Abstract
Background
Helicobacter pylori has been isolated from 10%–20% of human chronic cholecystitis specimens but the characteristics of “Helicobacter pylori positive cholecystitis” remains unclear. This study aims to compare the clinicopathological features between chronic cholecystitis patients with and without Helicobacter pylori infection in gallbladder mucosa.
Methods
Three hundred and twenty-six chronic cholecystitis patients were divided into two groups according to whether Helicobacter pylori could be detected by culture, staining or PCR for Helicobacter 16s rRNA gene in gallbladder mucosa. Positive samples were sequenced for Helicobacter pylori-specific identification. Clinical parameters as well as pathological characteristics including some premalignant lesions and the expression levels of iNOS and ROS in gallbladder were compared between the two groups.
Results
Helicobacter pylori infection in gallbladder mucosa was detected in 20.55% of cholecystitis patients. These patients had a higher prevalence of acid regurgitation symptoms (p = 0.001), more histories of chronic gastritis (p = 0.005), gastric ulcer (p = 0.042), duodenal ulcer (p = 0.026) and higher presence of Helicobacter pylori in the stomach as compared to patients without Helicobacter pylori infection in the gallbladder mucosa. Helicobacter pylori 16s rRNA in gallbladder and gastric-duodenal mucosa from the same individual patient had identical sequences. Also, higher incidences of adenomyomatosis (p = 0.012), metaplasia (p = 0.022) and higher enhanced expressions of iNOS and ROS were detected in Helicobacter pylori infected gallbladder mucosa (p<0.05).
Conclusions
Helicobacter pylori infection in gallbladder mucosa is strongly associated with Helicobacter pylori existed in stomach. Helicobacter pylori is also correlated with gallbladder premalignant lesions including metaplasia and adenomyomatosis. The potential mechanism might be related with higher ROS/RNS production but needs further investigation.
Introduction
Chronic cholecystitis is one of the most prevalent diseases requiring surgical intervention. In China, more than 90% of the cholecystitis cases are claimed to be caused by symptomatic cholelithiasis, the incidence of which is approximately 10% of the adult population. [1], [2] Histologically, chronic cholecystitis presents a large range of related inflammatory epithelial changes including mononuclear infiltrate, fibrosis, thickness of muscular layer, dysplasia, hyperplasia and metaplasia-the last three have been considered as premalignant lesions. [3]–[5].
The causes of chronic cholecystitis still remain unclear. Recently, many findings obtained from microbiological studies suggest that bacterial infection in biliary system might play a role. Our previous meta-analysis demonstrated that Helicobacter pylori (H.pylori) in human biliary system was correlated with chronic cholecystitis, especially in the regions with higher prevalence of this infectious agent such as South Asia, East Asia and Latin America. [6] Evidences supporting the association between H.pylori infection and chronic cholecystitis could be found by using direct culture or staining of H.pylori in gallbladder tissues as well as indirect techniques such as PCR, ELISA and serology for detecting H.pylori-specific genes or antibodies.[7]–[9] The positive rate of H.pylori in gallbladder is reported to be 10%–20% by culture. [10].
H.pylori can induce oxidative stress through producing reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are considered to be the important causes of chronic inflammation, ulcer and canceration of the stomach. [11] In H. pylori-infected stomach, possible sources of ROS/RNS include neutrophils, vascular endothelial cells, gastric mucosal cells, and H. pylori itself. One of the most important pathways of H.pylori-induced RNS is mediated by overproduction of endogenous synthesis nitric oxide (NO) through inducible NO synthase (iNOS) expression. [12] In benign inflammatory and malignant gallbladder diseases, ROS and iNOS also play an important role. [13] However, in biliary system, the correlation between H.pylori and ROS/RNS production still needs further investigation.
Two-thirds of the world population is infected with H.pylori. [14] The findings of H.pylori in biliary tract implicated that the stomach might not be the only arena of activity of this agent. However, few studies by far have specifically assessed the characteristics of “Helicobacter pylori positive cholecystitis”. Therefore, this study aims to compare the clinicopathological features between chronic cholecystitis patients with and without Helicobacter pylori infection in gallbladder mucosa.
Materials and Methods
Patients
Of 378 patients who underwent cholecystectomy in Department of General Surgery, Xinhua Hospital from December 2011 to July 2012, three hundred and twenty-six patients (97 males and 229 females, aged 21–87 years) who fulfilled the pathological criteria of chronic cholecystitis were enrolled in this study. The exclusion criteria were: (1) patients with history of hepato-biliary or pancreatic surgery which changed the normal structure and function of the biliary system, (2) patients who had previously received standard triple therapy for H. pylori eradication, (3) patients who had taken antibiotics or proton pump inhibitors 4–6 weeks prior to cholecystectomy. According to whether H. pylori was detected positive in gallbladder mucosa, patients were divided into two groups. The study protocol was approved by the Ethics Committee of Shanghai JiaoTong University, School of Medicine and signed informed consent was obtained from all the patients.
Gastroscopy
Before or after cholecystectomy, all patients enrolled in this study received gastroscopy with biopsy in order to clarify the infection status of H. pylori in their stomach. Gastroscopy was performed with video endoscopes that worked in high-resolution, white light mode and AFI mode (EVIS-FQ260Z; Olympus Medical Systems Co. Ltd, Tokyo, Japan). Two biopsy specimens were taken at each site from the greater curvature of the antrum, and the greater and lesser curvature of the corpus. Each of the two specimens from the above parts of the stomach were used respectively for culture and Warthin-Starry Staining of H. pylori.
Cholecystectomy and Gallbladder Biopsy
Laparoscopic cholecystectomy was performed by a single surgeon using a standardized 4-port technique (Laparoscopic Device, KARL STORZ GmbH, Tuttlingen, Germany). Two biopsy specimens were taken aseptically at each site from the fundus, body and neck of the gallbladder. Each of the two specimens from the above parts of the gallbladder were used respectively for culture and Warthin-Starry Staining of H. pylori.
The stomach and gallbladder specimens were aseptically transferred to the microbiology laboratory immediately after gastroscopy or cholecystectomy.
Verification of H. pylori Infection in Gallbladder and Stomach
The presence of H. pylori in gastric or gallbladder mucosa was determined by either positive culture, Warthin-Starry Staining or positive nest PCR for specific 16s rRNA of this bacterium. At least one positive test was regarded as confirmation of infection of this agent in gallbladder or gastric mucosa.
Culture of H. pylori
The gallbladder and gastric mucosa specimens were inoculated onto sterile plates containing endo agar and Brucella agar supplemented with 5% horse blood (Becton, Dickinson and Company, Sparks, Maryland, USA) for nonspecific bacterial and Campylobacter cultures, respectively. For isolation of H.pylori, we prepared specific media containing BHI agar supplemented with 7% sheep blood, 0.4% IsoVitaleX (Becton, Dickinson and Company, Sparks, Maryland, USA) and Skirrow-selective supplements (Oxoid Limited, Thermo Fisher Scientific, Hampshire, UK). H.pylori was incubated at a microaerophilic condition (5% O2, 10% CO2, 85% N2) for 3–7 days at 37°C. Bacteria colonies were examined with biochemical tests as well as microscopy for conformation. H. pylori colony was identified as showing the typical white, pin-point and transparent morphology. Oxidase and fast urease activity were also performed at colonies grown on H. pylori specific media. Bacterium with typical morphology, positive oxidase and urease activity were verified as H. pylori.
Warthin-Starry Staining of H. pylori
Warthin-Starry Staining was performed using the specific kit (Diagnostic BioSystems, Pleasanton, California, USA). Four-micrometer thick paraffin sections of gallbladder and gastric mucosa tissues from each patient were backed for 1 h at 60°C. After dewaxing and re-hydration, sections were incubated at 56°C for 1 h in 1% silver nitrate buffer in the dark box and then dipped in developer solution and stained for 5–8 min. Finally, sections were dehydrated with 100% alcohol, cleared with xylene. H. pylori was identified as stained into buffy or black color in a light yellow background under microscope with oil immersion lens (×1000). The results were also determined independently by the above two pathologists. Warthin-Starry staining and H. pylori culture were blindly assayed for all the specimens from each patient.
PCR for Helicobacter 16s rRNA Gene
The DNA extracts were prepared from the paraffin specimens as the kit (TIAN amp Micro DNA kit, TIANGEN Biotech, Beijing, China) instructed. A semi-nested PCR assay specific for Helicobacter 16s rRNA gene (16s rDNA) was amplified as previously described [15], using primers 1F (5′CTATGACGGGTATCCGGC3′), 1R (5′CTCACGACACGAGCTGAC3′) and 2R (5′TCGCCTTCGCAATGAGTATT3′). Primers 1F and 1R were used in the first step, whereas primers 1F and 2R were used in the second step. The PCR reaction mixture contained 1 µl of 10 µM each primer (1F and 1R), 2 µL of 10 mM dNTP, 1×PCR reaction buffer, 2.5 mM MgCl2, 0.05% casein, 0.05% formamid, 1.25 U rTaq DNA polymerase (Takara, Dalian, China), and 2 µL extracted DNA as templates. The amplification conditions for the first step were 94°C for 2 min; 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s; and finally 72°C for 10 min. The reaction mixture of the second step (25 µL) contained 0.5 µl of 10 µM each primer each primer (1F and 2R), 0.2 mmol/L each dNTP, 1×PCR reaction buffer, 2.5 mmol/L MgCl2, 1.0 U ExTaq DNA polymerase (Takara, Dalian, China), and 2 µL 10×diluted PCR product from the first step. The 416-bp PCR products were visualized by 1.2% agarose gel electrophoresis.
Sequence Analysis
The PCR products were subsequently ligated into pMD-19T simple vector (Takara, Dalian, China) for sequencing. The product was sequenced after PCR amplification and double enzyme digestion. The sequences were compared with the 16S rRNA gene sequences of the strains that have been registered in the GenBank database. Products of the sequence reaction were aligned and the closest homologous sequence was identified by Standard Nucleotide BLAST analysis tool on the internet (BLASTn, http://blast.ncbi.nlm. nih.gov).
Histological Analysis of Chronic Cholecystitis
Gallbladder specimens were fixed in 10% buffered formalin and embedded in paraffin. Four micrometer-thick sections were cut and stained with hematoxylin-eosin (HE). Sample slides were examined independently by two attending pathologists who specialized in biliary diseases and in the case of discrepancy, the decision was made by discussion or in consultation with a third experienced pathologist. Chronic inflammation was diagnosed in the presence of a predominantly mononuclear inflammatory infiltrate, fibrosis, or metaplastic changes. A scoring system proposed by Barcia JJ [16] was used to semi-quantitatively assess the histological changes of chronic cholecystitis (Table 1).
Table 1. Definitions of Pathological Changes of Chronic Cholecystitis.
| Pathological Changes of Chronic Cholecystitis | Definition |
| Inflammatory mononuclear infiltrate | |
| Mild | Diffuse, ≤10 inflammatory cells per HPF in any layer |
| Moderate | Diffuse, between 11 to 30 cells per HPF |
| Severe | Diffuse, more than 31 cells per HPF or follicular |
| Degree of fibrosis | |
| Mild | Uneven collagen deposition in ≤20% of material |
| Moderate | Uneven collagen deposition in 21% to 70% of material |
| Severe | Uneven collagen or lamellar fibroplasia in ≥71% of material |
| Thickness of the muscular layer | |
| Mild | Less than one third of the whole thickness |
| Moderate | One third to two thirds of the wall |
| Severe | More than two thirds of the wall thickness |
| Addipose tissue deposition | |
| Mild | Up to 10% of the material |
| Moderate | 11% to 60% of the material |
| Severe | More than 60% of the material |
| Degree of hyperplasia | |
| Diffuse | ≥70% of the whole sections |
| Focal | <70% of the whole sections |
| Degree of dysplasia | |
| Low-grade | Resemble tubular adenomas of the colon without intestinal metaplasia |
| High-grade | Markedly pleomorphic nuclei and/or prominent nucleoli |
| Metaplasia | |
| Pyloric type | Structures similar to the pyloric glands in the lamina propria |
| Intestinal type | Goblet cells and enterocitlike cells |
| Gastric surface type | Epithelial cells of gallbladder mucosa replaced by tall columnar cells with abundant mucin and basallylocated nuclei |
HPF: high power field.
Immunohistochemical Staining of iNOS and ROS
For iNOS and ROS detection, immunohistochemistry was performed on 4-µm thick, mounted on silane-coated slides of gallbladder mucosa tissue sections. Sections were deparaffinized and rehydrated, then washed in distilled water and 0.05 mol/L Tris buffer. After blocking the nonspecific binding sites by using Protein blocking agent (Coulter-Immunotech, Marseille, France), sections were incubated with the primary polyclonal rabbit anti-iNOS and ROS antibody (1∶200, Transduction Laboratories, kentucky, USA) at 4°C for 24 h. After sections were washed, a biotinylated immunoglobulin (anti-rabbit serum for iNOS and ROS) was applied for 30 min. Finally, all sections were incubated with the avidine-biotin-complex (ABC) with alkaline phosphatase (Vectastain, Vector Laboratories, Burlingame, California, USA). The staining was visualized with 3,3′-diaminobenzidine and hydrogen peroxide.
Semiquantitative analysis of the iNOS and ROS immunostaining was performed independently by the above two pathologists and in the case of discrepancy, the decision was made by discussion or in consultation with a third experienced pathologist. In ten randomly selected areas of the whole section, the number and percentage of positive cells were calculated for determining staining intensity and proportion of iNOS or ROS staining. A case without positive cells was considered negative. A case with less than 10% positive cells was scored 1, 10–50% was scored 2, 50–80% was scored 3 and more than 80% was scored 4. A staining intensity was classified as weak (I), moderate (II) and strong (III). A immunoreactive score was calculated as staining intensity×amount of positive cells (from lowest score 0 to highest 12) and specimen with a grade of more than 1 was defined as positive. [17].
Statistical Analysis
The software SAS 9.13 (SAS Institute, Gary, North Carolina, USA) was used for conducting statistical analysis. Student’s t-test was performed for comparing age and BMI. The immunoreactive score of iNOS and ROS were calculated and statistically compared between the two groups using Mann-Whitney U-test. Chi-square test or Fischer’s exact test was used to examine the rest clinicopathological parameters. For all statistical analyses, significance levels were set at p<0.05.
Results
Evidence of H. pylori in gallbadder mucosa was demonstrated by Warthin-Starry staining in 64 (19.63%) patients and H. pylori colonies were identified upon culture in 55 (16.87%) patients. Among them, 52 (77.61%) patients were both positive in staining and culture. In PCR test for Helicobacter-16s rRNA gene, 67 (20.55%) patients were positive. From all the gallbladder specimens, only the positive samples which detected by staining or culture were positive in nest PCR test. All samples positive for first-step amplicon were also positive for the nested PCR. Finally, H. pylori infection in gallbladder mucosa was detected in 20.55% (n = 67) of the cholecystitis patients (Figure 1 and 2). These patients had a higher prevalence of acid regurgitation symptoms (p = 0.001), more histories of chronic gastritis (p = 0.005), gastric ulcer (p = 0.042), duodenal ulcer (p = 0.026) and a higher positive rate of Helicobacter pylori (p<0.05) in the stomach as compared to patients without Helicobacter pylori infection in the gallbladder (Table 2). Of the 67 patients (20.55%) who were positive in H. pylori 16s rRNA detection in gallbladder mucosa, amplications of 16s rRNA in their gastric or duodenal specimens were also succeed in 42 patients (62.69%). These were 30 of 45 (66.67%) patients with chronic gastritis, 7 of 11 (63.64%) patients with gastric ulcer and 5 of 8 (62.50%) patients with duodenal ulcer (Figure 2). Consequently, we check the amplified PCR products by direct sequencing and BLAST search and confirmed that each sequence was 96–99% similar to a known H. pylori 16s rRNA gene registered in GenBank (Figure 3). No other kinds of Helicobacter species such as Helicobacter bilis, Helicobacter hepaticus or Helicobacter pullorum could be detected by PCR. Moreover, our data also revealed that H. pylori-16s rRNA in gallbladder and gastric (or duodenal) mucosa acquired from the same individual patient had identical sequences (Figure 4).
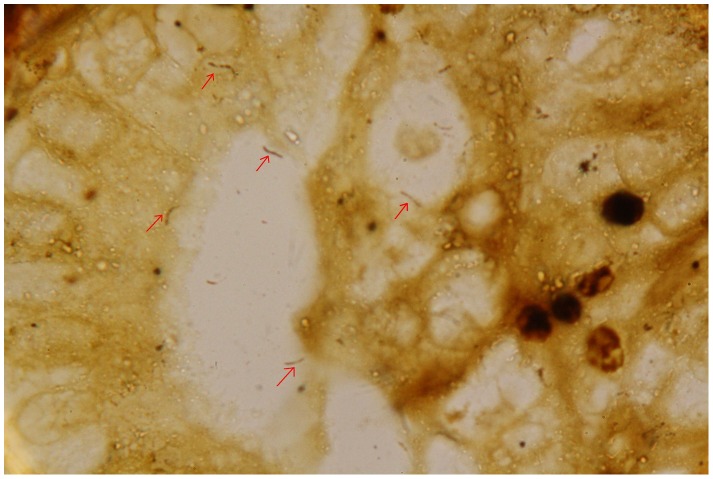
Figure 1. H.pylori infection in metaplastic gallbladder mucosa (oil immersion lens,×1000, red arrow indicates H.pylori).
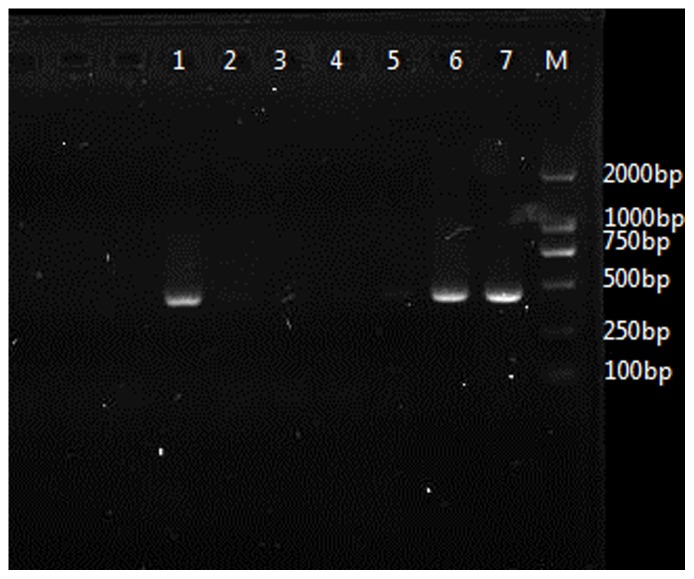
Figure 2. PCR products of Helicobacter specific 16s rRNA gene from gallbladder and gastric mucosa samples.
(lanes M: step-ladder marker; 1: positive control of gastric biopsy-derived H. pylori DNA; 2: negative control of gastric biopsy; 3: negative 16s rRNA gene in gallbladder; 4 and 5: negative 16s rRNA gene in gallbladder and gastric mucosa acquired from one individual patient; 6 and 7: positive 16s rRNA gene in gallbladder and gastric mucosa acquired from another individual patient).
Table 2. Clinical characteristics of H.pylori-positive and negative chronic cholecystitis.
| Characteristics | No. of Patients n (%) | ||
| H.pylori (+) in gallbladder mucosa (n = 67) | H.pylori (−) in gallbladder mucosa (n = 259) | p value | |
| Age (yr) | 45.54±12.58 | 47.82±11.56 | NS |
| Gender | |||
| Male: Female (% Male) | 19∶48(28.36) | 78∶181(30.12) | NS |
| BMI (kg/m2) | 23.10±2.24 | 22.72±1.85 | NS |
| Symptom | |||
| Mild Abdominal Pain | 44(65.67) | 149(57.53) | NS |
| Biliary Colic | 32(47.76) | 102(39.38) | NS |
| Loss of Appetite | 12(17.91) | 28(10.81) | NS |
| Acid Regurgitation | 22(32.84) | 39(15.06) | 0.001* |
| Heartburn | 20(29.85) | 50(19.31) | NS |
| Preoperative Ultrasound Diagnosis | |||
| Gallstone Disease | |||
| No. of Gallstones | |||
| Single: Multiple (% Single) | 21∶34(38.18) | 104∶108(49.06) | NS |
| Polypoid Lesion | |||
| No. of Polypoid Lesions | |||
| Single: Multiple (% Single) | 8∶4(66.67) | 18∶31(35.29) | NS |
| Gallbladder Wall Thickening | 12(17.91) | 39(15.06) | NS |
| Atrophic Gallbladder | 15(22.39) | 35(13.51) | NS |
| History of Other Gastrointestinal Diseases | |||
| Chronic Gastritis | 45(67.16) | 124(47.88) | 0.005* |
| Gastric Ulcer | 11(16.42) | 21(8.11) | 0.042▴ |
| Duodenal Ulcer | 8(11.94) | 12(4.63) | 0.026▴ |
| Reflux Esophagitis | 12(17.91) | 25(9.65) | NS |
| Chronic Enteritis | 3(4.48) | 8(3.09) | NS |
| H.pylori (+) in Gastric or Duodenal Mucosa | |||
| Warthin-Starry Stain | 39(58.21) | 106(40.93) | 0.011▴ |
| H.pylori Culture16s rRNA gene PCR | 33(49.25)42(62.69) | 90(34.75)115(44.40) | 0.029▴0.008* |
p<0.01.
p<0.05.
NS: not significant.
N/A: not applicable.
Figure 3. Comparison of complete sequence data of H. pylori 16s rRNA gene tested in gallbladder mucosa sample from published GenBank data: sequence ID ref|NR_044761.1|.
(nucleotides 263–695 were listed).
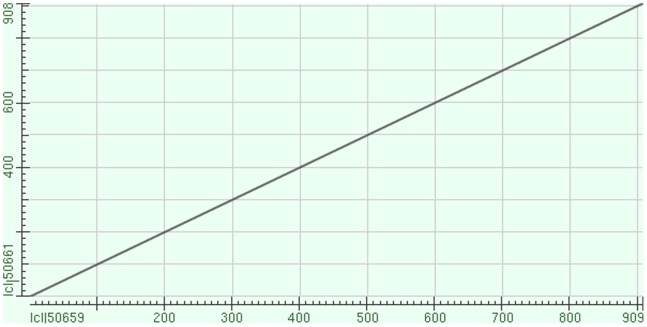
Figure 4. BLAST showed H. pylori 16s rRNA gene in gallbladder and gastric mucosa from the same individual patient had completely identical sequences.
(sequence ID50661: H. pylori 16s rRNA tested in gallbladder mucosa; sequence ID50659: H. pylori 16s rRNA tested in gastric mucosa).
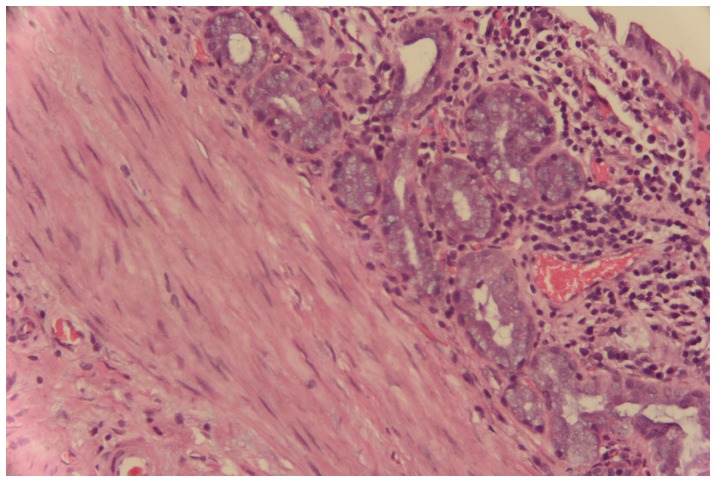
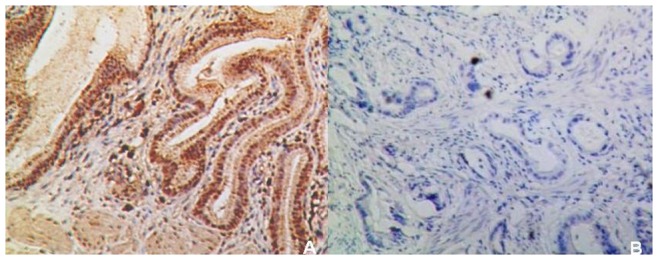
The results of comparison of pathological features between the two groups in gallbladder mucosa were demonstrated in Table 3. Higher incidences of adenomyomatosis (p = 0.012) and metaplasia (p = 0.022) were detected in H. pylori infected gallbladder mucosa (Figure 5). The metaplastic lesions were predominantly of pyloric type (21 cases, 84% from the total of metaplastic cases), characterized by structures similar to the pyloric glands in the lamina propria. Intestinal type, which characterized by the presence of goblet cells and enterocitlike cells, was detected in only 16% (4 cases) of all the metaplastic patients. No difference was found in the distribution of the two metaplastic types between the two groups (p = 0.602). Regarding iNOS and ROS expression, the immunoreactive scores were both significantly higher in H. pylori-positive gallbladder mucosa compared to H. pylori-negative mucosa (p = 0.012 and 0.000, respectively) (Figure 6 and 7). However, in our study, there were only 3% of the slides showed positive H. pylori staining and enhanced iNOS or ROS expressions occurring simultaneously in the same area.
Table 3. Pathological characteristics of H.pylori-positive and negative chronic cholecystitis.
| Characteristics | No. of Patients n(%) | ||
| H.pylori (+) in gallbladder mucosa (n = 67) | H.pylori (−) in gallbladder mucosan(n = 259) | p value | |
| Postoperative and Pathological Diagnosis | |||
| Gallstone | |||
| Cholesterol Stones | 14(25.45) | 68(32.08) | NS |
| Pigment Stones | 8(14.55) | 35(16.51) | NS |
| Mixed Stones | 33(60.00) | 109(51.42) | NS |
| Polypoid Lesion | |||
| Cholesterol Polyp: Inflammatory Polyp | 10∶2 | 45∶2 | NS |
| Gallbladder Adenomyomatosis | 35(52.24) | 92(35.52) | 0.012▴ |
| Xanthogranulomatous Cholecystitis | 5(7.46) | 12(4.63) | NS |
| Histological Analysis of Cholecystitis | |||
| Inflammatory mononuclear infiltrate | NS | ||
| Mild | 31(46.27) | 131(50.58) | |
| Moderate | 22(32.84) | 89(34.36) | |
| Severe | 14(20.90) | 39(15.06) | |
| Degree of Fibrosis | NS | ||
| Mild | 45(67.16) | 188(72.59) | |
| Moderate | 14(20.90) | 54(20.85) | |
| Severe | 8(11.94) | 17(6.56) | |
| Thickness of Muscular Layer | NS | ||
| Mild | 30(44.78) | 120(46.33) | |
| Moderate | 22(32.84) | 103(39.77) | |
| Severe | 15(22.39) | 36(13.90) | |
| Adipose Tissue Deposition | NS | ||
| Mild | 33(49.25) | 166(64.09) | |
| Moderate | 27(40.30) | 73(28.19) | |
| Severe | 7(10.45) | 20(7.72) | |
| Degree of Hyperplasia | NS | ||
| Diffuse | 25(37.31) | 115(44.40) | |
| Focal | 42(62.69) | 144(55.60) | |
| Dysplasia | NS | ||
| Low-grade | 3(4.48) | 8(3.09) | |
| High-grade | 0(0.00) | 0(0.00) | |
| Metaplasia | 9(13.43) | 14(5.41) | 0.022▴ |
| Immunoreactive score of iNOS | 6.06±1.59 | 5.12±1.34 | 0.012▴ |
| Immunoreactive score of ROS | 5.01±2.01 | 3.99±1.87 | 0.000* |
p<0.01,
p<0.05.
NS: not significant.
N/A: not applicable.
Figure 5. Metaplasia of Chronic Cholecystitis (hematoxylin-eosin stain,×100).
Figure 6. iNOS expression in gallbladder mucosa of chronic cholecystitis with H. pylori infection (A) and without H. pylori infection (B) (×100).
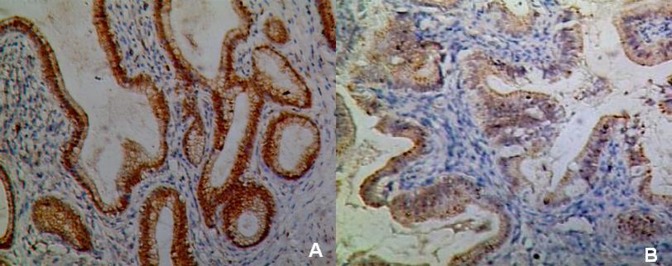
Figure 7. ROS expression in gallbladder mucosa of chronic cholecystitis with H. pylori infection (A) and without H. pylori infection (B) (×100).
Discussion
The presence of H.pylori in gallbladder mucosa was first confirmed by Kawaguchi et al in 1996. [18] However, although H.pylori has been found 3.5 times more frequently in presence of chronic cholecystitis, whether this agent contributes in the pathogenesis of this biliary disease is still poorly understood. [19] Firstly, it is difficult to verify the potential entry routes of H.pylori to the gallbladder including either ascending duodenum infection or the portal system circulation pathway. [20], [21] Secondly, since successful demonstration of H.pylori in gallbladder was mostly based on the indirect detection methods such as PCR for H.pylori-specific components rather than direct bacterial culture, some investigators believe that H.pylori is only a “stagger” but not an “invader” in biliary system. [22], [23].
Consistent with one previous report from Turkey [24] in our study, H.pylori was isolated in 20.55% (67/259) of the patients using culture, staining of gallbladder mucosa and PCR for specific 16s rRNA gene. Among the above three techniques, nest PCR still showed the highest sensitivity. We found that H.pylori in the stomach was strongly associated with the infection of this bacterium in gallbladder mucosa. Our data also showed a significant correlation between chronic cholecystitis and a few H.pylori-related diseases such as chronic gastritis, gastric ulcer and duodenal ulcer. Considering that H. pylori-16s rRNA in gallbladder and gastric-duodenal mucosa from the same individual patient had completely identical sequences, we hypothesize that H.pylori in the gastrointestinal system might be a potential candidate for increasing the risk of chronic inflammation of the gallbladder. H. pylori might reach the biliary system via sphincter of Oddi by the reflux mechanism. Bacteria colonized in the stomach and small intestine in patients with sphincterotomy and biliary enteric anastomoses, recurrent cholangitis and sphincter of Oddi dysfunction might be the cause of secondary gallstones and cholecystitis. [25], [26].
The presenting study revealed that H.pylori-infected gallbladder mucosa has a significantly higher prevalence of adenomyomatosis (GAM) than non-infected mucosa (52.24% versus 35.52%, p = 0.012). GAM is a benign, degenerative condition characterized by proliferation of the mucosal epithelium and hypertrophy of the muscularis mucosae accompanying with grossly formed mucosal invagination and intramural Rokitansky-Aschoff sinuses. [27] GAM can be diagnosed preoperatively through ultrasound, CT scan or MRI. [28], [29] Incidence rate of GAM is reported to be 25.8%–32% in chronic cholecystitis patients based on the cholecystectomy specimens. [30], [31] Some investigators strongly recommended cholecystectomy in case of GAM with gallstones or symptomatic GAM because stones and chronic inflammation secondary to GAM may lead to dysplasia, metaplasia and cancer. Although no similar finding has been reported with respect to H.pylori infection and its association with GAM, we speculate that H.pylori might be involved in the development of GAM by altering cell kinetics and proliferative activity which were verified in chronic gastritis and gastric carcinogenesis. [32].
According to literatures, metaplasia of gallbladder mucosa presents in 5%–39% of cholecystectomies. [33]–[35] In our study, metaplasia was identified in 7.67% of the included patients and it was shown to be statistically correlated with H. pylori infection in gallbladder mucosa (p = 0.047). Metaplasia is believed to be a strong histological sign for diagnosis of moderate or severe chronic cholecystitis because it is rarely observed in gallbladder autospy in which only mild inflammatory changes present. [36] Misra et al. [37] found that H. pylori colonises areas of metaplasia in gallbladder producing histological changes very similar to those seen in gastric mucosa. Chen et al. [38] demonstrated that metaplasia may provide suitable conditions for H. pylori colonization in the gallbladder. Their electron microscopy revealed at sites infected with H. pylori, the integrity of the cell-to-cell membrane of gallbladder epithelium was destructed, with swelling of mitochondria and dilatation of endoplasmic reticulum. In H.pylori-infected gallbladder nucosa, metaplasia lesions area accompanying with H.pylori colonization could be detected in 91.5% of the specimens. These morphological findings may indicate a potential direction for determining the role of H.pylori in the formation of metaplasia. Except metaplasia, hyperplasia and dysplasia were detected in 100% and 3.37% of the included patients, respectively. However, no significant correlation could be set up between these two kinds of pathological changes and H.pylori colonization in gallbladder mucosa.
H.pylori can damage gastrointestinal epithelial cells through mediating chronic inflammation. In the pathogenesis of gastric cancer, H.pylori is proved to promote the expression of ROS/RNS mediated by NF-κB, AP-1 and other pathways. [39], [40] High concentration of NO can lead to nitrative DNA damage and canceration of the epithelium. [41] In our study, the expression levels of ROS and iNOS were significantly increased in H.pylori infected gallbladder mucosa than that in non-infected mucosa. However, considering there were only 3% of the slides showed positive H. pylori staining and enhanced iNOS or ROS expressions occurring simultaneously in the same area, whether H. pylori could directly induce oxidative stress in gallbladder mucosa through increasing the expression of iNOS or ROS in gallbladder mucosa still needs further investigation. Recently, a study in vitro showed that H.pylori could significantly stimulate the growth of cholangiocarcinoma cell line (KKU-100) and DNA synthesis through iNOS pathway. [42] Unfortunately, no study so far has explored the role of H.pylori in the development of chronic cholecystitis in normal gallbladder epithelium with respect to cell proliferation, apoptosis, and inflammation.
Conclusions
In summary, our study indicated that Helicobacter pylori infection in gallbladder mucosa is strongly associated with Helicobacter pylori existed in the stomach. Helicobacter pylori is also correlated with gallbladder premalignant lesions including metaplasia and adenomyomatosis. The potential mechanism might be related with higher ROS/RNS production in Helicobacter pylori-positive gallbladder mucosa.
Acknowledgments
We thank Dr. Yong Yang and Dr. Ying-Bin Liu, Department of General Surgery, Xinhua Hospital, Shanghai JiaoTong University, School of Medicine, for their help of data collection.
Funding Statement
This work was supported by the National “Twelfth Five-Year” special science and technology major project (No.2012ZX10002016), (http://www.nmp.gov.cn/zxjs/crb/201012/t20101208_2127.htm); and the College Fund of Shanghai Jiaotong University School of Medicine (No.10XJ22003) (http://kjc.shsmu.edu.cn/Default.aspx). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda LC, et al. (2007) Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer 97: 1577–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andreotti G, Liu E, Gao YT, Safaeian M, Rashid A, et al. (2011) Medical history and the risk of biliary tract cancers in Shanghai, China: implications for a role of inflammation. Cancer Causes Control 22: 1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roa I, de Aretxabala X, Araya JC, Roa J (2006) Preneoplastic lesions in gallbladder cancer. J Surg Oncol 93: 615–623. [DOI] [PubMed] [Google Scholar]
- 4. Arora VK, Kumar S, Singh N, Bhatia A (2005) Intraoperative bile cytology of the dysplasia-carcinoma in situ sequence of gallbladder carcinoma. Cancer 105: 277–281. [DOI] [PubMed] [Google Scholar]
- 5. Duarte I, Llanos O, Domke H, Harz C, Valdivieso V (1993) Metaplasia and precursor lesions of gallbladder carcinoma. Frequency, distribution, and probability of detection in routine histologic samples. Cancer 72: 1878–1884. [DOI] [PubMed] [Google Scholar]
- 6. Zhou D, Zhang Y, Gong W, Mohamed SO, Ogbomo H, et al. (2011) Are Helicobacter pylori and other Helicobacter species infection associated with human biliary lithiasis? A meta-analysis. PLoS One 6: e27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee JW, Lee DH, Lee JI, Jeong S, Kwon KS, et al. (2010) Identification of Helicobacter pylori in Gallstone, Bile, and Other Hepatobiliary Tissues of Patients with Cholecystitis. Gut Liver 4: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yakoob J, Khan MR, Abbas Z, Jafri W, Azmi R, et al. (2011) Helicobacter pylori: association with gallbladder disorders in Pakistan. Br J Biomed Sci 68: 59–64. [DOI] [PubMed] [Google Scholar]
- 9. Pandey M (2007) Helicobacter species are associated with possible increase in risk of biliary lithiasis and benign biliary diseases. World J Surg Oncol 5: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen DF, Hu L, Yi P, Liu WW, Fang DC, et al. (2007) H. pylori exist in the gallbladder mucosa of patients with chronic cholecystitis. World J Gastroenterol 13: 1608–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Handa O, Naito Y, Yoshikawa T (2010) Helicobacter pylori: a ROS-inducing bacterial species in the stomach. Inflamm Res 59: 997–1003. [DOI] [PubMed] [Google Scholar]
- 12. Tonkic A, Tonkic M, Lehours P, Mégraud F (2012) Epidemiology and Diagnosis of Helicobacter pylori Infection. Helicobacter 17 Suppl 11–8. [DOI] [PubMed] [Google Scholar]
- 13. Zhang M, Pan JW, Ren TR, Zhu YF, Han YJ, et al. (2003) Correlated expression of inducible nitric oxide synthase and P53, Bax in benign and malignant diseased gallbladder. Ann Anat 185: 549–554. [DOI] [PubMed] [Google Scholar]
- 14. Correa P, Houghton J (2007) Carcinogenesis of Helicobacter pylori . Gastroenterology 133: 659–672. [DOI] [PubMed] [Google Scholar]
- 15. Karagin PH, Stenram U, Wadström T, Ljungh A (2010) Helicobacter species and common gut bacterial DNA in gallbladder with cholecystitis. World J Gastroenterol 16: 4817–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barcia JJ (2003) Histologic analysis of chronic inflammatory patterns in the gallbladder: diagnostic criteria for reporting cholecystitis. Ann Diagn Pathol 7: 147–153. [DOI] [PubMed] [Google Scholar]
- 17. Kasper HU, Wolf H, Drebber U, Wolf HK, Kern MA (2004) Expression of inducible nitric oxide synthase and cyclooxygenase-2 in pancreatic adenocarcinoma: correlation with microvessel density. World J Gastroenterol 10: 1918–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawaguchi M, Saito T, Ohno H, Midorikawa S, Sanji T, et al. (1996) Bacteria closely resembling Helicobacter pylori detected immunohistologically and genetically in resected gallbladder mucosa. J Gastroenterol 31: 294–298. [DOI] [PubMed] [Google Scholar]
- 19. Bulajic M, Maisonneuve P, Schneider-Brachert W, Müller P, Reischl U, et al. (2002) Helicobacter pylori and the risk of benign and malignant biliary tract disease. Cancer 95: 1946–1953. [DOI] [PubMed] [Google Scholar]
- 20. Pellicano R, Ménard A, Rizzetto M, Mégraud F (2008) Helicobacter species and liver diseases: association or causation? Lancet Infect Dis 8: 254–260. [DOI] [PubMed] [Google Scholar]
- 21. Tiwari SK, Khan AA, Ibrahim M, Habibullah CM (2006) Helicobacter pylori and other Helicobacter species DNA in human bile samples from patients with various hepato-biliary diseases. World J Gastroenterol 12: 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shukla HS, Tewari M (2012) Discovery of Helicobacter pylori in gallbladder. Indian J Gastroenterol 31: 55–56. [DOI] [PubMed] [Google Scholar]
- 23. Arnaout AH, Abbas SH, Shousha S (1990) Helicobacter pylori is not identified in areas of gastric metaplasia of gall bladder. J Pathol 160: 333–334. [DOI] [PubMed] [Google Scholar]
- 24. Abayli B, Colakoglu S, Serin M, Erdogan S, Isiksal YF, et al. (2005) Helicobacter pylori in the etiology of cholesterol gallstones. J Clin Gastroenterol 39: 134–137. [PubMed] [Google Scholar]
- 25. Cetta F (1993) Do surgical and endoscopic sphincterotomy prevent or facilitate recurrent common duct stone formation? Arch Surg 128: 329–336. [DOI] [PubMed] [Google Scholar]
- 26. Lary MA, Meier DE (1983) Sphincter incompetence caused by common bile duct stones. Surgery 93: 538–540. [PubMed] [Google Scholar]
- 27. Jutras JA (1960) Hyperplastic cholecystoses; Hickey lecture, 1960. Am J Roentgenol Radium Ther Nucl Med 83: 795–827. [PubMed] [Google Scholar]
- 28. Stunell H, Buckley O, Geoghegan T, O’Brien J, Ward E, et al. (2008) Imaging of adenomyomatosis of the gallbladder. J Med Imaging Radiat Oncol 52: 109–117. [DOI] [PubMed] [Google Scholar]
- 29. Poonam Y, Ashu S, Rohini G (2008) Clinics in diagnostic imaging (121). Gallbladder adenomyomatosis. Singapore Med J 49: 262–264. [PubMed] [Google Scholar]
- 30. Tanno S, Obara T, Maguchi H, Fujii T, Mizukami Y, et al. (1998) Association between anomalous pancreaticobiliary ductal union andadenomyomatosis of the gall-bladder. J Gastroenterol Hepatol 13: 175–180. [DOI] [PubMed] [Google Scholar]
- 31. Ootani T (1992) Relationship between gallbladder carcinoma and the segmental type of adenomyomatosis of the gallbladder. Cancer 69: 2647–2652. [DOI] [PubMed] [Google Scholar]
- 32. Bechi P, Balzi M, Becciolini A, Maugeri A, Raggi CC, et al. (1996) Helicobacter pylori and cell proliferation of the gastric mucosa: possible implications for gastric carcinogenesis. Am J Gastroenterol 91: 271–276. [PubMed] [Google Scholar]
- 33. Meirelles-Costa AL, Bresciani CJ, Perez RO, Bresciani BH, Siqueira SA, et al. (2010) Are histological alterations observed in the gallbladder precancerous lesions? Clinics 65: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stancu M, Căruntu ID, Giuşcă S, Dobrescu G (2007) Hyperplasia, metaplasia, dysplasia and neoplasia lesions in chronic cholecystitis - a morphologic study. Rom J Morphol Embryol 48: 335–342. [PubMed] [Google Scholar]
- 35. Khan MR, Raza SA, Ahmad Z, Naeem S, Pervez S, et al. (2011) Gallbladder intestinal metaplasia in Pakistani patients with gallstones. Int J Surg 9: 482–485. [DOI] [PubMed] [Google Scholar]
- 36. Fernandes JE, Franco MI, Suzuki RK, Bromberg SH (2008) Intestinal metaplasia in gallbladders: prevalence study. Sao Paulo Med J 126: 220–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Misra V, Misra SP, Dwivedi M, Shouche Y, Dharne M, et al. (2007) Helicobacter pylori in areas of gastric metaplasia in the gallbladder and isolation of H. pylori DNA from gallstones. Pathology 39: 419–424. [DOI] [PubMed] [Google Scholar]
- 38. Chen DF, Hu L, Yi P, Liu WW, Fang DC, et al. (2007) H pylori are associated with chronic cholecystitis. World J Gastroenterol 13: 1119–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee JS, Kim HS, Hahm KB, Sohn MW, Yoo M, et al. (2007) Inhibitory effects of 7-carboxymethyloxy -3′,4′,5-trimethoxyflavone (DA-6034) on Helicobacter pylori-induced NF-kappa B activation and iNOS expression in AGS cells. Ann N Y Acad Sci 1095: 527–535. [DOI] [PubMed] [Google Scholar]
- 40. Cho SO, Lim JW, Kim KH, Kim H (2010) Involvement of Ras and AP-1 in Helicobacter pylori-induced expression of COX-2 and iNOS in gastric epithelial AGS cells. Dig Dis Sci 55: 988–996. [DOI] [PubMed] [Google Scholar]
- 41. Naito Y, Takagi T, Okada H, Nukigi Y, Uchiyama K, et al. (2008) Expression of inducible nitric oxide synthase and nitric oxide-modified proteins in Helicobacter pylori-associated atrophic gastric mucosa. J Gastroenterol Hepatol 23 Suppl 2S250–257. [DOI] [PubMed] [Google Scholar]
- 42. Boonyanugomol W, Chomvarin C, Baik SC, Song JY, Hahnvajanawong C, et al. (2011) Role of cagA-positive Helicobacter pylori on cell proliferation, apoptosis, and inflammation in biliary cells. Dig Dis Sci 56: 1682–1692. [DOI] [PubMed] [Google Scholar]