Abstract
Objectives
We aimed to evaluate the efficacy and safety of combination therapy of Endostar (recombinant human endostatin) and S-1 combined with oxaliplatin (SOX) in patients with advanced gastric cancer.
Methods
In this randomized, controlled trial, 165 late-stage gastric cancer patients were assigned to the experimental arm with Endostar in combination with SOX (80 patients) and the control arm with SOX alone (85 patients). The end points of this study included progression-free survival, response rate, and disease-control rate.
Results
There was no statistically significant difference in response rate between the experimental arm and the control arm (53.8% vs 42.4%, P=0.188). The difference in disease-control rate was also statistically insignificant between the two arms (85.0% vs 72.9%, P=0.188). Progression-free survival in the experimental arm was significantly higher than that in the control arm (15.0 months vs 12.0 months, P=0.0001). Common adverse events included immunosuppression, gastrointestinal distress, and neuropathy. There was no statistical difference in the incidences of adverse events.
Conclusion
Combination therapy of Endostar and SOX provides therapeutic benefits to advanced gastric cancer patients, with tolerable adverse effects.
Keywords: endostatin, gastric cancer, SOX, oxaliplatin, Endostar, S-1
Introduction
Gastric adenocarcinoma is one of the most common malignant tumors in the gastrointestinal (GI) tract. Although surgical removal is frequently used for gastric adenocarcinoma at the early stage, advanced gastric adenocarcinoma does not benefit significantly from surgical operation. Chemotherapy for advanced gastric adenocarcinoma has been proven to be superior to best supportive care in terms of survival and quality of life.1–3 Chemotherapy is mainly used in early stage gastric cancer patients to prevent recurrent tumors and metastasis. For advanced-stage patients with inoperable gastric tumors, chemotherapy is considered the most effective treatment option.1
S-1 and oxaliplatin (SOX) is a chemotherapy regimen that has been shown to be an effective and safe treatment option for advanced gastric cancer patients.4 S-1 is an orally active drug that includes a combination of tegafur (a prodrug of fluorouracil), gimeracil (an inhibitor of fluorouracil degrader), and oteracil (a fluorouracil phosphorylation inhibitor in the GI tract, thus reducing the toxic GI effects of fluorouracil).5 Oxaliplatin is a platinum-based cytotoxic agent that has been well studied as a noninferior alternative of cisplatin in several clinical trials.6,7 Three recent clinical studies have demonstrated that the SOX regimen is a well-tolerated and effective treatment strategy for advanced gastric cancer patients.8–10
Endostar is a therapeutic recombinant human endostatin that is a C-terminal cleaved fragment of collagen type XVIII. Endostatin has been well studied as an endogenous antiangiogenic peptide that has multiple antitumor roles by modulating various receptors in the plasma membrane, such as suppression of angiogenesis and inhibition of tumor-cell migration and invasion.11–15 Because Endostar and the traditional cytotoxic chemotherapy drugs have distinct antitumor mechanisms, they are a potential combination that may demonstrate synergistic effects in inhibiting the development and progression of cancer. In fact, Endostar in combination with vinorelbine–cisplatin has been approved by the Chinese State Food and Drug Administration in 2005 as a first-line treatment for advanced non-small-cell lung cancer.16 The efficacy and safety of Endostar on other types of cancer are currently being evaluated in a number of ongoing clinical trials.17–19 However, Endostar has not been investigated in advanced gastric cancer.
In this study, we conducted a randomized, controlled clinical trial to compare the efficacy and safety of Endostar in combination with the SOX regimen to SOX regimen alone in patients with advanced gastric cancer.
Patients and methods
Patient selection
Eligible patients had histologically confirmed stage IV gastric adenocarcinoma, including patients with distant metastasis and recurrent gastric cancer. They had to be 25–75 years old, estimated life expectancy ≥3 months, with adequate hepatic, cardiac, renal, and bone marrow function, had never received chemotherapy or radiation therapy, and had a Karnofsky performance scale (KPS) score ≥60. All patients provided written consent. The institutional review board of the People’s Hospital of Xinjiang approved the protocol used in this study.
All eligible patients were enrolled in this study between June 1, 2009 and June 1, 2012, and randomly assigned into the experimental arm (80 patients) and the control arm (85 patients). There were no statistically significant differences in the baseline characteristics between the two arms (Table 1).
Table 1.
Demographic and baseline characteristics
| Experimental arm (n=80) | Control arm (n=85) | P-value | |
|---|---|---|---|
| Sex | 0.539 | ||
| Male | 55 | 58 | |
| Female | 25 | 27 | |
| Age (years) | 0.970 | ||
| 25–39 | 10 | 11 | |
| 40–69 | 42 | 43 | |
| 70–75 | 28 | 31 | |
| KPS score | 0.841 | ||
| 90–100 | 63 | 61 | |
| 60–80 | 17 | 14 | |
| Removal of primary tumor | 0.529 | ||
| Yes | 45 | 49 | |
| No | 30 | 41 | |
| Organs metastasized | 0.978 | ||
| 0 | 33 | 37 | |
| 1 | 27 | 26 | |
| 2 | 12 | 13 | |
| >2 | 8 | 9 |
Abbreviation: KPS, Karnofsky performance scale.
Treatments
For patients in the experimental arm, 15 mL of Endostar was injected intravenously from day 1 to day 14. For patients in both the control and experimental arms, oxaliplatin (130 mg/m2) was administered intravenously on day 1. S-1 (80 mg/m2/day) was given orally twice daily for 14 days. All patients then took 1 week’s rest before the next cycle. Physical examination and blood analysis were performed at each cycle during chemotherapy. Responses of chemotherapy were assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Responses were classified as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Response rate was calculated as the sum of CR and PR. The disease-control rate was the sum of CR, PR, and SD. Six cycles of chemotherapy were given to all patients who showed response. Computed tomography and magnetic resonance imaging were used to assess tumor size. Second-line chemotherapy was given to patients who did not show response to the SOX or SOX + Endostar treatment. For grade 3/4 adverse events, the chemotherapy dose could be reduced if the symptoms were alleviated after management. Chemotherapy could be postponed if a patient’s condition still did not meet the criteria for chemotherapy treatment after adverse-event management.
Patients were followed up every 3 months until death or until the cutoff date of this study on June 1, 2012. Quality of patient life was assessed by the KPS and recorded as much improved (≥+20), improved (+10−19), unchanged (+10 to −10), and decreased (≥−10). Toxicity was assessed according to World Health Organisation criteria.
Statistical analysis
End points of this study included response rate, disease-control rate, and progression-free survival (PFS). PFS was calculated from the date of randomization to the time of disease progression, death, or June 1, 2012. PFS was computed by the Kaplan–Meier method. The chi-squared test was used to compare the differences between arms. Statistical analyses were conducted using SPSS software, version 13.0 (IBM, Armonk, NY, USA).
Results
Response rate
The 85 patients in the control arm (SOX alone) received an average of 3.58 cycles of chemotherapy treatment while the 80 patients in the experimental arm (Endostar plus SOX) received an average of 3.22 cycles of chemotherapy treatment. There was no statistically significant difference in the average chemotherapy cycles received by patients between the control and experimental arms (P=0.068). The response rate in the experimental arm was 53.8%, which was higher than the 42.4% in the control arm, but failed to demonstrate statistical significance. The disease-control rate of the experimental arm and control arm was 85% and 72.9%, respectively (χ2=4.791, P=0.188) (Table 2).
Table 2.
Response rates of experimental and control arms
| Arm | n | CR | PR | SD | PD | RR (%) | DCR (%) |
|---|---|---|---|---|---|---|---|
| Experimental | 80 | 1 | 42 | 25 | 12 | 53.8 | 85.0 |
| Control | 85 | 0 | 36 | 26 | 23 | 42.4 | 72.9 |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; RR, response rate; DCR, disease-control rate.
Progression-free survival
Patients were followed up for 3 years between June 2009 and June 2012, when this study ended. PFS in the experimental arm was statistically higher than that in the control arm (15.0 months vs 12.0 months, P=0.0001, χ2=12.662). The experimental arm showed significant benefits in overall survival (Figure 1). The 1-year survival rate for the experimental arm was 68% compared to 52% in the control arm. The 2-year survival rate in the experimental arm was significantly higher than that in the control arm (32.0% vs 6.0%, P=0.0001).
Figure 1.
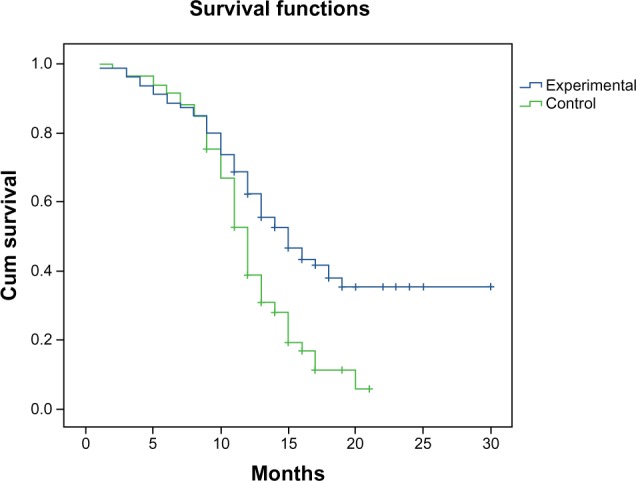
Progression-free survival of the experimental arm and the control arm.
Abbreviation: Cum, cumulative.
Adverse events
The most common grade 3/4 adverse events included bone marrow suppression, which leads to anemia, thrombocytopenia, and neutropenia. GI distress, such as nausea, vomiting, and diarrhea, was also frequently observed. Other recorded grade 3/4 adverse events were peripheral neuropathy, skin pigmentation, and toxicities in liver. There was no statistically significant difference in the incidences of adverse effects between the two arms (Table 3).
Table 3.
Adverse events
| Adverse effect | Experimental arm
|
Incidence | Control arm
|
Incidence | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | I | II | III | IV | ||||
| Neutropenia | 11 | 8 | 10 | 6 | 43.75 | 12 | 9 | 8 | 9 | 44.71 | 0.849 |
| Thrombocytopenia | 10 | 5 | 3 | 1 | 23.75 | 11 | 8 | 2 | 2 | 27.01 | 0.163 |
| Anemia | 12 | 7 | 3 | 1 | 28.75 | 15 | 7 | 2 | 1 | 29.41 | 0.877 |
| Nausea | 28 | 10 | 5 | 2 | 56.25 | 30 | 11 | 5 | 3 | 57.65 | 0.939 |
| Diarrhea | 7 | 1 | 1 | 0 | 11.25 | 8 | 2 | 1 | 0 | 12.94 | 0.488 |
| Skin pigmentation | 13 | 3 | 1 | 0 | 21.25 | 14 | 2 | 2 | 0 | 21.17 | 0.763 |
| Peripheral neuropathy | 24 | 8 | 2 | 0 | 42.50 | 26 | 7 | 3 | 0 | 42.35 | 0.865 |
| Nephrotoxicity | 13 | 4 | 0 | 0 | 21.25 | 15 | 3 | 1 | 0 | 22.35 | 0.555 |
| Hepatotoxicity | 15 | 3 | 1 | 1 | 23.75 | 17 | 4 | 2 | 0 | 27.06 | 0.706 |
| Cardiotoxicity | 8 | 2 | 0 | 0 | 12.50 | 2 | 1 | 0 | 0 | 3.53 | 0.455 |
Quality of life
Quality of life was assessed using the KPS. There was no statistically significant difference in the quality of patient life between the two arms (χ2=4.551, P=0.208, Table 4).
Table 4.
Changes in quality of life of patients
| Arm | n | Much improved | Improved | Stable | Decreased | Improvement rate (%) |
|---|---|---|---|---|---|---|
| Experimental | 80 | 1 | 32 | 35 | 12 | 41.3 |
| Control | 85 | 0 | 23 | 44 | 18 | 27.1 |
Discussion
Although the incidence of gastric cancer has decreased in the past decade, gastric adenocarcinoma is still one of the leading causes of cancer-related death in many countries, such as People’s Republic of China, South Korea, and Japan. The 5-year survival rate for gastric cancer is just 20%.20 For early stage gastric cancer, surgical dissection of tumor followed by adjuvant chemotherapy has become a standard treatment. For advanced-stage gastric cancer, chemotherapy is usually the most effective treatment option. However, the response rates of various chemotherapy regimens being tested in gastric cancer are still poor. Target therapy, such as trastuzumab, which targets human epidermal growth-factor receptor 2, has demonstrated promising results in treating advanced gastric cancer.21 In this study, we investigated the efficacy and safety of Endostar with the SOX regimen in advanced gastric cancer.
S-1 has become a first-line chemotherapy drug for advanced gastric cancer in Japan.22 S-1 monotherapy achieved response rates ranging from 26% to 49% for advanced gastric cancer.22 The SOX regimen has been evaluated in several clinical studies. Yamada et al reported in the 2010 American Society of Clinical Oncology meeting that the SOX regimen had a 59% response rate and 84% disease-control rate in treating inoperable and recurrent advanced gastric cancer. In this study, we reported a 42.2% response rate and 72.9% disease-control rate for the SOX regimen-alone arm, which did not show statistically significant difference with patients treated with Endostar combined with SOX. However, progression-free survival was significantly higher in the Endostar + SOX arm compared to the SOX-alone arm, indicating that Endostar has a synergistic effect with S-1 and oxaliplatin.
Endostar has been shown specifically to inhibit angiogenesis in in vivo and in vitro studies.23 Endostar suppresses vascular endothelial growth factor-mediated proliferation, migration, and tube formation of human umbilical vein endothelial cells.23 Endostar also can inhibit microvessel sprouting from rat aortic rings and prevent the growth of vessels in tumors.23 Endostar has been added to several standard chemotherapy regimens and did not show increased adverse events. In our study, we did not observe a statistical difference in such toxicities as immunosuppression, GI distress, or neuropathy between the experimental arm and the control arm. The most common toxicity of Endostar is cardiotoxicity. We noticed that ten patients in the Endostar + SOX arm developed grade 1 and 2 adverse events in cardiotoxicity, compared to three cases in the control SOX-alone arm. When proper management is used, cardiotoxicity can be well controlled. Therefore, patients taking Endostar should have their cardiac condition closely monitored, especially those with previous cardiac conditions.
In summary, Endostar in combination with the SOX regimen shows significant therapeutic benefits to patients with advanced gastric cancer. This finding provides the basis for further study to identify the patient subgroup that will be more likely to show response to Endostar treatment.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Glimelius B, Hoffman K, Haglund U, Nyrén O, Sjödén PO. Initial or delayed chemotherapy with best supportive care in advanced gastric cancer. Ann Oncol. 1994;5:189–190. doi: 10.1093/oxfordjournals.annonc.a058778. [DOI] [PubMed] [Google Scholar]
- 3.Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587–591. doi: 10.1038/bjc.1995.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao SG, Jia RN, Feng XS, et al. Therapeutic effects of combined oxaliplatin and S-1 in older patients with advanced gastric cardiac adenocarcinoma. World J Gastroenterol. 2011;17:5221–5226. doi: 10.3748/wjg.v17.i47.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 7.Al-Batran SE, Hartmann JT, Probst S, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–1442. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 8.Kim GM, Jeung HC, Rha SY, et al. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer. 2012;48:518–526. doi: 10.1016/j.ejca.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Oh SY, Kwon HC, Jeong SH, et al. A phase II study of S-1 and oxaliplatin (SOX) combination chemotherapy as a first-line therapy for patients with advanced gastric cancer. Invest New Drugs. 2012;30:350–356. doi: 10.1007/s10637-010-9507-2. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi W, Takiuchi H, Yamada Y, et al. Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer (G-SOX study) Ann Oncol. 2010;21:1001–1005. doi: 10.1093/annonc/mdp464. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 12.O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhuang HQ, Yuan ZY. Process in the mechanisms of endostatin combined with radiotherapy. Cancer Lett. 2009;282:9–13. doi: 10.1016/j.canlet.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J. Antiangiogenesis in cancer therapy – endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Grosios K. Endostatin (EntreMed) IDrugs. 2000;3:799–810. [PubMed] [Google Scholar]
- 16.Wang J, Sun Y, Liu Y. Results of randomised, multicentre, double-blind phase III trial of rh-endostantin (YH-16) in treatment of advanced non-small cell lung cancer patients. Chin J Lung Cancer. 2005;8:283–290. doi: 10.3779/j.issn.1009-3419.2005.04.07. [DOI] [PubMed] [Google Scholar]
- 17.Ke QH, Zhou SQ, Huang M, Lei Y, Du W, Yang JY. Early efficacy of Endostar combined with chemoradiotherapy for advanced cervical cancers. Asian Pac J Cancer Prev. 2012;13:923–926. doi: 10.7314/apjcp.2012.13.3.923. [DOI] [PubMed] [Google Scholar]
- 18.Zhou JF, Bai CM, Wang YZ, Li XY, Cheng YJ, Chen SC. Endostar combined with chemotherapy for treatment of metastatic colorectal and gastric cancer: a pilot study. Chin Med J (Engl) 2011;124:4299–4303. [PubMed] [Google Scholar]
- 19.Zhou ZT, Zhou FX, Wei Q, Zou LY, Qin BF, Peng XS. Phase II study of cisplatin/etoposide and Endostar for extensive-stage small-cell lung cancer. Cancer Chemother Pharmacol. 2011;68:1027–1032. doi: 10.1007/s00280-011-1576-1. [DOI] [PubMed] [Google Scholar]
- 20.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;28:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 22.Sato A, Ito T, Tomita T, Nakamura A, Taguchi S. Chemotherapy of gastric cancer – a review of clinical trials in Japan. Gan To Kagaku Ryoho. 2002;29:1522–1531. Japanese. [PubMed] [Google Scholar]
- 23.Ling Y, Yang Y, Lu N, et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun. 2007;361:79–84. doi: 10.1016/j.bbrc.2007.06.155. [DOI] [PubMed] [Google Scholar]


