The animals were immunosuppressed using daily oral cyclosporine (CsA). Three months later, viable grafts were observed at the injection sites in one animal, while no obvious grafts were present in the other two monkeys. The surviving grafts contained numerous GFP-positive cells that were positively labeled for nestin and MAP2 but not for glial fibrillary acidic protein (GFAP), NeuN, or tyrosine hydroxylase (TH). The grafted areas in all animals showed dense staining for GFAP, CD68, and CD45. These results indicated that xenografts of human stem cell derivatives in CsA-suppressed rhesus brain were mostly rejected. Our study suggests that immunological issues are obstacles for preclinical evaluation of hES cells and that improved immunosuppression paradigms and/or alternative cell sources that do not elicit immune rejection are needed for long-term preclinical studies.
Keywords: Parkinson’s disease (PD), Human embryonic stem (hES) cells, Dopamine (DA), Immune rejection, Cell transplantation, Cell differentiation
INTRODUCTION
Loss of dopaminergic neurons in the substantia nigra results in lower levels of dopamine (DA) in the striatum, which is associated with the classic motor symptoms of Parkinson’s disease (PD): resting tremor, altered gait, bradykinesia, muscular rigidity, and postural instability. Supplementation of DA via administration of L-DOPA and DA agonists remains the pharmacological mainstay therapy. Nevertheless, long-term use of L-DOPA almost always induces severe side effects, including motor fluctuations and dyskinesias (2). As an alternative to DA drugs, replacement of lost DA neurons with DA-producing cells, especially fetal midbrain tissues, has been proposed as a therapeutic alternative and has proven to be clinically effective in some patients (16,17). However, double-blinded clinical trials of fetal tissue transplantation showed variable outcomes (11,18). One of the reasons is the heterogeneity of the donor tissues between cases, as a few fetuses of a particular age are necessary for transplantation into a single patient, posing a logistical problem.
Human stem cells, including embryonic stem (ES) cells (23) and induced pluripotent stem (iPS) cells (22,27,28), can potentially become a consistent source of DA neurons as they can give rise to cell lines (for review, see 10). In particular, hES cells have been efficiently differentiated into DA-specific neural lineages (19,20,25). Transplantation of hES cell-derived DA neurons can significantly improve locomotion behavioral deficit in rodent models of PD (15,20,26,27). However, transplanted human cells sometimes result in large grafts in the rat brain (20). It is not clear whether this is due to the lack of response of human cells to the rat brain environment. Transplantation of human stem cells into the primate brain may address this question. Furthermore, this transplant paradigm may also allow to address additional issues, including immune responses, cell dosage, transplantation sites, and efficacy, before similar stem cell derivatives may be used in patients. In that regard, a recent publication reported in two MPTP-treated rhesus monkeys survival of hES cell striatal grafts, 1 month posttransplant (15). Interestingly, the grafts were composed of cells with immature neuroblast morphology and were infiltrated with microglial cells.
In the present study, we assessed in three MPTPinduced PD monkeys the survival and safety of transplanting into the nigrsotriatal system, hES cell-derived DA neuronal cultures that were prepared in the same way as that for our previous transplant study in PD rats (26). We found that the grafts survived in multiple brain regions but they were largely rejected 3 months posttransplantation, a time when most grafted cells become postmitotic neurons, suggesting that hES cell survival may depend on the immature phenotype.
MATERIALS AND METHODS
Animals
Three adult female rhesus monkeys (5.70–6.75 kg, 8–10 years old) were housed at the Wisconsin National Primate Research Center, University of Wisconsin- Madison, in single quarters with a 12-h light/dark cycle. Animals were provided with monkey chow and enrichment food twice a day and water ad libitum. The study followed federal guidelines for proper animal care and a protocol approved by the local institutional animal care and use committee.
MPTP Treatment
Under sterile surgical conditions, each monkey received a single unilateral intracarotid artery (ICA) infusion of 3 mg of MPTP-HCL in 20 ml of saline (rate: 1.33 ml/min), in accordance with previously published protocols (7,9).
Intracerebral Dopamine hES Cell Injections
Cells from human hES cell line H9 (NIH Registry, WA09, passage 16) were first infected with lentivirus containing the green fluorescent protein (GFP) gene driven by the EF1a promoter (detailed in 24). The GFP-hES cells were selected and expanded in an undifferentiated state (12,23). hES cell colonies were differentiated to an early pure population of neuroepithelial cells in an adherent colony culture (28) and then to DA neurons according to our previously established protocol (modified from 25).
Five months after MPTP administration, the three monkeys received seven MRI-guided stereotaxic intracerebral injections of differentiated hES cells according to previously published protocols (e.g., 4,6). The three monkeys were grafted on the same day (first, R96025; second, R96025; third, RH2290). The cells were delivered to the surgery room approximately 1 h (R96025 and 2099) and 2 h (R98067) before transplantation. All injections were administered unilaterally ipsilateral to the MPTP ICA infusion using a 10-µl Hamilton syringe. The animals received three injections in the caudate (volume: rostral 5 µl, medial 10 µl, caudal 10 µl), three in the putamen (volume: rostral 10 µl, medial 10 µl, caudal 5 µl), and one in the substantia nigra (volume: 5 µl) of a suspension containing 50,000 cells/µl. The motor-driven infusion was performed at a rate of 1 µl/min. The syringe remained in place for an additional 5 min after completion of the injection and before retrieval.
Immunosuppression treatment by daily administration of cyclosporine A (CsA; 40–50 mg/kg PO, bolus gavage) began 48 h before the stem cell implantation surgery and continued throughout the study. The monkeys were gently handled for drug administration using a pole and collar system as previously described (4,8). Periodic blood sampling was done to monitor drug levels, general chemistry, and hematological parameters. The samples were taken at “trough” CsA levels, meaning that the level was checked just prior to the next dose, at its lowest level, 24 h postdosing.
Necropsy, Preparation of Tissue
Three months after DA-hES cell injection, the monkeys were euthanized with pentobarbital (25 mg/kg IV) and perfused transcardially with heparinized normal saline followed by 4% paraformaldehyde. The brains were removed from the calvaria and slabbed in the coronal plane with a calibrated Lucite brain slice apparatus. The tissue was postfixed for approximately 18 h in 4% paraformaldehyde and cryoprotected by immersion in a graded (10–40%) sucrose/0.1 M phosphate-buffered saline (PBS, pH 7.2) solution. Frozen sections were cut on a sliding knife microtome to a thickness of 40 µm. All sections were stored in a cryoprotectant solution (30% sucrose, 30% ethylene glycol in PBS) until processing.
Immunohistochemistry
Coronal brain sections were used for immunohistochemical staining according to our previously published protocols (8), using antibodies against tyrosine hydroxylase (TH, LV1573151, 1:20,000; Chemicon, Inc., CA), glial fibrillary acid protein (GFAP, Z0334, 1:2,000; DakoCytomation, Glostrup, Denmark), CD68 (M0814, 1:3,000; DakoCytomation, Glostrup, Denmark), CD45 (M0701, 1:1,000; DakoCytomation, Glostrup, Denmark), and GFP (MAB3580, 1:200; Chemicon Inc., CA). Biotinylated goat secondary antibodies and ABC Vectastain kits (Vector Laboratories, Burlingame, CA) using DAB were used. Controls consisted of processing tissue in an identical manner except for using the primary antibody solvent or an irrelevant immunoglobulin G (IgG) in lieu of the primary antibody. Sections were mounted on gelatin-coated slides, dehydrated, and coverslipped with Permount. Nissl staining was also performed for general evaluation of brain structures.
Immunofluorescence and Colocalization
All procedures were done at room temperature unless otherwise stated. The collected tissue sections were washed in PBS/0.05% Triton-TX (dilution media) for 1 h; blocked in PBS/0.05% Triton-TX, 3% normal goat serum (NGS), 2% bovine serum albumin (BSA) for 1 h; and then incubated overnight in primary antibody solution containing blocking solution plus various combinations of the following antibodies: GFP (A11120, 1:500, Invitrogen, Carlsbad, CA), TH (P40101, 1:20,000, Pel-Freez, Inc., Rogers, AK), NeuN (MAB377, 1:500, Millipore, Billerica, MA), nestin (611658, 1:500, BD Transduction), GFAP (Z0334, 1:2,000, DakoCytomation, Glostrup, Denmark), MAP2 (AB5622, 1:1,000, Millipore, Billerica, MA), and β-tubulin (T8660, 1:500, Sigma, St. Louis, MO). The sections were washed again in dilution media and incubated in Alexa Fluor-conjugated goat secondary antibodies (Alexa 488, Alexa 546, and Alexa 633, Invitrogen) diluted in blocking solution in a dish covered with foil for 2 h. The tissue was then washed in dilution media, and the sections were mounted onto glass microscope slides with coverslips using PBS and fluorescence mounting medium (Fluoro Gel, Electron Microscopy Sciences) and stored in a dark place.
Confocal microscopy was done on a Nikon C1 confocal system with lasers at 488, 543, and 633 nm and captured using EZ-C1 software (Nikon, Melville, NY). Images shown are maximum projection volume renderings of z-stack images, approximately 24 images taken 4.5 µm apart. Each fluorophore was imaged separately/sequentially, and signal from the 633 laser channel was pseudocolored red for ease of viewing.
Optical Density (OD)
The density of TH-positive fibers was quantified within ventral, medial, and dorsal sections of both the caudate and putamen using NIH ImageJ software using our previously described method (8). Images of six coronal sections per monkey, approximately 2 mm apart, were captured using an Epson 1640XL-GA high-resolution digital scanner. ImageJ was calibrated using a step tablet, grayscale values were converted to OD units using the Rodbard function, and the mean OD for each area of interest was recorded. The amount of CD68 present was quantified by the same procedure in the caudate and putamen except that the area above a threshold of 0.10 OD unit was recorded.
Stereology
The number of TH-ir neurons in the SN and the number of GFP-ir cells in the caudate, putamen, and SN were estimated using unbiased stereologic cell counting methods as previously described (5,7). The stereology system consisted of a Zeiss Axioplan 2 imaging photomicroscope (Carl Zeiss, Inc.) coupled to a MAC5000 xyz motorized stage and a MicroFire CX9000 camera (Optronics, Goleta, CA). Cell counts were performed by the optical fractionator method using Stereo Investigator Version 7.5 (MicroBrightField, Williston, VT). Instruments were calibrated prior to each series of measurements. The grid size used was x = 385 µm and y = 320 µm, and the counting frame size was 71 µm × 71 µm. The SN was demarcated under low magnification by the caudal edge of the mammillary bodies rostrally, the cerebral peduncle ventrally and laterally, and the third cranial nerve rootlets medially. Areas of injection were similarly demarcated in GFP immunostained sections by the presence of GFP-ir cells and observation of adjacent coronal sections stained with Nissl for visualization of needle tracts. Cells were only counted within a 12-µm height of tissue, with guard zones above and below. The number of TH-ir or GFP-ir cells within the counting frame was counted using a 100× oil immersion objective with 1.4 numerical aperture. Equally spaced sections from each monkey containing the areas of interest were used for analysis.
RESULTS
Parkinsonism Is Induced in Rhesus Monkeys by MPTP
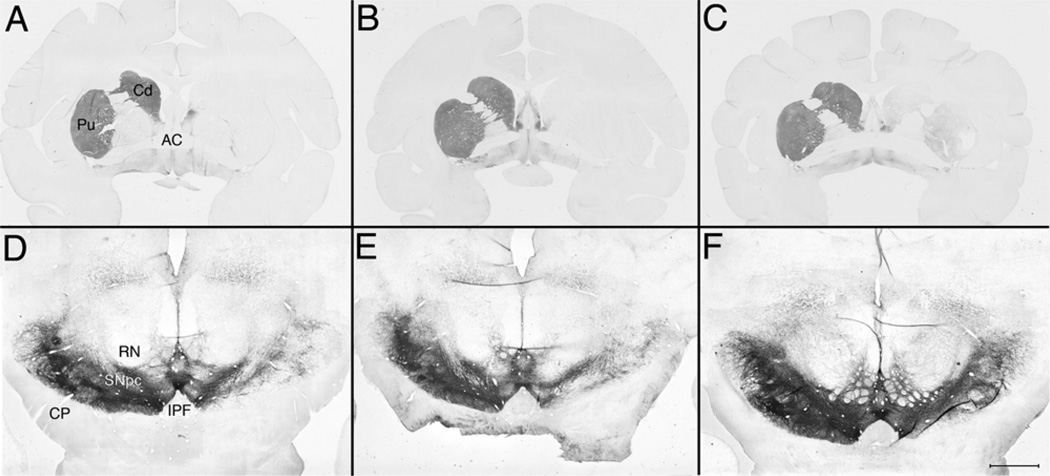
Histological evaluation of the three monkeys 3 months after stem cell transplantation indicated that at the level of the caudate and putamen there was a unilateral loss of TH-ir fibers ipsilateral to MPTP administration (Fig. 1). OD quantification of striatal TH immunostaining and unbiased stereological cell counts of nigral TH-ir cells both confirmed a substantial loss in all three animals, although some individual differences were identified (Table 1).
Figure 1.
Low-magnification images of TH-immunostained coronal sections at the level of the striatum (A–C) and the substantia nigra (D–F) for the three animals (A, D: R98067; B, E: R96025; C, F: RH2290). The three striata show a striking loss of TH immunoreactivity with individual differences. Note in Rh2290 (C) some TH immunoreactivity that is not present in the other animals. This difference in immunostaining is further seen in the substantia nigra. It should also be noted that, although the three nigral images are at the red nucleus level, (F) is in a slightly different plane showing more of the third cranial nerve emergence, which may have further emphasized in the picture the differences in nigral TH staining. Scale bar: 6.5 mm (A–C) and 2.0 mm (D–F). RN, red nucleus; CP, cerebral peduncle; SNpc, substantia nigra pars compacta; AC, anterior commissure; CC; corpus callosum; Cd, caudate nucleus; Pu, putamen nucleus.
Table 1.
Loss of Dopaminergic Innervation in the Nigrostriatal System of Hemiparkinsonian Rhesus Monkeys Treated With hES-Derived DA Cells
| Percentage of Loss | |||
|---|---|---|---|
| Area | RH2290 | R98067 | R96025 |
| Caudate (TH optical density) | 62.5 | 77.7 | 79.2 |
| Putamen (TH optical density) | 73.3 | 79.2 | 79.5 |
| Substantia nigra (TH cell number) | 70.1 | 81.7 | 86.2 |
The data are expressed as percentage of loss of TH immunostaining optical density or TH-positive cells in the side ipsilateralto a single intracarotid artery infusion of MPTP compared to the contralateral side.
hES Cells Survive in the MPTP-Treated Monkey Brain
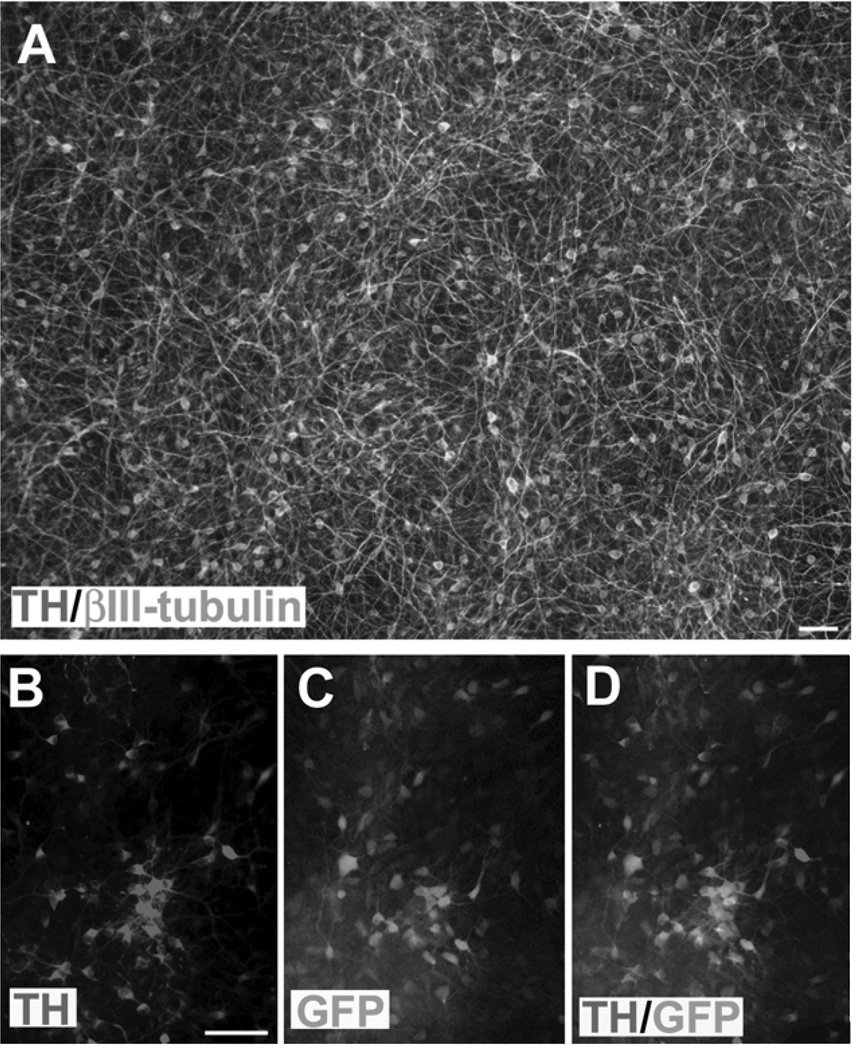
GFP-expressing hES cells (24) were differentiated to DA neurons in the same way as described before and in parallel to the rat studies (26). About 68% of the cells were βIII-tubulin+ neurons when the neural progenitors were differentiated in small clusters. That translates into approximately 29% of the total cells. About 43% of the total neurons were positive for TH by day 42, the time for transplantation (Fig. 2A). Many of the TH+ neurons retained GFP expression, although some had reduced levels of GFP (Fig. 2B–D).
Figure 2.
hESC-derived DA neurons for transplantation. (A) The GFP-hESCs were differentiated toward DA neurons for 42 days. About 43% of the βIII-tubulin+ neurons are expressing TH. Note that the staining for βIII-tubulin is pseudocolored for better visualization. (B–D) Many TH-expressing neurons retain GFP in culture. Scale bars: 50 µm.
As a pilot study to examine the survivability of the hES cell-derived DA neurons, we sacrificed the grafted animals 3 months posttransplantation. Two of the animals (R98067 and R96045) did not show grafts macroscopically and lacked obvious GFP-positive cells (with or without nickel enhancement), suggesting that the DA-hES cells had either been rejected early on or downregulated their transgenic GFP expression. Small numbers of weakly GFP-positive, round cells were found in and near the injection sites, but it was not feasible to count them due to the faintness of the staining. Nissl staining showed very small areas of abnormally dense nuclei near areas of necrosis, but no other evidence of engraftment (data not shown).
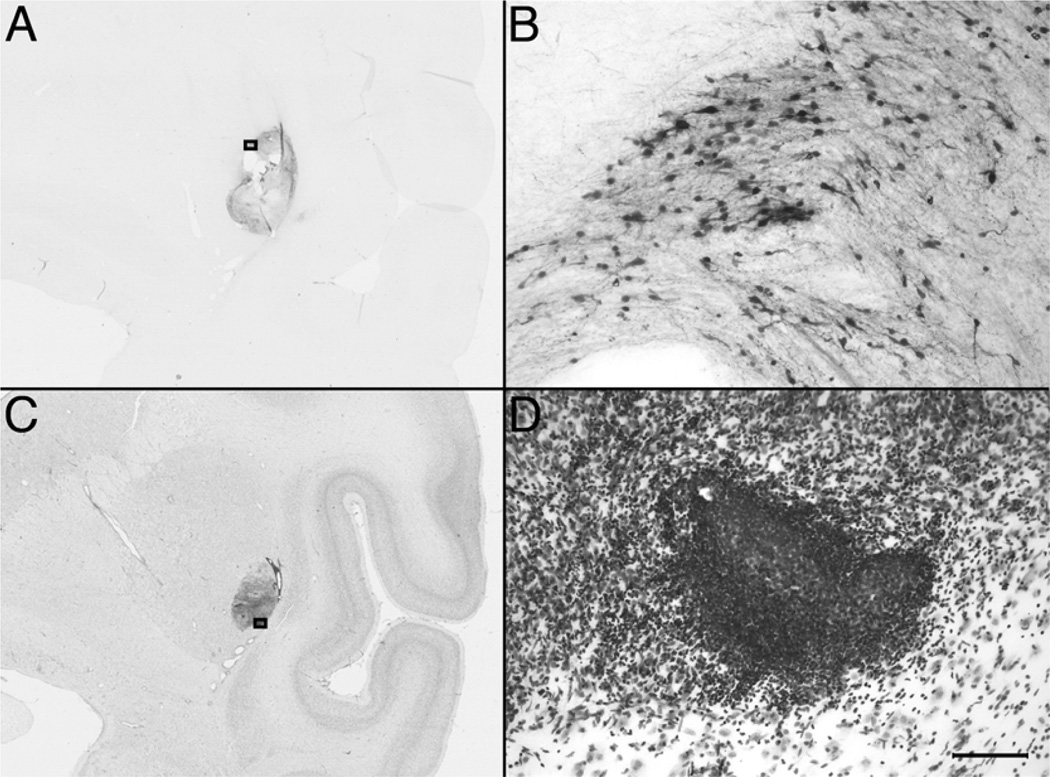
Only one of the three transplanted monkeys (RH2290) showed macroscopically visible grafts as oval-shaped areas that sometimes had holes or tears (up to 50 µm in diameter) that were attributed to necrosis. Microscopically, GFP-positive cells were located in the target areas of injection (caudate, putamen, and SN) and around regions of necrosis (Fig. 3A, B). Unbiased stereological counting of GFP-ir cells resulted in estimates of 347,360 cells (27.7% survival) in the caudate [Scheaffer CE (coefficient of error) 0.086], 434,668 cells (34.4%) in the putamen (Scheaffer CE 0.070), and 715 cells (2.9%) in the SN (Scheaffer CE 0.491).
Figure 3.
Low-magnification (A, C) and high-magnification (B, D) images of GFP-stained (A, B) and Nissl-stained (C, D) striatal coronal sections of monkey RH2290. Squares in (A) and (C) correspond to the area magnified in (B) and (D). Scale bars: 5.0 mm (A, C) and 100 µm (B, D).
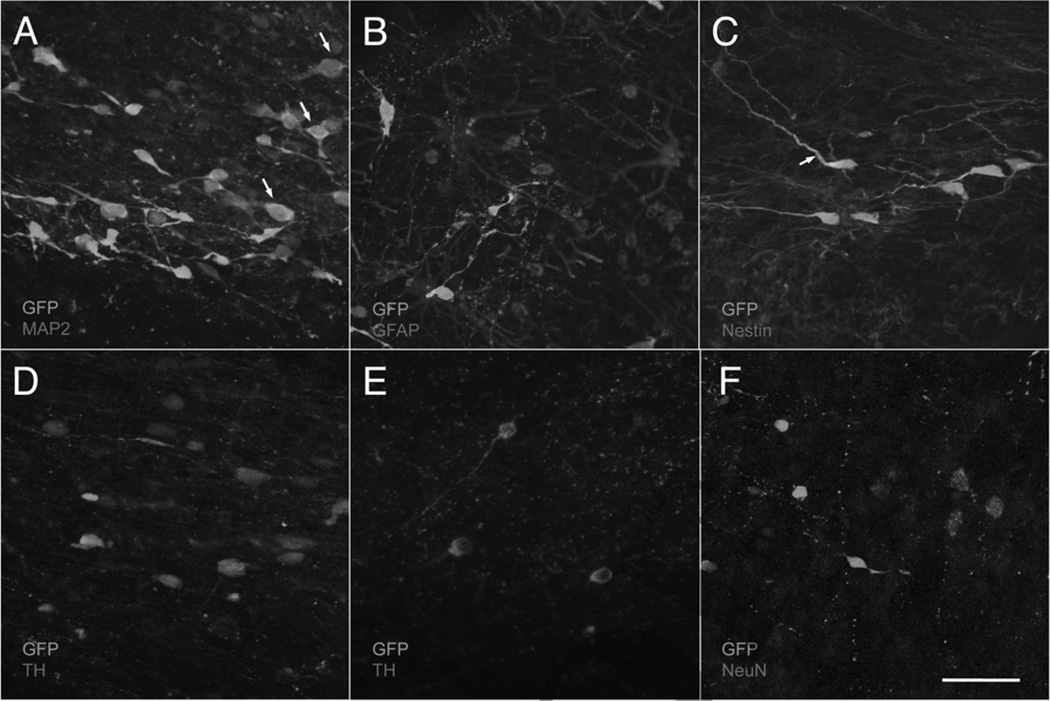
Confocal immunofluorescence microscopy revealed numerous GFP-positive cells with neuronal morphology in the grafted areas of RH2290 (Fig. 4). Colocalization of GFP and TH (Fig. 4D, E), GFP and NeuN (Fig. 4F), or GFP and GFAP (Fig. 4B) was not observed anywhere in the grafted tissue. However, many GFP-positive cells were also MAP2 positive (Fig. 4A), and occasionally a cell process was positive for both GFP and nestin (Fig. 4C). These results suggest that grafted human cells become mature neurons by 3 months postgraft and that mature neurons and glia have been rejected in the other two animals.
Figure 4.
Confocal micrographs of immunofluorescence in monkey RH2290. In each image, GFP is shown in green, while the other antigens are shown in red: MAP2A/B (A), GFAP (B), nestin (C), TH (D, E), and NeuN (F). The green signal could be attributed either to GFP excitation or to the use of an antibody labeled with a green secondary. Arrows in (A) correspond to cells with colocalization of both markers. Arrow in (C) corresponds to a single double-stained process. Neurons in (E) were found in the contralateral putamen, an ungrafted region, for comparison with (D). Scale bar: 50 µm.
Host Response to Xenografts
Overall, the animals tolerated the transplants and immunosuppression regimen without complications and maintained normal weight and clinical measures. No abnormalities were present in general chemistry and hematology panels at time of termination. Immunosuppression therapy using cyclosporine A (CsA) showed individual variations. Monkey R98067 had detectable but consistently lower blood levels of CsA as compared to the other monkeys (premortem value: R98067, 47 ng/ml; RH2290, 69 ng/ml; R96025, 107 ng/ml) even after the animal’s dosage was increased from 40 to 50 mg/kg PO.
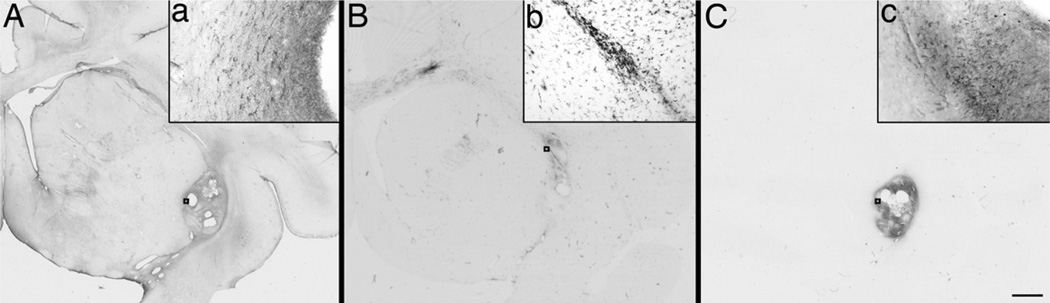
Nissl staining showed the presence of dense cells in the injection sites, particularly those with grafts in the animal RH2290. GFAP and CD68 immunostaining indicated mild staining in the SN ipsilateral to the ICA infusion of all the monkeys (Fig. 5). Moderate GFAP-ir cells were observed in the striatum and SN following the injection needle tracts and injection sites in all the monkeys (Fig. 5A). CD68-immunostained microglia/macrophages were also found following the injection needle tracts and injection sites in all the monkeys and from there spreading across white matter fibers of the corpus callosum (Fig. 5B).
Figure 5.
Low-magnification images of GFAP- (A), CD68- (B), and CD45-immunostained (C) coronal sections at the level of rostral striatum for RH2290. Scale bar: 5.0 mm. Insets (a–c) are high-magnification images of the regions indicated by boxes. Scale bar: 100 µm.
Monkey RH2290 showed evidence of necrosis in the rostral striatum that was surrounded by numerous CD68-positive cells. This monkey had the most intense CD68 staining overall, with pixel areas above threshold (from OD analysis) of 496 on the intact side and 3,042 on the MPTP side. R96025 and R98067 had pixel areas above threshold of 187 and 341 on the intact side and 558 and 1,060 on the MPTP side, respectively. RH2290 was also the only animal with CD45-positive leukocytes (Fig. 5C), which were localized mainly in the area of necrosis. These results suggest active immune response to the graft in this animal.
DISCUSSION
This is the first report evaluating the feasibility of transplantating DA-hES cells into a Parkinsonian nonhuman primate brain. Our results indicate that the grafted hES cell-derived neural cells survive in the PD monkey brain. However, the conventional immunosuppression with cyclosporine is not sufficient to prevent the rejection of xenografts.
Human ES cell-derived DA neuronal cultures have been shown to be effective in reversing the locomotive deficits in 6-OHDA lesioned rats (20,26). Unfortunately, in the PD monkey brain, the grafted cells were rejected in two of the animals and being rejected in another. In this animal, striatal compared to nigral grafts had a better survival rate. This is evidenced by the presence of large numbers of CD68 and CD45 cells in the injection sites and surrounding the grafts. We implemented the CsA dosing for this study based on our previous report transplanting human neuroprogenitor cells expressing GDNF (4), as a specific therapeutic dose of CsA for intracerebral grafting for rhesus monkeys has not yet been defined. This turned out to be not sufficient. The GDNF-expressing human neural progenitors usually remain as progenitors or immature, which express low levels of MHC antigens (1,21); hence, the regular oral administration of CsA is adequate. Human ES cell-derived neural progenitors, however, will mature to neurons and glia in the mouse or rat brain in about 3 months (12,26). Mature neurons and glia express higher levels of MHC antigens (3). Therefore, the immune system mounts response to the xenograft, and the conventional oral administration of CsA in monkey is not sufficient to prevent the rejection. Indeed, the timing corresponds to maturation of grafted hES-derived neural progenitors, with graft rejection in two animals and one being rejected. It is also evidenced by the fact that in the graft being rejected, the remaining cells in the grafts are mostly immature cells. While the experiment fails to achieve the initial goal, the report serves as a lesson for examination of human stem cells in monkey models. In that regard, successful hES grafts to two rhesus monkeys have been recently reported (15). CsA oral dosing in those monkeys started at 30 mg/kg and was tapered to 15 mg/kg; as individual circulating CsA levels were not described, immunosupression paradigms cannot be faithfully compared. Yet, it should be noted that the cell survival was observed 1 month posttransplantation and that the grafts were composed by cells with an immature neuroblast phenotype (15), further supporting our hypothesis relating cell maturation with rejection.
Transplanted hES-derived neural cells in the rodent brain often result in overgrowth of neural progenitors (20). One of the goals of this pilot study was to examine the tumorigenic potential of human stem cell derivatives in the primate brain. We reasoned that human cells may respond to the primate brain environment and differentiate to neurons and glia efficiently, thus preventing overgrowth of grafts. While the graft rejection prevents us from definitively assessing the issue, the results suggest that the hES-derived cells did differentiate to neurons and glia, which are then rejected. If the cells were to maintain in the progenitor stage and continue to divide, they will express a low level of MHC antigens, which will not elicit immune rejection in the presence of CsA. Of course, further experiments with effective immunosuppression will allow us to address this question.
Stem cell therapy may someday become useful for PD therapy, particularly in light of the recent development of iPS technology (22,27). Indeed, human iPS cells have been differentiated to DA neurons, which upon transplantation into 6-OHDA lesioned rats reverse locomotion deficits (14). While immunological issue may be irrelevant for developing a clinical therapy involving autologous transplantation using iPS cells, it is likely that human iPS derivatives need to be tested in nonhuman primates. In that regard, our present finding provides a stepping stone for examining the safety and efficacy of human stem cells in nonhuman primate models (13). In particular, we propose that a rigorous regimen of immune suppression is needed for long-term preclinical observation of human stem cell derivatives in nonhuman primate models. An alternative is to derive monkey iPS cells, differentiate to target cells such as DA neurons, and transplant the differentiated cells back to the monkey, realizing autologous graft. This model system will not only mimic future use of human iPS cells for therapy in patients but also allow long-term observations on safety, efficacy, and side effects without immunosuppression.
ACKNOWLEDGMENTS
This project was supported in part by grant P51 RR000167 (NIH-NCRR) to the Wisconsin National Primate Research Center, University of Wisconsin-Madison, start-up funds from the Department of Medical Physics, University of Wisconsin-Madison (M.E.E.), and in part by NINDS (NS 045926 to S.C.Z.). This research was conducted in part at a facility constructed with support grants RR15459-01 and RR020141-01. We are grateful to Dr. Jolien Connor for thoughtful discussions.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Behrstock S, Ebert AD, Klein S, Schmitt M, Moore JM, Svendsen CN. Lesion-induced increase in survival and migration of human neural progenitor cells releasing GDNF. Cell Transplant. 2008;17:753–762. doi: 10.3727/096368908786516819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: Potential for new therapies. Nat. Rev. Neurosci. 2001;2:577–588. doi: 10.1038/35086062. [DOI] [PubMed] [Google Scholar]
- 3.Drukker M. Immunogenicity of human embryonic stem cells: Can we achieve tolerance? Springer Semin. Immunopathol. 2004;26:201–213. doi: 10.1007/s00281-004-0163-5. [DOI] [PubMed] [Google Scholar]
- 4.Emborg ME, Ebert AD, Moirano J, Peng S, Suzuki M, Capowski E, Joers V, Roitberg BZ, Aebischer P, Svendsen CN. GDNF-secreting human neural progenitor cells increase tyrosine hydroxylase and VMAT2 expression in MPTP-treated cynomolgus monkeys. Cell Transplant. 2008;17:383–395. [PubMed] [Google Scholar]
- 5.Emborg ME, Ma SY, Mufson EJ, Levey AI, Taylor MD, Brown WD, Holden JE, Kordower JH. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J. Comp. Neurol. 1998;401:253–265. [PubMed] [Google Scholar]
- 6.Emborg ME, Moirano J, Raschke J, Bondarenko V, Zufferey R, Peng S, Ebert AD, Joers V, Roitberg B, Holden JE, Koprich J, Lipton J, Kordower JH, Aebischer P. Response of aged parkinsonian monkeys to in vivo gene transfer of GDNF. Neurobiol. Dis. 2009;36:303–311. doi: 10.1016/j.nbd.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emborg ME, Moirano J, Schafernak KT, Moirano M, Evans M, Konecny T, Roitberg B, Ambarish P, Mangubat E, Ma Y, Eidelberg D, Holden J, Kordower JH, Leestma JE. Basal ganglia lesions after MPTP administration in rhesus monkeys. Neurobiol. Dis. 2006;23:281–289. doi: 10.1016/j.nbd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Emborg ME, Shin P, Roitberg B, Sramek JG, Chu Y, Stebbins GT, Hamilton JS, Suzdak PD, Steiner JP, Kordower JH. Systemic administration of the immunophilin ligand GPI 1046 in MPTP-treated monkeys. Exp. Neurol. 2001;168:171–182. doi: 10.1006/exnr.2000.7592. [DOI] [PubMed] [Google Scholar]
- 9.Emborg-Knott ME, Domino EF. MPTP-induced hemi-parkinsonism in nonhuman primates 6–8 years after a single unilateral intracarotid dose. Exp. Neurol. 1998;152:214–220. doi: 10.1006/exnr.1998.6845. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick KM, Raschke J, Emborg ME. Cell-based therapies for Parkinson’s disease: Past, present, and future. Antioxid. Redox. Signal. 2009;11:2189–2208. doi: 10.1089/ars.2009.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 12.Guillaume DJ, Johnson MA, Li XJ, Zhang SC. Human embryonic stem cell-derived neural precursors develop into neurons and integrate into the host brain. J. Neurosci. Res. 2006;84:1165–1176. doi: 10.1002/jnr.21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halme DG, Kessler DA. FDA regulation of stem-cell-based therapies. N. Engl. J. Med. 2006;355:1730–1735. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- 14.Hargus G, Cooper O, Deleidi M, Levy A, Lee K, Marlow E, Yow A, Soldner F, Hockemeyer D, Hallett PJ, Osborn T, Jaenisch R, Isacson O. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc. Natl. Acad. Sci. USA. 2010;107:15921, 5926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011 doi: 10.1038/nature10648. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, Widner H, Revesz T, Lindvall O, Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 17.Mendez I, Viñuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat. Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olanow CW, Goetz CG, Kordower JH, Stoessel AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 19.Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. U S A. 2004;101:12543, 2548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One. 2007;2:e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluri-potent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 24.Xia X, Zhang Y, Zieth CR, Zhang SC. Transgenes delivered by lentiviral vector are suppressed in human embryonic stem cells in a promoter-dependent manner. Stem Cells Dev. 2007;16:167–176. doi: 10.1089/scd.2006.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D, Zhang ZJ, Oldenburg M, Ayala M, Zhang SC. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells. 2008;26:55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz- Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 28.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]