Abstract
Pregnancy complications such as preeclampsia and diabetes affect approximately 5 to 10 % of all pregnancies and compromise maternal and fetal health during gestation. Complications during pregnancy may also contribute to the development of hypertension and future cardiovascular risk in the mother. Moreover, fetal exposure to hypertension and diabetes during pregnancy can program hypertension and cardiovascular disease in the offspring. Transgenerational transmission of programmed cardiovascular risk highlights the importance of understanding the mechanisms that link complications during pregnancy with later hypertension in her offspring and subsequent generations. However, experimental studies are needed to investigate the cause and effect of increased blood pressure in the mother following a complicated pregnancy and provide insight into the development of preventative measures that may improve the long-term cardiovascular health of women and their offspring.
Keywords: Pregnancy complications, Hypertension, Preeclampsia, Diabetes, Fetal programming, Transgenerational
Introduction
Pregnancy is associated with profound physiological and anatomical changes in the mother that occur in order to meet the growing metabolic needs of the developing fetus and placenta [1]. However, complications such as hypertension and diabetes that occur during pregnancy compromise the health of the mother and the fetus [2, 3] (Fig. 1). Recent epidemiological studies suggest that adverse consequences of complications during pregnancy can persist well beyond the gestational period in the mother and child [4, 5] (Fig. 1). Experimental studies providing mechanistic insight into the causative factors linked to future risk of hypertension in the mother are limited. However, experimental studies investigating the mechanisms that contribute to the development of hypertension in the offspring of mothers with complications during pregnancy are providing significant insight into the etiology of fetal programming of chronic disease. This review highlights the impact of complications during pregnancy on future hypertension and cardiovascular (CV) in both the mother and child with a particular emphasis on potential mechanism.
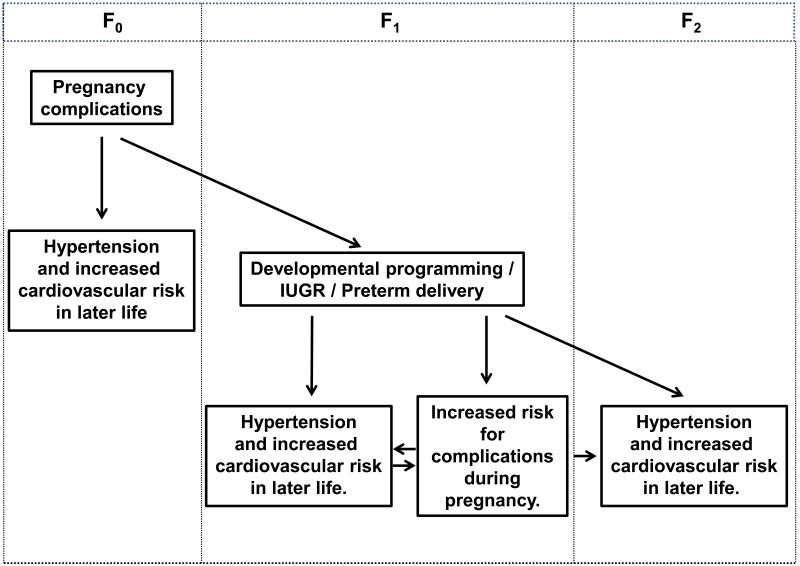
Fig. 1.
This figure highlights the impact of complications during pregnancy on future hypertension and cardiovascular risk in the mother and the fetal programming of hypertension and adverse gestational health in the offspring
Pregnancy Complications and Maternal Hypertension and Cardiovascular Health
Preeclampsia is a pregnancy-specific disorder that is a leading cause of maternal and fetal mortality and morbidity [3]. Preeclampsia is defined as new onset of an elevated blood pressure of 140/90 mmHg or more in the presence of at least 0.3 grams of protein in a 24 hour urine sample [6]. Risk factors for the development of preeclampsia include extreme ages, history of diabetes, hypertension, and obesity [7.]. Obesity is also a risk factor for the development of diabetes during pregnancy [8]. Gestational diabetes mellitus (GDM), defined as any degree of glucose intolerance with onset or first recognition during pregnancy [9], is also a common complication during pregnancy [2].
Epidemiologic Studies
Recent studies indicate that women with preeclampsia exhibit an increased risk for hypertension and CV disease after pregnancy [4, 10-12], preterm delivery and/or intrauterine growth restriction (IUGR) magnify this risk [13, 14]. Moreover, the risk of death from CV disease is higher in women that develop early-onset (< 32 weeks gestation) preeclampsia compared to women with late-onset (>32 weeks gestation) preeclampsia [10]. The association of hypertension in pregnancy and future CV risk is significantly enhanced in women that experience hypertension in more than one pregnancy [12]. Moreover, IUGR, similar to preeclampsia, is a condition related to placental health, and a pregnancy complicated by IUGR can serve as a risk factor for future CV disease in the mother [15]. GDM is also associated with higher blood pressure [16] and increased CV risk following delivery [4, 17]. However, the exact mechanisms linking complications during pregnancy with later CV risk in the mother including hypertension remain unclear. Moreover, whether complications during pregnancy associated with future hypertension and increased CV disease in the mother share a common genetic or environmental cause or whether postpartum hypertension and increased CV risk originate as a consequence of the adverse events that occur during a complicated pregnancy is not clear.
Potential Mechanisms
Preeclampsia and CV disease share many pathophysiological mechanisms including high blood pressure, increased body mass index (BMI) and endothelial dysfunction [18]. These CV risk factors may be present prior to pregnancy [19], and thus, not only predispose a woman to hypertension during pregnancy [12], but may also be a more important determinant in the development of hypertension in later life than the existence of increased blood pressure during pregnancy [18]. Although within the normotensive range, blood pressure is higher following a pregnancy complicated by new-onset hypertension [20] relative to women with a history of a normotensive pregnancy. Furthermore, elevations in blood pressure are reported as early as 12 weeks [21] and up to 10 [22, 23] to 20 years postpartum [24, 25]. Often, hypertension in women with a history of a preeclamptic pregnancy is associated with a higher BMI [12, 22]. Postpartum increases in blood pressure are also associated with impaired flow-mediated vasodilation [26]. Yet, whether endothelial dysfunction persists following a preeclamptic pregnancy is controversial. Evans et al. reported that stress-induced endothelial function is impaired in conjunction with an increase in MAP at 16 months post-partum in women with prior preeclampsia [27]. However, mediators of endothelial dysfunction such as cellular fibronectin or E-selectin were not altered in this study [27]. Yinon et al. reported that impaired flow-mediated vasodilation is noted only in women with early-onset preeclampsia or delivery of an IUGR infant [28]. However, this impairment was not associated with a change in expression of angiogenic factors such as sFlt-1 [28], a critical mediator of preeclampsia [3]. Yet, other studies noted that flow-mediated dilatation is normalized by 12 weeks postpartum [21], or that despite a marked increase in SBP, endothelial function does not differ at 5 to 8 years postpartum in women with prior preeclampsia unless complicated by delivery of an SGA infant [29]. Thus preeclampsia is associated with risk factors linked to CV disease. However, whether preeclampsia serves as an independent risk factor for later hypertension and CV disease due to the development of factors that develop as a consequence of a preeclamptic pregnancy is not yet defined.
The etiology of preeclampsia involves activation of several pathways that contribute to the development of hypertension in this pregnancy-specific disorder [3]. Increases in anti-angiogenic factors such as soluble fms-like Tyrosine Kinase 1 (sFlt-1) and soluble Endoglin (sEng) are observed during preeclampsia and the relevance for an increase in these angiogenic factors in the etiology of preeclampsia is supported by experimental studies [30]. Abnormal activation of maternal inflammatory responses and the formation of reactive oxygen species are also thought to contribute to the pathogenesis of the hypertension in preeclampsia [3]. Increased risk for hypertension and CV disease in women with a prior history of preeclampsia is proposed to involve sustained activation of these pathways. However, whether alterations in angiogenic factors persist after a preeclamptic pregnancy is not clear. Kvehaugen et al. noted that a marked elevation in s-Flt-1 in women 5 to 8 years post-partum of a preeclamptic pregnancy is associated with a marked increase in blood pressure [29]. However, in other studies an increase in blood pressure is not associated with an increase in sFlt-1 levels [20] regardless of the length of follow-up; 12 weeks [21] or 10 years postpartum [31]. Investigation into the importance of oxidative stress in the development of hypertension in women with a history of preeclampsia is very limited but one study reports a marked elevation in mean arterial pressure at 16 months postpartum in women with prior preeclampsia is not associated with an increase in markers of oxidative stress [27]. Evidence from different experimental models of hypertension implicates a role for oxidative stress in mediating increased blood pressure in males, but not females [32]. Thus, oxidative stress may not be a contributory factor in the development of hypertension in women following a preeclamptic pregnancy. Inflammatory cytokines such as tumor necrosis factor (TNF)-alpha are elevated in the maternal serum of pregnancies complicated by preeclampsia [33] and experimental studies demonstrate a direct causative role for TNF-alpha in the etiology of hypertension during pregnancy [34]. TNF-alpha levels remain elevated in women following a preeclamptic pregnancy [35]. However, experimental studies indicate that placental factors associated with the hormonal environment of a pregnancy are required for cytokine-induced hypertension in females [34, 36] indicating that an increase in an inflammatory cytokine such as TNF-alpha may not be sufficient to induce hypertension in the absence of other confounding factors.
Increased sympathetic nervous system (SNS) [37] and/or renin angiotensin system (RAS) [38] activation contribute to the etiology of hypertension in many experimental models of hypertension. Yet, the exact contribution of these regulatory systems to the development of hypertension and increased CV risk following a hypertensive disorder during pregnancy has not been fully elucidated. Two studies suggest that chronic activation of the SNS does not contribute to the increase in blood pressure observed in later life in women with a history of a hypertensive pregnancy [12, 39]. However, alterations in sensitivity to the RAS may be a key mediator. Enhanced sensitivity to angiotensin II (Ang II), a hallmark of preeclampsia, is present at 8 months postpartum following a pregnancy complicated by preeclampsia [20]. Circulating agnostic angiotensin II type 1 autoantibodies (AAT-AAS) that bind to the angiotensin type 1 receptor (AT1R) develop in women with preeclampsia [40] and are reported to remain elevated in a subset of women 18 months after a preeclamptic pregnancy [41]. Thus, the mechanism involved in mediating enhanced sensitivity to Ang II in women with a history of preeclampsia is not yet apparent, but the etiology may entail a sustained increase in the AAT-AAS which may also serve as a component of the increased risk for hypertension and CV disease in women following preeclampsia.
Recent studies indicate that epigenetic mechanisms may contribute to complications during pregnancy [42]. Epigenetic processes alter heritable changes in gene function without changing the nucleotide sequence [43]. Only a few studies have directly tested the importance of epigenetics in the pathophysiology of preeclampsia [44, 45] and the exact physiological relevance of these findings is unclear. However, epigenetic modifications that contribute to preeclampsia may also underlie the development of hypertension and increased CV risk in women with a history of preeclampsia.
Diabetes in Pregnancy and Vascular Dysfunction
Although glucose tolerance returns to normal in most women with GDM, GDM is a known risk factor for the development of later CV disease [46]. Only a few studies have investigated potential mechanisms related to later hypertension and increased CV risk following a pregnancy complicated by diabetes; however, endothelial dysfunction, a marker of CV risk [47], may be a contributing factor. Endothelial dysfunction is observed in women with prior GDM despite current normal glucose tolerance and regardless of current BMI [48]. Impaired small artery function is observed as early as 2 years postpartum after GDM in women with normal glycemic control [49] suggesting that risk for later CV disease may have its origins long before adverse CV events transpire. The mechanisms mediating impaired vascular function following GDM may involve alterations in angiogenic factors. Pro-atherogenic markers such as plasminogen activator inhibitor-1 (PAI-1) are elevated in women with GDM relative to age-matched controls in late gestation [50]. Vascular dysfunction in women with prior GDM is associated with sustained increases in PAI-1 [51] suggesting that events initiated during GDM, may persist into later life and contribute to later CV risk.
To summarize, complications during pregnancy that alter the health of the mother can also lead to the development of hypertension and thus, increased CV risk in later life (Fig. 1). Additional studies are needed to identify the exact mechanisms involved. Moreover, use of experimental models will allow investigation into cause and effect and provide insight into preventative measures that may improve the long-term CV health of women that experience complications such as hypertension or diabetes during pregnancy.
Pregnancy Complications and Offspring Cardiovascular Health
The maternal-fetal interface ensures an environment during pregnancy that is able to maintain and nurture the development of the fetus [52]. Alterations in the formation of the maternal-fetal interface can occur during pregnancy-specific disorders such as preeclampsia and lead to IUGR [53] or preterm delivery [54]. IUGR can also be a consequence of a diabetic pregnancy [2]. Numerous studies now implicate the impact of a pregnancy complicated by hypertension or diabetes on the development of hypertension and CV risk in the offspring (Fig. 1). Importantly, experimental studies are providing significant insight into the mechanisms that link adverse influences during fetal life and the etiology of fetal programming of hypertension [55].
Epidemiological Studies
In addition to an increased CV risk in the mother, children of preeclamptic pregnancies are at an increased risk to develop hypertension and CV complications in later life [5, 56, 57]. CV risk factors such as increased blood pressure [5, 56] and BMI are observed in children of preeclamptic pregnancies [5]. In addition, hypertension during pregnancy is a risk factor for intrauterine growth restriction (IUGR) or preterm delivery [58]. Higher blood pressure in adult life is observed in individuals born with IUGR relative to normal birth weight for gestation [57]. Preterm birth (<37 weeks gestation) is also associated with higher systolic blood pressure [59]. Low birth weight is not only a risk factor for increased blood pressure [60], but is also associated with an increased risk for diabetes, stroke, heart attack or heart disease in offspring of complicated pregnancies [61]. Children exposed to diabetes during fetal life also exhibit an increase in blood pressure associated with an increase in BMI [62, 63], an association that remains after adjustment for current body weight [64]. Thus, fetal exposure to complications during pregnancy programs hypertension and increased CV risk in the offspring. Experimental studies substantiate the associations noted by epidemiological reports and are providing insight into the mechanisms linking complications during pregnancy and hypertension and increased CV risk in the offspring.
Potenial Mechanisms
Numerous experimental models are utilized to study the impact of an abnormal intrauterine milieu on later CV health [55]. These include models induced via placental insufficiency, under-nutrition, diabetes, or obesity [65]. Insight into the mechanisms leading to the fetal programming of hypertension and later CV risk are provided by these diverse experimental models and despite the experimental model utilized, implicate an important role for the RAS [66, 67], the SNS [68, 69], oxidative stress [70-72] and impaired vascular function [70] (Extensive reviews are provided by [5, 55, 73-75].). Recent studies indicate that alterations in blood pressure and vascular dysfunction are transmitted to the F2 generation in the absence of a maternal insult in the programmed F1 generation [76, 77]. In addition to serving as a mediator of later maternal health in response to complications during pregnancy, recent studies indicate that epigenetics may also contribute to programmed hypertension and increased CV risk in the offspring of complicated pregnancies [78, 79] (review [80]). Moreover, epigenetic mechanisms may also be responsible for the transgenerational passage of hypertension and vascular dysfunction from the F1 to the F2 generation [81]. Several studies indicate that the fetal response to complications during pregnancy results in sex differences in adult blood pressure (for extensive reviews see: [82, 83]). Aging also impacts CV risk programmed by in utero insults [84]. Thus, these findings highlight the need for additional experimental studies to elucidate the mechanisms and additional physiological influences that mediate programming of hypertension and CV risk in offspring of a complicated pregnancy.
Risk for a Complicated Pregnancy in Women Born with low Birth Weight
The risk of preeclampsia is increased in women born preterm or in low birth weight women [85, 86], obesity amplifies this risk [87]. Women with low birth weight are also at risk for glucose intolerance and diabetes during gestation [85, 88]. Moreover, women born with low birth weight exhibit a greater risk of preterm delivery [89], an observation enhanced by obesity [90]. Experimental studies are limited but Gallo et al. report that rats born small exhibit glucose intolerance during late pregnancy [91] that may be linked to enhanced basal hepatic insulin sensitivity [92]. Thus, exposure to complications during pregnancy in one generation has far reaching consequences on the gestational health of future generations. When the additive effect of developmental programming of later CV risk is considered, the impact of a complicated pregnancy in one generation is a significant event that transcends the effect on maternal health to include the gestational and CV health of future generations (Fig. 1).
Conclusions
Despite the significant number of studies that implicate increased blood pressure and CV risk in women following a pregnancy complicated by hypertension or diabetes, little is known regarding the mechanisms that link this association. Insight into the mechanisms that mediate the development of hypertension and increased CV risk as it accumulates across the lifespan following a complicated pregnancy will provide a basis for the development of future preventative strategies to alleviate the adverse influence of a complicated pregnancy on later maternal health. The adverse effect of a complicated pregnancy on the CV health of the offspring is well documented and significant insight into the potential mechanisms that link insults during fetal life with the development of hypertension in offspring are being elucidated by experimental studies. However, evidence indicating the transgenerational transmission of hypertension highlights the importance of understanding how risk transmits to the next generation. Insight into the mechanisms that mediate the development of hypertension in offspring programmed in response to a complicated pregnancy may alleviate the transmission of increased CV disease risk to subsequent generations.
Acknowledgments
Dr. Alexander is supported by NIH grants HL074927 and HL51971. Dr. Intapad is supported by an American Heart Association, Post-doctoral Fellowship grant, 12POST11980021.
Footnotes
Conflict of Interest:
Suttira Intapad declares that she has no conflict of interest.
Barbara T. Alexander declares that she has no conflict of interest.
References
- 1.Chang J, Streitman D. Physiologic adaptations to pregnancy. Neurol Clin. 2012;30(3):781–9. doi: 10.1016/j.ncl.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham FG, Gant NF, Leveno KJ, et al. Diabetes. In: Cunningham FG, Gant NF, Leveno KJ, et al., editors. Williams Obstetrics. 21st ed. McGraw-Hill; New York, NY: 2001. pp. 1359–81. [Google Scholar]
- 3.George EM, Granger JP. Mechanisms and potential therapies for preeclampsia. Curr Hypertens Rep. 2011;13(4):269–75. doi: 10.1007/s11906-011-0204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125(11):1367–80. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552–61. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 6.ACOG Practice Bulletin, Clinical Management Guidelines for Obstetrician-Gynecologists. 2002 Jan;(33) [Google Scholar]
- 7.Wagner SJ, Barac S, Garovic VD. Hypertensive pregnancy disorders: current concepts. J Clin Hypertens (Greenwich) 2007;9(7):560–6. doi: 10.1111/j.1524-6175.2007.06695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salihu HM, De La Cruz C, Rahman S, August EM. Does maternal obesity cause preeclampsia? A systematic review of the evidence. Minerva Ginecol. 2012;64(4):259–80. [PubMed] [Google Scholar]
- 9.American Diabetes Association Diagnosis and classification of diabetes mellitis. Diabetes Care. 2006;29:s43–s48. [PubMed] [Google Scholar]
- 10.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010;56(1):166–71. doi: 10.1161/HYPERTENSIONAHA.110.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009;114(5):961–70. doi: 10.1097/AOG.0b013e3181bb0dfc. [DOI] [PubMed] [Google Scholar]
- 12.Mangos GJ, Spaan JJ, Pirabhahar S, Brown MA. Markers of cardiovascular disease risk after hypertension in pregnancy. J Hypertens. 2012;30(2):351–8. doi: 10.1097/HJH.0b013e32834e5ac7. [DOI] [PubMed] [Google Scholar]
- 13.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 14.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis. 2004;175(2):189–202. doi: 10.1016/j.atherosclerosis.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 15.Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Maternal cardiovascular impairment in pregnancies complicated by severe fetal growth restriction. Hypertension. 2012;60(2):437–43. doi: 10.1161/HYPERTENSIONAHA.112.194159. [DOI] [PubMed] [Google Scholar]
- 16.Meyers-Seifer CH, Vohr BR. Lipid levels in former gestational diabetic mothers. Diabetes Care. 1996;19(12):1351–6. doi: 10.2337/diacare.19.12.1351. [DOI] [PubMed] [Google Scholar]
- 17.Freibert SM, Mannino DM, Bush H, Crofford LJ. The association of adverse pregnancy events and cardiovascular disease in women 50 years of age and older. J Women Health (Larchmt) 2011;20(2):287–93. doi: 10.1089/jwh.2010.2097. [DOI] [PubMed] [Google Scholar]
- 18.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation. 2010;122(6):579–84. doi: 10.1161/CIRCULATIONAHA.110.943407. [DOI] [PubMed] [Google Scholar]
- 19.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161(5):1200–4. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 20.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension. 2010;55(5):1239–45. doi: 10.1161/HYPERTENSIONAHA.109.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122(5):478–87. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 22.Canti IC, Komlos M, Martins-Costa SH, Ramos JG, Capp E, Corleta H. Risk factors for cardiovascular disease ten years after preeclampsia. Sao Paulo Med J. 2010;128(1):10–3. doi: 10.1590/S1516-31802010000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drost JT, Arpaci G, Ottervanger JP, de Boer MJ, van Eyck J, van der Schouw YT, et al. Cardiovascular risk factors in women 10 years post early preeclampsia: the Preeclampsia Risk EValuation in FEMales study (PREVFEM) Eur J Prev Cardiol. 2012;19(5):1138–44. doi: 10.1177/1741826711421079. [DOI] [PubMed] [Google Scholar]
- 24.Marin R, Gorostidi M, Portal CG, Sanchez M, Sanchez E, Alvarez J. Long-term prognosis of hypertension in pregnancy. Hypertens Pregnancy. 2000;19(2):199–209. doi: 10.1081/prg-100100136. [DOI] [PubMed] [Google Scholar]
- 25.Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42(1):39–42. doi: 10.1161/01.HYP.0000074428.11168.EE. [DOI] [PubMed] [Google Scholar]
- 26.Hamad RR, Eriksson MJ, Silveira A, Hamsten A, Bremme K. Decreased flow-mediated dilation is present 1 year after a pre-eclamptic pregnancy. J Hypertens. 2007;25(11):2301–7. doi: 10.1097/HJH.0b013e3282ef5fc0. [DOI] [PubMed] [Google Scholar]
- 27.Evans CS, Gooch L, Flotta D, Lykins D, Powers RW, Landsittel D, et al. Cardiovascular system during the postpartum state in women with a history of preeclampsia. Hypertension. 2011;58(1):57–62. doi: 10.1161/HYPERTENSIONAHA.111.173278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yinon Y, Kingdom JC, Odutayo A, Moineddin R, Drewlo S, Lai V, et al. Vascular dysfunction in women with a history of preeclampsia and intrauterine growth restriction: insights into future vascular risk. Circulation. 2010;122(18):1846–53. doi: 10.1161/CIRCULATIONAHA.110.948455. [DOI] [PubMed] [Google Scholar]
- 29.Kvehaugen AS, Dechend R, Ramstad HB, Troisi R, Fugelseth D, Staff AC. Endothelial function and circulating biomarkers are disturbed in women and children after preeclampsia. Hypertension. 2011;58(1):63–9. doi: 10.1161/HYPERTENSIONAHA.111.172387. [DOI] [PubMed] [Google Scholar]
- 30.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 31.Gaugler-Senden IP, Tamsma JT, van der Bent C, Kusters R, Steegers EA, de Groot CJ. Angiogenic factors in women ten years after severe very early onset preeclampsia. PLoS One. 2012;7(8):e43637. doi: 10.1371/journal.pone.0043637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Ruiz A, Sartori-Valinotti J, Yanes LL, Iliescu R, Reckelhoff JF. Sex differences in control of blood pressure: role of oxidative stress in hypertension in females. Am J Physiol Heart Circ Physiol. 2008;295(2):H466–74. doi: 10.1152/ajpheart.01232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tosun M, Celik H, Avci B, Yavuz E, Alper T, Malatyalioglu E. Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-alpha in normal pregnancies and in pregnancies complicated by preeclampsia. J Matern Fetal Neonatal Med. 2010;23(8):880–6. doi: 10.3109/14767051003774942. [DOI] [PubMed] [Google Scholar]
- 34.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens. 2002;15(2 Pt 1):170–5. doi: 10.1016/s0895-7061(01)02255-5. [DOI] [PubMed] [Google Scholar]
- 35.Vitoratos N, Economou E, Iavazzo C, Panoulis K, Creatsas G. Maternal serum levels of TNF-alpha and IL-6 long after delivery in preeclamptic and normotensive pregnant women. Mediators Inflamm. 2010;2010:908649. doi: 10.1155/2010/908649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep. 2007;9(6):480–5. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- 37.DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11(2):197–200. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Crowley SD, Coffman TM. Recent advances involving the renin-angiotensin system. Exp Cell Res. 2012;318(9):1049–56. doi: 10.1016/j.yexcr.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collen AC, Manhem K, Sverrisdottir YB. Sympathetic nerve activity in women 40 years after a hypertensive pregnancy. J Hypertens. 2012;30(6):1203–10. doi: 10.1097/HJH.0b013e3283531ed2. [DOI] [PubMed] [Google Scholar]
- 40.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–52. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hubel CA, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts JM, et al. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension. 2007;49(3):612–7. doi: 10.1161/01.HYP.0000256565.20983.d4. [DOI] [PubMed] [Google Scholar]
- 42.Choudhury M, Friedman JE. Epigenetics and microRNAs in preeclampsia. Clin Exp Hypertens. 2012;34(5):334–41. doi: 10.3109/10641963.2011.649931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinhold B. Epigenetics: the science of change. Environ Health Perspect. 2006;114(3):A160–7. doi: 10.1289/ehp.114-a160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulkarni A, Chavan-Gautam P, Mehendale S, Yadav H, Joshi S. Global DNA methylation patterns in placenta and its association with maternal hypertension in pre-eclampsia. DNA Cell Biol. 2011;30(2):79–84. doi: 10.1089/dna.2010.1084. [DOI] [PubMed] [Google Scholar]
- 45.Mousa AA, Archer KJ, Cappello R, Estrada-Gutierrez G, Isaacs CR, Strauss JF, et al. DNA methylation is altered in maternal blood vessels of women with preeclampsia. Reprod Sci. (3rd) 2012;19(12):1332–42. doi: 10.1177/1933719112450336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan SD, Umans JG, Ratner R. Gestational diabetes: implications for cardiovascular health. Curr Diab Rep. 2012;12(1):43–52. doi: 10.1007/s11892-011-0238-3. [DOI] [PubMed] [Google Scholar]
- 47.Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomark Med. 2010;4(3):351–60. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anastasiou E, Lekakis JP, Alevizaki M, Papamichael CM, Megas J, Souvatzoglou A, et al. Impaired endothelium-dependent vasodilatation in women with previous gestational diabetes. Diabetes Care. 1998;21(12):2111–5. doi: 10.2337/diacare.21.12.2111. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee M, Anderson SG, Malik RA, Austin CE, Cruickshank JK. Small artery function 2 years postpartum in women with altered glycaemic distributions in their preceding pregnancy. Clin Sci (Lond) 2012;122(2):53–61. doi: 10.1042/CS20110033. [DOI] [PubMed] [Google Scholar]
- 50.Salmi AA, Zaki NM, Zakaria R, Nor Aliza AG, Rasool AH. Arterial stiffness, inflammatory and pro-atherogenic markers in gestational diabetes mellitus. Vasa. 2012;41(2):96–104. doi: 10.1024/0301-1526/a000171. [DOI] [PubMed] [Google Scholar]
- 51.Heitritter SM, Solomon CG, Mitchell GF, Skali-Ounis N, Seely EW. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. 2005;90(7):3983–8. doi: 10.1210/jc.2004-2494. doi:jc.2004-2494. [DOI] [PubMed] [Google Scholar]
- 52.Audus KL, Soares MJ, Hunt JS. Characteristics of the fetal/maternal interface with potential usefulness in the development of future immunological and pharmacological strategies. J Pharmacol Exp Ther. 2002;301(2):402–9. doi: 10.1124/jpet.301.2.402. [DOI] [PubMed] [Google Scholar]
- 53.Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51(4):970–5. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 54.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 55.Ojeda NB, Grigore D, Alexander BT. Developmental programming of hypertension: insight from animal models of nutritional manipulation. Hypertension. 2008;52(1):44–50. doi: 10.1161/HYPERTENSIONAHA.107.092890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey Smith G, Sattar N, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 2010;122(12):1192–9. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spence D, Stewart MC, Alderdice FA, Patterson CC, Halliday HL. Intra-uterine growth restriction and increased risk of hypertension in adult life: a follow-up study of 50-year-olds. Public Health. 2012;126(7):561–5. doi: 10.1016/j.puhe.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Orbach H, Matok I, Gorodischer R, Sheiner E, Daniel S, Wiznitzer A, et al. Hypertension and antihypertensive drugs in pregnancy and perinatal outcomes. Am J Obstet Gynecol. 2012 doi: 10.1016/j.ajog.2012.11.011. doi:S0002-9378(12)02066-2 [pii] [DOI] [PubMed] [Google Scholar]
- 59.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59(2):226–34. doi: 10.1161/HYPERTENSIONAHA.111.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mu M, Wang SF, Sheng J, Zhao Y, Li HZ, Hu CL, et al. Birth weight and subsequent blood pressure: a meta-analysis. Arch Cardiovasc Dis. 2012;105(2):99–113. doi: 10.1016/j.acvd.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Johnson RC, Schoeni RF. Early-life origins of adult disease: national longitudinal population-based study of the United States. Am J Public Health. 2011;101(12):2317–24. doi: 10.2105/AJPH.2011.300252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 2011;54(3):504–7. doi: 10.1007/s00125-010-2008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aceti A, Santhakumaran S, Logan KM, Philipps LH, Prior E, Gale C, et al. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta-analysis. Diabetologia. 2012;55(11):3114–27. doi: 10.1007/s00125-012-2689-8. [DOI] [PubMed] [Google Scholar]
- 64.Tsadok MA, Friedlander Y, Paltiel O, Manor O, Meiner V, Hochner H, et al. Obesity and blood pressure in 17-year-old offspring of mothers with gestational diabetes: insights from the Jerusalem Perinatal Study. Exp Diabetes Res. 2011;2011:906154. doi: 10.1155/2011/906154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ojeda NB, Grigore D, Alexander BT. Intrauterine growth restriction: fetal programming of hypertension and kidney disease. Adv Chronic Kidney Dis. 2008;15(2):101–6. doi: 10.1053/j.ackd.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, et al. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R758–63. doi: 10.1152/ajpregu.00311.2006. [DOI] [PubMed] [Google Scholar]
- 67.Manning J, Vehaskari VM. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R80–4. doi: 10.1152/ajpregu.00309.2004. [DOI] [PubMed] [Google Scholar]
- 68.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45(4):754–8. doi: 10.1161/01.HYP.0000153319.20340.2a. [DOI] [PubMed] [Google Scholar]
- 69.Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol. 2008;295(1):F29–34. doi: 10.1152/ajprenal.00123.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodford JL, Torrens C, Siow RC, Mann GE, Hanson MA, Clough GF. Endothelial dysfunction and reduced antioxidant protection in an animal model of the developmental origins of cardiovascular disease. J Physiol. 2008;586(Pt 19):4709–20. doi: 10.1113/jphysiol.2008.156976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 2005;68(5):2180–8. doi: 10.1111/j.1523-1755.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 72.Ojeda NB, Hennington BS, Williamson DT, Hill ML, Betson NE, Sartori-Valinotti JC, et al. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension. 2012;60(1):114–22. doi: 10.1161/HYPERTENSIONAHA.112.192955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poston L. Influence of maternal nutritional status on vascular function in the offspring. Microcirculation. 2011;18(4):256–62. doi: 10.1111/j.1549-8719.2011.00086.x. [DOI] [PubMed] [Google Scholar]
- 74.Nuyt AM. Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: evidence from human studies and experimental animal models. Clin Sci (Lond) 2008;114(1):1–17. doi: 10.1042/CS20070113. [DOI] [PubMed] [Google Scholar]
- 75.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59(3):279–89. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 76.Torrens C, Poston L, Hanson MA. Transmission of raised blood pressure and endothelial dysfunction to the F2 generation induced by maternal protein restriction in the F0, in the absence of dietary challenge in the F1 generation. Br J Nutr. 2008;100(4):760–6. doi: 10.1017/S0007114508921747. [DOI] [PubMed] [Google Scholar]
- 77.Ponzio BF, Carvalho MH, Fortes ZB, do Carmo Franco M. Implications of maternal nutrient restriction in transgenerational programming of hypertension and endothelial dysfunction across F1-F3 offspring. Life Sci. 2012;90(15-16):571–7. doi: 10.1016/j.lfs.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 78.Drake AJ, McPherson RC, Godfrey KM, Cooper C, Lillycrop KA, Hanson MA, et al. An unbalanced maternal diet in pregnancy associates with offspring epigenetic changes in genes controlling glucocorticoid action and foetal growth. Clin Endocrinol (Oxf) 2012;77(6):808–15. doi: 10.1111/j.1365-2265.2012.04453.x. [DOI] [PubMed] [Google Scholar]
- 79.Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res. 2007;100(4):520–6. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li CC, Maloney CA, Cropley JE, Suter CM. Epigenetic programming by maternal nutrition: shaping future generations. Epigenomics. 2010;2(4):539–49. doi: 10.2217/epi.10.33. [DOI] [PubMed] [Google Scholar]
- 81.Burdge GC, Slater-Jefferies J, Torrens C, Phillips ES, Hanson MA, Lillycrop KA. Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr. 2007;97(3):435–9. doi: 10.1017/S0007114507352392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moritz KM, Cuffe JS, Wilson LB, Dickinson H, Wlodek ME, Simmons DG, et al. Review: Sex specific programming: a critical role for the renal renin-angiotensin system. Placenta. 2010;31:S40–6. doi: 10.1016/j.placenta.2010.01.006. Suppl. [DOI] [PubMed] [Google Scholar]
- 83.Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med. 2008;5(Suppl A):S121–32. doi: 10.1016/j.genm.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen W, Srinivasan SR, Berenson GS. Amplification of the association between birthweight and blood pressure with age: the Bogalusa Heart Study. J Hypertens. 2010;28(10):2046–52. doi: 10.1097/HJH.0b013e32833cd31f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boivin A, Luo ZC, Audibert F, Masse B, Lefebvre F, Tessier R, et al. Pregnancy complications among women born preterm. CMAJ. 2012;184(16):1777–84. doi: 10.1503/cmaj.120143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Innes KE, Marshall JA, Byers TE, Calonge N. A woman’s own birth weight and gestational age predict her later risk of developing preeclampsia, a precursor of chronic disease. Epidemiology. 1999;10(2):153–60. [PubMed] [Google Scholar]
- 87.Dempsey JC, Williams MA, Luthy DA, Emanuel I, Shy K. Weight at birth and subsequent risk of preeclampsia as an adult. Am J Obstet Gynecol. 2003;189(2):494–500. doi: 10.1067/s0002-9378(03)00491-5. [DOI] [PubMed] [Google Scholar]
- 88.Bo S, Marchisio B, Volpiano M, Menato G, Pagano G. Maternal low birth weight and gestational hyperglycemia. Gynecol Endocrinol. 2003;17(2):133–6. [PubMed] [Google Scholar]
- 89.Innes KE, Byers TE, Marshall JA, Baron A, Orleans M, Hamman RF. Association of a woman’s own birth weight with subsequent risk for gestational diabetes. JAMA. 2002;287(19):2534–41. doi: 10.1001/jama.287.19.2534. [DOI] [PubMed] [Google Scholar]
- 90.De B, Lin S, Lohsoonthorn V, Williams MA. Risk of preterm delivery in relation to maternal low birth weight. Acta Obstet Gynecol Scand. 2007;86(5):565–71. doi: 10.1080/00016340701223127. [DOI] [PubMed] [Google Scholar]
- 91.Gallo LA, Denton KM, Moritz KM, Tare M, Parkington HC, Davies M, et al. Long-term alteration in maternal blood pressure and renal function after pregnancy in normal and growth-restricted rats. Hypertension. 2012;60(1):206–13. doi: 10.1161/HYPERTENSIONAHA.112.195578. [DOI] [PubMed] [Google Scholar]
- 92.Tran M, Gallo LA, Wadley GD, Jefferies AJ, Moritz KM, Wlodek ME. Effect of pregnancy for females born small on later life metabolic disease risk. PLoS One. 2012;7(9):e45188. doi: 10.1371/journal.pone.0045188. [DOI] [PMC free article] [PubMed] [Google Scholar]