Abstract
Background
The Eastern experience has reported the safety of laparoscopic assisted gastrectomy (LAG) for gastric cancer. Its use in Western countries is still debated owing to concerns about its oncologic equivalence to open gastrectomy (OG). We sought to review and compare their operative outcomes and oncologic specimen quality (number of harvested lymph nodes and surgical margins) for gastric adenocarcinoma (GA).
Methods
We reviewed the charts of all patients undergoing LAG (2007–2010) and OG (2000–2010) for GA in a single institution. Several surgeons performed the OGs, whereas 1 fellowship-trained laparoscopic surgeon performed LAGs. The primary outcome was quality of the surgical specimen, assessed by the number of harvested lymph nodes (LNs) and margin status. Secondary outcomes were perioperative events. Data were analyzed as intention to treat.
Results
We retrieved 60 cases (47 OGs, 13 LAGs). The conversion rate was 23%. Mean operative time was 115 minutes longer and blood loss was 425 mL less (both p < 0.001) for LAGs. A mean of 14.4 (standard deviation [SD] 9.8) and 11.2 (SD 8.2) LNs were harvested for OGs and LAGs, respectively (p = 0.29). Negative margins were achieved for all patients. Mean length of stay was similar (LAG: 19 d v. OG: 18.9 d; p = 0.91). The groups did not differ on major postoperative complications (12.7% v. 23.1%; p = 0.39) or operative mortality (2.1% v. 7.7%; p = 0.32).
Conclusion
Laparoscopic assisted gastrectomy is a challenging but safe and feasible procedure in experienced hands. It offers the same radical resection as OG regarding negative margins and LN retrieval. Long-term follow-up is warranted.
Abstract
Contexte
La gastrectomie assistée par laparoscopie (GAL) a été démontrée dans des études orientales comme sécuritaire dans le traitement de l’adénocarcinome gastrique (AG). En occident, l’équivalence oncologique de la GAL avec la gastrectomie ouverte (GO) demeure cependant controversée. Cette étude vise à comparer le devenir post-opératoire et la qualité des spécimens oncologiques de ces interventions, dans le traitement de l’AG.
Méthodes
Tous les dossiers de patients opérés par GAL (2007–2010) ou GO (2000–2010) pour AG dans un seul centre hospitalier ont été revus. Différents chirurgiens ont réalisé les GO. Toutes les GAL ont été réalisées par un seul chirurgien sur-spécialisé en chirurgie laparoscopique. Le critère d’évaluation principal était la qualité du spécimen chirurgical évaluée par le nombre de ganglions lymphatiques (GL) prélevés et le statut des marges de résection. Le critère d’évaluation secondaire était la morbidité post-opératoire. Les données ont été analysées en fonction de l’intention de traiter.
Résultats
Soixante cas ont été inclus (47 GO, 13 GAL). Le taux de conversion s’est établi à 23 %. Le temps opératoire moyen était plus long de 115 minutes, et les pertes sanguines moyennes moindres de 425 mL (p < 0,001 dans les 2 cas) pour la GAL que pour la GO. Une moyenne de 14,4 (écart-type [ET] 9,8) et 11,2 (ET 8,2) GL ont été prélevés respectivement pour la GO et la GAL (p = 0.29). On a obtenu des marges négatives pour tous les patients. La durée moyenne du séjour ne différait pas (GAL 19 j c. GO 18,9 j; p = 0,91). Les complications majeures (12,7 % c. 23,1 %; p = 0,39) et la mortalité post-opératoires (2,1 % c. 7,7 %; p = 0,32) étaient similaires.
Conclusion
La gastrectomie assistée par laparoscopie est une intervention complexe, mais peut être réalisée de manière sécuritaire en des mains expertes. Elle offre un spécimen oncologique comparable à la gastrectomie ouverte, en termes de lymphadénectomie et de marges de résection. Un suivi à long terme demeure nécessaire.
Since Dubois and colleagues1 reported the first experience with laparoscopic cholecystectomy, beginning in 1988, laparoscopic surgery has rapidly gained popularity. Many reports have highlighted the benefits of the laparoscopic approach over the open one for the treatment of a variety of abdominal conditions, mainly owing to a decrease in pain, blood loss, length of hospital stay and complications. However, its use for the treatment of malignant conditions has been more slowly accepted because of concerns about the possibility to achieve an equivalent oncologic procedure.
The only possible curative treatment for gastric adenocarcinoma is surgical resection. The first laparoscopic gastric resection was reported in 1993.2 Since then, minimally invasive gastric surgery has gained general acceptance for benign conditions3–5 and some malignant ones for which extended resection or lymphadenectomy is not required.3,6 Its role in the treatment of gastric adenocarcinoma is still debated. The extensive Asian experience, where gastric cancer is diagnosed at an early stage, has confirmed the adequacy of laparoscopic resection with regards to postoperative outcomes, technical feasibility of appropriate lymphadenectomy and oncologic outcomes.7–12 In Western countries, where gastric cancer is less prevalent and diagnosed at an advanced stage in 75% of patients,13 laparoscopic gastrectomy has not yet been accepted as a curative treatment. Therefore, conclusions regarding the treatment in large Asian trials cannot be generalized to Western patients. Western data comparing laparoscopic to open gastrectomy are challenged by small sample sizes and limited follow-up.14–17 To our knowledge, no study has compared both techniques specifically for malignant disease in the Canadian setting.
The aim of this study was to review and compare the quality of the oncological specimens obtained by laparoscopic assisted gastrectomy (LAG) versus open gastrectomy (OG) for gastric adenocarcinoma in a Canadian academic health centre. We hypothesized that the margin status and the number of harvested lymph nodes (LNs) obtained with LAG would be similar to that with OG.
Methods
We conducted a retrospective cohort study to compare LAG and OG.
Selection of participants
All gastric resections performed from January 2000 to November 2010 in a single academic institution (Centre Hospitalier Universitaire de Québec, Québec, Que.) were identified through the hospital administrative database. Laparoscopic assisted gastrectomy was introduced in 2007. Afterwards, both techniques were used, and the choice of approach was left to the surgeon’s discretion. We included all adult patients (age ≥ 18 yr) with confirmed gastric adenocarcinoma of all anatomic localizations submitted to either LAG or OG with a curative intent. We excluded patients treated for benign conditions or malignant diseases other than adenocarcinoma. Reviewers not involved in the treatment process obtained clinical and pathological data from the patients’ charts.
Outcome measures and data collection
The primary outcome was the oncological quality of the resected gastric specimen, as assessed by the number of harvested LNs and margin status. Secondary outcomes included operative duration, blood loss, length of stay and postoperative morbidity.
Demographic and diagnostic data included age, sex, body mass index (BMI), comorbidities and the use of a neoadjuvant or adjuvant treatment. Operative data included operative duration; estimated blood loss; gastrointestinal reconstruction; length of stay; conversion rates; and major complications, including cardiac (acute coronary syndrome, arrhythmia, congestive heart failure) and respiratory events (respiratory failure requiring reintubation), pancreatic fistula, intra-abdominal abscess, anastomotic leak and postoperative mortality (within 30 d). We reviewed pathology reports for final pathologic diagnosis, tumour size, number of retrieved LNs, distal and proximal margins status and pathologic TNM staging. Analysis of the lesion size, margins status and harvested LNs was performed only for curative intent resection; these elements were not taken into consideration during palliative surgery.
Technical information
Four surgeons performed the procedures; all of them performed OG, whereas only 1 of them, a fellowship-trained laparoscopic surgeon, performed all LAGs. All surgeries were booked with a curative intent. If curative resection was deemed impossible, palliative surgery was carried on based on the surgeon’s perioperative decision.
Open gastrectomies were performed through a midline laparotomy. The type of gastric resection performed was determined based on the tumour location, its size and the depth of invasion; it was performed in a standard fashion with a D1 lymphadenectomy, including LN stations 1–6, according to the Japanese Research Society for Gastric Cancer.18
For LAGs, patients under general anesthesia were placed in the supine split leg position with the surgeon standing between the legs. A 5-trocar technique was used to perform subtotal or total gastrectomy and D1 lymphadenectomy. Pneumoperitoneum at 15 mm Hg was established through an open umbilical approach. After careful exploration of the peritoneal cavity, gastric dissection began with mobilization of the greater curvature. The greater omentum was included in the specimen. Dissection was then continued toward the spleen with division of the short gastric vessels using LigaSure (Covidien) and toward the pylorus to include infrapyloric LNs and divide the right gastroepiploic artery and vein at the level of the pancreatic border. The dissection continued on the lesser curvature; the lesser omentum was opened, and the right gastric artery was exposed and divided between metallic clips. Dissection then continued toward the gastresophageal junction. The left gastric artery was exposed and divided at its root. All perigastric LNs were carefully dissected along the lesser curvature, the left gastric artery and the distal portion of the hepatic artery. The duodenum was then divided 3 cm distal to the pylorus, immediately above the gastroduodenal artery. Through a small 5 cm upper-midline incision protected by an Alexis retractor (Applied Medical), the proximal stomach was transected obliquely at a distance depending on the tumour location using multiple firings of a 3.5 mm Endo GIA stapler (Covidien). The resected specimen was then extracted, with the wound protected from tumour spillage by the Alexis retractor, and gastrointestinal reconstruction was completed. An anterior retrocolic Billroth II technique was used for subtotal gastrectomies. For total gastrectomies, Roux-en-Y was combined with the transoral anvil placement of a CEEA (Covidien) for the esophagojejunal anastomosis.
Pathologists specializing in gastrointestinal tumours analyzed all specimens. Tumours were classified according to the pathologic stage, following the guidelines of the AJCC Cancer Staging Manual, 6th edition.19 The number of LNs in the specimen was assessed by naked-eye dissection.
Statistical analysis
We performed our analyses using XLSTAT version 2010.6 (Addinsoft SARL) for Microsoft Excel. Continuous data are expressed as means with standard deviations (SD) or medians with interquartile ranges (IQR), as appropriate. Categorical data are reported as proportions. We compared OG and LAG groups using a 2-sample t test, Fisher exact test or Pearson χ2 test, as appropriate. Missing data were estimated by the mean for quantitative variables and by the mode for categorical variables. We applied the intention-to-treat principle to the data analysis, with the data of patients converted from LAG to OG analyzed in the LAG group. We considered results to be significant at p < 0.05.
Results
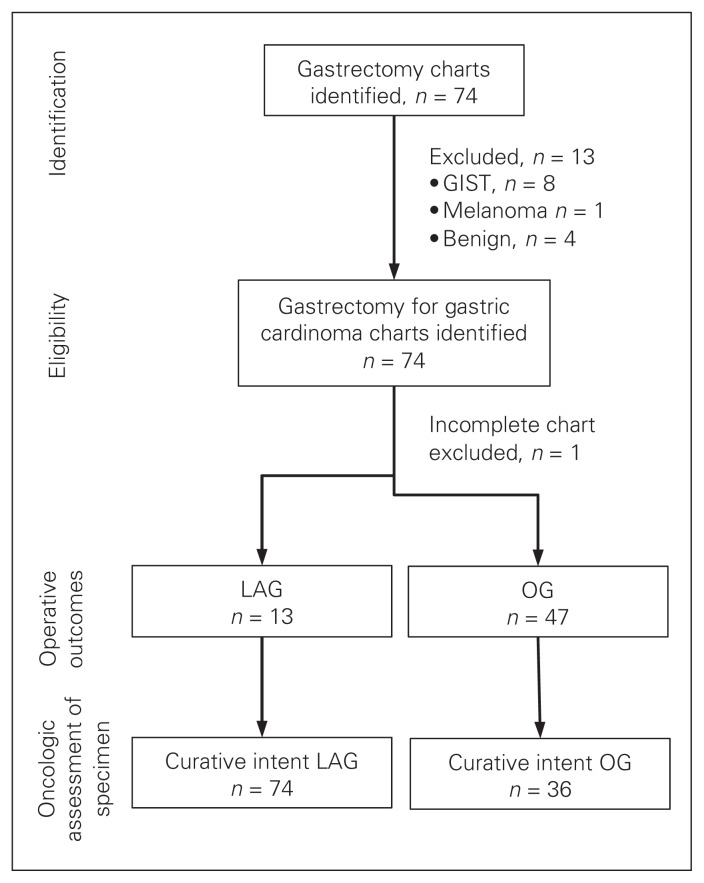
Seventy-four gastrectomies were performed in our institution during the study period (Fig. 1). Of these, 14 cases were excluded (8 gastrointestinal stromal tumour, 1 melanoma, 4 benign lesions, 1 incomplete chart rendering data collection impossible). Thus, 60 cases (13 LAGs and 47 OGs) were included in our analyses. Curative treatment was intended for 10 (76.9%) patients who underwent LAG and 36 (76.6%) who underwent OG; these patients were included in our analysis of the oncologic assessment of the specimens.
Fig. 1.
Patient identification and inclusion. GIST = gastrointestinal stromal tumour; LAG = laparoscopic assisted gastrectomy; OG = open gastrectomy.
Demographic data and preoperative characteristics for all patients are presented in Table 1. The age, sex and BMI distribution did not differ between the groups. More patients in the LAG than the OG group had cardiovascular disease (30.7 v. 0%) and diabetes (30.7 v. 6.4%, p = 0.034). There was no difference in the use of neoadjuvant chemotherapy.
Table 1.
Demographic and preoperative characteristics of patients undergoing gastrectomy
| Characteristic | Surgical approach; no. (%)* | p value | |
|---|---|---|---|
| OG | LAG | ||
| Patients, no. | 47 | 13 | |
| Age, mean (SD) yr | 74.6 (8.1) | 68.6 (12.4) | 0.12 |
| Sex, no. male:female | 28:19 | 5:8 | 0.18 |
| BMI, mean (SD) | 20.9 (9.7) | 25.2 (4.9) | 0.18 |
| Comorbidity | |||
| Hypertension | 24 (51.1) | 4 (30.7) | 0.23 |
| Cardiac disease | 0 (0) | 4 (30.7) | — |
| Diabetes | 3 (6.4) | 4 (30.7) | 0.034 |
| Chronic renal failure | 7 (14.9) | 4 (30.7) | 0.23 |
| Stage according to pTNM† | |||
| I | 13 (27.7) | 3 (23.1) | > 0.99 |
| II | 5 (10.6) | 5 (38.5) | 0.031 |
| III | 21 (47.7) | 3 (23.1) | 0.21 |
| IV | 8 (17.0) | 2 (15.4) | > 0.99 |
| Neoadjuvant chemotherapy‡ | 1 (2.1) | 2 (15.4) | 0.12 |
BMI = body mass index; LAG = laparoscipcal assisted gastrectomy; OG = open gastrectomy; pTNM = pathological tumour, node, metastasis; SD = standard deviation.
Unless otherwise indicated.
AJCC Cancer Staging Manual, 6th edition.19
MAGIC protocol.
Operative characteristics and postoperative follow-up are summarized in Table 2. The median operative duration was 115 minutes longer for laparoscopic than the open cases (p < 0.001), and the median estimated blood loss was 425 mL greater in the open than the LAG group (p < 0.001). There were 3 (23.1%) conversions in the LAG group. The extent of resection was not statistically different between the groups. However, there was a trend toward fewer total gastrectomies in the LAG group (7.7% v. 36.2%). Billroth II reconstruction was used more often in the LAG than the OG group (92.3 v. 61.7%, p = 0.045).
Table 2.
Operative characteristics and postoperative follow-up of patients undergoing gastrectomy
| Characteristic | Surgical approach; no. (%)* | p value | |
|---|---|---|---|
| OG | LAG | ||
| Curative intent treatment | 36 (76.6) | 10 (76.9) | > 0.99 |
| Operative time, median (IQR) min | 192 (172.5–262.5) | 307.5 (283.5–350.5) | < 0.001 |
| EBL, median (SD) mL | 550 (300–910) | 125 (100–300) | < 0.001 |
| Conversion to open | — | 3 (23.1) | — |
| Procedure performed | |||
| Total gastrectomy | 17 (36.2) | 1 (7.7) | 0.08 |
| Subtotal gastrectomy | 27 (57.4) | 9 (69.2) | 0.53 |
| Limited resection† | 3 (6.4) | 3 (23.1) | 0.11 |
| Gastrointestinal reconstruction | |||
| Billroth I | 1 (2.1) | 0 (0) | — |
| Billroth II | 29 (61.7) | 12 (92.3) | 0.045 |
| Roux-en-Y | 17 (36.2) | 1 (7.7) | 0.08 |
| Major postoperative morbidity | 6 (12.7) | 3 (23.1) | 0.39 |
| Cardiac | 2 (4.3) | 2 (15.4) | 0.16 |
| Respiratory failure | 1 (2.1) | 1 (7.7) | 0.32 |
| Intra-abdominal abscess | 4 (2.1) | 1 (7.7) | > 0.99 |
| Pancreatic fistula | 1 (2.1) | 1 (7.7) | 0.39 |
| Anastomotic leak | 0 (0) | 1 (7.7) | — |
| Duodenal stump leak | 0 (0) | 1 (7.7) | — |
| LOS, median (IQR) d | 13.0 (10.5–23.0) | 10.0 (7.0–22.7) | 0.91 |
| Postoperative mortality‡ | 1 (2.1) | 1 (7.7) | 0.32 |
| Follow-up, median (IQR) d | 550 (248.5–1117.7) | 286.5 (170.25–334.25) | < 0.001 |
EBL = estimated blood loss; IQR = interquartile range; LAG = laparoscopic assisted gastrectomy; LOS = length of stay; OG = open gastrectomy; SD = standard deviation.
Unless otherwise indicated.
Antrectomy or wedge resection for palliative purpose.
< 30 postoperative days.
We observed no difference in the median length of stay (10.0 d in the LAG v. 13.0 d in the OG group, p = 0.91). One postoperative death (< 30 d) occurred in each group (p = 0.39). Overall there was no difference in postoperative morbidity (23.1 in the LAG v. 12.7% in the OG group, p = 0.39). However, 2 leaks (15.4%; 1 at the gastrojejunal anastomosis in the immediate postoperative period, 1 at the duodenal stump) occurred in the LAG group compared with none in the OG group. The median follow-up was different between the groups (286.5 d in the LAG v. 550 d in the OG group, p < 0.001).
Oncologic assessment of the surgical specimen is presented in Table 3. More patients with stage II disease were treated with LAG than OG (38.5 v. 10.6%, p = 0.031). Overall, 56.7% of patients presented with advanced gastric cancer (stage III or IV). The mean lesion size was 4.5 (SD 2.0) cm in the LAG group and 5.3 (SD 2.5) cm in the OG group (p = 0.12). Negative margins were obtained for all cases with a curative intent. A mean of 11.2 (SD 8.2) LNs were collected in the LAG group compared with 14.4 (SD 9.8) in the OG group (p = 0.29).
Table 3.
Pathologic characteristics after gastrectomy with curative intent
| Characteristic | Surgical approach | p value | |
|---|---|---|---|
| OG | LAG | ||
| Tumour size, mean (SD) cm | 5.5 (2.5) | 4.5 (2.0) | 0.28 |
| R0 resection rate, no. (%) | 36 (100) | 10 (100) | > 0.99 |
| No. of harvested LNs, mean (SD) | 14.4 (9.8) | 11.2 (8.2) | 0.29 |
LAG = laparoscopic assisted gastrectomy; LN = lymph node; OG = open gastrectomy; SD = standard deviation.
Discussion
To our knowledge, this is the first report of a Canadian experience that compares LAG to OG for gastric adenocarcinoma exclusively. We chose to consider only adenocarcinoma because resections for other gastric lesions do not imply the same need for lymphadenectomy. The oncologic quality of the specimens did not differ between LAG and OG, with a similar number of negative margins and mean of harvested LNs for a D1 dissection. Estimated blood loss was lower in the LAG than the OG group. The mean operative duration was longer for the laparoscopic approach, probably because of the technical challenge of LAG and the introduction phase of the technique. However, it did not translate to a difference in overall postoperative morbidity. Three conversions from LAG to OG were necessary: 2 for suspected invasion of the pancreatic head and 1 for a challenging distal pyloric margin. More total gastrectomies were performed in the OG than the LAG group, which may reflect the technical challenge associated with LAG. Two leaks occurred in the LAG group, whereas none was recorded after OG. The first leak was an early occurrence at the gastrojejunal anastomosis and was attributed to technical failure. This obese patient had an antecolic Billroth II stapled reconstruction. Unfortunately, she fell in the immediate postoperative period and presented a bilious leakage from a trocar wound on postoperative day 1. Our impression is that the anastomosis was torn apart by the weight of the heavy unprepared transverse colon. The second leak was observed at the duodenal stump when resuming chemotherapy 6 weeks after surgery (MAGIC protocol). This patient was seen for routine follow-up at 3 weeks postoperatively and was then asymptomatic. Overall, the clinical characteristics of the groups were similar at baseline except for cardiovascular disease and diabetes, which disfavoured the LAG group. More early stage lesions (stage II) were treated with LAG than OG, which could reflect either a preference for a laparoscopic approach to treat earlier lesions or a tendency to diagnose gastric cancer earlier in the latter part of the study period, since LAG was performed starting in 2007.
As previously stated, the laparoscopic approach for gastric diseases is now accepted for benign lesions or malignant ones that do not require extensive lymphadenectomy.3–6 The technique is, however, more controversial for gastric adenocarcinoma. A large body of evidence comes from the Asian literature. Laparoscopic assisted gastrectomy was first used for early gastric cancer, for which lymphadenectomy is not as essential, and was shown to be as safe as OG in addition to providing postoperative benefits such as a shorter hospital stay, earlier mobilization, fewer pulmonary complications and an earlier functional recovery.20–22,25 Retrospective evidence followed for treatment of advanced gastric cancer, with studies reporting equivalent radical oncology resection and improved postoperative suites and recovery.23,24 However, the portrait of gastric cancer in Western countries differs. Prevalence is lower, and more advanced lesions are treated, with 75% being stage III to IV at diagnosis.13 Therefore, the Asian data cannot be readily applied to Western patients.
Few Western studies are available to address the many issues that have been raised regarding the technical and oncological safety of LAG. Comparative studies consisted of retrospective designs,14,15,26–32 2 case-matched cohorts17,33 and a single prospective randomized controlled trial (RCT) by Huscher and colleagues.16 Sample sizes were limited owing to the Western gastric cancer reality. Francescutti and colleagues27 have reported the only previous Canadian experience, but they included patients with benign lesions as well as those with malignant ones. All comparative studies have reported decreased or similar early postoperative morbidity with LAG ranging from 0% to 26%,15–17,26–29,31 which compares with our results. Other benefits of the laparoscopic approach, including decreased time to ambulation and resumed diet, decreased consumption of analgesia and a shorter length of stay, have been reported in these studies.16,17,33 However, we found no difference in length of stay. We performed a laparoscopic assisted technique that included a limited median incision for digestive reconstruction. This may have affected the length of stay. Since this is an early experience, postoperative management was probably more cautious and did not reflect a fast track philosophy. The information available in the chart did not allow us to capture sufficient, consistent details regarding analgesia usage or diet resumption to report them.
From an oncologic perspective, many are worried that LAG would not offer the same radical resection as OG. Issues regarding the pneumoperitoneum and peritoneal implants have already been addressed and refuted by studies on colorectal cancer.34 Fear remains that these results would not apply to the different biological behaviour of gastric cancer. In a large retrospective cohort study of 1417 patients treated with LAG for gastric cancer, Song and colleagues10 reported a 13.4% recurrence for advanced gastric cancer and observed a pattern and timing of recurrence similar to that described after open surgery.35,36 Another concern regards the feasibility of an adequate lymphadenectomy. Strong and colleagues17 reported a significantly decreased number of harvested LNs with LAG in a case-matched study of 60 patients (18 with LAG v. 21 with OG, p = 0.03). Others reported no difference between the laparoscopic and open approaches.15,16,26–31,33 In a meta-analysis of RCTs comparing laparoscopic and open approaches for early gastric cancer, Ohtani and colleagues9 concluded that there was a lesser number of LNs with laparoscopy. Debate is still ongoing about the extent of the lymphadenectomy and the number of LNs needed in the specimen. Gastrectomy with D2 dissection is the standard of care for curable gastric cancer in Asia. In Western countries, no survival benefit has been revealed by 2 RCTs comparing D2 to D1 dissection.37,38 However, 15-year follow-up data from the Dutch trial39 have recently shown a significant decrease in gastric cancer–related death with D2 dissection.39 At this time, the current Western guidelines recommend including at least 15 LNs in the dissection to allow proper staging, without mentioning the level of LN dissection.40 We reported no difference in the number of LNs between groups, but neither achieved the 15 LNs required, despite a meticulous D1 surgical technique. The pathology team at our institution relies on standard manual LN dissection after formalin fixation. This approach is known to retrieve significantly fewer LNs in patients with colorectal cancer.41 Furthermore, our results are consistent with most published results of D1 dissection. Francescutti and colleagues27 retrieved a similar mean of 11.8 LNs in LAG compared with 7.3 in OG (p = 0.21). Weber and colleagues33 obtained a mean of 8 and 11 LNs, respectively, when comparing LAG and OG. Overall, we were able to harvest a reasonable and comparable number of LNs with LAG and OG, considering the standard of care at the time of study. The level of LN dissection for gastric adenocarcinoma is a rapidly evolving topic. Considering the recent data, D2 dissection will probably become a standard of care, and laparoscopic D2 will need to be further developed. No margins were positive for patients treated with a curative intent, so no difference was observed between groups, which is consistent with the current Western literature.15,16,26–31,33 However, obtaining a safe distal margin laparoscopically can be challenging for lesions seating close to the pylorus. This was the reason for 1 of the conversions in the LAG group in our study.
Short follow-up and small sample size in the LAG group precluded proper recurrence or survival analysis in the present study. Huscher and colleagues,16 in the only Western prospective RCT, revealed no significant difference between LAG and OG in 5-year overall survival (58.9% v. 55.7%) and recurrence-free survival (57.3% v. 54.8%). This has also been observed in retrospective studies. Moisan and colleagues28 observed no difference in 3-year overall survival comparing LAG and OG (74% v. 75%, p = 0.88) and 3-year disease-free survival (77.8% v. 68.8%, p = 0.90). In a case-matched study from the Memorial Sloan Kettering Cancer Center, Strong and colleagues17 also found no difference in 3-year survival.
Limitations
Because of its retrospective design and the small sample size, our study has several limitations. A selection bias cannot be overlooked. All gastric cancers were treated with OG before 2007. Afterwards, both OG and LAG were used depending on the surgeon’s preference, which inevitably led to early stage tumours being approached laparoscopically. A historical bias can also be considered, since neoadjuvant chemotherapy protocols were introduced in 2006,42 but both groups were comparable on that aspect, and survival was not an outcome of interest. As all cases were booked with curative intent, we chose to include palliative surgeries in the perioperative outcomes analysis to reflect the clinical reality of the surgical treatment of gastric adenocarcinoma. Laparoscopic assisted gastrectomies were performed by a single surgeon, which limits the generalizability of our results. Laparoscopic gastric surgery remains a challenging technique with a steep learning curve. Solid laparoscopic experience is necessary to perform complex gastric procedures, such as lymphadenectomy for cancer. Considering the small number of cases of gastric cancers in Western countries and the growing complexity of their management, it is not expected that these results will need to be reproduced outside of expert centres. Also, at the moment, LAG appears more suited for early stage lesions, which represent the minority of cases in Western countries; this may further affect the widespread use of LAG.
Conclusion
This study reports the feasibility and surgical specimen equivalence of LAG compared with OG in a Canadian setting. This approach for gastric adenocarcinoma resection cannot be applied to every patient. Contraindications could include prior abdominal surgeries, prior radiotherapy in the surgical field, invasion of other organs and linitis plastica. Considering the low incidence of gastric cancer in Western countries, the issues regarding LAG will hardly be addressed by RCTs. Therefore, we believe that, as long as basic surgical principles are followed, it is reasonable to expect similar oncologic long-term results with the open and the laparoscopic approaches. Notwithstanding the flaws previously addressed, our study reports no difference in morbidity and quality of the oncological specimen between LAG and OG for gastric adenocarcinoma. Laparoscopic assisted gastrectomy is a technically safe and feasible procedure in experienced hands. It satisfies the oncologic requirement by offering the same radical resection as the open approach in terms of negative margins and adequate LN retrieval. Longer follow-up in prospective trials is warranted to definitely conclude on survival outcomes in the Western setting. In the meantime, the use of LAG in experienced laparoscopic centres appears justified in a selected population.
Footnotes
Work presented at the Canadian Surgery Forum, Québec, Que., Sept. 2–5, 2010.
Competing interests: None declared.
Contributors: J. Hallet and J.-P. Gagné designed the study and wrote the article. J. Hallet, S. Labidi and A. Clairoux acquired the data, which J. Hallet, A. Bouchard-Fortier and J.-P. Gagné analyzed. All authors reviewed the article and approved its publication.
References
- 1.Dubois F, Berthelot G, Levard H. Coelioscopic cholecystectomy: experience with 2006 cases. World J Surg. 1995;19:748–52. doi: 10.1007/BF00295921. [DOI] [PubMed] [Google Scholar]
- 2.Lacy AM, Tabet J, Grande L, et al. Laparoscopic-assisted resection of a gastric lipoma. Surg Endosc. 1995;9:995–7. doi: 10.1007/BF00188458. [DOI] [PubMed] [Google Scholar]
- 3.Amin AT, Kono Y, Shiraishi N, et al. Long-term outcomes of laparoscopic wedge resection for gastrointestinal stromal tumors of the stomach of less than 5 cm in diameter. Surg Laparosc Endosc Percutan Tech. 2011;21:260–3. doi: 10.1097/SLE.0b013e318220f1c7. [DOI] [PubMed] [Google Scholar]
- 4.Perdikis G, Hinder RA, Lund RJ, et al. Laparoscopic Nissen fundoplication: Where do we stand? Surg Laparosc Endosc. 1997;7:17–21. [PubMed] [Google Scholar]
- 5.Nguyen NT, Ho HS, Palmer LS, et al. A comparison study of laparoscopic versus open gastric bypass for morbid obesity. Am Coll Surg. 2000;191:149–155. doi: 10.1016/s1072-7515(00)00276-3. discussion 155–7. [DOI] [PubMed] [Google Scholar]
- 6.Novitsky YW, Kercher KW, Sing RF, et al. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006;243:738–45. doi: 10.1097/01.sla.0000219739.11758.27. discussion 745–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Memon MA, Khan S, Yunus RM, et al. Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc. 2008;22:1781–9. doi: 10.1007/s00464-008-9925-9. [DOI] [PubMed] [Google Scholar]
- 8.Kim H-H, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report — a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–20. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 9.Ohtani H, Tamamori Y, Noguchi K, et al. A meta-analysis of randomized controlled trials that compared laparoscopy-assisted and open distal gastrectomy for early gastric cancer. J Gastrointest Surg. 2010;14:958–64. doi: 10.1007/s11605-010-1195-x. [DOI] [PubMed] [Google Scholar]
- 10.Song J, Lee H-J, Cho GS, et al. Recurrence following laparoscopy-assisted gastrectomy for gastric cancer: a multicenter retrospective analysis of 1,417 patients. Ann Surg Oncol. 2010;17:1777–86. doi: 10.1245/s10434-010-0932-4. [DOI] [PubMed] [Google Scholar]
- 11.Lee S-W, Nomura E, Bouras G, et al. Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg. 2010;211:33–40. doi: 10.1016/j.jamcollsurg.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 12.An JY, Heo G-U, Cheong J-H, et al. Assessment of open versus laparoscopy-assisted gastrectomy in lymph node-positive early gastric cancer: a retrospective cohort analysis. J Surg Oncol. 2010;102:77–81. doi: 10.1002/jso.21554. [DOI] [PubMed] [Google Scholar]
- 13.Al-Refaie W, Abdalla E, Ahmad S. The MD Anderson Surgical Oncology Handbook. 4th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2006. pp. 205–40. [Google Scholar]
- 14.Reyes CD, Weber KJ, Gagner M, et al. Laparoscopic vs open gastrectomy. Surg Endosc. 2001;15:928–31. doi: 10.1007/s004640080185. [DOI] [PubMed] [Google Scholar]
- 15.Dulucq JL, Wintringer P, Stabilini C, et al. Laparoscopic and open gastric resections for malignant lesions: a prospective comparative study. Surg Endosc. 2005;19:933–8. doi: 10.1007/s00464-004-2172-9. [DOI] [PubMed] [Google Scholar]
- 16.Huscher CGS, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer. Ann Surg. 2005;241:232–7. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strong VE, Devaud N, Allen PJ, et al. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case–control study. Ann Surg Oncol. 2009;16:1507–13. doi: 10.1245/s10434-009-0386-8. [DOI] [PubMed] [Google Scholar]
- 18.Association of Japanese Gastric Cancer. Japanese Classification of Gastric Carcinoma — 2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 19.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 20.Adachi Y, Shiraishi N, Shiromizu A, et al. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg. 2000;135:806–10. doi: 10.1001/archsurg.135.7.806. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Han H-S, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–73. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 22.Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131(Suppl):S306–11. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 23.Hwang SI, Kim HO, Yoo CH, et al. Laparoscopic-assisted distal gastrectomy versus open distal gastrectomy for advanced gastric cancer. Surg Endosc. 2009;23:1252–8. doi: 10.1007/s00464-008-0140-5. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Ramos D, Miralles-Tena J, Cuesta AM, et al. Laparoscopy versus open surgery for advanced and resectable gastric cancer: a meta-analysis. Rev Esp Enferm Dig. 2011;103:133–41. doi: 10.4321/s1130-01082011000300005. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi H, Ochiai T, Shimada H, et al. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172–6. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 26.Chouillard E, Gumbs AA, Meyer F, et al. Laparoscopic versus open gastrectomy for adenocarcinoma: a prospective comparative analysis. Minerva Chir. 2010;65:243–50. [PubMed] [Google Scholar]
- 27.Francescutti V, Choy I, Biertho L, et al. Gastrectomy and esophago-gastrectomy for proximal and distal gastric lesions: a comparison of open and laparoscopic procedures. Surg Innov. 2009;16:134–9. doi: 10.1177/1553350609336738. [DOI] [PubMed] [Google Scholar]
- 28.Moisan F, Norero E, Slako M, et al. Completely laparoscopic versus open gastrectomy for early and advanced gastric cancer: a matched cohort study. Surg Endosc. 2012;26:661–72. doi: 10.1007/s00464-011-1933-5. [DOI] [PubMed] [Google Scholar]
- 29.Pugliese R, Maggioni D, Sansonna F, et al. Total and subtotal laparoscopic gastrectomy for adenocarcinoma. Surg Endosc. 2007;21:21–7. doi: 10.1007/s00464-005-0409-x. [DOI] [PubMed] [Google Scholar]
- 30.Sarela AI. Entirely laparoscopic radical gastrectomy for adenocarcinoma: lymph node yield and resection margins. Surg Endosc. 2009;23:153–60. doi: 10.1007/s00464-008-0072-0. [DOI] [PubMed] [Google Scholar]
- 31.Scatizzi M, Kröning KC, Lenzi E, et al. Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer: a case–control study. Updates Surg. 2011;63:17–23. doi: 10.1007/s13304-011-0043-1. [DOI] [PubMed] [Google Scholar]
- 32.Varela JE, Hiyashi M, Nguyen T, et al. Comparison of laparoscopic and open gastrectomy for gastric cancer. Am J Surg. 2006;192:837–42. doi: 10.1016/j.amjsurg.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 33.Weber KJ, Reyes CD, Gagner M, et al. Comparison of laparoscopic and open gastrectomy for malignant disease. Surg Endosc. 2003;17:968–71. doi: 10.1007/s00464-002-8738-5. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Milsom JW, Gramlich TL, et al. Does laparoscopic vs. conventional surgery increase exfoliated cancer cells in the peritoneal cavity during resection of colorectal cancer? Dis Colon Rectum. 1998;41:971–8. doi: 10.1007/BF02237382. [DOI] [PubMed] [Google Scholar]
- 35.D’Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–16. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–42. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 37.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–14. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 38.Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Br J Cancer. 1999;79:1522–30. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Songun I, Putter H, Kranenbarg EM-K, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 40.NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines): Gastric Cancer (including cancer in the proximal 5 cm of the stomach) Fort Washington (OPA): National Comprehensive Cancer Network, Inc.; 2011. [accessed 2013 June 11]. Available: www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. [Google Scholar]
- 41.Poller DN. Method of specimen fixation and pathological dissection of colorectal cancer influences retrieval of lymph nodes and tumour nodal stage. Eur J Surg Oncol. 2000;26:758–62. doi: 10.1053/ejso.2000.0999. [DOI] [PubMed] [Google Scholar]
- 42.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]