Abstract
Understanding the genetic architecture of any quantitative trait requires identifying the genes involved in its expression in different environmental conditions. This goal can be achieved by mutagenesis screens in genetically tractable model organisms such as Drosophila melanogaster. Temperature during ontogenesis is an important environmental factor affecting development and phenotypic variation in holometabolous insects. In spite of the importance of phenotypic plasticity and genotype by environment interaction (GEI) for fitness related traits, its genetic basis has remained elusive. In this context, we analyzed five different adult morphological traits (face width, head width, thorax length, wing size and wing shape) in 42 co-isogenic single P-element insertional lines of Drosophila melanogaster raised at 17°C and 25°C. Our analyses showed that all lines differed from the control for at least one trait in males or females at either temperature. However, no line showed those differences for all traits in both sexes and temperatures simultaneously. In this sense, the most pleiotropic candidate genes were CG34460, Lsd-2 and Spn. Our analyses also revealed extensive genetic variation for all the characters mostly indicated by strong GEIs. Further, our results indicate that GEIs were predominantly explained by changes in ranking order in all cases suggesting that a moderate number of genes are involved in the expression of each character at both temperatures. Most lines displayed a plastic response for at least one trait in either sex. In this regard, P-element insertions affecting plasticity of a large number of traits were associated to the candidate genes Btk29A, CG43340, Drak and jim. Further studies will help to elucidate the relevance of these genes on the morphogenesis of different body structures in natural populations of D. melanogaster.
Introduction
Understanding the genetic architecture of a quantitative trait requires identifying the genes implicated in its expression in different environmental conditions [1]–[3]. This goal can be achieved by mutagenesis screens in genetically tractable model organisms such as Drosophila melanogaster. In fact, quantitative genetic analysis of the effects of P-element mutations induced in an isogenic background [4], [5] is a reliable method for functional genomic analyses [6]–[11]. In this sense, we have previously identified candidate genes related to variation of different morphological traits using 191 P-element insertion lines raised at 25°C [12], [13]. In general, our previous results indicate that the genetic architecture of morphological traits involves a large fraction of the genome and is largely sex- and trait-specific [12], [13].
One of the most important environmental factors affecting body size in ectothermic animals is temperature [14]. Generally, insects grown at lower temperatures are bigger [15]. The effect of temperature on different morphological traits has been profoundly studied in Drosophila [16]–[25]. Several of such studies were performed using Drosophila’s wing as a model and, while some of them showed that thermal changes affected wing shape predominantly in the posterior compartment, most of them showed that wings elongated disproportionately as temperature decreased [26]–[33].
Most of the mentioned work has been done using isofemale lines raised at different temperatures or flies derived from natural populations distributed along geographic gradients (i.e., clines). Regarding morphological traits, only a recent work has addressed the effect of genetic and environmental manipulations in Drosophila’s wing [34]. In particular, these authors used heterozygous insertional mutations of 16 genes involved in the formation of the wing, raising flies at two developmental temperatures [34]. Their results showed that the phenotypic effects of mutations depended on developmental temperature [34].
The phenotypic response to a change in an environmental variable (i.e., phenotypic plasticity) may vary among genotypes which may be manifested as a genotype by environment interaction (GEI) [35]–[37]. Abundant experimental and theoretical work gives strong support to the idea that GEI may be involved in the maintenance of phenotypic plasticity, genetic variation and the evolution of fitness related traits [38]–[40]. In spite of the importance of phenotypic plasticity and GEI for fitness related traits in variable environments, its genetic basis has remained elusive. In this sense, for a given trait, it is necessary to identify the genes responding to changes in different environmental variables (i.e., plasticity genes) and to establish whether they are the same genes affecting trait expression in particular conditions [1], [3], [41].
In this article, we studied different morphological traits in 42 mutants that have been previously analyzed under a different thermal treatment [12], [13] in order to investigate the genetic basis of their phenotypic plasticity. In the light of the results obtained by Debat et al. [34], we expected the phenotypic effects to depend on temperature. Further, and according to our previous results [12], [13], we expected this dependence to be trait- and sex-specific. Simultaneously, the experimental design employed allowed us to perform a study of GEI for each character and sex to asses if it may be involved in the maintenance of phenotypic plasticity and genetic variation. Finally, it enabled us to identify candidate genes involved in plasticity in relation to the morphological traits studied.
Materials and Methods
Drosophila Stocks
We used 42 independent homozygous viable single p[GT1]-element insertion lines, constructed in a coisogenic Canton-S background [4], to identify candidate genes affecting different morphological traits at 17°C. These lines, which are publicly available at the Bloomington Drosophila Stock Center (Berkeley Drosophila Genome Project website. Available: http://www.fruitfly.org. Accessed 2013 July 3), represent a random sample of the 191 lines that have been used to study the same traits at 25°C [12], [13]. All lines screened at 17°C were simultaneously assessed with one of the batches reared at 25°C. In fact, 20 out of the 42 lines were raised at both temperatures (17° and 25°C) at the same time while the remaining 22 lines were raised at 25°C within the previous six months. To account for environmental variation in morphological traits between batches, a control strain (a co-isogenic P-element insertion free line with the same genetic background) was run in parallel with each batch.
Experimental Design
For each temperature, 300 pairs of sexually mature flies corresponding to each line were placed for 8 hours in separate oviposition chambers. Eggs were allowed to hatch and batches of 30 first-instar larvae were transferred to culture vials containing a standard cornmeal-agar-molasses medium (4 replicates per mutant line and 4–8 replicates for the control for each temperature). Larvae were raised at controlled temperature (4–8 replicates per line at 17±1°C and 4–8 replicates per line at 25±1°C) and 60–70% of humidity with a 12∶12 light:dark photoperiod until adult emergence. All adults emerged from each vial were preserved in a freezer at −20°C until quantification of morphological traits was performed.
Morphological Traits
Five flies of each sex were randomly chosen from each vial (20 males and 20 females per line) and the head, the thorax and the wings of each individual were removed and placed on a slide conserving their relative position. Separate images for 3-D structures (i.e., head and thorax) and flat structures (i.e., wings) were captured using a binocular microscope (10×) and an attached digital camera connected to a computer. Different morphological traits were estimated using tpsDig [42], exactly as in previous works in which they were studied at 25°C [12], [13]. Face width (FW, the smallest distance between the eyes), head width (HW, the distance between the right and the left side of the head capsule), and thorax length (TL, the distance between the anterior margin of the thorax and the tip of the scutellum) were estimated directly from the pictures (Figure S1). For the estimation of wing size (WSi) and wing shape (WSh), 11 landmarks were digitized on the ventral face of the left wing of each fly (Figure S2). A single WSi measure (centroid size) was calculated by taking the square root of the sum of squared distances between each landmark and the centroid (the point whose coordinates are the means of the x and y coordinates of all landmarks) of each wing. Wing shape was analyzed using the Procrustes generalized least squares procedure which eliminates variation in size, position and orientation for the examination of differences in the position of landmarks [43]. This procedure generated 22 procrustes coordinates which were subsequently transformed into 18 new shape variables (relative warps, RWs) [44] using tpsRelw [45]. These variables constitute a multivariate approximation to the study of wing shape. Additionally, we estimated the Procrustes distance which represents an univariate approximation to the study of this trait.
Statistical and Morphometrical Analyses
Identification of significant lines and associated candidate genes
Dunnett contrasts were performed in males and females separately, to detect significant differences (induced by p[GT1] insertions) between the mutants and the control in body size related traits. For WSh, one MANOVA was conducted with the RWs scores corresponding to each line and the respective control in males and females separately. Those lines that exhibited significant differences relative to the control were considered as lines bearing an insertion in a candidate gene. In order to identify these genes, we conducted homology searches of sequences flanking the P-element insertion against release 5 of the published D. melanogaster genomic sequence (http://flybase.bio.indiana.edu/). Only the gene nearest to the insertion was selected as candidate gene, except in those cases in which two genes were closer than 1 Kb to the P-element insertion site and neither disruption occurred in the gene.
Genetic correlation analyses between body size related traits
In order to include all data (estimations of different morphological traits corresponding to flies of each sex raised at 17°C and 25°C) in the same analyses, the values corresponding to each variable were transformed by subtracting from each individual value the mean value of the respective control line and dividing it by the same value.
A correlation analysis was performed between each pair of size traits within each sex and with each variable between sexes. The mean of the values corresponding to each line was used in all correlation analyses. In addition, we carried out Mantel tests to compare correlation matrices between temperatures for each sex separately using Infostat [46]. Since the diagonals of both matrices must be filled with zeros, we constructed each matrix with the respective 1 - r (correlation coefficient) values.
Visualization of wing shape deformations
Differences in wing shape between each mutant line and the respective control were estimated performing a thin-plate spline analysis using the respective Procrustes coordinates [44]. Particularly, shape changes of each line respect to the control were shown as vectors diagrams obtained with tpsSplin [47].
Quantitative genetic analyses
Transformed values (see above) corresponding to each univariate variable (FW, HW, TL, WSi and WSh estimated by the Procrustes distance) were analyzed using an ANOVA with line (random) and temperature (fixed) as factors in each sex separately. This procedure allowed us to estimate variance components corresponding to random sources of variation and, consequently, the percentage of total variance explained by the genetic factors (line and line by temperature interaction).
A significant GEI can arise as a consequence of differences in among-lines variance in separate environments (change in scale) and/or deviations from unity of the cross-environment genetic correlation (change in ranking order). The contribution of the two sources of variation to GEI was analyzed by means of the equation derived by Robertson [48]: VGEI = [(σE1 − σE2)2+2×σE1×σE2× (1 − rGEI)]/2; where VGEI is the GEI variance component, rGEI is the cross-environment genetic correlation and, σE1 and σE2 are the square roots of the among-lines variance components at 17°C and 25°C (which were obtained after performing ANOVAs for each temperature separately). The first term corresponds to differences in among-lines variance whereas the second to deviations from the perfect correlation across environments (rGEI <1). The cross-environment genetic correlation is the genetic correlation of measurements of the same trait in different environments and here reflects the degree in which the same genes control trait expression across temperatures. rGEI was estimated for each trait as: rGEI = COVE1 E2/σE1 σE2; where COVE1E2 is the covariance of lines means for each sex measured in different temperatures.
Identification of candidate plasticity genes
Finally, we studied the phenotypic effect of thermal change in each mutant, sex and trait separately with a fixed ANOVA. Those lines that exhibited significant differences between temperatures were considered as lines bearing a mutation in a candidate gene involved in the plastic response of the respective character to temperature variation. A homogeneity test was conducted to compare the number of candidate lines associated to the plasticity of each trait between sexes.
In general, statistical analyses were performed using the STATISTICA software package [49]. Bonferroni correction for multiple tests was applied whenever results from multiple tests were combined in one final conclusion.
Results
Phenotypic Effects of Mutants at Different Temperatures and Associated Candidate Genes
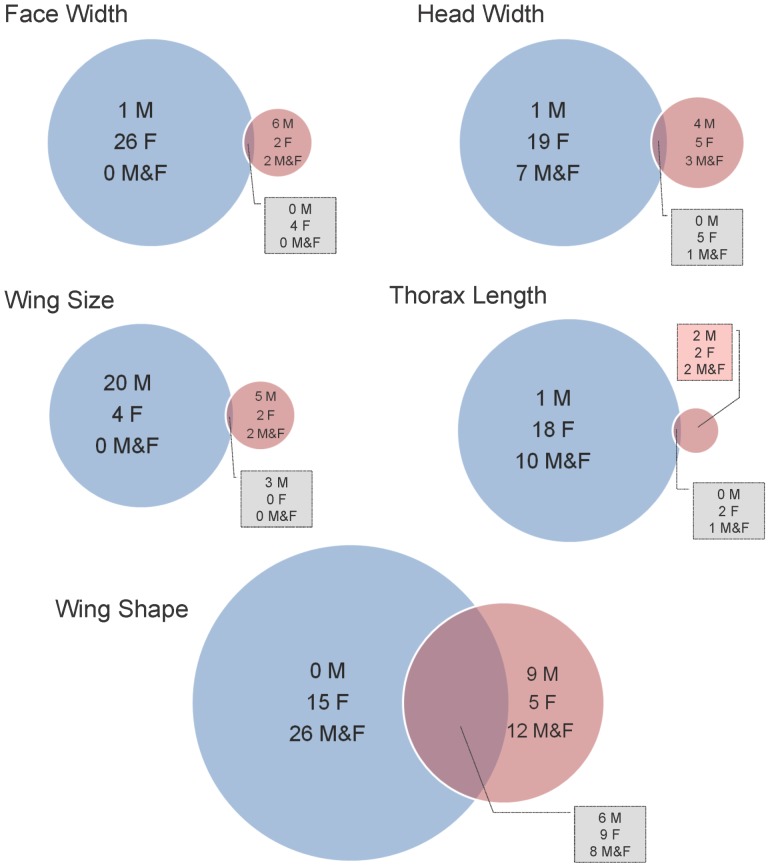
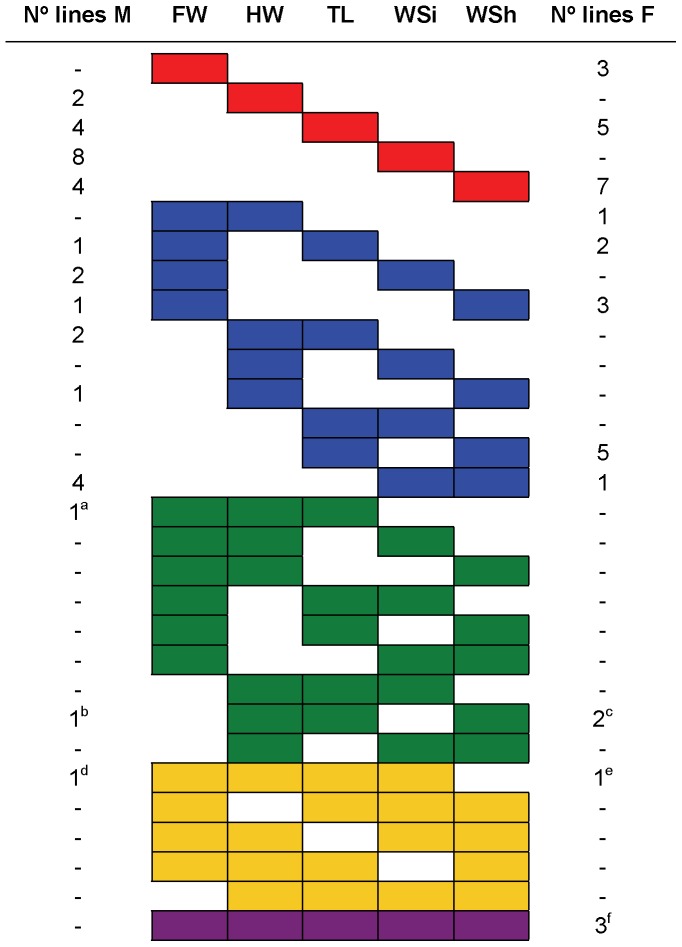
The analyses revealed that all 42 lines differed from the control for at least one trait in either sex at 17°C (Table S1). Further, most of the measurements were smaller in the mutants than in the control at this developmental temperature (Table S1). Interestingly, the number of lines showing significant results at 17°C was larger than the number associated to the higher temperature (25°C, Figure 1).
Figure 1. Lines showing significant effects for each trait, temperature and sex.
Number of mutant lines showing significant differences with respect to the control for each morphological trait. We enumerated the lines showing significant effects at 17°C (blue circles), at 25°C (pink circles) and at both temperatures simultaneously (intersections between blue and pink circles). Also, for each one of the mentioned categories, we enumerated the lines showing significant effects only in males (M), only in females (F) and in both sexes simultanously (M&F).
We identified 46 candidate genes (39 protein coding genes) based on insertion of the P-element within 5 Kb from the transcription initiation site (Table S1). Nine of them (CG13333, CG13334, CG14591, CG17574, clt, Imp, rut, SCAP and scyl; Table S1) affected morphological traits only at 17°C. However, no line showed differences with respect to the control for all traits in both sexes and temperatures simultaneously. In this sense, the most pleiotropic lines were BG01011 (Spn), BG02462 (CG34460) and BG02830 (Lsd-2), which showed at least 13 significant differences considering all traits in both sexes and temperatures (Table S1).
As the effects of mutations on size traits were apparently different between 17°C and 25°C (Figure 1, Tables S1 and S2), we compared the genetic correlation matrices corresponding to each sex between temperatures. Results derived from Mantel tests were not significant for both sexes. These results indicate that relationships among size traits showed different patterns at 17°C and 25°C suggesting that their genetic basis differ between temperatures. Finally, results regarding WSh showed that most lines differed from the control at least in one sex and temperature (Figure 1, Table S1). However, only eight of them showed those differences in both sexes and temperatures simultaneously (Figure 1, Table S1). Even though some of these lines (BG00373, BG01014, BG02462 and BG02830) showed larger wing shape deformations than the others (BG01028, BG01354, BG01548 and BG02690), the largest changes were observed at 17°C in females (Figures S3 and S4). In general, most of the mutations displaced the posterior cross-vein (Figure S2) causing an enlargement of the distal portion of the wing at expense of the proximal part of the organ. In some cases the anterior cross-vein (Figure S2) also moved reducing the proximal region even more. Finally, some mutations produced slight veins displacements causing an anterior-posterior expansion. To conclude, it is important to note that the lines that showed larger differences in wing shape with respect to the control also exhibited significant results for other body size related traits in males and/or females grown at 17°C and/or 25°C (i.e., these mutations caused more important pleiotropic effects than the others; Table S1).
Analyses of GEI and Identification of Candidate Plasticity Genes
In general, transformed values in flies raised at 25°C were larger than in individuals grown at 17°C (Table 1). Considering the transformation implemented, these results indicate that the mutant lines grew less than the control at 17°C while the opposite occurred at 25°C. The line factor was significant in a few cases but the line by temperature interaction was highly significant for all traits in both sexes implying a significant contribution of the genetic factors to total phenotypic variation (Table 1).
Table 1. Principal results of the ANOVAs for morphological traits at 17°C and 25°C in each sex separately.
| FaceWidth | HeadWidth | ThoraxLength | WingSize | WingShape | |
| Females | |||||
| L | 1.25 | 1.36 | 1.15 | 1.82* (10)§ | 1.49 |
| T | 63.72*** | 17.99*** | 40.54*** | 1.07 | 159.97*** |
| L×T | 4.40*** (18) | 5.24*** (21) | 6.86*** (27) | 4.88*** (18) | 3.13*** (12) |
| Males | |||||
| L | 1.56 | 1.94* (12) | 2.56** (14) | 1.71* (11)§ | 2.88*** (12) |
| T | 11.70** | 4.13* § | 42.28*** | 30.62*** † | 114.78*** |
| L×T | 5.03*** (19) | 6.09*** (22) | 3.92*** (14) | 7.65*** (27) | 2.38*** (7) |
The F value and its significance as well as the percentage of total phenotypic variance explained by each random source of variation (between parentheses) are shown. L: Line, T: Temperature.
p<0.05,
p<0.01,
p<0.001.
Not significant after Bonferroni correction for multiple tests (PB = 0.025). The mean of the transformed values at 25°C was larger than the mean at 17°C in all cases except for †.
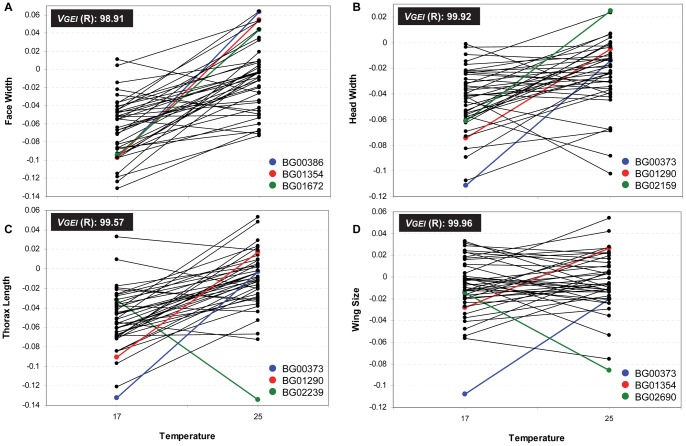
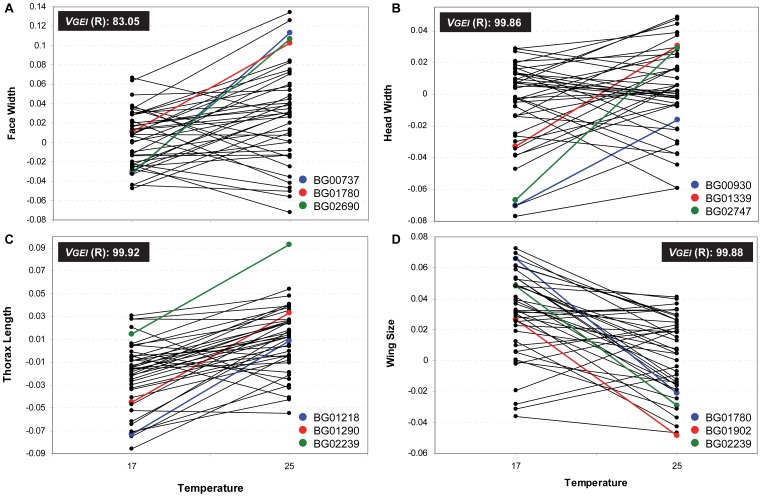
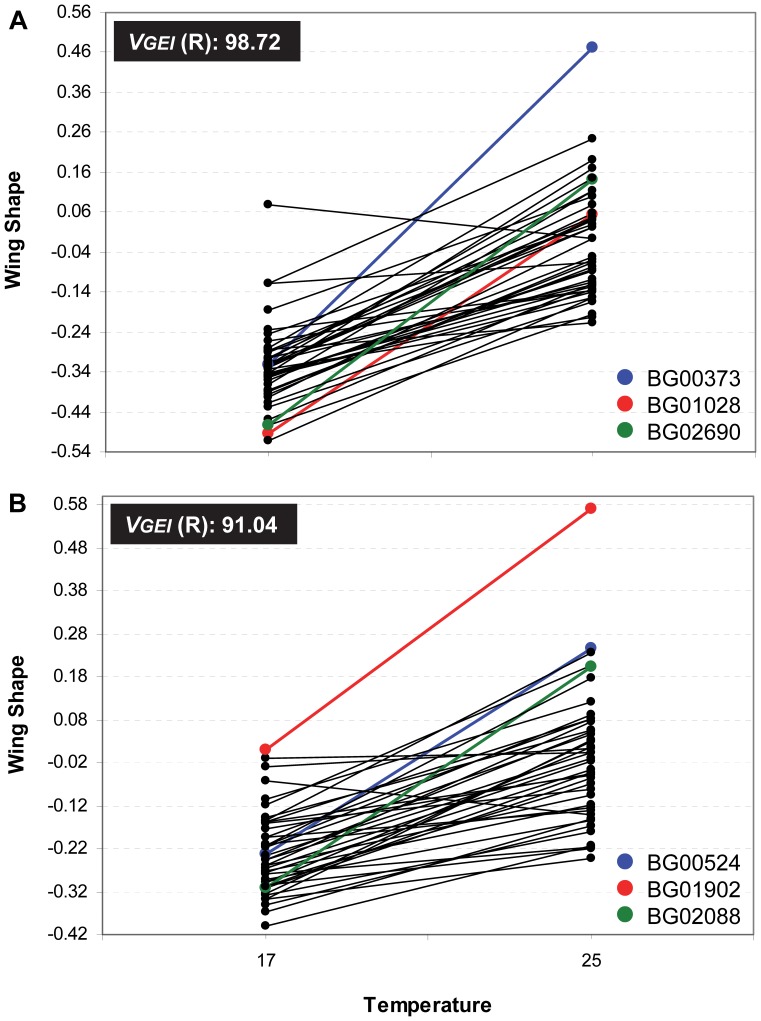
We observed low genetic correlations (rGxE) between measurements of each trait in different environments (17°C and 25°C; Table S3). Further, the change in ranking order (i.e., the deviation from perfect correlation between temperatures) explained a percentage of GEI’s variation much larger than the change in scale (i.e., the difference in variance among lines between temperatures; Table S3). This pattern may be easily seen in Figures 2, 3 and 4 which show the mean of the transformed values corresponding to each line at both temperatures for every morphological trait in females and males.
Figure 2. Genotype by environment interaction for each body size related trait in females.
Line by temperature interaction in females for A) Face Width, B) Head Width, C) Thorax Length and D) Wing Size. Each dot corresponds to the average of the transformed values. VGEI (R) is the percentage of GEI’s variance explained by changes in ranking order. The three lines showing the largest significant differences between temperatures are coloured.
Figure 3. Genotype by environment interaction for each body size related trait in males.
Line by temperature interaction in males for A) Face Width, B) Head Width, C) Thorax Length and D) Wing Size. Each dot corresponds to the average of the transformed values. VGEI (R) is the percentage of GEI’s variance explained by changes in ranking order. The three lines showing the largest significant differences between temperatures are coloured.
Figure 4. Genotype by environment interaction for wing shape in both sexes.
Line by temperature interaction for wing shape (estimated by the procrustes distance) in A) females and B) males. Each dot corresponds to the average of the transformed values. VGEI (R) is the percentage of GEI’s variance explained by changes in ranking order. The three lines showing the largest significant differences between temperatures are coloured.
We identified 39 lines displaying a plastic response for at least one morphological trait in either sex (Table S4). According to the homogeneity test, the number of lines showing plasticity for each trait differed between sexes (χ2 4 = 11.5, p = 0.02) suggesting that the genetic basis underlying plasticity for these characters is sexually dimorphic.
The line that showed a plastic response for more morphological traits in both sexes is BG02159 (Figure 5, Table S4), in which the P-element insertion ocurred in Drak (Table S1). In females, BG00373 (jim) as well as BG01354 (CG43340) displayed a plastic response for all five morphological traits whereas BG01290 (Btk29A) showed differences between temperatures in four of them (Figure 5, Table S4). In contrast, males did not present any line displaying a plastic response for all traits although BG02239 showed differences between thermal treatments in four of them (Figure 5, Table S4). It is important to note that these lines are among those that showed the largest significant differences between temperatures (Figures 2, 3 and 4; Table S4).
Figure 5. Lines showing phenotypic plasticity for different traits in each sex.
Number of lines showing phenotypic plasticity for one, two, three, four or all five traits in females (F) and males (M). FW: Face Width, HW: Head Width, TL: Thorax Length, WSi: Wing Size and WSh: Wing Shape. The candidate genes (or the name of the lines when the respective genes could not be identified) affecting plasticity of a large number of traits in each sex are: a Drak; b rut; c BG00930, CG13333, CG13334; d BG02239; e Btk29A; f CG43340, Drak, jim.
Discussion
Genetic Basis of Morphological Traits at Different Temperatures
Our results suggest that many genes are related to variation of morphological traits at 17°C and that their effect depends extensively on the sex, according to observations made at 25°C [12], [13]. However, analyses comparing results obtained at different temperatures indicate that the relationships among body size related traits as well as their genetic basis differ between 17°C and 25°C. These results suggest that relations among growth rates of different imaginal discs may change with temperature of development in flies. Therefore, our results are in line with those obtained by Shingleton et al. [50], who have found that different environmental factors, including temperature, might regulate body and trait size as well as the relationship between them (i.e., allometry) through different developmental mechanisms. Further, our results indicate that the genetic basis involved in this process might differ between distinct environmental states (i.e., 17°C and 25°C).
Regarding wing shape, our results are in agreement with previous studies [51], [52] in that most mutations analyzed determined small but significant phenotypic effects and that morphometric variation show, simultaneously, a high degree of integration across the wing as well as certain local specificity [53]–[59]. In particular, each mutation might affect wing shape more globally or locally depending on the position of the gene in the genetic hierarchy that determine the development of the organ and the capacity of the system to buffer the effects in different instances of that process [13]. In this sense, previous studies suggest that developmental buffering is a trait-specific process [60], [61] that may be altered by environmental as well as genetic factors [34]. Finally, there seems to be a great resemblance between our results and those obtained by Debat et al. [34]. Furthermore, other authors have found similar wing shape changes when studying Drosophila populations located along different latitudinal gradients [26], [57], [62]–[63]. Therefore, these observations suggest that the same pathways may be involved in phenotypic variation observed in nature and in experimental populations, as it was observed for developmental time [64].
Candidate Genes for Plasticity of Morphological Traits
The analyses revealed that the control line tended to grow significantly more than the mutant lines at 17°C, while the control flies reared at 25°C showed sizes lying in the middle of the range of genotypic effects. This is interesting, especially because the control line was raised together with 20 lines at both temperatures at the same time, giving support to the idea that the insertions effect could be responsible for the plastic response. However, it should be noticed that the control line has been kept for 10 years as an isogenic stock and, during this period of time, it might have accumulated novel mutations affecting its thermal plastic response. In spite of this, our observations are remarkably similar to those made by Debat et al. using a different set of mutant lines raised at 18°C and 28°C [34]. Beyond this strange pattern, flies were generally larger at lower temperatures, according to multiple findings [16], [17], [19], [21], [25]. Particularly, size increment showed by the control line was comparable to that observed in isofemales lines of D. melanogaster studied in the mentioned works. Finally, as WSh analysis presented analogous results, we stress that both, the traits and the effects of the mutations, exhibited differences between temperatures (i.e., plasticity was observed at two levels of analysis).
Analyses of GEI showed that the genetic correlation between the values of each trait measured at different temperatures was relatively low indicating that a moderate number of genes are associated to variation of each character at both temperatures. The type of GEI observed indicate that, if natural populations present analogous genetic variants, selection might favor different genotypes in environments with distinct temperatures contributing to maintain intra-specific genetic variability [38], [65]. Finally, this might help to explain the clinal patterns repeatedly observed in Drosophila for different traits, including the morphological ones [66]–[75]. This would occur through the process of genetic assimilation [76] which seems to be an appealing mechanism for body size evolution based on abundant evidence of GEIs for traits in general and body size in particular [77].
Results of the line-specific analyses suggest that most genes involved in the genetic basis of morphological traits cause disparate phenotypic effects at different temperatures (i.e., plastic effects) although these effects are highly trait- and sex-specific. This seems to contradict results derived from a recent study of variation in genome-wide gene expression of an outbred D. melanogaster population under 20 different environments [78] which revealed that only ∼15% of the transcriptome is environmentally plastic. However, discrepancies might be explained by differences in the experimental designs implemented (i.e., plasticity might be due to differences in post-translational modifications between environments instead of transcriptional differences which can not be assesed by the methodology implemented in the mentioned work). Furthermore, the mentioned work analyzed the transcriptome in adults, whereas the developmental stages relevant for our study are the larval and pupal stages, when the growth of imaginal discs occurs. Setting these differences aside, the cited work grouped transcripts showing phenotipic plasticity into two categories: Class I, in which transcripts are genetically variable and Class II, in which transcripts have low genetic variance and show sexually dimorphic expression [78]. In particular, only four of our candidate genes showed significant results in the mentioned investigation: on one side CG13333, which was assigned to Class II in males and, on the other side, Hsp 27, CG17574 and Lsd-2, which were nominated as Class I genes in both sexes [78]. Furthermore, concerning these genes, Lsd-2 was the only one which was associated with developmental time and starvation resistance [78]. This is interesting because we have found that this gene displayed highly pleiotropic effects on morphological traits closely associated to body size, a character also related to the mentioned life history traits [79]–[84]. It is worth pointing out that this gene did not show outstanding results with respect to phenotypic plasticity, a pattern which was also shown by Spn and CG34460, the other most pleiotropic genes for morphological traits. These observations are somewhat in line with preliminary results that showed that Lsd-2 present low nucleotidic polymorphism as well as little genetic differentiation among natural populations of D. melanogaster (Carreira, unpublished data). Furthermore, an exploratory analysis of the sequence of this gene in different Drosophila species indicates that its evolution has occurred according to the postulates of the neutral theory (Carreira, unpublished data) which is in agreement with recent works that did not find evidence of positive selection for Lsd-2 in D. melanogaster [41] and related species [85].
On the contrary, the few genes that affected plasticity of a large number of traits did not affect many characters in each temperature. This is the case of Btk29A, CG43340 and jim in females; the unidentified gene affected by the P-element insertion in BG02239 in males and Drak in both sexes. It would be interesting to investigate if these genes also present important nucleotidic variability in natural populations and thus are less conserved than the others. However, some genes might present high levels of genetic variability for a trait and, concurrently, low levels of plasticity for it which might indicate that the character is subjected to environmental canalization. Studies on the molecular genetics of populations of these candidate genes might help to clarify this and other issues regarding evolution of morphological traits.
Supporting Information
Head and thorax of a fly and related morphological traits. Picture showing the positioning of 3-D body structures on a slide and related measurements taken with tpsDig.
(TIF)
Ventral view of left wing and positioning of landmarks. LV: longitudinal vein, HCV: humeral cross vein, ACV: anterior cross-vein, PCV: posterior cross-vein.
(TIF)
Lines showing wing shape deformations at both temperatures in females. Lines showing significant wing shape deformations with respect to the control line in females raised at 17°C (in blue) and 25°C (in red). The gene affected by the P-element insertion is shown between parentheses for each line. Arrows indicate the magnitude and direction of landmarks displacement with respect to the corresponding control line. Arrows size has been magnified three times to show more clearly wing shape changes.
(TIF)
Lines showing wing shape deformations at both temperatures in males. Lines showing significant wing shape deformations with respect to the control line in males raised at 17°C (in blue) and 25°C (in red). The gene affected by the P-element insertion is shown between parentheses for each line. Arrows indicate the magnitude and direction of landmarks displacement with respect to the corresponding control line. Arrows size has been magnified three times to show more clearly wing shape changes.
(TIF)
Lines in which the P -element insertion affected one or more morphological traits in either sex at 17°C. The candidate gene, the site of the mutation and its morphological effect are given for each line.
(PDF)
Principal results of genetic correlation analyses between body size related traits at 17°C and 25°C. Correlation coefficients corresponding to the analyses within each sex and between sexes for each variable are shown.
(PDF)
Principal results of ANOVAs for morphological traits in each temperature and sex separately and analyses of GEI. The F values and the genetic variance components derived from the ANOVAs are shown. Also, the correlation coefficients and the components explaining the interaction between temperatures are given.
(PDF)
Principal results of the ANOVAs performed to study the change of the phenotypic effect of the P -element insertion with thermal change in each line, sex and trait separately. The unsigned difference between the means of the transformed values at 17°C and 25°C for each morphological trait are given.
(PDF)
Acknowledgments
The authors wish to thank T.F.C. Mackay (North Carolina State University, Raleigh, N.C., U.S.A.) for providing p[GT1] insertion lines. They also wish to thank two anonymous reviewers as well as the academic editors T. Flatt and A.W. Shingleton for comments and suggestions that greatly helped to improve previous versions of this paper.
Funding Statement
This work was supported by the University of Buenos Aires (grants UBACyT 20020100300039 and UBACyT 20020100100482) and the Consejo Nacional de Investigaciones Científicas y Técnicas (grant PIP 11220080102042). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schlichting CD, Smith H (2002) Phenotypic plasticity: linking molecular mechanisms with evolutionary outcomes. Evol Ecol 16: 189–211. [Google Scholar]
- 2. Mackay TFC (2004) The genetic architecture of quantitative traits: lessons from Drosophila. . Curr Opin Genet & Dev 14: 253–257. [DOI] [PubMed] [Google Scholar]
- 3. Auld JR, Agrawal AA, Relyea RA (2009) Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc R Soc B 277: 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lukacsovich T, Asztalos Z, Awano W, Baba K, Kondo S, et al. (2001) Dual-tagging gene trap of novel genes in Drosophila melanogaster. . Genetics 157: 727–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellen HJ, O’Kane CJ, Wilson C, Grossniklaus U, Pearson RK, et al. (2004) The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics 167: 761–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anholt RRH, Lyman RL, Mackay TFC (1996) Effects of single P-element insertions on olfactory behavior in Drosophila melanogaster . Genetics 143: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lyman RF, Lawrence F, Nuzhdin SV, Mackay TFC (1996) Effects of single P-element insertions on bristle number and viability in Drosophila melanogaster . Genetics 143: 277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norga KK, Gurganus MC, Dilda CL, Yamamoto A, Lyman RF, et al. (2003) Quantitative analysis of bristle number in Drosophila mutants identifies genes involved in neural development. Curr. Biol. 13: 1388–1397. [DOI] [PubMed] [Google Scholar]
- 9. Harbison ST, Yamamoto AH, Fanara JJ, Norga KK, Mackay TFC (2004) Quantitative trait loci affecting starvation resistance in Drosophila melanogaster . Genetics 166: 1807–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sambandan D, Yamamoto A, Fanara JJ, Mackay TFC, Anholt RR (2006) Dynamic genetic interactions determine odor-guided behavior in Drosophila melanogaster . Genetics 174: 1349–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mensch J, Lavagnino N, Carreira VP, Massaldi A, Hasson E, et al. (2008) Identifying candidate genes affecting developmental time in Drosophila melanogaster: pervasive pleiotropy and gene-by-environment interaction. BMC Dev. Biol. 8: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carreira VP, Mensch J, Fanara JJ (2009) Body size in Drosophila: genetic architecture, allometries and sexual dimorphism. Heredity 102: 246–256. [DOI] [PubMed] [Google Scholar]
- 13. Carreira VP, Soto IM, Mensch J, Fanara JJ (2011) Genetic basis of wing morphogenesis in Drosophila: sexual dimorphism and non-allometric effects of shape variation. BMC Dev Biol 11: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atkinson D, Sibly RM (1997) Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol Evol 12: 235–239. [DOI] [PubMed] [Google Scholar]
- 15. Atkinson D (1994) Temperature and organism size: a biological law for ectotherms? Adv Ecol Res 25: 1–58. [Google Scholar]
- 16. Scheiner SM, Caplan RL, Lyman RF (1991) The genetics of phenotypic plasticity. III. Genetic correlations and fluctuating aymmetries. J Evol Biol 4: 51–68. [Google Scholar]
- 17. Gebhardt MD, Stearns SC (1993) Phenotypic plasticity for life history traits in Drosophila melanogaster. I. Effect on phenotypic and environmental correlations. J Evol Biol 6: 1–16. [Google Scholar]
- 18. Barker JSF, Krebs RA (1995) Genetic variation and plasticity of thorax length and wing length in Drosophila aldrichi and D. buzzatii. . J Evol Biol 8: 689–709. [Google Scholar]
- 19. Noach EJK, de Jong G, Scharloo W (1996) Phenotypic plasticity in morphological traits in two populations of Drosophila melanogaster. . J Evol Biol 9: 831–844. [Google Scholar]
- 20. Morin JP, Moreteau B, Pétavy G, Parkash R, David JR (1997) Reaction norms of morphological traits in Drosophila: adaptive shape changes in a stenotherm circumtropical species? Evolution 51: 1140–1148. [DOI] [PubMed] [Google Scholar]
- 21. Nunney L, Cheung W (1997) The effect of temperature on body size and fecundity in female Drosophila melanogaster: evidence for adaptative plasticity. Evolution 51: 1529–1535. [DOI] [PubMed] [Google Scholar]
- 22. Loeschcke V, Bungaard J, Barker JSF (1999) Reaction norms across and genetic parameters at different temperatures for thorax and wing size traits in Drosophila aldrichi and D. buzzatii. . J Evol Biol 12: 605–623. [Google Scholar]
- 23. Wolf LL, Starmer WT, Polak M, Barker JSF (2000) Genetic architecture of a wing size measure in Drosophila hibisci from two populations in eastern Australia. Heredity 85: 521–529. [DOI] [PubMed] [Google Scholar]
- 24. Gilchrist GW, Huey RB (2004) Plastic and genetic variation in wing loading as a function of temperature within and among parallel clines in Drosophila subobscura . Integr Comp Biol 44: 461–470. [DOI] [PubMed] [Google Scholar]
- 25. David JR, Legout H, Moreteau B (2006) Phenotypic plasticity of body size in a temperate population of Drosophila melanogaster: when the temperature-size rule does not apply. J Genet 85: 9–23. [DOI] [PubMed] [Google Scholar]
- 26. Azevedo BR, James AC, McCabe J, Partridge L (1998) Latitudinal variation of wing:thorax size ratio and wing-aspect ratio in Drosophila melanogaster. . Evolution 52: 1353–1362. [DOI] [PubMed] [Google Scholar]
- 27. Moreteau B, Imasheva AG, Morin JP, David JR (1998) Wing shape and developmental temperature in two Drosophila sibling species: different wing regions exhibit different norms of reaction. Russ J Genet 34: 183–192. [Google Scholar]
- 28. Bitner-Mathé BC, Klaczko LB (1999) Plasticity of Drosophila melanogaster wing morphology: effects of sex, temperature and density. Genetica 105: 203–210. [DOI] [PubMed] [Google Scholar]
- 29. Birdsall K, Zimmerman E, Teeter K, Gibson G (2000) Genetic variation for the positioning of wing veins in Drosophila melanogaster. . Evol Dev 2(1): 16–24. [DOI] [PubMed] [Google Scholar]
- 30. Imasheva AG, Moreteau B, David JR (2000) Growth temperature and genetic variability of wing dimensions in Drosophila: opposite trends in two sibling species. Genet Res (Camb) 76: 237–247. [DOI] [PubMed] [Google Scholar]
- 31. Debat V, Bégin M, Legout H, David JR (2003) Allometric and non allometric components of Drosophila wing shape respond differently to developmental temperature. Evolution 57: 2773–2784. [DOI] [PubMed] [Google Scholar]
- 32. Matta BP, Bitner-Mathé BC (2004) Genetic architecture of wing morphology in Drosophila simulans and an analysis of temperature effects on genetic parameter estimates. Heredity 93: 330–341. [DOI] [PubMed] [Google Scholar]
- 33. Kjærsgaard A, Faurby S, Andersen DH, Pertoldi C, David JR, et al. (2007) Effects of temperature and maternal and grandmaternal age on wing shape in parthenogenetic Drosophila mercatorum. . J Therm Biol 32: 59–65. [Google Scholar]
- 34. Debat V, Debelle A, Dworkin I (2009) Plasticity, canalization, and developmental stability of the Drosophila wing: joint effects of mutations and developmental temperature. Evolution 63(11): 2864–2876. [DOI] [PubMed] [Google Scholar]
- 35. Via S, Lande R (1985) Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39: 505–522. [DOI] [PubMed] [Google Scholar]
- 36. Falconer DS (1990) Selection in different environments: effects on environmental sensitivity (reaction norm) and on mean performance. Genet Res 56: 57–70. [Google Scholar]
- 37.Lynch M, Walsh B (1998) Genetics and Analysis of Quantitative Traits. Sunderland: Sinauer Associates. 980 p.
- 38. Ungerer MC, Halldorsdottir SS, Purugganan MD, Mackay TFC (2003) Genotype-environment interactions at quantitative trait loci affecting inflorescence development in Arabidopsis thaliana. . Genetics 165: 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carreira VP, Soto IM, Hasson E, Fanara JJ (2006) Patterns of variation in wing morphology in the cactophilic Drosophila buzzatii and its sibling D. koepferae. . J Evol Biol 19: 1275–1282. [DOI] [PubMed] [Google Scholar]
- 40. Fanara JJ, Folguera G, Fernández Iriarte P, Mensch J, Hasson E (2006) Genotype by environment interactions in viability and developmental time in populations of cactophilic Drosophila. . J Evol Biol 19: 900–903. [DOI] [PubMed] [Google Scholar]
- 41. Mackay TFC, Richards S, Stone EA, Barbadilla A, Ayroles JF, et al. (2012) The Drosophila melanogaster Genetic Reference Panel. Nature 482: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohlf FJ (2001) tpsDig©. Version 1.31. Free software available on the SB Morphometrics website: http://life.bio.sunysb.edu/morph/. Accessed 2013 July 3. Department of Ecology and Evolution, State University of New York, Stony Brook, New York.
- 43. Rohlf FJ, Slice D (1990) Extensions of the procrustes method for the superimposition of landmarks. Syst Zool 39: 40–59. [Google Scholar]
- 44.Bookstein FL (1991) Morphometric tools for landmark data: geometry and biology. Cambridge: Cambridge University Press. 455 p.
- 45.Rohlf FJ (2003) tpsRelw©. Version 1.31. Free software available on the SB Morphometrics website: http://life.bio.sunysb.edu/morph/. Accessed 2013 July 3. Department of Ecology and Evolution, State University of New York, Stony Brook, New York.
- 46.Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, et al. (2011) InfoStat version 2011. Software available on the Infostat website: http://www.infostat.com.ar. Accessed 2013 July 3. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina.
- 47.Rohlf FJ (2004) tpsSplin©. Version 1.18. Free software available on the SB Morphometrics website: http://life.bio.sunysb.edu/morph/. Accessed 2013 July 3. Department of Ecology and Evolution, State University of New York, Stony Brook, New York.
- 48. Robertson A (1959) Experimental design in the evaluation of genetic parameters. Biometrics 15(2): 219–226. [Google Scholar]
- 49.StatSoft Inc (2004) STATISTICA. Version 7. Software available on the StatSoft website: http://www.statsoft.com. Accessed 2013 July 3.
- 50. Shingleton AW, Estep CM, Driscoll MV, Dworkin I (2009) Many ways to be small: different environmental regulators of size generate distinct scaling relationships in Drosophila melanogaster. . Proc R Soc B 276: 2625–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weber K, Johnson N, Champlin D, Patty A (2005) Many P-element insertions affect wing shape in Drosophila melanogaster. . Genetics 169: 1461–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dworkin I, Gibson G (2006) Epidermal growth factor receptor and transforming growth factor-beta signaling contributes to variation for wing shape in Drosophila melanogaster. . Genetics 173: 1417–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cowley DE, Atchley WR (1990) Development and quantitative genetics of correlation structure among body parts of Drosophila melanogaster . Am Nat 135: 242–268. [Google Scholar]
- 54. Cavicchi S, Pezzoli C, Giorgi G (1981) Correlation between characters as related to developmental pattern in Drosophila. Genetica 56: 189–195. [Google Scholar]
- 55. Weber KE (1990) Selection on wing allometry in Drosophila melanogaster . Genetics 126: 975–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weber KE (1992) How small are the smallest selectable domains of forms? Genetics 130: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Imasheva AG, Bubli OA, Lazebny OE, Zhivotovsky LA (1995) Geographic differentiation in wing shape in Drosophila melanogaster. . Genetica 96: 303–306. [DOI] [PubMed] [Google Scholar]
- 58. Guerra D, Pezzoli MC, Georgi G, Garoia F, Cavicchi S (1997) Developmental constraints in the Drosophila wing. Heredity 79: 564–571. [DOI] [PubMed] [Google Scholar]
- 59. Klingenberg CP, Zaklan SD (2000) Morphological integration between developmental compartments in the Drosophila wing. Evolution 54(4): 1273–1285. [DOI] [PubMed] [Google Scholar]
- 60. Breuker CJ, Patterson JS, Klingenberg CP (2006) A single basis for developmental buffering of Drosophila wing shape. PLoS One 1: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dreyer AP, Shingleton AW (2011) The effect of genetic and environmental variation on genital size in male Drosophila: canalized but developmentally unstable. PLoS One 6 (12): e28278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gilchrist AS, Azevedo RBR, Partridge L, O’Higgins PP (2000) Adaptation and constraint in the evolution of Drosophila melanogaster wing shape. Evol Dev 2: 114–124. [DOI] [PubMed] [Google Scholar]
- 63. Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L (2000) Rapid evolution of a geographic cline in size in an introduced fly. Science 287: 308–309. [DOI] [PubMed] [Google Scholar]
- 64. Mensch J, Carreira V, Lavagnino N, Goenaga J, Folguera G, et al. (2010) Stage-specific effects of candidate heterochronic genes on variation in developmental time along an altitudinal cline of Drosophila melanogaster . PLoS One 5 (6): e11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thomas RH, Barker JSF (1993) Quantitative genetic analysis of the body size and shape of Drosophila buzzatii . Theor Appl Genet 85: 598–608. [DOI] [PubMed] [Google Scholar]
- 66. Stalker HD, Carson HL (1948) An altitudinal transect of Drosophila robusta Sturtevant. Evolution 2: 295–305. [DOI] [PubMed] [Google Scholar]
- 67. Misra RR, Reeve ECR (1964) Clines in body dimensions in populations of Drosophila subobscura. . Genet Res 5: 240–256. [Google Scholar]
- 68. Sokoloff A (1966) Morphological variation in natural and experimental populations of Drosophila pseudoobscura and Drosophila persimilis. . Evolution 20: 47–71. [DOI] [PubMed] [Google Scholar]
- 69. Bitner-Mathe BC, Peixoto AA, Klaczko LB (1995) Morphological variation in a natural population of Drosophila mediopunctata: altitudinal cline, temporal changes and influence of chromosome inversions. Heredity 75: 54–61. [DOI] [PubMed] [Google Scholar]
- 70. Parkash R, Karan D, Kataria SK, Munjal AK (1999) Phenotypic variability of quantitative traits in Indian populations of Drosophila kikkawai. . J Zool Syst Evol Res 37: 13–17. [Google Scholar]
- 71. Hallas R, Schiffer M, Hoffmann AA (2002) Clinal variation in Drosophila serrata for stress resistance and body size. Genet Res 79 (2): 141–148. [DOI] [PubMed] [Google Scholar]
- 72. de Jong G, Bochdanovits Z (2003) Latitudinal clines in Drosophila melanogaster: body size, allozyme frequencies, inversion frequencies, and the insulin-signalling pathway. J Genet 82: 207–223. [DOI] [PubMed] [Google Scholar]
- 73. Hoffmann AA, Weeks AR (2007) Climatic selection on genes and traits after a 100 year-old invasion: a critical look at the temperate-tropical clines in Drosophila melanogaster from eastern Australia. Genetica 129: 133–147. [DOI] [PubMed] [Google Scholar]
- 74. Arthur AL, Weeks AR, Sgrò CM (2008) Investigating latitudinal clines for life history and stress resistance traits in Drosophila simulans from eastern Australia. J Evol Biol 21: 1470–1479. [DOI] [PubMed] [Google Scholar]
- 75. Folguera G, Ceballos S, Spezzi L, Fanara JJ, Hasson E (2008) Clinal variation in developmental time and viability and the response to thermal treatments in two species of Drosophila. Biol. J. Linn Soc. 95: 233–245. [Google Scholar]
- 76.West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford: Oxford Univ. Press. 916 p.
- 77.Shingleton AW (2011) The regulation and evolution of growth and body size. In: Flatt T, Heyland A, editors. Mechanisms of Life History Evolution. Oxford: Oxford Univ. Press. 43–55.
- 78. Zhou S, Campbell TG, Stone EA, Mackay TFC, Anholt RRH (2012) Phenotypic plasticity of the Drosophila transcriptome. PLoS Genet 8 (3): e1002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmidt-Nielsen K (1984) Scaling: why is animal size so important? Cambridge: Cambridge University Press. 256 p. [Google Scholar]
- 80.Roff DA (1992) The Evolution of life histories. New York: Chapman and Hall. 548 p. [Google Scholar]
- 81.Stearns SC (1992) The evolution of Life Histories. Oxford: Oxford Univ. Press. 264 p. [Google Scholar]
- 82. Partridge L, Fowler K (1993) Responses and correlated responses to artificial selection on thorax length in Drosophila melanogaster . Evolution 47: 213–226. [DOI] [PubMed] [Google Scholar]
- 83. Betrán E, Santos M, Ruiz A (1998) Antagonistic pleiotropic effect of second-chromosome inversions on body size and early life-history traits in Drosophila buzzatii . Evolution 52: 144–154. [DOI] [PubMed] [Google Scholar]
- 84. Fernández Iriarte PE, Hasson E (2000) The role of antagonistic pleiotropy and different cactus host in the maintenance of the inversion polymorphism in Drosophila buzzatii . Evolution 54: 743–748. [DOI] [PubMed] [Google Scholar]
- 85. Serra F, Arbiza L, Dopazo J, Dopazo H (2011) Natural selection on functional modules, a genome-wide analysis. PLoS Comput Biol 7 (3): e1001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Head and thorax of a fly and related morphological traits. Picture showing the positioning of 3-D body structures on a slide and related measurements taken with tpsDig.
(TIF)
Ventral view of left wing and positioning of landmarks. LV: longitudinal vein, HCV: humeral cross vein, ACV: anterior cross-vein, PCV: posterior cross-vein.
(TIF)
Lines showing wing shape deformations at both temperatures in females. Lines showing significant wing shape deformations with respect to the control line in females raised at 17°C (in blue) and 25°C (in red). The gene affected by the P-element insertion is shown between parentheses for each line. Arrows indicate the magnitude and direction of landmarks displacement with respect to the corresponding control line. Arrows size has been magnified three times to show more clearly wing shape changes.
(TIF)
Lines showing wing shape deformations at both temperatures in males. Lines showing significant wing shape deformations with respect to the control line in males raised at 17°C (in blue) and 25°C (in red). The gene affected by the P-element insertion is shown between parentheses for each line. Arrows indicate the magnitude and direction of landmarks displacement with respect to the corresponding control line. Arrows size has been magnified three times to show more clearly wing shape changes.
(TIF)
Lines in which the P -element insertion affected one or more morphological traits in either sex at 17°C. The candidate gene, the site of the mutation and its morphological effect are given for each line.
(PDF)
Principal results of genetic correlation analyses between body size related traits at 17°C and 25°C. Correlation coefficients corresponding to the analyses within each sex and between sexes for each variable are shown.
(PDF)
Principal results of ANOVAs for morphological traits in each temperature and sex separately and analyses of GEI. The F values and the genetic variance components derived from the ANOVAs are shown. Also, the correlation coefficients and the components explaining the interaction between temperatures are given.
(PDF)
Principal results of the ANOVAs performed to study the change of the phenotypic effect of the P -element insertion with thermal change in each line, sex and trait separately. The unsigned difference between the means of the transformed values at 17°C and 25°C for each morphological trait are given.
(PDF)