Abstract
Background
Anal dilation during tumour excision with transanal endoscopic micro-surgery (TEM) has caused concerns regarding postoperative anal function. We sought to determine whether TEM affects anorectal function and quality of life.
Methods
All patients undergoing TEM between March 2007 and December 2008 were considered for inclusion. We excluded patients who were treated with subsequent radical resection, unavailable for interview or deceased. Patients were interviewed by phone to measure the preoperative and postoperative function using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 (EORTC QLQ-C30) and Core 38 (CR38) instruments, the Fecal Incontinence Severity Index (FISI) and the Fecal Incontinence Quality of Life (FIQL) questionnaires. Statistical analysis involved the Wilcoxon signed rank test and Spearman rank correlation coefficient.
Results
Forty patients received TEM; 30 of them met all inclusion criteria and agreed to participate. The median age was 70 (42–93) years, and median follow-up time between the interview and the operation was 365 (55–712) days. Tumours excised included 19 adenomas, 8 carcinomas and 3 carcinoid tumours. The median distance from the tumour to the anal verge was 6.5 (2–13) cm. Median length of stay was 1 (0–12) day. For most aspects of quality of life, there were no detectable differences after surgery. The EORTC QLQ-C30 showed a significant improvement in diarrhea (27.8 v. 10, p = 0.002). The FIQL scores improved with surgery (3.59 v. 3.85, p = 0.020). There was no difference in pre-versus postoperative FISI scores (6.7 v. 6.3, p = 0.93).
Conclusion
Despite a large operating rectoscope, TEM improves quality of life related to fecal incontinence and does not have a negative impact on fecal continence.
Abstract
Contexte
La dilatation de l’anus au cours de l’excision d’une tumeur par micro-chirurgie endoscopique transanale (MET) soulève des préoccupations quant à la fonction anale postopératoire. Nous avons cherché à déterminer si la MET a un effet sur la fonction anorectale et la qualité de vie.
Méthodes
Nous avons envisagé d’inclure tous les patients ayant subi une MET entre mars 2007 et décembre 2008. Nous avons exclu les patients qui ont été traités par résection radicale subséquente, qui n’étaient pas disponibles pour une entrevue ou qui étaient décédés. Nous avons interviewé les patients par téléphone pour mesurer la fonction préopératoire et postopératoire au moyen du Questionnaire sur la qualité de vie — Base 30 de l’Organisation européenne de recherche sur le traitement du cancer (EORTC QLQ-C30) et Base 38 (CR38), l’Indice de sévérité de l’incontinence fécale (ISIF) et la qualité de vie liée à l’incontinence fécale (QVIF). L’analyse statistique a comporté le test de Wilcoxon pour observations appariées et le coefficient de corrélation de rang de Spearman.
Résultats
Sur les 40 patients qui ont subi une MET, 30 répondaient à tous les critères d’inclusion et ont consenti à participer. L’âge médian était de 70 (42–93) ans et le temps médian du suivi qui s’est écoulé entre l’entrevue et l’opération s’est établi à 365 (55–712) jours. Les tumeurs excisées comportaient 19 adénomes, 8 carcinomes et 3 tumeurs carcinoïdes. La distance moyenne entre la tumeur et la marge de l’anus était de 6,5 (2–13) cm. La durée médiane du séjour était de 1 (0–12) jour. Pour la plupart des aspects de la qualité de vie, il n’y avait pas de différence détectable après l’intervention chirurgicale. Le questionnaire EORTC QLQ-C30 a révélé une amélioration importante au niveau de la diarrhée (27,8 c. 10, p = 0,002). Les scores ISIF se sont améliorés après l’intervention chirurgicale (3,59 c. 3,85, p = 0,020). Il n’y avait pas de différence au niveau des scores ISIF préopératoires et postopératoires (6,7 c. 6,3, p = 0,93).
Conclusion
En dépit de la grosseur du rectoscope utilisé pendant l’intervention, la MET améliore la qualité de vie liée à l’incontinence fécale et n’a pas d’effet négatif sur la continence fécale.
Transanal endoscopic microsurgery (TEM) is a minimally invasive technique for local resection of rectal tumours and was first developed in the 1980s by Buess in Germany.1,2 The technique is safe and effective for removal of rectal adenomas and is an alternative to radical rectal resection in carefully selected patients with rectal cancer.3–5
Transanal endoscopic microsurgery involves prolonged anal dilation with insertion of a rectoscope 40 mm in diameter and insufflation of the rectum. Dilation of the anal canal can cause problems with postoperative anal function and continence.6 A key theoretical advantage of TEM is avoidance of postoperative functional impairment seen in patients after radical rectal resection.7 To date, few studies have evaluated long-term functional outcomes after TEM.
We sought to determine the effects of TEM on anorectal function and quality of life at a subspecialty colorectal surgery centre in Canada.
Methods
Patient selection
Between March 2007 and December 2008, all patients treated by 3 subspecialty colorectal surgeons using TEM at St. Paul’s Hospital in Vancouver, Canada, were considered for inclusion in this study. Patients were excluded if they did not consent to participate, if they were unable to complete the interview or if they were treated with subsequent radical resection. All patients provided informed consent, and the research ethics board of the University of British Columbia approved our study protocol.
Surgical approach
All patients were evaluated preoperatively with colonoscopy, tumour biopsy and, in cases of suspected cancer, endorectal ultrasound. Rigid rectoscopy was performed in most patients to confirm the height and location of the tumour. The TEM procedure was performed with patients in a lithotomy or prone position, depending on the location of the tumour. We used the Richard Wolf Medical Instrument Corporation TEM instrument system and the KARL STORTZ GmbH & Co. insufflator with pressures set to 15 mm Hg. Full thickness rectal wall excision was performed for all malignant lesions and selectively for adenomas. The method of closure of the rectal wall defect was left to the discretion of the surgeon.
Data collection
For quality monitoring purposes, perioperative data, including patient demographics, preoperative diagnostic testing details, surgery details, pathology and postoperative complications, are prospectively collected and maintained in the St. Paul’s Hospital TEM Database.
All patients were interviewed in January and February of 2009 by telephone using standardized and validated questionnaires to determine patient satisfaction with the operation, quality of life and functional results before and up to 2 years after TEM. Each patient completed the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 (EORTC QLQ-C30),8 Core 38 (EORTC QLQ-CR38)9 and the Fecal Incontinence Severity Index (FISI).10 All patients with fecal incontinence except for 2 patients with unchanged incontinence for flatus completed the Fecal Incontinence Quality of Life Index (FIQL).11 For scoring the FISI we used patient self-reported ratings, which better reflect the degree of incontinence experienced by the patient.10,12 We asked patients about their satisfaction with the procedure and their preference regarding TEM.
Statistical analysis
We performed the Wilcoxon signed rank test and Spearman rank correlation coefficient. We compared pre- and postoperative questionnaire scores, with each patient serving as their own control. We tested for correlations between patient characteristics, operative details and quality of life and function. We considered results to be significant at p < 0.05.
Results
Between March 2007 and December 2008, 40 patients received TEM, 30 of whom were eligible for the study and provided informed consent. Ten patients were excluded: 3 patients died during follow-up from non–cancer related causes, 4 patients had subsequent radical resection, 1 patient could not complete the surveys owing to comorbid health issues, and 2 patients declined to participate.
Surgical indications and operative details
Patients who received TEM were representative of patients treated with transanal excision at our centre (Table 1). The median age of the included patients was 70 (interquartile range [IQR] 62.5–80) years, and the male:female ratio was 16:14. Patients were treated for villous adenoma (n = 19), adenocarcinoma (n = 8) and carcinoid tumour (n = 3). In the patients treated for adenocarcinoma, the pathological stage was pT1 in 3 patients, pT2 in 4 patients and pT3 in 1 patient. The patients with pT2 and pT3 tumours were high-risk patients not eligible for radical resection or refused radical resection. One of them was a young, high-risk patient who underwent neoadjuvant radiotherapy. The 3 patients with carcinoid tumours received preoperative diagnoses by colonoscopy, and the TEM was used for a full-thickness resection of the base of the carcinoid.
Table 1.
Patient, tumour and surgical characteristics
| Characteristic | No.* |
|---|---|
| Patients | |
| Male:female | 16:14 |
| Median age, mean (SD) yr | 69.8 (12.5) |
| Diagnosis | |
| Adenoma | 19 |
| Adenocarcinoma | 8 |
| T1 | 3 |
| T2 | 4 |
| T3 | 1 |
| Carcinoid | 3 |
| Tumour description | |
| Tumour distance, mean (SD) cm | 6.5 (3.0) |
| Median tumour size, mean (SD) cm | 3.4 (1.6) |
| Surgery | |
| Median duration of surgery, mean (SD) min | 80 (33) |
| Full thickness excision | 24 |
| Follow up, mean (SD) d | 338 (183) |
| Conversion to conventional transanal excision | 3 |
| Hospital stay, mean (SD) d | 1.8 (2.5) |
| Closure of defect | 16 |
| Complications | 6 |
| Intra-abdominal perforation | 3 |
| Bleeding | 2 |
| TIA | 1 |
| Residual disease | |
| R0 | 26 |
| R1 | 3 |
| R2 | 1 |
SD = standard deviation; TIA = transient ischemic attack.
Unless indicated otherwise.
The mean distance of the tumour from the anal verge was 6.5 (range 2–13) cm. The median time interval between the operation and the interview was 1 year (range 2 mo to 2 yr). The median length of stay in hospital was 1 (0–12) day, and the median duration of surgery was 80 (29–169) minutes.
Perioperative complications
Six patients (20%) experienced complications. Three patients had postoperative peritonitis due to intra-abdominal perforation diagnosed by computed tomography, which showed small amounts of free air in the abdominal cavity. All 3 patients were successfully treated with antibiotics and bowel rest. Two patients had postoperative bleeding, 1 of whom required blood transfusion. Neither patient required reoperation. One patient had a transient ischemic attack characterized by muscle weakness and sensory loss in the right arm. The patient recovered to full function and was discharged on the first postoperative day.
Quality of life
There were no significant differences in pre- and postoperative scores on most subscales of the EORTC QLQ-C30 and CR38 (Tables 2 and 3). However, the subscale regarding occurrence of diarrhea improved significantly after TEM (27.8 v. 10, p = 0.002). This was not associated with a decrease in gastrointestinal problems, as measured with the EORTC QLQ-CR38.
Table 2.
EORTC QLQ-C30 scores of patients who underwent transanal endoscopic microsurgery
| Scale | Time; mean (SD) | p value | |
|---|---|---|---|
| Preoperative | Postoperative | ||
| Function* | |||
| Physical | 90.0 (16.3) | 90.7 (15.7) | 0.48 |
| Role | 92.8 (22.2) | 97.2 (10.8) | 0.50 |
| Emotional | 75.9 (25.1) | 80.0 (23.4) | 0.32 |
| Cognitive | 92.8 (18.9) | 93.3 (12.1) | 0.77 |
| Social | 91.7 (20.9) | 96 (10.4) | 0.25 |
| Global health status | 73.9 (22.0) | 78.6 (17.7) | 0.10 |
| Symptom† | |||
| Fatigue | 5.9 (11.6) | 7.8 (12.4) | 0.69 |
| Nausea/vomiting | 6.1 (20.2) | 4.4 (19.0) | 0.25 |
| Pain | 10.1 (24.1) | 10.0 (20.8) | > 0.99 |
| Dyspnoea | 10.1 (23.7) | 13.3 (24.1) | 0.50 |
| Sleep disturbance | 76.7 (27.9) | 74.4 (27.2) | 0.75 |
| Appetite loss | 95.4 (11.7) | 92.2 (16.8) | 0.50 |
| Constipation | 91.1 (17.4) | 86.7 (22.5) | 0.34 |
| Diarrhea | 72.2 (31.2) | 90.0 (15.5) | 0.002 |
| Financial worries | 93.3 (22.1) | 95.6 (19.0) | > 0.99 |
EORTC QLQ-C30 = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30;8 SD = standard deviation.
High score reflects better function.
Low score indicates fewer symptoms.
Table 3.
EORTC QLQ-C38 scores of patients who underwent transanal endoscopic microsurgery
| Measure | Time; mean (SD) | p value | |
|---|---|---|---|
| Preoperative | Postoperative | ||
| Micturition problems | 91.9 (13.7) | 85.6 (20.6) | 0.12 |
| Gastrointestinal problems | 92.0 (14.0) | 91.7 (12.8) | 0.85 |
| Weight loss | 88.9 (23.7) | 93.3 (18.4) | 0.51 |
| Body image | 99.6 (2.0) | 97.8 (7.4) | 0.25 |
| Defecation problems | 76.1 (18.5) | 81.5 (14.8) | 0.20 |
| Chemotherapy side effects | 93.0 (15.0) | 93.0 (9.9) | > 0.99 |
| Sexual function | 80.0 (26.0) | 78.7 (25.9) | > 0.99 |
| Sexual enjoyment | 66.7 (40.8) | 69.2 (41.9) | > 0.99 |
| Male sexual problems, n = 10 | 81.7 (20.0) | 81.7 (20.0) | — |
| Female sexual problems, n = 2 | 83.3 (23.6) | 83.3 (23.6) | — |
| Future perspective | 82.2 (27.3) | 87.8 (20.5) | 0.24 |
EORTC QLQ-C38 = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Core 38;9 SD = standard deviation.
Anorectal function
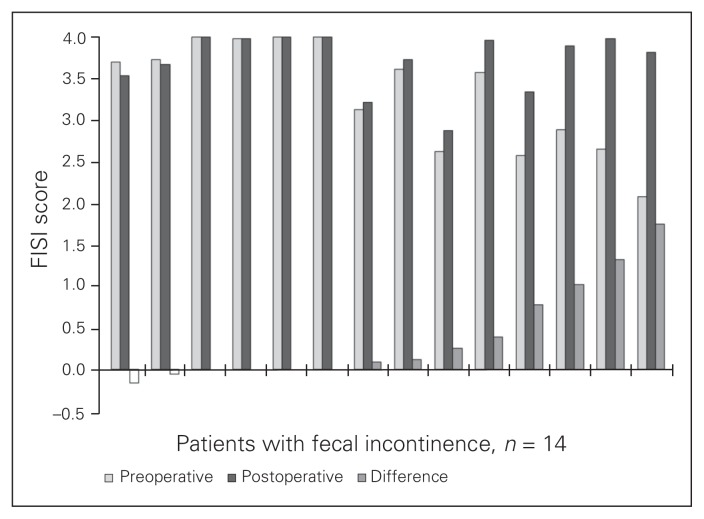
The TEM procedure did not influence the FISI score (6.7 vs. 6.3, p = 0.92). After TEM, 5 of 30 (17%) patients experienced improvement in fecal continence (as demonstrated by a reduced FISI score), 7 of 30 (23%) patients had decreased fecal continence, and 18 of 30 (60%) patients had no change in FISI scores (Fig. 1). Tumour height, tumour size and duration of surgery were not associated with FISI outcomes.
Fig. 1.
Fecal Incontinence Severity Index10 (FISI) scores.
Fourteen patients with fecal incontinence completed the FIQL questionnaire (higher score means better quality of life). Eight of 14 (57%) patients showed improvement, 1 patient had a slightly lowered FIQL score and the remaining 5 patients reported no differences. The FIQL scores (Table 4) were improved after surgery (3.59 vs. 3.85, p = 0.020). The subscales of coping (2.95 vs. 3.60, p = 0.008), depression (3.21 vs. 3.61, p = 0.047) and embarrassment (3.48 vs. 3.74, p = 0.031) showed a significant improvement with surgery.
Table 4.
FIQL scores of the 14 patients with preoperative incontinence who underwent transanal endoscopic microsurgery
| Measure | Time; mean (SD) | p value | |
|---|---|---|---|
| Preoperative | Postoperative | ||
| Lifestyle | 3.64 (0.54) | 3.89 (0.28) | 0.12 |
| Coping | 2.95 (1.02) | 3.60 (0.52) | 0.008 |
| Depression | 3.21 (0.80) | 3.61 (0.48) | 0.047 |
| Embarrassment | 3.48 (0.47) | 3.74 (0.42) | 0.031 |
| Total | 3.32 (0.65) | 3.71 (0.35) | 0.20 |
FIQL = Fecal Incontinence Quality of Life Index;11 SD = standard deviation.
Changes in function and quality of life were not related to complications, duration of surgery, distance from the anal verge or to whether the rectal wound was sutured or left open.
In addition to the standardized questionnaires, we asked all patients to indicate their satisfaction with the procedure (Table 5). Overall, most patients were content with the procedure. The 2 patients who were unsatisfied had a postoperative bleed (n = 1) and slow recovery with prolonged hospital stay for reasons not related to the TEM procedure itself (n = 1).
Table 5.
Satisfaction of patients who underwent transanal endoscopic microsurgery with the procedure
| Question | Yes/very | Probably would/a little | No opinion | Probably not/not | No/very unsatisfied |
|---|---|---|---|---|---|
| Would you recommend this operation to someone else with the same diagnosis? | 27 | 2 | 1 | 0 | 0 |
| Would you have made the same decision to undergo this surgery again? | 24 | 4 | 1 | 0 | 1 |
| How satisfied are you with this operation? | 27 | 1 | 0 | 1 | 1 |
Discussion
Transanal endoscopic microsurgery is a safe, effective and minimally invasive alternative to radical resection for rectal adenomas. The role of TEM in the treatment of adenocarcinoma is controversial.13–17 Generally, TEM has been used for adenomas not amenable to endoscopic or transanal excision, for T1N0 rectal carcinomas in patients who accept a higher risk of local recurrence to avoid radical rectal resection, and for palliative resection for more advanced lesions in medically unfit patients.
We investigated the functional and quality of life outcomes of TEM. We found that despite anal dilation required for insertion of the operating rectoscope, TEM actually improved fecal incontinence quality of life. There was no negative impact on overall quality of life and fecal incontinence, and patient satisfaction was high.
We observed significant improvements in quality of life related to fecal continence in our patients with preoperative fecal incontinence. A reason for this may be the alleviation of diarrhea related to rectal adenomas and cancers. The excision of a mucous-producing tumour that causes preoperative diarrhea can improve continence. While there were no significant changes in the FISI scores, quality of life measures may be more sensitive to subtle changes in continence.18
The FISI scores did not show significant changes after surgery in our patients. Of note, 7 patients had a higher FISI score postoperatively than preoperatively. In 3 patients with pre-existing fecal incontinence, there was a minor, nonsignificant worsening of overall continence reflected by the FISI score. Four patients had new onset of fecal incontinence after surgery; 2 of them were postoperatively treated with radiotherapy, which may have contributed to incontinence. In an earlier publication, we reported that radiotherapy has an independent negative influence on fecal incontinence in patients treated with low anterior resection.19 A third patient in the present study had follow-up of only 2 months and was still recovering from surgery; the last episode of incontinence was 3 weeks before the interview, and it is possible that her incontinence will improve in subsequent evaluation. The remaining patient had a small increase in FISI score. Despite these patients’ increases in FISI scores, we did not observe a negative effect on quality of life or satisfaction in any of them.
We did not find a correlation between the duration of surgery, tumour distance from the anal verge or tumour size and fecal incontinence outcomes. Doornebosch and colleagues20 found a correlation between the tumour height and the FISI score. In their study, patients with a tumour in the distal 7 cm of the rectum had a significant improvement in FISI score (16 v. 5, p = 0.01). Dafnis and colleagues21 found that patients who experienced post-TEM incontinence had longer surgery durations (175 v. 117 min, p = 0.002). Similarly, Kennedy and colleagues22 described a correlation between duration of surgery and functional results.
To our knowledge, only 2 other studies have assessed the functional and quality of life outcomes of TEM. Cataldo and colleagues23 reported the outcomes of 41 patients who received TEM for villous adenoma and rectal cancer. All patients completed the FISI and FIQL questionnaires before and 6 weeks after surgery, and the number of bowel movements and urgency were recorded. There were no significant alterations in the measured parameters. The mean pre- and postoperative FISI scores were the same (2.4 v. 2.4). There was an improvement in 8 patients, worsening in 4 patients and no change in 37 patients. The FIQL scores were taken from all patients, but no significant changes were found. The authors attributed these outcomes to good preoperative function and short operative durations (mean 69 min).
Similarly, Doornebosch and colleagues20 performed TEM excision in 47 patients with preoperatively diagnosed villous adenoma. For assessment of continence and quality of life, the FISI, FIQL and the EuroQoL EQ-5D were administered preoperatively and at least 6 months postoperatively. The postoperative FISI scores were found to be significantly improved (10 v. 7, p = 0.01). There was an improvement in 24 (65%) patients. There was a significant improvement in the mean quality of life score (p = 0.02), but these changes did not correspond with the improvement of FISI scores. Also, significant improvements were found in the FIQL subscales for embarrassment (p = 0.03) and lifestyle (p = 0.05). The authors attributed the improvement in fecal continence to preoperative tumour symptoms.
Doornebosch and colleagues24 also compared TEM to total mesorectal excision. They demonstrated no significant difference between the 2 strategies of transanal excision in postoperative quality of life, as measured with the EORTC-QLQ C30 and EQ-VAS scales. However, patients who received TEM had less defecation problems, as measured by the EORTC-QLQ CR38. The postoperative EORTC scores in our series are slightly better than those reported by Doornebosch and colleagues.24 A possible explanation is that all their patients had a T1 adenocarcinoma.
Functional and quality of life outcomes of conventional transanal excision (TAE) have been investigated by Fenech and colleagues.18 This study used the FIQL and retrospectively measured preoperative continence with the Wexner continence scale. They found continence to be impaired after TAE; however, patients had a good quality of life.
Limitations
The major limitation of our study is the retrospective collection of preoperative quality of life and anorectal function scores. Anecdotally, patients were confident in their ability to recall their preoperative function. However, recall bias is certainly possible. We continue to monitor pre- and postoperative fecal continence in a prospective manner, and we do find it useful from a clinical perspective. The few patients with marginal deterioration of continence are counselled about Kegel exercises and physiotherapy options.
Our study is consistent with the current literature in that, despite a large-calibre operating rectoscope, TEM does not have a negative impact on early fecal incontinence and quality of life in most patients. However, this evidence is based on several small case series. A large randomized controlled trial is needed to provide better evidence about the appropriate use of TEM in patients with rectal cancer, and it would be important to include fecal incontinence and quality of life outcomes in such a trial.
Conclusion
Despite a large operating rectoscope, TEM does not have a negative impact on fecal incontinence and improves fecal incontinence–related quality of life. Transanal endoscopic microsurgery is a safe and well-tolerated alternative to radical resection for excision of rectal adenomas and favourable T1 adenocarcinomas in selected patients.
Footnotes
Presented as a poster at the Canadian Surgery Forum in Victoria, BC, Sept. 11–13, 2009.
Competing interests: None declared.
Contributors: All authors conceived and designed the study, analyzed and interpreted data, drafted and revised the article, and approved its publication. A. Planting aquired the data.
References
- 1.Buess G, Mentges B, Manncke K, et al. Technique and results of transanal endoscopic microsurgery in early rectal cancer. Am J Surg. 1992;163:63–9. doi: 10.1016/0002-9610(92)90254-o. [DOI] [PubMed] [Google Scholar]
- 2.Buess G, Theiss R, Gunther M, et al. Endoscopic surgery in the rectum. Endoscopy. 1985;17:31–5. doi: 10.1055/s-2007-1018451. [DOI] [PubMed] [Google Scholar]
- 3.Floyd ND, Saclarides TJ. Transanal endoscopic microsurgical resection of pT1 rectal tumors. Dis Colon Rectum. 2006;49:164–8. doi: 10.1007/s10350-005-0269-4. [DOI] [PubMed] [Google Scholar]
- 4.Lev-Chelouche D, Margel D, Goldman G, et al. Transanal endoscopic microsurgery: experience with 75 rectal neoplasms. Dis Colon Rectum. 2000;43:662–7. doi: 10.1007/BF02235583. [DOI] [PubMed] [Google Scholar]
- 5.Moore JS, Cataldo PA, Osler T, et al. Transanal endoscopic micro-surgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum. 2008;51:1026–30. doi: 10.1007/s10350-008-9337-x. [DOI] [PubMed] [Google Scholar]
- 6.Konsten J, Baeten CG. Hemorrhoidectomy vs. Lord’s method: 17-year follow-up of a prospective, randomized trial. Dis Colon Rectum. 2000;43:503–6. doi: 10.1007/BF02237194. [DOI] [PubMed] [Google Scholar]
- 7.Chatwin NA, Ribordy M, Givel JC. Clinical outcomes and quality of life after low anterior resection for rectal cancer. Eur J Surg. 2002;168:297–301. doi: 10.1002/ejs.49. [DOI] [PubMed] [Google Scholar]
- 8.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 9.Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer. 1999;35:238–47. doi: 10.1016/s0959-8049(98)00357-8. [DOI] [PubMed] [Google Scholar]
- 10.Rockwood TH, Church JM, Fleshman JW, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999;42:1525–32. doi: 10.1007/BF02236199. [DOI] [PubMed] [Google Scholar]
- 11.Rockwood TH, Church JM, Fleshman JW, et al. Fecal Incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000;43:9–16. doi: 10.1007/BF02237236. [DOI] [PubMed] [Google Scholar]
- 12.Rockwood TH. Incontinence severity and QOL scales for fecal incontinence. Gastroenterology. 2004;126(Suppl 1):S106–13. doi: 10.1053/j.gastro.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 13.Doornebosch PG, Ferenschild FTJ, de Wilt JHW, et al. Treatment of recurrence after transanal endoscopic microsurgery (TEM) for T1 rectal cancer. Dis Colon Rectum. 2010;53:1234–9. doi: 10.1007/DCR.0b013e3181e73f33. [DOI] [PubMed] [Google Scholar]
- 14.Bentrem DJ, Okabe S, Wong WD, et al. T1 adenocarcinoma of the rectum: Transanal excision or radical surgery? Ann Surg. 2005;242:472–7. doi: 10.1097/01.sla.0000183355.94322.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellgren A, Sirivongs P, Rothenberger DA, et al. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064–71. doi: 10.1007/BF02236551. [DOI] [PubMed] [Google Scholar]
- 16.Nascimbeni R, Nivatvongs S, Larson DR, et al. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum. 2004;47:1773–9. doi: 10.1007/s10350-004-0706-9. [DOI] [PubMed] [Google Scholar]
- 17.Suppiah A, Maslekar S, Alabi A, et al. Transanal endoscopic micro-surgery in early rectal cancer: Time for a trial? Colorectal Dis. 2008;10:314–27. doi: 10.1111/j.1463-1318.2007.01448.x. [DOI] [PubMed] [Google Scholar]
- 18.Fenech DS, Takahashi T, Liu M, et al. Function and quality of life after transanal excision of rectal polyps and cancers. Dis Colon Rectum. 2007;50:598–603. doi: 10.1007/s10350-006-0865-y. [DOI] [PubMed] [Google Scholar]
- 19.Murata A, Brown CJ, Raval M, et al. Impact of short-course radio-therapy and low anterior resection on quality of life and bowel function in primary rectal cancer. Am J Surg. 2008;195:611–5. doi: 10.1016/j.amjsurg.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Doornebosch PG, Gosselink MP, Neijenhuis PA, et al. Impact of transanal endoscopic microsurgery on functional outcome and quality of life. Int J Colorectal Dis. 2008;23:709–13. doi: 10.1007/s00384-008-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dafnis G, Pahlman L, Raab Y, et al. Transanal endoscopic micro-surgery: clinical and functional results. Colorectal Dis. 2004;6:336–42. doi: 10.1111/j.1463-1318.2004.00629.x. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy ML, Lubowski DZ, King DW. Transanal endoscopic microsurgery excision: Is anorectal function compromised? Dis Colon Rectum. 2002;45:601–4. doi: 10.1007/s10350-004-6252-7. [DOI] [PubMed] [Google Scholar]
- 23.Cataldo PA, O’Brien S, Osler T. Transanal endoscopic microsurgery: a prospective evaluation of functional results. Dis Colon Rectum. 2005;48:1366–71. doi: 10.1007/s10350-005-0031-y. [DOI] [PubMed] [Google Scholar]
- 24.Doornebosch PG, Tollenaar RA, Gosselink MP, et al. Quality of life after transanal endoscopic microsurgery and total mesorectal excision in early rectal cancer. Colorectal Dis. 2007;9:553–8. doi: 10.1111/j.1463-1318.2006.01186.x. [DOI] [PubMed] [Google Scholar]