Abstract
Purpose
The indications for treatment of brain metastases from non-small cell lung cancer (NSCLC) with stereotactic radiosurgery (SRS) remain controversial. Here, we studied patterns, predictors, and cost of SRS utilization in elderly patients with NSCLC.
Methods and Materials
Using the Surveillance, Epidemiology, and End Results-Medicare (SEER-Medicare) database, we identified patients with NSCLC, who were diagnosed with brain metastases between 2000 and 2007. Our analytic cohort included patients treated with radiation therapy, and not surgical resection, as initial treatment for brain metastases.
Results
We identified 7684 patients treated with radiation therapy within 2 months after brain metastases diagnosis, of whom 469 (6.1%) had billing codes for SRS. Annual SRS use increased from 3.0% in 2000 to 8.2% in 2005 and varied from 3.4% to 12.5% by specific registry site. After controlling for clinical and sociodemographic characteristics, SRS use was significantly associated with increasing year of diagnosis, specific SEER registry, higher socioeconomic status, admission at a teaching hospital, no history of participation in low-income state buy-in programs, no extracranial metastases, and longer interval from NSCLC diagnosis. The average cost per patient associated with radiation therapy was 2.19 times greater for those who received SRS compared to those who did not.
Conclusions
The use of SRS in patients with metastatic NSCLC increased almost 3- fold from 2000 to 2005. In addition, we found significant variation of SRS utilization across SEER registries and socioeconomic quartiles. National practice patterns suggest both a lack of consensus and overall limited use of the approach among elderly patients before 2008.
Keywords: Stereotactic Radiosurgery, Health Services Research, Cost Analysis, Brain Metastases, Non-Small Cell Lung Cancer
Introduction
Approximately 20% of patients with non-small cell lung cancer (NSCLC) will develop brain metastases in their lifetime. Even with treatment, the prognosis of these patients remains poor with a median survival of 7 months, though certain subgroups experience a median survival up to 15 months.(1) Whole brain radiation therapy (WBRT) with or without surgical resection has traditionally been the mainstay of treatment for brain metastases.
Since the 1980s, stereotactic radiosurgery (SRS) has been available as an additional treatment modality for brain metastases. Unlike WBRT, SRS delivers a high dose of radiation to a focal target while minimizing dose to normal surrounding brain tissue. Advantages of SRS include a one-day treatment course and avoiding or delaying side effects from WBRT. Advantages of WBRT include decreased intracranial relapse, the ability to start radiation treatment quickly, and lower cost.
Controversy exists regarding the optimal treatment of brain metastases. Subset analysis of a randomized trial demonstrated improved survival with the addition of SRS to WBRT in patients with a single brain metastasis and in patients younger than 65 with good performance status, controlled primary tumor, and no extracranial metastases compared to WBRT alone.(2)
More recently, randomized trials comparing SRS alone to WBRT and SRS combined have shown conflicting results for patients with 1–4 brain metastases. Two studies demonstrated a reduction in intracranial relapses with the addition of WBRT, with one of these studies also demonstrating reduced neurological death.(3, 4) In contrast, another study showed worsened overall survival and neurocognition at four months following WBRT compared to patients treated with SRS alone.(5) Additionally, questions about the cost-effectiveness of SRS have fueled the controversy regarding its indications.(6–11)
Despite ongoing debate, no study has examined the utilization rates, patterns of care, and cost of SRS for brain metastases in the United States. Therefore, we sought to analyze utilization patterns, and identify predictors and cost of SRS use in the treatment of brain metastases in a population-based database that is nationally representative of patients with non-small cell lung cancer (NSCLC).
Methods and Materials
Data Sources
We used the linked Surveillance, Epidemiology, and End Results–Medicare (SEER-Medicare) database as our data source.(12) The SEER database includes population-based tumor registries in 17 geographic areas: Alaska (since 1999), Atlanta, Connecticut, Detroit, Greater California (since 2000), Hawaii, Iowa, Kentucky (since 2000), Los Angeles, Louisiana (since 2000), New Jersey (since 2000), New Mexico, Rural Georgia, San Francisco, San Jose, Seattle, and Utah. These registries cover approximately 25% of the US population. Sociodemographic information at the census tract level for each patient is included. Inpatient and outpatient Medicare claims, physician, laboratory, durable medical equipment, home health, and hospice billings have been linked to SEER.(13) The Institutional Review Board of XXX approved this study.
Cohort Selection
The cohort consisted of patients with NSCLC diagnosed in a SEER region between January 1, 1995, and December 31, 2005, and a diagnosis of brain metastases indicated by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code for secondary malignant neoplasm of brain and spinal cord (198.3) in any of the following inpatient or outpatient claims data files: hospice, home health (HHA), Medicare Provider Analysis and Review (MedPAR) (inpatient), outpatient, carrier or durable medical equiprment (DME) between January 1, 2000 and December 31, 2007.(14) The SEER portion of the SEER-Medicare merged database does not include patients with NSCLC diagnosed between 2005 and 2007. However, we wanted to include all SRS treatments for the available Medicare years between 2000 and 2007, and adjusted for this issue in our multivariable analysis.
Exclusion criteria included patients under age 66 (since they would not have a full year of data pre-diagnosis for comorbidity assessment), enrollment in Medicare for end-stage renal disease or disability; more than one diagnosis of cancer; death date different by more than 3 months between SEER and Medicare; diagnosis made from autopsy or death certificates; and unknown date of diagnosis. We also excluded patients without continuous Medicare enrollment (Part A and Part B) or who were enrolled in a health maintenance organization (HMO) any time from 13 months before NSCLC diagnosis through death or end of study on December 31, 2007.
Our cohort consisted of patients who underwent radiation therapy and not neurosurgical resection within 1 month prior through 2 months after initial diagnosis of brain metastases, which we defined as the first instance of ICD-9-CM diagnosis code 198.3 occurring any time from 1 month pre-NSCLC diagnosis through death/end of study. We used the window of 1 month prior to capture any claims that were delayed for logistical billing reasons and the window of 2 months after because we felt this was a reasonable time frame for a patient to complete initial treatment for brain metastases. Radiation therapy administration and neurosurgical resection were identified in the Medicare claims files as outlined in Table 1. For identifying neurosurgical resection, we used any code pertaining to craniotomy to allow for miscoding.
Table 1.
Medicare treatment codes. ICD9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; CPT, Current Procedural Terminology; HCPCS, Healthcare Common Procedure Coding System.
| Radiation therapy administration | |
|---|---|
| ICD9-CM | 92.23–92.24, 92.30–92.33, and 92.39. |
| CPT | 61793, 61796–61800, 77261–77263, 77280, 77285, 77290, 77295, 77299–77301, 77305, 77310, 77315, 77321, 77332- 77334, 77336–77337, 77370–77372, 77399, 77402–77414, 77416, 77418–77420, 77425, 77427, 77430, 77432, and 0073T. |
| HCPCS | G0173-G0174, G0242-G0243, G0251, and G0338-G0340. |
| Neurosurgical resection | |
| ICD9-CM | 01.21–01.25, 01.31, 01.51, and 01.59. |
| CPT | 61304–61305, 61312–61315, 61320–61321, 61330, 61332–61334, 61340, 61343, 61345, 61440, 61450, 61458, 61460, 61470, 61500–61501, 61510, 61512, 61514, 61516, 61518, 61519- 61522, 61524, 61526, 61530–61531, 61533–61536, 61538- 61539, 61541–61546, 61550, 61552, 61556–61559, 61563- 61564, 61570–61571, 61575–61576, 61580–61586, 61590–61592, 61596–61598, 61600–61601, 61605–61613, and 61615–61616. |
| Stereotactic radiosurgery administration | |
| ICD9-CM | 92.30–92.33 and 92.39. |
| CPT | 61793, 61796–61800, 77371–77372, and 77432. |
| HCPCS | G0173, G0242, G0243, G0251, G0338, G0339, and G0340. |
The specific anatomic site targeted with radiation therapy is not discernible from Medicare claims data. Therefore, we considered any radiation treatment within our window of 1 month before to 2 months after brain metastases diagnosis to be treatment for brain metastases. We tested the validity of this assumption using data from the National Comprehensive Cancer Network Outcomes Database and found that fewer than 7% of NSCLC patients meeting these criteria actually received radiation therapy to anatomic sites other than the brain for the time period of 1 month prior to 2 months after diagnosis of brain metastases (data not shown).
Patient Characteristics
Time since initial cancer diagnosis was calculated as the interval between date of initial NSCLC diagnosis in SEER and first date of brain metastases diagnosis (occurance of diagnosis code 198.3). The presence of extracranial metastases was determined by specific ICD-9-CM diagnosis codes for secondary malignant neoplasms other than brain and spinal cord (197.x, 198.x, except 198.3) occurring any time before through 1 month after the first brain metastases diagnosis in any of the Medicare claims files.
Year of brain metastasis diagnosis was studied as a categorical variable (2000 as referent) to identify any nonlinear trends. A patient was considered to be enrolled in hospice post-diagnosis if they had at least one claim in the Medicare hospice file occurring any time after NSCLC diagnosis. A patient was considered admitted to a teaching hospital if there was an institutional payment for indirect medical education during their hospitalization.
Sociodemographic factors including race, ethnicity, and marital status were determined from SEER data at the time of NSCLC diagnosis. All SEER registries were included except for Rural Georgia, which had no claims for SRS, and Alaska, which is not included in the SEER-Medicare dataset. New Jersey was used as the reference group because it had the largest cohort. Socioeconomic quartiles were developed on the basis of median income in the census tract where the patient lived according to SEER data, using census data from the year 2000. Education quartiles were similarly developed on the basis of percent college educated in the patient’s census tract. If census tract information was missing (≤ 1% of cohort), the patient was classified in the lowest socioeconomic quartile.(15) A patient was classified as having a history of low income if enrolled in the state buy-in program between 1986 and 2007.
Comorbidities were identified by looking for diagnostic billing codes for specific health conditions during the year before the first diagnosis of brain metastases using the Deyo implementation(16) of the Charlson score(17) applied to inpatient and outpatient claims as described by Klabunde et al.(18) The Charlson score was then categorized as 0, 1, or 2 or more.
Outcome Studied
SRS administration within the window of 1 month before through 2 months after brain metastases diagnosis was identified in the Medicare claims files (See Table 1). The majority (98%) of patients with claims for SRS also had claims for other general radiation therapy codes so we could not distinguish between patients who received SRS alone and those who received SRS as well as WBRT.
Cost Analysis
Costs associated with radiation therapy were calculated from a payer’s perspective (total amount reimbursed by Medicare to providers) for the window between 1 month before and 2 months after the brain metastases diagnosis. These costs included Medicare payment aggregated from claims in the Outpatient (hospital-based outpatient) and Carrier claims (individual physician) files that included CPT codes pertaining to radiation therapy and/or SRS delivery, management, and planning, as listed in Table 1. Fewer than 1% (n=66) of patients had no costs associated with radiation therapy codes in the Outpatient or Carrier claims files. We then determined the ratio of radiation therapy costs per patient receiving SRS to radiation therapy costs per patient not receiving SRS within each SEER region. Utah, Hawaii, San Jose, and New Mexico were not included in the analysis since fewer than 20 patients received SRS in those regions.
Statistical Analyses
Statistical analyses were conducted using SAS for Windows (Version 9.2; SAS Institute, Cary, NC). Utilization rates of SRS among patients with brain metastases were examined by demographic, clinical and treatment characteristics. Rates of SRS use were calculated for all of the aforementioned variables. A Chi-Square test was used to assess the relationship between these variables and receipt of SRS. Forward and backward stepwise logistic regression was used to identify significant variables as well as patient age and comorbidity score to determine the final multivariable model. Results are presented as odds ratios (ORs) with 95% confidence intervals (CIs) and p values.
Results
Among patients diagnosed with NSCLC between 1995 and 2005, 7684 were treated with radiation therapy without neurosurgical resection within 1 month before through 2 months after diagnosis with brain metastases between 2000 and 2007. In this cohort of patients receiving radiation therapy, 469 (6.1%) received SRS.
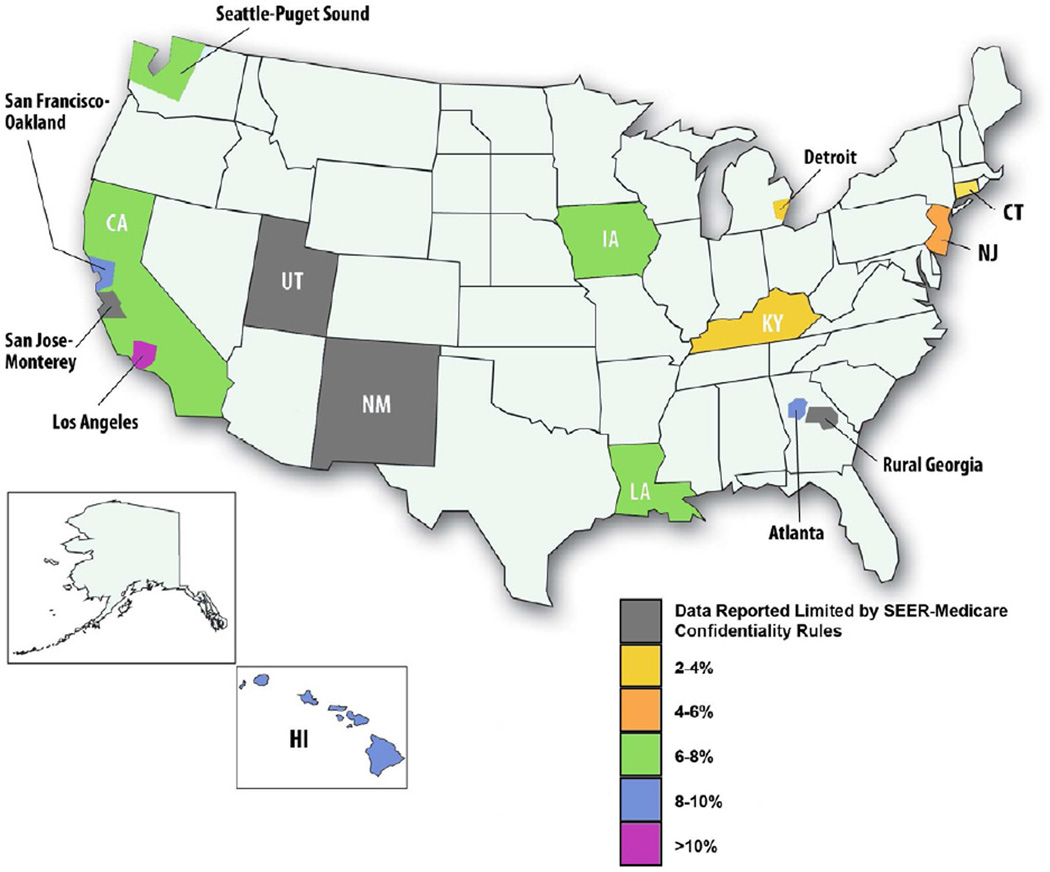
In addition to clinical characteristics, including longer time from initial NSCLC diagnosis (p<0.0001) and lack of extracranial metastases (p<0.0001), race (p=0.02), increasing year of brain metastases diagnosis (p<0.0001), SEER registry (p<0.0001), higher socioeconomic status (p<0.0001), no low income history (p=0.04), marital married at diagnosis (p=0.002), higher education level (p<0.0001), and admission to a teaching hospital (p<0.0001) were significantly associated with SRS use in univariate analyses (Table 2). Annual SRS use increased from 3.0% in 2000 to 8.2% in 2005 and overall use varied across SEER registry ranging from 3.4% (Detroit and Kentucky) to 12.5% (Los Angeles) (Figure 1).
Table 2.
Univariate predictors of stereotactic radiosurgery use
| Total | No SRS (%) |
SRS (%) |
p | |
|---|---|---|---|---|
| Demographics | ||||
| Age at diagnosis with brain mets | 0.76 | |||
| 65–69 | 1,756 | 93.8 | 6.2 | |
| 70–74 | 2,507 | 93.5 | 6.5 | |
| 75–79 | 2,036 | 94.3 | 5.7 | |
| 80+ | 1,385 | 94.0 | 6.0 | |
| Race | 0.02 | |||
| White | 6,657 | 93.8 | 6.2 | |
| Black | 635 | 95.9 | 4.1 | |
| Asian or Pacific Islander | 371 | 91.4 | 8.6 | |
| Other | 21 | >47.6* | <52.4* | |
| Ethnicity | 0.33 | |||
| Non-Hispanic | 7,354 | 93.8 | 6.2 | |
| Hispanic | 330 | 95.2 | 4.8 | |
| SEER registry | <0.0001 | |||
| Seattle | 435 | 92.0 | 8.0 | |
| Greater California | 1,140 | 92.7 | 7.3 | |
| San Francisco | 269 | 91.4 | 8.6 | |
| San Jose | 151 | >92.7* | <7.3* | |
| Los Angeles | 497 | 87.5 | 12.5 | |
| Utah | 100 | >89.0* | <11.0* | |
| New Mexico | 139 | >92.1* | <7.9* | |
| Hawaii | 125 | 91.2 | 8.8 | |
| Iowa | 516 | 93.2 | 6.8 | |
| Detroit | 772 | 96.6 | 3.4 | |
| Louisiana | 613 | 92.5 | 7.5 | |
| Kentucky | 783 | 96.6 | 3.4 | |
| Atlanta | 244 | 90.6 | 9.4 | |
| Connecticut | 597 | 96.1 | 3.9 | |
| New Jersey | 1,303 | 95.9 | 4.1 | |
| Socioeconomic status | <0.0001 | |||
| Lowest quartile | 1,924 | 95.5 | 4.5 | |
| Second quartile | 1,920 | 94.9 | 5.1 | |
| Third quartile | 1,918 | 93.1 | 6.9 | |
| Highest quartile | 1,922 | 92.1 | 7.9 | |
| Low income history | 0.04 | |||
| No | 6,452 | 93.6 | 6.4 | |
| Yes | 1,232 | 95.2 | 4.8 | |
| Comorbidity Score | 0.25 | |||
| 0 | 3,315 | 93.5 | 6.5 | |
| 1 | 2,537 | 93.9 | 6.1 | |
| 2+ | 1,832 | 94.7 | 5.3 | |
| Gender | 0.90 | |||
| Female | 3,709 | 93.9 | 6.1 | |
| Male | 3,975 | 93.9 | 6.1 | |
| Married at diagnosis | 0.002 | |||
| No | 3,466 | 94.8 | 5.2 | |
| Yes | 4,218 | 93.1 | 6.9 | |
| % College educated in census tract | <0.0001 | |||
| Lowest quartile | 1,953 | 94.6 | 5.4 | |
| Second quartile | 1,910 | 95.1 | 4.9 | |
| Third quartile | 1,906 | 92.1 | 7.9 | |
| Highest quartile | 1,915 | 93.8 | 6.2 | |
| Clinical characteristics | ||||
| Time since initial NSCLC diagnosis | <0.0001 | |||
| < 3 months | 4,622 | 95.1 | 4.9 | |
| 3 –12 months | 1,751 | 93.7 | 6.3 | |
| ≥ 12 months | 1,311 | 90.0 | 10.0 | |
| Extracranial metastases | <0.0001 | |||
| Yes | 5,000 | 94.7 | 5.3 | |
| No | 2,684 | 92.4 | 7.6 | |
| Treatment characteristics | ||||
| Year of brain metastases diagnosis | <0.0001 | |||
| 2000 | 942 | 97.0 | 3.0 | |
| 2001 | 1,134 | 96.3 | 3.7 | |
| 2002 | 1,207 | 95.6 | 4.4 | |
| 2003 | 1,285 | 94.2 | 5.8 | |
| 2004 | 1,286 | 93.0 | 7.0 | |
| 2005 | 1,250 | 91.8 | 8.2 | |
| 2006 | 436 | 86.5 | 13.5 | |
| 2007 | 144 | 86.1 | 13.9 | |
| Hospice enrollment | 0.21 | |||
| No | 3,162 | 93.5 | 6.5 | |
| Yes | 4,522 | 94.2 | 5.8 | |
| Admission to a teaching hospital | <0.0001 | |||
| Never admitted | 1018 | 96.8 | 3.2 | |
| Admitted, not to a teaching hospital | 2838 | 96.0 | 4.0 | |
| Admitted, to a teaching hospital | 3,828 | 91.6 | 8.4 | |
| Living in an urban area | 0.13 | |||
| Yes | 6,959 | 93.8 | 6.2 | |
| No | 725 | 95.2 | 4.8 |
Data reported limited by SEER-Medicare confidentiality rules
Figure 1. Stereotactic radiosurgery use by SEER region.
Percentage of study cohort receiving stereotactic radiosurgery within each SEER region.
After controlling for significant clinical and sociodemographic characteristics, SRS use was significantly associated with increasing year of brain metastases diagnosis (p<0.0001) and specific SEER registry (p<0.0001) (Table 3). The SEER region with the greatest use of SRS (Los Angeles) had an increased odds of 4.25 compared to the SEER region with the largest cohort (New Jersey). Admission to a teaching hospital was also associated with SRS use (odds ratio (OR) 2.82; p<0.0001).
Table 3.
Factors significantly associated with stereotactic radiosurgery receipt in multivariable analysis.
| Factor | Odds Ratio |
95% Confidence Interval |
p |
|---|---|---|---|
| Demographics | |||
| Year of diagnosis with brain metastases | <0.0001 | ||
| 2000 | 1.00 | ||
| 2001 | 1.28 | 0.78 to 2.09 | |
| 2002 | 1.47 | 0.92 to 2.37 | |
| 2003 | 2.00 | 1.27 to 3.14 | |
| 2004 | 2.34 | 1.50 to 3.63 | |
| 2005 | 3.10 | 2.01 to 4.79 | |
| 2006 | 4.28 | 2.60 to 7.04 | |
| 2007 | 4.00 | 2.06 to 7.77 | |
| Age at diagnosis with brain metastases | 0.82 | ||
| 65–69 | 1.00 | ||
| 70–74 | 0.99 | 0.76 to 1.28 | |
| 75–79 | 0.89 | 0.67 to 1.18 | |
| 80+ | 0.93 | 0.68 to 1.28 | |
| Comorbidity Score | 0.35 | ||
| 0 | 1.20 | 0.85 to 1.70 | |
| 1 | 1.09 | 0.76 to 1.55 | |
| 2+ | 0.91 | 0.60 to 1.39 | |
| Marital status | 0.07 | ||
| Unmarried | 1.00 | ||
| Married | 1.21 | 0.98 to 1.48 | |
| SEER registry | <0.0001 | ||
| Seattle | 2.26 | 1.39 to 3.68 | |
| Greater California | 2.93 | 1.94 to 4.42 | |
| San Francisco | 2.61 | 1.53 to 4.45 | |
| San Jose | 2.32 | 1.08 to 4.96 | |
| Los Angeles | 4.25 | 2.82 to 6.41 | |
| Utah | 1.04 | 0.36 to 3.07 | |
| New Mexico | 1.64 | 0.74 to 3.64 | |
| Hawaii | 2.24 | 1.11 to 4.51 | |
| Iowa | 1.87 | 1.16 to 3.01 | |
| Detroit | 0.65 | 0.40 to 1.08 | |
| Louisiana | 2.88 | 1.86 to 4.46 | |
| Kentucky | 1.11 | 0.67 to 1.83 | |
| Atlanta | 3.33 | 1.95 to 5.68 | |
| Connecticut | 0.80 | 0.48 to 1.33 | |
| New Jersey | 1.00 | ||
| Socioeconomic status | 0.008 | ||
| Lowest quartile | 1.00 | ||
| Second quartile | 1.07 | 0.78 to 1.48 | |
| Third quartile | 1.55 | 1.12 to 2.15 | |
| Highest quartile | 1.54 | 1.11 to 2.14 | |
| Low income history | 0.01 | ||
| No | 1.00 | ||
| Yes | 0.67 | 0.49 to 0.91 | |
| % College educated in census tract | 0.01 | ||
| Lowest quartile | 1.00 | ||
| Second quartile | 0.84 | 0.32 to 1.14 | |
| Third quartile | 1.24 | 0.93 to 1.66 | |
| Highest quartile | 0.83 | 0.59 to 1.17 | |
| Clinical characteristics | |||
| Time since initial NSCLC diagnosis | 0.05 | ||
| < 3 months | 1.00 | ||
| 3 –12 months | 1.13 | 0.88 to 1.46 | |
| ≥ 12 months | 1.41 | 1.08 to 1.84 | |
| Extracranial metastases | <0.0001 | ||
| Yes | 1.00 | ||
| No | 1.65 | 1.35 to 2.01 | |
| Treatment characteristics | |||
| Admission to a teaching hospital | <0.0001 | ||
| Never admitted | 0.91 | 0.61 to 1.37 | |
| Admitted, not to a teaching hospital | 1.00 | ||
| Admitted, to a teaching hospital | 2.83 | 2.23 to 3.60 |
Patients living in higher socioeconomic status census tracts (third and fourth quartiles) were more likely to receive SRS relative to those in the lowest quartile (OR, 1.58 and 1.61, respectively; p=0.004). In addition, lack of extracranial metastases, longer interval from initial NSCLC diagnosis, and absence of history of low-income status were independently associated with increased odds of receiving SRS. Higher educational level was significantly associated with SRS use in the multivariable model (p=0.01), however, there was no clear trend. Patient age, comorbidity score, race, and marital status were not significantly associated with SRS use on multivariable analysis.
The average cost per patient treated with SRS was 2.19 times greater than the average cost per patient without SRS. This cost ratio of the average cost per patient with and without SRS ranged from 1.40 (Seattle) to 2.52 (Atlanta) across specific SEER registries (Table 4). Cost ratio and percent SRS use within each SEER region did not appear correlated on scatter plot (data not shown).
Table 4.
Ratio of average cost per patient with and without stereotactic radiosurgery, by SEER registry.
| SEER region | Cost ratio |
|---|---|
| Seattle | 1.40 |
| Connecticut | 1.75 |
| Detroit | 1.83 |
| San Francisco | 1.85 |
| Los Angeles | 1.92 |
| Louisiana | 1.93 |
| Kentucky | 2.09 |
| Iowa | 2.10 |
| Greater California | 2.20 |
| New Jersey | 2.31 |
| Atlanta | 2.52 |
| Hawaii | * |
| New Mexico | * |
| San Jose | * |
| Utah | * |
<20 patients with stereotactic radiosurgery
Discussion
Overall, the use of SRS for treatment of brain metastases in this population-based cohort of elderly Medicare enrollees with NSCLC increased over the study period from 3.0% in 2000 to 8.2% in 2005 and varied by SEER regions and measures of patient socioeconomic status.
The National Comprehensive Cancer Network (NCCN) guidelines recommend consideration of SRS for patients with one to three brain metastases with stable systemic disease or reasonable systemic treatment options.(19) Indeed, the randomized trial Radiation Therapy Oncology Group 9408 revealed improved survival only when SRS is added to WBRT in patients with a single brain metastasis and good prognosis (Karnofsky Performance Status ≥ 70, age < 65 years, controlled primary tumor, and no extracranial metastases).(2) In our analysis, patients with extracranial metastases were less likely to receive SRS consistent with these recommendations. However, age did not influence SRS use. This perhaps reflects that physicians are choosing SRS out of a desire to minimize side effects rather than with the goal of improved local control.
We also found significant disparities in the use of SRS. Patients living in areas with higher socioeconomic status were more likely to receive SRS. SRS utilization also varied by geographic region and the teaching status of the admitting hospital. It is unclear whether this variation is due to provider preference, treatment availability and/or other patient characteristics not captured in the dataset.
It is important to note that we cannot determine whether the overall rate of 6.1% SRS use represents under-use, over-use or misuse, as the SEER-Medicare dataset lacks information on the number and size of the brain metastases. Thus, it is not possible to determine how many patients in our cohort qualified for SRS treatment per NCCN guidelines. We acknowledge that the absolute number of SRS cases may be low; however we included all possible codes specific to radiosurgery within the cpt manual. Although we may not have captured all cases of radiosurgery, the financial incentives to bill codes for radiosurgery make it unlikely that we have missed a significant number of cases. We did observe a 2–3 fold increase in SRS utilization from 2000 to 2005, which is slower than the adoption of intensity modulated radiation therapy (IMRT), which increased more than 35-fold in the treatment of head and neck cancer and 10-fold in the treatment of breast cancer over the same period.(20–22) Though technology for SRS has been available longer than for IMRT, establishing a program capable of delivering SRS may require a wider range of technical expertise (imaging, physics, neurosurgery) and start-up costs, leading to a slower adoption overall. It is also possible that our study period ended before more rapid adoption occurred. Since the Japanese Radiation Oncology Study Group trial was published in 2006, adoption of SRS as an alternative to WBRT rather than an adjunctive treatment has likely increased.(3) In addition, surveys suggest that many radiosurgeons are now willing to extend the use of SRS as an initial treatment to more than 5 metastases.(23)
Finally, though the cost of radiosurgery is often at the forefront of the debate regarding the optimal treatment of brain metastases,(6–11) there are limited studies that address the incremental costs of SRS. We determined that radiation therapy costs over the 2-month period after diagnosis with brain metastases was 2.19 times higher for patients who received SRS. This estimate does not include the cost of surveillance imaging, which may be associated with SRS use. In addition, the incremental cost of SRS within each SEER region was not associated with SRS use.
Our study must be interpreted in the context of its design, which is an observational study using administrative data on Medicare enrollees. Since the SEER data do not include information about patients who develop brain metastases after initial diagnosis of NSCLC, we relied on claims data to identify our brain metastases population. This is limited by 198.3 pertaining to both brain and spinal cord, so that patients treated with radiation therapy or SRS to the spine may also be included. However, we surmise that this represents a small minority of patients given that Eichler and Lamont showed the occurrence of at least one ICD-9-CM code 198.3 for brain metastasis had favorable sensitivity, specificity, and positive predictive value for the diagnosis of brain metastasis in claims from a single institution. They also found that the claims-based date of diagnosis was accurate, with 92% of dates falling within 30 days using the gold standard of chart review. Because this methodology has not yet been validated in multiple institutions, there may be selection bias introduced by defining our cohort using these claims which may affect our observed patterns of care. However, given that patients who received no procedures for their brain metastases are those that are less likely to have a claim with a secondary metastases code, this bias may be mitigated as we restricted our cohort to patients with claims for radiation therapy(14) In addition, though it is likely that most patients with a claim for radiation therapy procedures near a diagnosis of brain metastases received radiation therapy for the brain, we acknowledge that this is an assumption and therefore performed no direct comparisons between patients receiving SRS and patients receiving WBRT.
In conclusion, we have identified geographic and sociodemographic variability in SRS use for patients treated with radiation therapy within 2 months of diagnosis with brain metastases. Though use of SRS more than doubled between 2000 and 2005, the adoption of this technique was slower in comparison to the adoption of IMRT for other disease sites. This perhaps reflects the considerable expertise needed to establish SRS programs and/or the continued controversy over the optimal use of SRS in this population. Overall, given the variability of use and increased radiation therapy costs associated with SRS, our findings suggest that further comparative effectiveness research is needed to better define patient populations who may have improved quality of life and/or overall survival with SRS.
Summary.
Analyzing data from SEER-Medicare, we examine the practice patterns, predictors, and cost of stereotactic radiosurgery (SRS) for treatment of brain metastases in elderly patients with non-small cell lung cancer in the United States. We found significant geographic and socioeconomic variability, as well as a 2.19-fold increase in radiation therapy cost, associated with SRS use.
Acknowledgments
This study was supported by a grant from the Joint Center for Radiation Therapy, Boston, MA, and presented at the 53rd Annual Meeting of the American Society For Therapeutic Radiology and Radiation Oncology (ASTRO) in Miami Beach, FL, October 2–6, 2011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. International journal of radiation oncology, biology, physics. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 3.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 4.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. The lancet oncology. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 6.Flickinger JC, Kondziolka D. Radiosurgery instead of resection for solitary brain metastasis: the gold standard redefined. International journal of radiation oncology, biology, physics. 1996;35:185–186. doi: 10.1016/s0360-3016(96)85028-0. [DOI] [PubMed] [Google Scholar]
- 7.Lal LS, Byfield SD, Chang EL, et al. Cost-effectiveness Analysis of a Randomized Study Comparing Radiosurgery With Radiosurgery and Whole Brain Radiation Therapy in Patients With 1 to 3 Brain Metastases. American journal of clinical oncology. 2011 doi: 10.1097/COC.0b013e3182005a8f. [DOI] [PubMed] [Google Scholar]
- 8.Larson D, Sahgal A. Adjuvant whole brain radiotherapy: strong emotions decide but rationale studies are needed: in regard to Brown et al. International journal of radiation oncology, biology, physics. 2008;72:959. doi: 10.1016/j.ijrobp.2008.06.1932. Int J Radiat Oncol Biol Phys 2008 70 1305–1309. [DOI] [PubMed] [Google Scholar]
- 9.Mehta M, Noyes W, Craig B, et al. A cost-effectiveness and cost-utility analysis of radiosurgery vs. resection for single-brain metastases. International journal of radiation oncology, biology, physics. 1997;39:445–454. doi: 10.1016/s0360-3016(97)00071-0. [DOI] [PubMed] [Google Scholar]
- 10.Sperduto PW, Hall WA. Radiosurgery, cost-effectiveness, gold standards, the scientific method, cavalier cowboys, and the cost of hope. International journal of radiation oncology, biology, physics. 1996;36:511–513. doi: 10.1016/s0360-3016(96)00347-1. [DOI] [PubMed] [Google Scholar]
- 11.Rutigliano MJ, Lunsford LD, Kondziolka D, et al. The cost effectiveness of stereotactic radiosurgery versus surgical resection in the treatment of solitary metastatic brain tumors. Neurosurgery. 1995;37:445–453. doi: 10.1227/00006123-199509000-00012. discussion 453–445. [DOI] [PubMed] [Google Scholar]
- 12.Surveillance Epidemiology and End Results. http://seer.cancer.gov/index.html.
- 13.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Medical care. 1993;31:732–748. [PubMed] [Google Scholar]
- 14.Eichler AF, Lamont EB. Utility of administrative claims data for the study of brain metastases: a validation study. Journal of neuro-oncology. 2009;95:427–431. doi: 10.1007/s11060-009-9943-z. [DOI] [PubMed] [Google Scholar]
- 15.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. American journal of public health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. Journal of clinical epidemiology. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. Central Nervous System. Practice Guidelines in Oncology. 2011 www.nccn.org.
- 20.Guadagnolo BA, Liu CC, Cormier JN, et al. Evaluation of trends in the use of intensity-modulated radiotherapy for head and neck cancer from 2000 through 2005: socioeconomic disparity and geographic variation in a large population-based cohort. Cancer. 2010;116:3505–3512. doi: 10.1002/cncr.25205. [DOI] [PubMed] [Google Scholar]
- 21.Sher DJ, Neville BA, Chen AB, et al. Predictors of IMRT and conformal radiotherapy use in head and neck squamous cell carcinoma: a SEER-Medicare analysis. International journal of radiation oncology, biology, physics. 2011;81:e197–e206. doi: 10.1016/j.ijrobp.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Smith BD, Pan IW, Shih YC, et al. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. Journal of the National Cancer Institute. 2011;103:798–809. doi: 10.1093/jnci/djr100. [DOI] [PubMed] [Google Scholar]
- 23.Knisely JP, Yamamoto M, Gross CP, et al. Radiosurgery alone for 5 or more brain metastases: expert opinion survey. Journal of neurosurgery. 2010;113(Suppl):84–89. doi: 10.3171/2010.8.GKS10999. [DOI] [PubMed] [Google Scholar]