Abstract
Objective
Left ventricular (LV) function and dyssynchrony parameters measured from serial gated single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) using blinded processing had a poorer repeatability than when manual side-by-side processing was used. The objective of this study was to validate whether an automatic alignment tool can reduce the variability of LV function and dyssynchrony parameters in serial gated SPECT MPI.
Methods
Thirty patients who had undergone serial gated SPECT MPI were prospectively enrolled in this study. Thirty minutes after the first acquisition, each patient was repositioned and a gated SPECT MPI image was reacquired. The two data sets were first processed blinded from each other by the same technologist in different weeks. These processed data were then realigned by the automatic tool, and manual side-by-side processing was carried out. All processing methods used standard iterative reconstruction and Butterworth filtering. The Emory Cardiac Toolbox was used to measure the LV function and dyssynchrony parameters.
Results
The automatic tool failed in one patient, who had a large, severe scar in the inferobasal wall. In the remaining 29 patients, the repeatability of the LV function and dyssynchrony parameters after automatic alignment was significantly improved from blinded processing and was comparable to manual side-by-side processing.
Conclusion
The automatic alignment tool can be an alternative method to manual side-by-side processing to improve the repeatability of LV function and dyssynchrony measurements by serial gated SPECT MPI.
Keywords: gated myocardial perfusion single-photon emission computed tomography, left ventricular dyssynchrony, left ventricular function
Introduction
Cardiac resynchronization therapy (CRT) has been reported to improve the quality of life, left ventricular (LV) function, and mortality in patients with New York Heart Association class III–IV heart failure, left ventricular ejection fraction (LVEF) less than or equal to 35%, and QRS duration on the surface ECG of at least 120 ms [1–3]. However, 20–40% of the patients having CRT do not respond to this expensive and invasive procedure [1]. Previous studies showed that the main reasons for nonresponse to CRT were: (a) selection of patients without significant baseline LV mechanical dyssynchrony [4]; (b) inappropriate position of the LV pacing lead [5]; and (c) large myocardial scar burden [6]. In those studies CRT response was defined as either a change in LV function parameters [LVEF and LV end-systolic volume (LVESV)] [7] or clinical improvement at 6-month follow-up [8]. In addition, changes in LV mechanical dyssynchrony after CRT have been shown to correlate independently with LV reverse remodeling (changes in LV volumes and LVEF) [9]. As CRT response is assessed by comparing LV function and dyssynchrony parameters before and after CRT, the repeatability of the measurements of those parameters is very important.
Echocardiography is the most widely used modality for measuring LV function and dyssynchrony parameters for CRT. However, echocardiography requires expertise to obtain reliable results. Echocardiography has been found to have poor reproducibility when applied in a multi-center trial setting, such as the Predictors of Responders to Cardiac Resynchronization Therapy (PROSPECT) trial [10].
Gated single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is an alternative approach to measuring LV function and dyssynchrony [11–13]. Importantly, it has been shown that LV function and dyssynchrony parameters measured by gated SPECT MPI are highly repeatable in patients with standard indications for CRT [14]. In that study the LV function and dyssynchrony parameters obtained by blinded processing had a poorer repeatability than those obtained by side-by-side processing, because blinded processing resulted in inconsistent reorientation and apical/basal slice selection between serial images.
A software tool has been developed to automatically align serial gated SPECT images and improve consistency in apex/base selections. The objective of this study was to prospectively validate whether this tool could improve the repeatability of LV function and dyssynchrony parameters measured by serial gated SPECT.
Materials and methods
Patient studies
This study enrolled 30 patients referred for clinically indicated stress testing to the University of Pittsburgh Medical Center who consented to participate in a study evaluating the repeatability of LVEF measurements on two serial gated SPECT MPI studies. The average age was 63±12 years, and 19 (63%) were men. The primary indication for stress testing was preoperative cardiovascular evaluation in 12 (40%), chest pains in nine (30%), exertional dyspnea in five (17%), and evaluation of known CAD in two (7%), syncope in one (3%), and congestive heart failure in one (3%). Twelve patients (40%) had abnormal perfusion on SPECT. The summed stress score from the clinical readings was 10.5±8.1 (range 3–30). The resting ECG showed left bundle branch block, right bundle branch block, and right ventricular paced rhythm in 1, 2, and 3 patients, respectively. Emory University served as the core laboratory for image analysis. This study was approved by the Institutional Review Boards of University of Pittsburgh Medical Center and Emory University School of Medicine.
Imaging acquisition and processing
All patients underwent same-day, rest–stress 99mTc sestamibi gated SPECT MPI on a dual-headed camera (Philips Medical Systems, Milpitas, California, USA) using standard imaging acquisition parameters (step and shoot format with 25 s per each of 64 stops, 1801 orbit, and low-energy high-resolution collimators) and 16-bin gating. Thirty minutes after the first poststress acquisition, patients were repositioned on the table by a separate technologist, and a gated SPECT MPI image was reacquired. The two poststress gated SPECT MPI images were analyzed in this study.
The Emory University Nuclear Cardiology R&D laboratory served as the core laboratory for this study. All patient studies were sent to Emory for image processing and analysis. All processing methods used standard iterative reconstruction and Butterworth filtering. Three methods were used to process the patients’ gated SPECT images. The first method was manual blinded processing by an experienced technologist. The technologist processed the first poststress data set weeks before processing the second poststress data set. The second method was manual side-by-side processing to minimize potential inconsistency in oblique reorientation and apical/basal slice selection between the two serial gated SPECT studies. The third method was automatic alignment of the two poststress data sets using the developed automatic alignment tool. After processing, the aligned data sets were sent to the Emory Cardiac Toolbox (Emory University, Atlanta, Georgia, USA) to measure ejection fraction (EF), ESV, end-diastolic volume, phase standard deviation, and phase histogram bandwidth (PHB).
Automatic alignment tool
The approach was to first rigidly align the ventricles in summed (static) versions of both studies and then adjust the apical and basal slices on each frame so that they are consistent. The alignment methods have been described previously [15]. Briefly, the LV epicardial boundaries estimated from automatic perfusion analysis were used to create a binary mask containing the LV. These masks were dilated nonsymmetrically and then rolled off at their edges using a Gaussian function to eliminate sharp edges. The summed perfusion images were multiplied by this mask to remove extracardiac structures. Following this step, the two LV images were aligned over three-dimensional translations and rotations by minimizing their normalized mutual information. The minimization was performed using the direction set method of Powell [16]. As a resampled image requires interpolation that may slightly reduce resolution, this alignment was performed in two steps. Study 1 was first aligned to study 2. Study 2 was then aligned to the resampled version of study 1, and study 2 was then resampled. The two transformations were applied to each of the original, unmasked gates of the appropriate studies, which were then resampled to create aligned gated studies.
Apical and basal slice selections were then refined so that the gated polar maps were similar for each gate. First, a new apical/basal slice was chosen for the end-diastolic gate of study 1 by finding the best slice that matched ‘template’ apical and basal slices. The templates were created by averaging apical and basal slices chosen by an expert from 10 randomly chosen studies taken from our clinical database, not included in the rest of the analysis. This ‘template’ set consisted of images from six female and four male patients; four of the studies had inferior defects not reaching the base, and two had septal perfusion defects, again not affecting the base. Five studies were normal; thus, one study had both septal and inferior defects. To create the basal template, the base slice chosen by the expert for each patient was extracted from the study, and these 10 basal slices were averaged, pixel by pixel, to create a gray-level ‘average’ base slice image. The template was normalized to a maximum of 100. The apical template was created similarly using the expert’s chosen apex slices. Candidate apical and basal slices of the end-diastolic frame were normalized using their maximum within the radius of search used for perfusion quantification and then translated and rotated in 2 days until each best matched the template using normalized least squares as the cost function and the Powell method for optimization [16]. Then the slice with the lower overall cost was accepted as the most likely apical/basal slice for the end-diastolic frame. The end-diastolic frame was resampled to create a polar map using the new apex/base; this plot was normalized by dividing it by its mean and multiplying by 100. For each subsequent frame, apical and basal slices were adjusted and new polar plots were generated; each of these was also normalized by its mean. For each frame, the apex and base that created a new normalized polar plot that best matched the normalized ED plot in the least squares sense were accepted as the best parameters. For study 2, the apex/base of the end-diastolic frame was adjusted until the polar map for study 2 best matched the polar map of the end-diastolic frame of study 1. The rest of the gate’s apical and basal slices were then chosen such that the polar plots matched that of the end-diastolic frame in study 2. This process and its results are shown in Fig. 1.
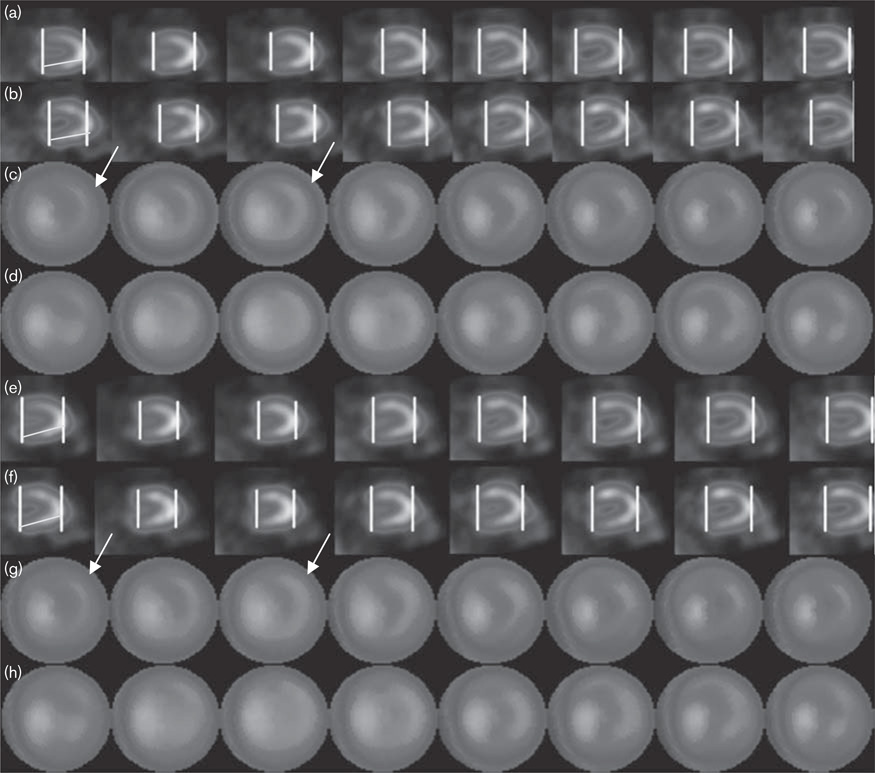
Fig. 1.
Example of alignment and consistent apex/base selection. (a) Study 1 before processing. (b) Study 2 before processing. (c) Gated polar maps for (a). (d) Gated polar maps for (b). (e) Study 1 after processing. (f) Study 2 after processing. (g) Gated polar maps for (e). (h) Gated polar maps for (f). Note that, after alignment, gated studies are better aligned, particularly about the vertical axis. Polar maps are more consistent particularly toward the base. To help demonstrate this, lines were drawn to nearly bisect the inferior wall of the first frame of study 1, and identically angled lines were copied onto frame 1 of study 2. Polar maps are more consistent particularly toward the base; arrows showing the basal differences before and after the new parameter finding are drawn on frames 1 and 3. Although these differences are admittedly subtle, even in this patient the average phase standard deviation decreased from 53 to 8.1, and the repeatability of phase standard deviation decreased from 9 to 1.2 after alignment.
Statistical analysis
Paired t-tests were used to compare the difference in LV function and dyssynchrony parameters between the two serial gated SPECT studies. The mean differences (μ) and SD of the differences (σ) were calculated to describe the repeatability of the LV function and dyssynchrony measurements. The F-test was used to compare variability of these parameters among blinded processing, manual side-by-side processing, and automatic alignment.
Results
The automatic tool failed in one patient who had a large, severe perfusion defect in the inferobasal wall, coupled with significant gut uptake. Therefore, the final analysis of this study was based on 29 patients.
The mean difference and SD of difference in the LV function and dyssynchrony parameters between the serial scans were calculated and are listed in Table 1. EF, end-diastolic volume, and PHB were significantly different between the two serial scans for blinded processing by the paired t-test. There were no significant differences in any parameters between the two serial scans for manual side-by-side processing or automatic alignment. With the F-test, the variations in ESV, phase standard deviation, and PHB between the two serial scans were significantly larger for blinded processing compared with automatic alignment. The variations in any parameters between the two serial scans were not significantly different between manual side-by-side processing and automatic alignment.
Table 1.
Differences in LV function and dyssynchrony parameters between serial gated SPECT studies given by blinded processing, manual side-by-side processing, and automatic alignment
| EF (%) | ESV (ml) | EDV (ml) | PSD (deg.) | PHB (deg.) | |
|---|---|---|---|---|---|
| Blinded processing | |||||
| µ | 3.0 | − 2.1 | 6.6 | 2.1 | 6.6 |
| σ | 5.0 | 10.1 | 13.4 | 5.8 | 13.9 |
| P (paired t-test between the two serial data sets) | 0.003 | 0.273 | 0.012 | 0.065 | 0.020 |
| Manual side-by-side processing | |||||
| µ | 1.1 | − 2.1 | − 1.3 | − 0.1 | − 0.5 |
| σ | 4.8 | 8.9 | 10.8 | 2.6 | 8.4 |
| P (paired t-test between the two serial data sets) | 0.233 | 0.215 | 0.516 | 0.903 | 0.747 |
| Automatic alignment | |||||
| µ | − 0.7 | 0.8 | − 1.4 | 0.2 | 0.2 |
| σ | 4.3 | 5.8 | 9.7 | 2.2 | 8.0 |
| P (paired t-test between the two serial data sets) | 0.373 | 0.470 | 0.451 | 0.678 | 0.905 |
| P (F-test on σ vs. blinded processing) | 0.430 | 0.005 | 0.093 | < 0.001 | 0.005 |
| P (F-test on σ vs. manual side-by-side processing) | 0.564 | 0.027 | 0.574 | 0.382 | 0.798 |
EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; LV, left ventricular; PHB, phase histogram bandwidth; PSD, phase standard deviation; SPECT, single-photon emission computed tomography; μ, mean difference; σ, standard deviation of difference.
P < 0.05, statistical significance.
Figure 2 shows two serial scans of a patient example processed by independent, manual alignment and automatic alignment. Manual side-by-side processing and automatic alignment had more consistent reorientation and apical/basal slice selection as compared with blinded processing. The better consistency led to less variability in the LV function and dyssynchrony parameters between the two serial gated SPECT studies.
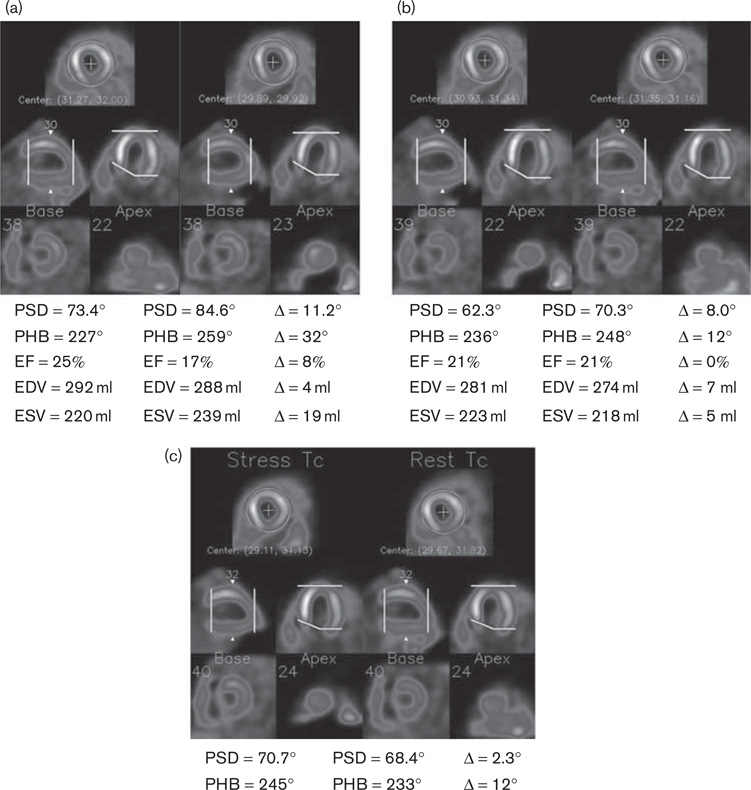
Fig. 2.
A patient example obtained by blinded processing (a), manual side-by-side processing (b), and automatic alignment (c). When processed blinded, the two serial images had inconsistent oblique reorientation [shown on the horizontal long-axis images in (a)] and apical slice selection [shown on the vertical long-axis images in (a)]. Manual side-by-side processing and automatic alignment better aligned the two images and improved the repeatability of the measured parameters. EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; PHB, phase histogram bandwidth; PSD, phase standard deviation.
Discussion
The main finding of this study was that the variability in the LV function and dyssynchrony parameters obtained by automatic alignment was significantly less than that obtained by blinded processing and was comparable to that obtained by manual side-by-side processing, validating that the automatic alignment tool can improve the repeatability of LV function and dyssynchrony measurements by serial gated SPECT. Such improvement was achieved by consistent reorientation and apical/basal slice selection between serial images.
Other studies have shown that precise alignment between serial scans, rest and stress scans, or even perfusion and metabolism images improves the comparison between them and allows better analysis of the difference [15, 17, 18]. Consistent parameters are also intuitively important for reproducible processing. Apex/base selection, in particular, is critical for accurate volume and EF analysis, as well as for dyssynchrony measurements. As phase analysis looks at the time course of three-dimensional-sampled myocardial points, and the coordinate system of these points can change from frame to frame depending on placement of the apex and base, including or excluding slices even from a single frame of the cardiac cycle may cause changes in the phase calculations toward the base. In turn, these phase measurements will generally affect the bandwidth and SD in the dyssynchrony analysis. Improving consistency in the appearance of the gated polar maps themselves helps to ensure that these errors are minimized. In essence, the template matching for end-diastolic apex/base selection improves accuracy, and the polar map matching for consecutive frames improves the consistency of the apex/base on a frame-by-frame basis.
One type of CRT response is defined as positive long-term change in LV function parameters, such as improvement in LVESV and LVEF at 6-month follow-up [7]. In addition, acute changes in LV dyssynchrony parameters have been shown to be predictive of CRT long-term response [19] and patient outcome [20]. Therefore, improving the repeatability of LV function and dyssynchrony measurements by serial gated SPECT is important, because more repeatable techniques can measure smaller changes in these parameters before and after CRT. Lin et al. [14] reported that processing serial gated SPECT studies side-by-side is desirable, as this approach can reduce variation resulting from image processing. However, automatic alignment has multiple advantages over manual side-by-side processing. It is objective and less dependent on the operator’s experience. It is automatic and less time-consuming. It is convenient to use, as it does not require reprocessing of raw gated SPECT data.
The main limitation of this study is that automatic alignment failed in one patient with a large inferobasal scar and significant bowel uptake. In this patient, the original parameters for perfusion quantification did not find the heart in one of the studies, resulting in extracardiac regions in the masked image. Consequently, the alignment was between the LV in one study and the liver in the other. This indicates that the alignment tool needs manual supervision to ensure correct alignment, especially for cases with significant extracardiac uptake. Other limitations of the alignment tool could be: (a) apex/base finding may fail in the case of severe apical/basal abnormalities, in that the basal slices of the study do not look sufficiently like the template and/or the apex is nonexistent; and (b) in severely dyssynchronous ventricles, the gated polar maps may change appearance during the cardiac cycle, and this may hinder consistent apex/base selection. These problems did not occur in this population and thus could not be evaluated further.
Conclusion
The automatic alignment tool can be an alternative method to manual realignment to improve the repeatability of LV function and dyssynchrony measurements by serial gated SPECT MPI. Manual supervision is needed for cases with severe perfusion defects and/or significant overlapping extracardiac uptakes.
Acknowledgements
This study was supported in part by an NIH grant (1R01HL094438, PI: Ji Chen, PhD). Dr Faber, Dr Folks, Dr Garcia, and Dr Chen receive royalties from the sale of the Emory Cardiac Toolbox with SyncTool. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict-of-interest practice.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 4.Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–1840. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Norisada K, Kawai H, Tanaka H, Tatsumi K, Onishi T, Fukuzawa K, et al. Myocardial contractile function in the region of the left ventricular pacing lead predicts the response to cardiac resynchronization therapy assessed by two-dimensional speckle tracking echocardiography. J Am Soc Echocardiogr. 2010;23:181–189. doi: 10.1016/j.echo.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, de Roos A, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–976. doi: 10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]
- 7.Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, et al. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005;112:1580–1586. doi: 10.1161/CIRCULATIONAHA.105.538272. [DOI] [PubMed] [Google Scholar]
- 8.Bleeker GB, Bax JJ, Fung JWH, van der Wall EE, Zhang Q, Schalij MJ, et al. Clinical versus echocardiographic parameters to assess response to cardiac resynchronization therapy. Am J Cardiol. 2006;97:260–263. doi: 10.1016/j.amjcard.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Knappe D, Pouleur AC, Shah AM, Cheng S, Uno H, Hall WJ, et al. Dyssynchrony, contractile function, and response to cardiac resynchronization therapy. Circ Heart Fail. 2011;4:433–440. doi: 10.1161/CIRCHEARTFAILURE.111.962902. [DOI] [PubMed] [Google Scholar]
- 10.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, et al. Results of predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 11.Abidov A, Germano G, Hachamovitch R, Berman DS. Gated SPECT in assessment of regional and global left ventricular function: major tool of modern nuclear imaging. J Nucl Cardiol. 2006;13:261–279. doi: 10.1007/BF02971251. [DOI] [PubMed] [Google Scholar]
- 12.Faber TL, Vansant JP, Pettigrew RI, Galt JR, Blais M, Chatzimavroudis G, et al. Evaluation of left ventricular endocardial volumes and ejection fractions computed from gated perfusion SPECT with magnetic resonance imaging: comparison of two methods. J Nucl Cardiol. 2001;8:645–651. doi: 10.1067/mnc.2001.117173. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687–695. doi: 10.1016/j.nuclcard.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 14.Lin X, Xu H, Zhao X, Folks RD, Garcia EV, Soman P, et al. Repeatability of left ventricular dyssynchrony and function parameters in serial gated myocardial perfusion SPECT studies. J Nucl Cardiol. 2010;17:811–816. doi: 10.1007/s12350-010-9238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faber TL, Modersitzki J, Folks RD, Garcia EV. Detecting changes in serial myocardial perfusion SPECT: a simulation study. J Nucl Cardiol. 2005;12:302–310. doi: 10.1016/j.nuclcard.2004.12.299. [DOI] [PubMed] [Google Scholar]
- 16.Powell MJD. An efficient method for finding the minimum of a function of several variables without calculating derivatives. Comput J. 1964;7:52–162. [Google Scholar]
- 17.Slomka PJ, Hurwitz GA, Stephenson J, Cradduck T. Automated alignment and sizing of myocardial stress and rest scans to three-dimensional normal templates using an image registration algorithm. J Nucl Med. 1995;36:1115–1122. [PubMed] [Google Scholar]
- 18.Marinelli M, Martinez-Moller A, Jensen B, Positano V, Weismuller S, Navab N, et al. Registration of myocardial PET and SPECT for viability assessment using mutual information. Med Phys. 2010;37:2414–2424. doi: 10.1118/1.3395554. [DOI] [PubMed] [Google Scholar]
- 19.Bleeker GB, Mollema SA, Holman ER, Van d, Ypenburg C, Boersma E, et al. Left ventricular resynchronization is mandatory for response to cardiac resynchronization therapy: analysis in patients with echocardiographic evidence of left ventricular dyssynchrony at baseline. Circulation. 2007;116:1440–1448. doi: 10.1161/CIRCULATIONAHA.106.677005. [DOI] [PubMed] [Google Scholar]
- 20.Friehling M, Chen J, Saba S, Bazaz R, Schwartzman D, Adelstein EC, et al. A prospective pilot study to evaluate the relationship between acute change in LV synchrony after cardiac resynchronization therapy and patient outcome using a single-injection gated SPECT protocol. Circ Cardiovasc Imaging. 2011;4:532–539. doi: 10.1161/CIRCIMAGING.111.965459. [DOI] [PMC free article] [PubMed] [Google Scholar]