Abstract
Objective
To describe the long-term effects (10 years) of the Age-Related Eye Disease Study (AREDS) formulation of high-dose antioxidants and zinc supplement on progression of age-related macular degeneration (AMD).
Design
Multi-centered, randomized, controlled clinical trial; followed by epidemiologic follow-up study.
Participants
4757 participants with varying severity of AMD were enrolled in the clinical trial. 3549 surviving participants consented to the follow-up study.
Methods
Participants were randomly assigned to antioxidants C, E, and beta-carotene and/or zinc vs. placebo during the clinical trial. In participants with intermediate AMD or advanced AMD in one eye, the AREDS formulation delayed the progression to advanced AMD. Participants were then enrolled in a follow-up study. Eye exams were conducted with annual fundus photographs and best-corrected visual acuity assessments. Medical histories and mortality were obtained for safety monitoring. Repeated measures logistic regression was used in the primary analyses.
Main Outcome Measurement
(1) Photographic assessment of progression to, or history of treatment for, advanced AMD [neovascular (NV) or central geographic atrophy (CGA)], and (2) moderate visual acuity loss from baseline (≥ 15 letters).
Results
Comparison of the participants originally assigned to placebo in AREDS categories 3 and 4 at baseline with those originally assigned to AREDS formulation at 10 years demonstrated a statistically significant (p<0.001) odds reduction in the risk of developing advanced AMD or the development of NV AMD (odds ratios and 99% confidence intervals: OR 0.66, CI: (0.53–0.83) and OR 0.60, CI: (0.47–0. 78), respectively). No statistically significant reduction (p=0.93) was seen for the CGA (OR 1.02, CI: 0.71–1.45). A statistically significant reduction (p=0.002) for the development of moderate vision loss was seen (OR 0.71, CI: 0.57–0.88). No adverse effects were associated with the AREDS formulation. Mortality was reduced in participants assigned to zinc, especially death from circulatory diseases.
Conclusion
Five years after the clinical trial ended, the beneficial effects of the AREDS formulation persisted for development of NV AMD but not for CGA. These results are consistent with the original recommendations that persons with intermediate AMD or advanced AMD in one eye should consider taking the AREDS formulation.
Introduction
In 2001, the Age-Related Eye Disease Study (AREDS) Research Group reported results from a randomized, controlled clinical trial showing that a high-dose antioxidant vitamins plus zinc formulation was effective in retarding the progression of age-related macular degeneration.1 Use of the formulation was recommended for patients at moderate to high risk of progression to advanced age-related macular degeneration (AMD) (AREDS categories 3 and 4).
Following the cessation of the clinical trial in 2001, the participants were followed until 2005 to observe the subsequent natural history of AMD in the cohort. This report describes the long-term effects of the AREDS formulation on progression of AMD during 10 years of follow-up, in particular the effects on persons for whom treatment with the AREDS formulation has been recommended. Long-term possible adverse effects associated with the original treatment assignments in the clinical trial were also examined. The effect of the treatments on mortality was also evaluated.
Materials and Methods
Study Population
Details of the design and methods of the AREDS Study have been presented elsewhere2 but are briefly summarized here. Eleven retinal specialty clinics enrolled 4757 participants in AREDS from 1992 through 1998. Participants were 55 to 80 years of age at enrollment and had best-corrected visual acuity of 20/32 or better in at least one eye. Media were sufficiently clear to obtain adequate quality stereoscopic fundus photographs of the macula. The Institutional Review Board for each clinical center approved the protocol, and informed consent was obtained from all participants.
Participants were recruited based upon the severity of AMD and were placed into four AREDS AMD categories according to the size and extent of drusen in each eye, the presence of advanced AMD and visual acuity, as previously described. AREDS AMD category 1 consisted of persons free of AMD with less than 5 small drusen (< 63 μm). Category 2 participants had early AMD with multiple small drusen or non-extensive intermediate drusen (63 to 124 μm), pigment abnormalities or a combination of the two. Category 3 participants had no advanced AMD but had at least 1 large drusen (125 μm), extensive area of intermediate drusen or geographic atrophy (GA) not involving the center of macula. Category 4 participants had advanced AMD, central geographic atrophy (CGA) or neovascular AMD in one eye. The fellow eye of Category 4 participants and both eyes of participants in the other categories were the study eyes.
The 4757 participants enrolled for a clinical trial of antioxidant vitamins and zinc were followed until 2001 when the trial was completed. 3549 of the 4203 (84.4%) who were alive at the end of the trial subsequently consented for additional follow-up through 2005.
Study Drug Assignment
Participants in the clinical trial were randomly assigned to one of four treatment groups: placebo, zinc, antioxidants or antioxidants plus zinc. The antioxidants consisted of vitamins C (500 mg), E (400 IU), and beta-carotene (15 mg). Zinc was given as zinc oxide (80 mg) along with copper as cupric oxide (2 mg) daily. The study medications were matching tablets in size, shape and color in all four treatment groups. In addition, participants were offered a multi-vitamin-mineral supplement with recommended dietary allowance (RDA) doses (Centrum) that was provided by the study. Median follow-up in the randomized trial was 6.5 years. Following the termination of the clinical trial, participants were invited to continue in a follow-up observational study. When the AREDS formulation became available for distribution, participants with at least intermediate AMD (AREDS category 3) were offered the antioxidant plus zinc formulation and treatment use was monitored.
Procedures
Eye examinations were conducted at baseline and semi-annually throughout the clinical trial which ended in 2001. Only annual visits were conducted through 2005. Best-corrected visual acuity (BCVA) was assessed by certified examiners using the Early Treatment of Diabetic Retinopathy Study (ETDRS) logarithm of the minimum angle of resolution chart and a standardized protocol. Demographic information and medical history were obtained at baseline. Data were collected on age, race, gender, education, smoking, body mass index, use of medications, and history of diabetes, hypertension, angina, and arthritis. Stereoscopic fundus photographs of the macula were taken at baseline and annually beginning two years after enrollment and continuing through the follow-up study. Photographs were graded centrally at a reading center using standardized grading procedures. Mortality data were collected from hospital records, death certificates and a national death index search.
Outcomes
The two primary outcomes evaluated were: 1) progression to advanced AMD and 2) visual acuity loss of ≥15 letters from baseline in study eyes. Progression to neovascular AMD was based on clinical center reports of photocoagulation or other therapies such as photodynamic therapy for choroidal neovascularization or photographic documentation at the reading center of any of the following: non-drusenoid retinal pigment epithelial detachment, serious or hemorrhagic retinal detachment, hemorrhage under the retina or the retinal pigment epithelium (RPE) and/or subretinal fibrosis. Central geographic atrophy (CGA) was present if the center subfield is involved (approximately 500 microns diameter centered on the fovea). Such eyes did not count as CGA when subretinal fibrosis was diagnosed in an eye at the same visit.
Analyses
Primary comparisons for the development of advanced AMD and for a visual acuity decrease were conducted on persons in AREDS categories 3 and 4, the group for whom treatment with the AREDS formulation has been recommended. Although persons in category 2 were at low risk of developing advanced AMD at 10 years, treatment effects were also examined for the entire AMD cohort that included participants in AREDS categories 2, 3 and 4 at baseline. Repeated-measures logistic regression incorporating the generalized estimating equations (GEE) methodology was used to assess the association of the primary outcomes and the AREDS treatment. The analysis was adjusted for visit and AMD category. Covariate adjusted Cox proportional hazards models predicting mortality were created with AMD category, visual acuity status, nuclear opacity status, cortical opacity status, posterior subcapsular cataract (PSC) status, history of cataract surgery and assigned AREDS treatment at baseline as independent variables.
Results
At baseline, 4757 participants were enrolled in the clinical trial from 1992 to 1998. The baseline characteristics of the participants included in the present analyses are displayed in Table 1. Following the cessation of the clinical trial in April 2001, the follow-up study enrolled 3549 of the 4203 (84.4%) surviving participants. Annual visits for the follow-up study started in 2001 and ended November 30, 2005. Participants who enrolled in the follow-up study were more likely to be white, younger, non-smokers, non-diabetics and to have less severe AMD, higher educational level, and lower blood pressure than those who were not active participants. The rates of loss to follow-up in the clinical trial and the follow-up study were 2% and 4%, respectively, with no differences among the treatment groups. Compliance with the treatment assignments was approximately 75% (at least 75% of the study medications were taken according to pill count) during the clinical trial. At the end of the trial, use of a supplement of antioxidants plus zinc such as that used in AREDS was recommended for persons with intermediate AMD (AREDS Category 3) or worse. Unfortunately, the AREDS formulation was not available immediately after the clinical trial ended. When it became available in 2003 the formulation was supplied to participants in the study at no cost. The proportion of the participants in AMD categories 3 and 4 taking the AREDS formulation increased from near zero in the first 2 years following the end of the randomized clinical trial to about 70% in the last years of follow-up. The proportions of participants taking the AREDS supplements in the follow-up study were similar in participants originally randomized to placebo and those randomized to each of the active AREDS formulations. The treatment groups also had similar demographic characteristics in the follow-up study.
Table 1.
Baseline characteristics of Age-Related Eye Disease Study (AREDS) participants included in analyses
| Analysis
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Mortality Analysisψ | Treatment Analysis of Categories 2, 3, 4 | Treatment Analysis of Categories 3, 4 | Treatment Analysis of Category 4 | |||||
|
| ||||||||
| N | % | N | % | N | % | N | % | |
| Total | 4753 | 100.0 | 3476 | 100.0 | 2459 | 100.0 | 901 | 100.0 |
| Age-Related Macular Degeneration (AMD) category | ||||||||
| 1 | 1116 | 23.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 1060 | 22.3 | 1017 | 29.3 | 0 | 0 | 0 | 0 |
| 3 | 1620 | 34.1 | 1558 | 44.8 | 1558 | 63.4 | 0 | 0 |
| 4 | 957 | 20.1 | 901 | 25.9 | 901 | 36.6 | 901 | 100.0 |
| AREDS treatment | ||||||||
| Placebo | 1483 | 31.2 | 861 | 24.8 | 598 | 24.3 | 215 | 23.9 |
| Antioxidants | 1480 | 31.1 | 891 | 25.6 | 636 | 25.9 | 239 | 26.5 |
| Zinc | 903 | 19.0 | 865 | 24.9 | 607 | 24.7 | 223 | 24.8 |
| Antioxidants + Zinc | 887 | 18.7 | 859 | 24.7 | 618 | 25.1 | 224 | 24.9 |
| Age | ||||||||
| <65 | 1000 | 21.0 | 654 | 18.8 | 415 | 16.9 | 135 | 15.0 |
| 65-69 | 1577 | 33.2 | 1109 | 31.9 | 720 | 29.3 | 213 | 23.6 |
| ≥70 | 2176 | 45.8 | 1713 | 49.3 | 1324 | 53.8 | 553 | 61.4 |
| Sex | ||||||||
| Female | 2655 | 55.9 | 1964 | 56.5 | 1353 | 55.0 | 470 | 52.2 |
| Male | 2098 | 44.1 | 1512 | 43.5 | 1106 | 45.0 | 431 | 47.8 |
| Educationa | ||||||||
| High school or less | 1705 | 35.9 | 1298 | 37.4 | 973 | 39.6 | 420 | 46.6 |
| Some college | 1409 | 29.7 | 1042 | 30.0 | 743 | 30.2 | 265 | 29.4 |
| College graduate | 1636 | 34.4 | 1134 | 32.6 | 741 | 30.2 | 216 | 24.0 |
| Race | ||||||||
| Non-White | 207 | 4.4 | 121 | 3.5 | 65 | 2.6 | 14 | 1.6 |
| White | 4546 | 95.6 | 3355 | 96.5 | 2394 | 97.4 | 887 | 98.4 |
| Smoking status | ||||||||
| Never | 2105 | 44.3 | 1507 | 43.4 | 1003 | 40.8 | 316 | 35.1 |
| Former | 2273 | 47.8 | 1686 | 48.5 | 1225 | 49.8 | 464 | 51.5 |
| Current | 375 | 7.9 | 283 | 8.1 | 231 | 9.4 | 121 | 13.4 |
| Body Mass Index (BMI)a | ||||||||
| <24.9 | 1550 | 32.6 | 1122 | 32.3 | 772 | 31.4 | 250 | 27.7 |
| 25-29.9 | 1984 | 41.8 | 1458 | 42.0 | 1029 | 41.9 | 368 | 40.8 |
| ≥30 | 1216 | 25.6 | 895 | 25.8 | 657 | 26.7 | 283 | 31.4 |
| Hypertension | ||||||||
| Normal | 2869 | 60.4 | 2076 | 59.7 | 1419 | 57.7 | 479 | 53.2 |
| Controlled | 1177 | 24.8 | 877 | 25.2 | 653 | 26.6 | 264 | 29.3 |
| Uncontrolled and treated | 346 | 7.3 | 252 | 7.2 | 187 | 7.6 | 76 | 8.4 |
| Uncontrolled and untreated | 361 | 7.6 | 271 | 7.8 | 200 | 8.1 | 82 | 9.1 |
| Angina | ||||||||
| No | 4264 | 89.7 | 3114 | 89.6 | 2168 | 88.2 | 774 | 85.9 |
| Yes | 489 | 10.3 | 362 | 10.4 | 291 | 11.8 | 127 | 14.1 |
| Diabetes | ||||||||
| No | 4357 | 91.7 | 3190 | 91.8 | 2249 | 91.5 | 814 | 90.3 |
| Yes | 396 | 8.3 | 286 | 8.2 | 210 | 8.5 | 87 | 9.7 |
3 with missing education, and 2 with missing BMI data
This study cohort includes AMD Category 1, 2, 3, and 4 participants
Effects of AREDS Formulation
Progression to Advanced AMD
Five years after the trial ended, assignment to the antioxidant plus zinc formulation in the AREDS clinical trial compared with assignment to placebo continued to be associated with a significantly reduced odds of developing advanced AMD in participants in AREDS categories 2, 3 and 4 at baseline (odds ratio [OR]0.69, 99% Confidence interval [CI]: 0.56-0.86, p=0.001) (Table 2). The odds ratio for the development of NV AMD was 0.64, 99% CI: 0.50–0.82, p<0.001 and the odds ratio for the development of CGA was 0.99, 99% CI: 0.69–1.43, p=0.975. In category 2, 3 and 4 participants randomly assigned to antioxidants alone at baseline, the ORs for the development of advanced AMD and, especially for the development of neovascular AMD, were also in the direction of benefit and statistically significant (Table 2).
Table 2.
Results of the Long Term (10 year) treatment with Antioxidants, Zinc, and Combination, The Age-Related Eye Disease Study (AREDS) Formulation
| Age-related Macular Degeneration (AMD) | AMD Categories 2, 3 and 4 | AMD Categories 3 and 4 | AMD Category 4 | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Outcome | Treatment | Odds Ratio (OR) (99% confidence Intervals (CI)) | P | OR (99% CI) | P | OR (99% CI) | P |
| Advanced AMD | Antioxidants | 0.74 (0.59-0.92) | 0.007 | 0.70 (0.56-0.88) | 0.002 | 0.64 (0.46-0.91) | 0.012 |
| Zinc | 0.87 (0.70-1.07) | 0.183 | 0.82 (0.66-1.02) | 0.081 | 0.68 (0.49-0.96) | 0.026 | |
| Antioxidants+Zinc | 0.69 (0.56-0.86) | 0.001 | 0.66 (0.53-0.83) | <.001 | 0.56 (0.40-0.79) | <.001 | |
| Neovascular AMD | Antioxidants | 0.73 (0.58-0.93) | 0.011 | 0.71 (0.56-0.91) | 0.007 | 0.69 (0.48-0.98) | 0.041 |
| Zinc | 0.83 (0.65-1.05) | 0.119 | 0.79 (0.62-1.00) | 0.054 | 0.65 (0.45-0.93) | 0.018 | |
| Antioxidants+Zinc | 0.64 (0.50-0.82) | <.001 | 0.60 (0.47-0.78) | <.001 | 0.44 (0.30-0.65) | <.001 | |
| Central Geographic Atrophy | Antioxidants | 0.76 (0.52-1.10) | 0.146 | 0.77 (0.53-1.11) | 0.162 | 0.73 (0.40-1.31) | 0.292 |
| Zinc | 1.12 (0.79-1.61) | 0.520 | 1.12 (0.79-1.59) | 0.528 | 1.16 (0.67-2.02) | 0.601 | |
| Antioxidants+Zinc | 0.99 (0.69-1.43) | 0.975 | 1.02 (0.71-1.45) | 0.927 | 1.43 (0.83-2.47) | 0.195 | |
| 15+ Letters Visual Acuity Loss | Antioxidants | 0.88 (0.73-1.06) | 0.185 | 0.83 (0.67-1.02) | 0.078 | 0.75 (0.53-1.06) | 0.106 |
| Zinc | 0.89 (0.74-1.08) | 0.232 | 0.86 (0.70-1.07) | 0.174 | 0.68 (0.48-0.96) | 0.031 | |
| Antioxidants+Zinc | 0.76 (0.63-0.93) | 0.007 | 0.71 (0.57-0.88) | 0.002 | 0.54 (0.38-0.78) | <.001 | |
| Visual Acuity <20/100 | Antioxidants | 0.87 (0.68-1.11) | 0.247 | 0.82 (0.64-1.07) | 0.140 | 0.76 (0.52-1.12) | 0.163 |
| Zinc | 0.91 (0.71-1.15) | 0.420 | 0.88 (0.69-1.14) | 0.331 | 0.66 (0.45-0.98) | 0.038 | |
| Antioxidants+Zinc | 0.75 (0.58-0.97) | 0.026 | 0.72 (0.56-0.94) | 0.015 | 0.58 (0.38-0.86) | 0.007 | |
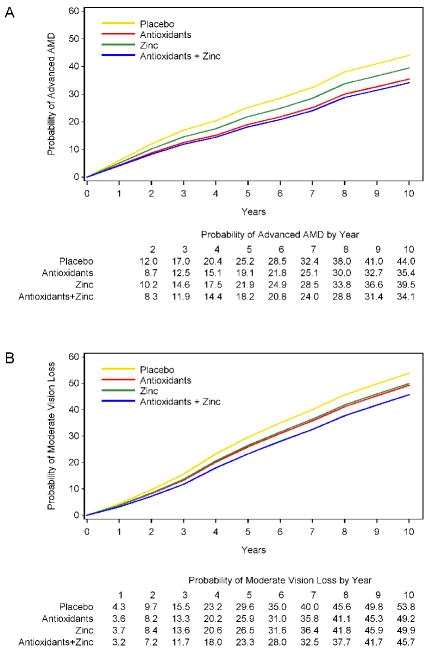
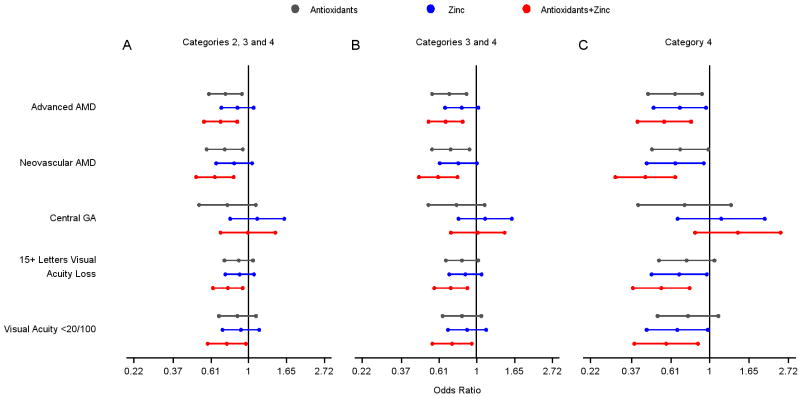
For participants in AREDS categories 3 and 4 (the group for whom treatment with the AREDS formulation has been recommended), assignment to the antioxidant plus zinc formulation in the clinical trial continued to be associated with significantly reduced odds of developing advanced AMD (OR 0.66, 99% CI: 0.53-0.83, p<.001) (Table 2 and Figure 1A). The rates of progression to advanced AMD at 10 years were 44% and 34% for participants assigned to placebo and the AREDS formulation (combined antioxidants and zinc), respectively (Figure 1A). The OR for developing NV AMD was 0.60, 99% CI: 0.47-0.78, p=<.001 (Figure 2 and Table 2). For CGA, the OR was 1.02, (99% CI: 0.71-1.45, p=0.927 (Figure 2 and Table 2). For AREDS category 3 and 4 participants there was also a statistically significant reduction in the odds of advanced AMD and neovascular AMD in those originally assigned to antioxidants alone.
Figure 1.
A. Repeated-measures estimates of the probability of development of advanced age-related macular degeneration (AMD) in at least 1 study eye of participants in Categories 3 and 4 adjusted by Age-Related Eye Disease Study (AREDS) categories and study visits. The study eye is an eye without evidence of advanced AMD, and with a visual acuity score of greater than 73 letters (20/32 or better) at baseline.
B. Repeated-measures estimates of the probability of a loss in the visual acuity score of at least 15 letters in at least 1 study eye of participants in age-related macular degeneration (AMD) Categories 3 and 4 by adjusted by AMD categories and study visits. The study eye is an eye without evidence of advanced AMD and with a visual acuity score greater than 73 letters (20/32 or better) at baseline.
Figure 2.
A, B, C. Odds ratios (central dot) and 99% confidence intervals (colored bars) for each original treatment assignment compared with placebo for participants in the following Age-Related Eye Disease Study (AREDS) AMD Categories: Figure A. Categories 2, 3 and 4; Figure B. Categories 3 and 4; and Figure C. Category 4. AMD is age-related macular degeneration, GA is geographic atrophy.
In separate analyses of category 3 and category 4 participants, only those in AMD category 4 had statistically significant reduced odds of developing advanced AMD (OR 0.56, 99% CI: 0.40-0.79, P0<.001) and neovascular AMD (OR of 0.44 (99% CI: 0.30-0.65, P<0.001) with assignment to the antioxidant plus zinc formulation (Figure 2 and Table2). For category 4 participants assigned to the zinc only arm or the antioxidant only arm, odds ratios for the development of advanced AMD were also in the direction of benefit.
Visual acuity loss
For participants in AREDS AMD Categories 2, 3, and 4 the risk of moderate vision loss, defined as 3 or more lines of vision loss, was reduced in those assigned to the antioxidant plus zinc supplement compared with those assigned to placebo; (OR 0.76, 99% CI: 0.63-0.93, p=0.007) (Figure 2 and Table 2). For more severe vision loss, worse than 20/100, the corresponding odds ratio was 0.75, 99% CI: 0.58-0.97, p=0.026 (Figure 2 and Table 2). In analyses restricted to participants in AMD categories 3 and 4, the rates of moderate vision loss were 53.8% for the placebo group and 45.7% for the AREDS formulation group at 10 years (Figure 1B). The odds ratio for developing moderate vision loss in the AREDS formulation versus placebo comparison was 0.71, 99% CI: 0.57-0.88, p=0.002. The corresponding odds ratio for the development of more severe vision loss (worse than 20/100) was 0.72 (99% CI: 0.56-0.94, p=0.015) (Figure 2 and Table 2) Again, in separate analyses of category 3 and category 4 participants the beneficial effects of the AREDS formulation and of zinc alone in the reduction of moderate vision loss or more severe vision loss were demonstrated only in the AREDS AMD category 4 group. For moderate vision loss, the OR for the AREDS formulation was 0.54 (99% CI: 0.38-0.78, P<0.001) and the OR for zinc alone was 0.68 (99% CI: 0.48-0.96, p=0.031). For more severe vision loss, the OR for the AREDS formulation was 0.58 (99% CI: 0.38-0.86, p=0.007) and for zinc alone was 0.66 (99% CI: 0.45-0.98, p=0.038).
Adverse Effects
No statistically significant increase in hospitalizations was associated with assignment to any of the AREDS supplements in the clinical trial during the 10-year follow-up in logistic regression analyses adjusted for age, sex, smoking status and treatment.
Morbidity and Mortality
With 10 years of follow-up, associations between mortality and baseline ocular and treatment characteristics were similar to those noted in the 2001 report on the AREDS clinical trial.3 In analyses that examined the main effects of antioxidants and zinc there was no statistically significant effect of antioxidant vitamins on mortality, HR (hazard ratio): 1.06, 95% CI: 0.93–1.21, p=0.39 (Table 3). However, participants randomized to zinc continued to show a reduction in all-cause mortality (HR: 0.83, 95% CI 0.73-0.95, p=0.008), largely related to a decrease in deaths from diseases of the circulatory system. Advanced AMD at baseline was again found to be associated with increased mortality, particularly death from cardiovascular disease (hazard ratio [HR]: 1.27, 95% CI: 1.05-1.54, p=0.01). Both nuclear cataract (HR: 1.29, 95% CI: 1.10-1.52, p=0.002) and cataract surgery HR: 1.30, 95% CI: 1.05-1.61 p=0.02 were again associated with increased all-cause mortality.
Table 3.
Associations of All-Cause and Cause-Specific Mortality with Baseline Ocular and Treatment Characteristics
| All-Cause Mortality | Circulatory System | Neoplasms | Other Causes | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Characteristic | Deaths/Total | Mortality Ratea | Hazard Ratiob (95%Confidence Interval (CI)) | Hazard Ratioc (95%CI) | Risk Ratioc (95%CI) | Risk Ratioc (95%CI) | Risk Ratioc (95%CI) |
| Age-Related Macular Degeneration (AMD) category | |||||||
| 1 | 191/1116 | 14 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 2 | 197/1060 | 16 | 1.02 (0.84–1.25) | 1.01 (0.83–1.24) | 1.15 (0.81–1.64) | 0.91 (0.64–1.29) | 0.94 (0.66–1.35) |
| 3 | 349/1620 | 19 | 1.09 (0.91–1.30) | 1.01 (0.85-1.21) | 1.28 (0.93–1.75) | 0.80 (0.58–1.10) | 0.99 (0.72–1.36) |
| 4 | 320/957 | 28 | 1.62 (1.35–1.95) | 1.27 (1.05–1.54) | 1.58 (1.14–2.20) | 1.12 (0.80–1.56) | 1.18 (0.84–1.86) |
| Nuclear opacityd | |||||||
| Grade <4 in available eye(s) | 819/4001 | 17 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Grade ≥4 in at least 1 eye | 191/617 | 28 | 1.30 (1.10–1.53) | 1.29 (1.10–1.52) | 1.31 (1.00–1.71) | 1.29 (0.95–1.75) | 1.29 (0.97–1.71) |
| Cortical opacityd | |||||||
| ≤5% in available eye(s) | 870/4090 | 17 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| >5% in at least 1 eye | 139/524 | 26 | 1.13 (0.94–1.36) | 1.06 (0.88–1.27) | 1.15 (0.86–1.54) | 1.10 (0.78–1.55) | 0.89 (0.83–1.26) |
| Posterior Subcapsular Cataract (PSC)d | |||||||
| ≤5% in available eye(s) | 979/4503 | 18 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| >5% in at least 1 eye | 30/111 | 26 | 1.20 (0.83–1.73) | 1.11 (0.77–1.61) | 1.14 (0.64–2.03) | 1.22 (0.63–2.38) | 1.08 (0.56-2.11) |
| Cataract surgery | |||||||
| No | 961/4464 | 18 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 96/289 | 32 | 1.38 (1.11–1.70) | 1.30 (1.05–1.61) | 1.38 (0.99–1.92) | 1.49 (1.01–2.20) | 1.01 (0.66–1.55) |
| AREDS treatmente | |||||||
| Antioxidants main effect | |||||||
| No antioxidants | 427/1806 | 20 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Antioxidants | 439/1831 | 20 | 1.06 (0.93–1.21) | 1.06 (0.93–1.21) | 1.20 (0.97–1.49) | 1.07 (0.83–1.38) | 0.94 (0.74–1.20) |
| Zinc main effect | |||||||
| No zinc | 465/1847 | 21 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Zinc | 401/1790 | 19 | 0.87 (0.76–0.99) | 0.83 (0.73–0.95) | 0.80 (0.64–0.99) | 0.84 (0.65–1.08) | 0.93 (0.73–1.18) |
Age and sex- adjusted mortality for median follow-up (10.5 years)
Age and sex-adjusted risk ratios
Adjusted for significant covariates: age, sex, race, education, smoking status, body mass index, diabetes, angina, cancer, and hypertension
135 participants without slit-lamp photographs and 139 participants without retroillumination photographs
AMD category 2, 3 and 4 participants only
Discussion
The Age-Related Eye Disease Study (AREDS) was an 11-center double-masked clinical trial designed to evaluate the effect of high-dose vitamins and zinc on AMD progression and visual acuity. In 2001, after an average follow-up time of 6.3 years, the study reported that treatment with a combination of antioxidants and zinc reduced the risk of progression to advanced AMD in participants in AREDS categories 2, 3 and 4 [OR 0.72, 99% confidence interval [CI]: 0.52-0.98, P=0.007. The risk reduction for those taking the formulation was about 25%. Because so few events were noted for category 2 participants (1.3% by year 5), analyses were also done for those most likely to benefit from an effective treatment, AMD categories 3 and 4, for whom the 5 year event rates were 18% and 43%, respectively. Comparisons with placebo found a statistically significant risk reduction for antioxidants plus zinc (OR 0.66, 99% CI: 0.47-0.91, P=0.001). At the conclusion of the trial, the study group recommended that persons with at least moderate risk of progression to advanced AMD (categories 3 and 4) should consider taking a supplement similar to the AREDS antioxidant plus zinc formulation.
The AREDS cohort continued to be followed until 2005 with careful monitoring of visual acuity and progression of AMD. Because the AREDS formulation was not available immediately after the trial ended, few participants took the AREDS formulation during the first two years of the observational period. The AREDS supplement became available in 2003 and, by the end of follow-up in 2005, about 70% of participants were taking the supplement which was provided by the study. Equal proportions of participants across the four original treatment groups took the supplement in the follow-up phase. Despite the non-availability of the AREDS supplement in the first 2 years of follow-up and then the use of the supplement by the majority of participants in the last three years of follow-up, the beneficial effect of original assignment to antioxidant plus zinc formulation persisted. By year 10, 44% of category 3 and 4 participants originally randomized to placebo had progressed to advanced AMD compared with 34% of those originally randomized to antioxidants and zinc. Also, by the end of the follow-up study participants originally assigned to the antioxidant and zinc formulation compared with those assigned to placebo had a reduced risk of both at least moderate vision loss (≥ 3 lines) and more severe vision loss (worse than 20/100).
Separate analyses of category 3 and category 4 participants showed that much of the beneficial effect of the AREDS formulation on progression to advanced AMD and vision loss was driven by the category 4 participants. Point estimates for category 3 participants were in a beneficial direction but not statistically significant. The smaller number of AMD and vision loss events in category 3 participants may have limited the power to detect associations for this group.
Analyses of the components of the AREDS definition of advanced AMD, development of neovascular disease and GA involving the center of the macula, were performed on participants in categories 3 and 4. A statistically significant benefit of treatment with antioxidants plus zinc compared with placebo was observed for neovascular AMD outcomes but not for the development of GA involving the center of the macula. These results are similar to those reported after the clinical trial ended.
It is interesting to note that the persistence of the beneficial effect of tested therapy or therapies in extended follow-up after the cessation of a randomized controlled clinical trial has been demonstrated in other trials when the follow-up exceeded the length of the clinical trial. The Diabetes Control and Complications Trial found a beneficial effect of intensive glycemic control compared with the conventional glycemic control in reducing both the development and progression of diabetic retinopathy.4 This study was extended as an epidemiologic study with additional follow-up through year 10. During follow-up, the measures of glycemic control of both treatment groups became almost equivalent but yet the beneficial effects of the intensive glycemic control persisted, albeit somewhat attenuated at 10 years.5 Investigators speculated that this may be due to a “metabolic imprinting” which may be secondary to a slow accumulation and subsequent slow degradation of glycation endproducts.6 Alternatively, it could be an epigenetic phenomenon effects or a combination of these 2 speculations. Following stopping the randomized clinical trial portion of the study, continued follow-up also resulted in persistence of beneficial effects of both focal and scattered laser photocoagulation for diabetic retinopathy.6,7 Similar beneficial results were also found in the longer follow-up of participants originally enrolled in a study of aspirin use for the prevention of cardiovascular morbidity and mortality.8
Possible long-term adverse effects of the original AREDS treatment assignments were evaluated out to 10 years of follow-up also. No serious adverse effects were noted. No statistically significant effect on mortality was seen in participants randomized to antioxidant vitamins. In those who were randomized to zinc, there appears to be a statistically significant reduction in mortality, mostly accounted for by a reduction in cardiovascular deaths. Further investigation in this area is warranted.
Several factors need to be considered when interpreting results from the follow-up study. First, the AREDS population differs from the general population in several respects. It is better nourished, more highly educated and healthier. The effect of this on generalizability of the results to the general population is unknown. Secondly, the treatment effect is relatively modest and AMD and vision loss events continue to occur in participants taking the AREDS formulation. Thirdly, we still do not know how long someone at risk of advanced AMD should take the supplements. Finally, it is important to remember that the results reported here are from an observational follow-up study of original treatment assignments in the clinical trial. By the end of follow-up about 70% of the cohort was taking the AREDS formulation. It is not possible to determine the effect of this on the results. However, it is encouraging that the results of the original randomized contrast persisted long after the clinical trial ended.
Strengths of the follow-up study are many. The study followed a very large cohort of subjects at moderate to high risk of progression to advanced AMD. Approximately 12% of the AREDS population died prior to the beginning of the follow-up study with no differential survival according to the treatment group. Approximately 84% of the surviving cohort was followed in the observational portion of the study. The rates of loss to follow-up were extraordinarily low in both the clinical trial (2%) and in the follow-up study (4%). Major ocular outcome measurements were determined centrally using standardized procedures. The rigorous design of the original clinical trial, the high proportion of participants who participated in the follow-up study, the low rate of losses to follow-up and the careful monitoring of end points suggest that the long term findings are representative of this clinic population.
In summary, participants in the AREDS clinical trial who had been assigned to the antioxidant and zinc formulation continued to show a reduced odds of developing advanced AMD, especially neovascular AMD, 5 years after the clinical trial ended. We continue to recommend the use of the AREDS formulation in persons with intermediate AMD or advanced AMD in one eye, persons at moderate to high risk of developing advanced AMD. Although much of the benefit of the AREDS formulation is driven by efficacy in decreasing the development of NV AMD and not CGA, we believe that all participants with AREDS AMD category 3 and 4 characteristics should consider taking the AREDS formulation. The development of neovascularization in patients with CGA may occur as frequently as 40% in 10 years. (AREDS data, submitted for publication). Thus the simultaneous occurrence of both forms of advanced AMD is common.
Further evaluation of the AREDS formulation is currently underway in the AREDS2 study. AREDS2 is randomized controlled clinical trial primarily designed to determine the effects of lutein/zeaxanthin and omega-3 long-chain polyunsaturated fatty acids on progression of AMD. Until the results from AREDS2 become available, the AREDS formulation remains the treatment of choice for persons with intermediate AMD and advanced AMD in one eye.
Acknowledgments
Financial Support: This study is supported by the intramural program funds and contracts AREDS (Contract NOI-EY-0-2127) from the National Eye Institute/National Institutes of Health, Department of Health and Human Services, Bethesda, MD.
Footnotes
No authors have any financial/conflicting interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Age-Related Eye Disease Study Group. A randomized, placebo-controlled clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1. Control Clin Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.AREDS Research Group. Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study: AREDS report no. 13. Arch Ophthalmol. 2004;122:716–26. doi: 10.1001/archopht.122.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes: 10 years after the Diabetes Control and Complications Trial. Arch Ophthalmol. 2008;126:1707–15. doi: 10.1001/archopht.126.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;98(suppl):766–85. [PubMed] [Google Scholar]
- 7.Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy (DRS) findings, DRS report number 8. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- 8.Cook NR, Hebert PR, Manson JE, et al. Self-selected posttrial aspirin use and subsequent cardiovascular disease and mortality in the Physicians’ Health Study. Arch Intern Med. 2000;160:921–8. doi: 10.1001/archinte.160.7.921. [DOI] [PubMed] [Google Scholar]