Abstract
Leafy green produce has been associated with numerous outbreaks of foodborne illness caused by strains of Escherichia coli O157:H7. While the amounts of culturable E. coli O157:H7 rapidly decline after introduction onto lettuce in the field, it remains to be determined whether the reduction in cell numbers is due to losses in cell viability, cell injury and a subsequent inability to be detected by standard laboratory culturing methods, or a lack of adherence and hence rapid removal of the organism from the plants during application. To assess which of these options is most relevant for E. coli O157:H7 on leafy green produce, we developed and applied a propidium monoazide (PMA) real-time PCR assay to quantify viable (with PMA) and total (without PMA) E. coli O157:H7 cells on growth chamber and field-grown lettuce. E. coli O157:H7, suspended in 0.1% peptone, was inoculated onto 4-week-old lettuce plants at a level of approximately 106 CFU/plant. In the growth chamber at low relative humidity (30%), culturable amounts of the nontoxigenic E. coli O157:H7 strain ATCC 700728 and the virulent strain EC4045 declined 100 to 1000-fold in 24 h. Fewer E. coli O157:H7 cells survived when applied onto plants in droplets with a pipette compared with a fine spray inoculation. Total cells for both strains were equivalent to inoculum levels for 7 days after application, and viable cell quantities determined by PMA real-time PCR were approximately 104 greater than found by colony enumeration. Within 2 h after application onto plants in the field, the number of culturable E. coli ATCC 700728 was reduced by up to 1000-fold, whereas PCR-based assessments showed that total cell amounts were equivalent to inoculum levels. These findings show that shortly after inoculation onto plants, the majority of E. coli O157:H7 cells either die or are no longer culturable.
Introduction
Fresh and minimally processed fruit and vegetables contribute significantly to the burden of foodborne illness in the U.S. [1]. While introduction of pathogens can occur at any step from production through consumption, preharvest contamination of leafy greens has been identified in a number of recent outbreaks [2], [3]. Recurrent outbreaks and product recalls associated with leafy greens and the isolation of Salmonella or Escherichia coli O157:H7 from implicated product suggest that specific plant-bacteria interactions or environmental conditions might favor the introduction and persistence of these pathogens in the production environment.
To evaluate the risks of preharvest contamination of leafy greens, the ability of E. coli O157:H7 to colonize or survive on plants has been assessed under both laboratory and field conditions. On plants in growth chambers, E. coli O157:H7 was found to grow and survive under specific conditions on lettuce seedlings and mature plants [4]–[8]. Growth of E. coli O157:H7 was generally associated with the phyllosphere, or the aerial portions of plants [9], [10]. Endophytic growth has also been demonstrated, but mainly for plants in hydroponic solution [10]. Young lettuce leaves were found to sustain higher populations of the pathogen than older leaves [11].
Although controlled conditions in the laboratory provide an opportunity to study virulent strains of human pathogens, such investigations do not fully mimic the (a)biotic conditions plants are exposed to in the field. Shortly after application of (non-toxigenic) E. coli O157:H7 onto lettuce in production regions, culturable populations of the pathogen rapidly declined [12]–[14], regardless of the season [14] or location (e.g. Salinas Valley, California, USA [14]; Tifton, Georgia, USA [12]; or Summerland, B.C., and Kentville, Nova Scotia, Canada [13]). However, E. coli O157:H7 can apparently survive, at least in low quantities, on field-grown plants, and viable cells were found on a fraction of lettuce plants for several weeks after inoculation [12]–[15].
Studies of E. coli O157:H7 survival on plants in the field have generally relied on colony-based assessments coupled with enrichment methods to detect the presence/absence of the pathogen. Such approaches do not permit measurements of total (viable and non-viable) cell amounts or the detection of individual cells that are viable but injured and no longer recovered on standard laboratory growth medium. These sub-lethally injured cells may still be infectious or capable of resuming growth under favorable conditions. The latter possibility is relevant to leaf surface habitats, which are characterized as stressful environments for microorganisms and are subject to fluctuating temperatures and moisture levels as well as other environmental insults [16]. Therefore, culture-independent methods, such as fluorescence-based cell detection or genetic approaches, might be complementary for measurements of pathogen presence and survival on plants. In particular, combining membrane-impermeable, DNA-intercalating compounds, such as propidium monoazide (PMA), with quantitative real-time PCR might offer novel opportunities to sensitively and selectively detect pathogens among a background of other microorganisms in environmental samples [17]. PMA efficiently enters cells with compromised but not intact membranes, and, upon photoactivation, PMA is irreversibly cross-linked to DNA and prevents PCR amplification of target sequences.
In this study, we developed a PMA real-time PCR assay to quantify both viable and total E. coli O157:H7 recovered from lettuce plants in the laboratory and field; these results were compared with quantities of this organism determined by culture methods. We also used this approach to design plant inoculation and incubation conditions in the laboratory that more closely mimic field environments and could be used to study the plant-associated behaviors of virulent outbreak-associated strains of the pathogen.
Materials and Methods
Bacterial Strains and Culture Conditions
E. coli O157:H7 strain EC4045 was kindly provided by Thomas A. Cebula (John Hopkins University, USA). This strain was isolated from spinach during a 2006 E. coli O157:H7 spinach-associated outbreak [18]. A spontaneous rifampicin-resistant mutant of EC4045 was isolated by selecting colonies resistant to 120 µg/ml rifampicin (Gold Biotechnology, St. Louis, MO). Rifampicin-resistant E. coli O157:H7 ATCC 700728 lacks stx1 and stx2 genes and was used in previous field trials [14]. The cells were routinely cultured on tryptic soy broth (TSB) or tryptic soy agar (TSA) from BD (Franklin Lakes, USA) at 37°C. For selective growth of E. coli O157:H7 strains ATCC 700728 and EC4045, rifampicin was added to the medium at 50 µg/ml.
To prepare the E. coli O157:H7 cells for inoculation onto plants, 20 µl of an overnight bacterial liquid culture was spread plated with an automated spiral plater (Autoplate 4000, Spiral Biotech Inc., Boston, MA) onto TSA and incubated at 37°C for 12 h to produce a bacterial lawn. Cells were dislodged with 5 ml of 0.1% peptone, and then the suspension was diluted in 0.1% peptone to the desired concentration.
Inoculation of E. coli O157:H7 onto Lettuce Grown in the Laboratory
Seeds of Romaine lettuce (Lactuca sativa) cv. Parris Island were planted in Sunshine mix potting soil (Sun Gro Horticulture Distribution, Bellevue, WA) and grown in an environmental chamber (PGR15, Conviron, Pembina, ND) with a light intensity of 230 µmol m−2 s−2. The chamber was maintained at a constant relative humidity (60% RH) and daily temperatures of 22°C (for 12 h, with light) and 18°C (for 12 h, without light). Starting 2 weeks after emergence, the plants were fertilized weekly with Hoagland nutrient water.
Four weeks after plant emergence, 10 2-µl drops of E. coli O157:H7 ATCC 700728 or EC4045 were inoculated onto the leaves. The bacterial suspension was dispensed with an Eppendorf repeater pipette (Eppendorf, Hauppauge, NY) onto the interveinal spaces of two leaves per plant to yield approximately 106 CFU/leaf. E. coli O157:H7 ATCC 700728 was also inoculated onto lettuce plants using a spray bottle as described previously for field studies [14]. Spray bottles were calibrated to deliver a dose adjusted to 106 CFU of E. coli O157:H7 ATCC700728 per spray, and a single spray was applied to each plant from a distance of 20 cm and with a dispersal area of approximately 0.3 m2. Immediately after inoculation, plants were transferred to a growth chamber (Percival, Geneva Scientific LLC, Fontana, WI) maintained with a 12-h photoperiod (light intensity 230 µmol m−2 s−2), constant relative humidity (30% RH), and temperatures of 18°C (12 h) and 23°C (12 h). Within 24 h after application, free water was no longer visible on the surface of the plants. Cross-contamination of strains EC4045 and ATCC 700728 was prevented by inoculating one strain per plant and keeping plants spatially separated. Five leaves (drop inoculation) or five plants (spray inoculation) were collected for measurement of E. coli O157:H7 amounts at the time of inoculation and then 1, 2 and 7 days after application of the pathogen onto the plants.
Field Trials
Field trials were conducted in the Salinas Valley during spring 2010 (5/4/10 to 7/5/10) and summer 2010 (7/28/10 to 10/4/10). Permits and approvals for use of United States-owned land were granted by the United States Department of Agriculture. All beds were direct seeded according to standard commercial practice with two rows of Romaine lettuce (Lactuca sativa) cv. Green Towers per bed. The field trials were conducted exactly as described in Moyne et al. [14] on 4-week old lettuce plants after thinning. Inoculation was carried out in the morning (when no dew was visible) by delivering one spray (107 CFU) per 4-week-old lettuce plant just after thinning. For E. coli O157:H7 enumeration on lettuce, entire lettuce heads were removed by cutting at the base with a sterile scalpel approximately 3 cm above the ground; each head was bagged individually at the time of collection. Twelve lettuce heads were sampled immediately after inoculation, and 24 lettuce plants were then randomly sampled among the inoculated beds at 2 h and 2 and 7 days. Twenty-four non-inoculated lettuce heads (controls) were collected concurrently at the time of inoculation. The lettuce was brought to the laboratory from the field in a cooler on ice and analyzed within 24 h.
Enumeration of Culturable E. coli O157:H7 Populations on Lettuce
One leaf (laboratory drop-inoculated sample) or the entire lettuce head (laboratory spray-inoculated and field-inoculated sample) was placed into a sterile Whirl-Pak bag (Nasco, Modesto, CA) with 50 ml of 0.1% peptone water. The lettuce was homogenized in a Stomacher 400 laboratory blender (Seward, Westbury, NY) at medium speed for 2 min. A total of 500 µl of the resulting cell suspension was used for PMA real-time PCR and 1 ml was serial diluted for CFU enumerations on TSA supplemented with 50 µg/ml rifampicin. To improve the limit of detection of culturable cells, the remaining cell suspension was filtered through disposable analytical filter units (0.45 µm; Nalgene, Rochester, NY). Filter membranes were then laid onto CHROMagar O157 (CHROMagar, Paris, France) [19] and incubated at 37°C overnight. The detection limit by this approach was 1 CFU per leaf or plant in the laboratory experiments and 10 CFU per plant in the field experiments. In addition to plating for CFU enumeration, the lettuce heads were mixed at a ratio of 1∶2 (wt lettuce:vol culture medium) with TSB supplemented with rifampicin at 50 µg/ml and incubated at 42°C for 18 h. When CFU estimates were low, the enrichment broth culture was spiral plated onto CHROMagar O157 supplemented rifampicin at 50 mg/ml and incubated at 37°C overnight. The enrichment was considered positive if mauve colonies typical of E. coli O157:H7 on this medium were observed.
PMA Assay, DNA Extractions, and Real-time PCR Quantification
PMA (Biotum, Hayward, CA) was dissolved in water to obtain a 20 mM stock solution and stored at −20°C in the dark. For comparisons of different PMA concentrations, 40, 50, or 100 µM PMA was added to 500 µl cells of E. coli O157:H7 in clear transparent 2-ml microtubes and incubated, with shaking at 400 rpm, at room temperature in the dark for 15, 30, or 60 min. After incubation with PMA, samples were exposed to a 500W halogen light at a distance of 20 cm for 3 min with occasional mixing. To avoid overheating, the cultures were kept on ice during light exposure.
Assessment of PMA for distinguishing live and dead cells were performed by incubating one half of a 12-h E. coli O157:H7 culture in 0.1% peptone (live) and suspending the other cell fraction in 70% isopropanol for 10 min (dead). Both live and dead fractions were then washed twice in 0.1% peptone and suspended in 0.1% peptone for exposure to PMA.
DNA was extracted from cells recovered from laboratory cultures and lettuce with or without prior exposure to PMA. First, 500 µl of the cell suspension was concentrated by centrifugation at 10,000×g for 2 min. The resulting cell pellet was then washed once with 0.1% peptone, suspended in 100 µl of Prepman solution (Applied Biosystems, Foster City, CA), and boiled for 5 min. Debris was removed by centrifugation at 10,000×g for 2 min, and 1 µl of the supernatant was used for real-time PCR. High quality E. coli O157:H7 genomic DNA for use in the real-time PCR assay development was obtained using the Wizard Genomic Purification kit (Promega, Madison, WI) as described by the manufacturer.
Real-time PCR was performed on an ABI 7500 Fast Real-Time PCR system (Applied Biosystems) using 0.25 µM of forward and reverse primers, 1X Ssofast Evagreen Supermix (Bio-Rad, Hercules, CA) and 1 µl of prepared DNA template. Cycling conditions included an initial activation step at 95°C for 10 s, followed by 40 cycles of denaturation at 95°C for 5 s, and annealing/extension temperatures between 60 to 68°C for 30 s [20], [21] for the primers shown in Table S1, supporting information. After 40 PCR cycles, melting curves were generated by increasing the temperature from 60 to 95°C at 0.2°C/10 s and recording the fluorescence. Threshold cycle (Ct) values were automatically generated by the 7500 Fast Real-Time PCR software.
To quantify the number of E. coli O157:H7 cells in the samples, a standard curve was included for each plate with three replicates for each DNA concentration. Standard curves were generated by amplifying a 5-log DNA dilution series from a known concentration of E. coli O157:H7 cells.
Statistical Analysis
Microbial data (CFU per plant or leaf) were log transformed before being statistically analyzed with JMP software (SAS Institute Inc., Cary, NC). Analysis of variance (ANOVA) was performed to compare the survival of E. coli O157:H7 strains at each time point after inoculation. Differences between means were considered significant at P<0.05.
Results
Development of PMA Real-time PCR Assay for Quantification of Live and Dead E. coli O157:H7 Cells on Lettuce
The use of PMA to prevent amplification of target DNA from dead cells in real-time PCR assays has been applied successfully for a variety of organisms and environments [22]–[27]. However, PMA real-time PCR assays should be optimized for each strain and condition of interest. Therefore, we investigated several parameters for quantification of low amounts of viable and total E. coli O157:H7 cells on lettuce.
Primer selection for E. coli ATCC 700728
The stx1 or stx2 genes coding for Shiga-toxin 1 and 2 are typically used as genetic targets for detection or quantification of E. coli O157:H7. Because these genes are absent from the nontoxigenic strain ATCC 700728 and a genome sequence for this strain was not available, real-time PCR primers designed for other genes in E. coli O157:H7 were compared including enterocyte effacement loci genes csgA (curli fimbriae), eae (intimin), espA (extracellular protein), fliC (flagellar antigen), ler (positive regulator of LEE), and lpfA (long polar fimbriae) [20] (Tables S1 and S2, supporting information).
Our goal was to develop a method capable of detecting small amounts of E. coli O157:H7 ATCC 700728 cells among high numbers of endogenous bacteria present on lettuce leaves (between 104 to 105 cells/g [14]). Therefore, primers were selected based on minimum Ct (a measure of sensitivity) for amplifying 1 ng of E. coli O157:H7 ATCC 700728 genomic DNA (the equivalent of 105 genomes) and maximum Ct (a measure of selectivity) for amplifying 1 ng of DNA isolated from the closely-related bacterial strain E. coli K12. These comparisons were performed because non-pathogenic E. coli were detected on some of the lettuce heads sampled in previous field studies [14], albeit in much lower amounts than evaluated in this assay.
E. coli K12 DNA was amplified in low quantities with all primer sets when the annealing temperature was less than 65°C (Table S2, supporting information). Although increasing the annealing temperature reduced the non-specific amplification of E. coli K12 DNA, the sensitivity of E. coli ATCC 700728 DNA detection also decreased (indicated by higher Ct values) and lowered the dynamic range of the assay (Table S2, supporting information). Based on these comparisons, we selected primers targeting the lpfA gene and an annealing temperature of 60°C because this combination resulted in the earliest detectable amplification (lowest Ct) of target E. coli O157:H7 ATCC 700728 genomic DNA and yielded the lowest levels of non-specific amplification (highest Ct) of E. coli K12 genomic DNA. The efficiency of the PCR with lpfA primers was between 92 to 100% and the correlation coefficient was between 0.98 and 0.99.
Optimization of PMA concentration and incubation time with cell cultures
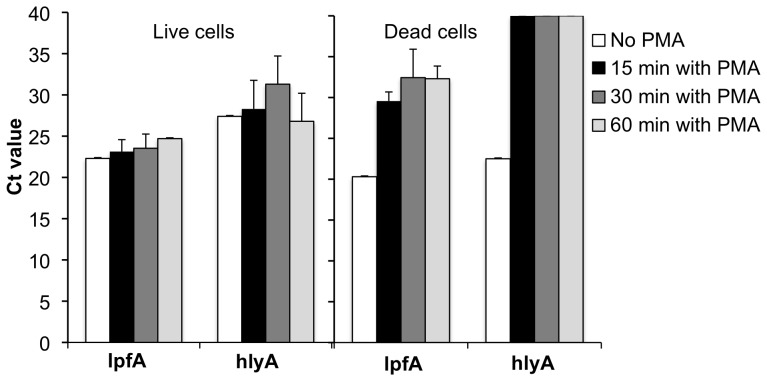
To accurately quantify live and dead cells in a mixture, PMA should completely inhibit amplification of DNA from dead cells without affecting the enumeration of live cells. Real-time PCR amplification of nucleic acids from dead cells of E. coli O157:H7 ATCC 700728 was significantly reduced, but not completely inhibited, after treatment with PMA at concentrations of 50 and 100 µM, as indicated by an increase in the Ct values (Fig. S1, supporting information). These concentrations of PMA were high enough to influence the accurate detection of viable cells in the real-time PCR assay by increasing the Ct values for the viable cells. In contrast, PMA at a concentration of 40 µM did not interfere with DNA amplification from viable cells in the real-time PCR assay (Fig. 1). Amplification of DNA from dead cells was also significantly inhibited by over 9 Ct values at that concentration of PMA (Fig. 1). Increasing the exposure to PMA from 15 to 60 min yielded only modest effects (Fig. 1), and therefore, we used an incubation time of 30 min for subsequent studies.
Figure 1. Effect of incubation time and PCR primer on detection of live and dead cells by real-time PCR.
Live or isopropanol-inactivated dead cells of E. coli O157:H7 ATCC 700728 (5×107 CFU) were incubated in the dark with or without 40 µM PMA for 15, 30, or 60. Real-time PCR was performed using the lpfA or hlyA primer sets [20], [21]. Each bar represents the mean ± stdev for three independent replicates.
To fully investigate the factors that resulted in albeit low, but still measurable, amounts of DNA amplification from dead cells after incubating in PMA, we examined the effects of long versus short PCR products on distinguishing cell viability. In contrast to the 165 bp PCR product resulting from the lfpA primers, PCR amplification was completely inhibited from ATCC 700728 cells using hlyA primers (528 bp product size) (Fig. 1). However, DNA amplification using the hlyA primers was 10-fold less sensitive for detecting low quantities of E. coli O157:H7 cells (Fig. S2A and S2B, supporting information). The limit of detection for both E. coli O157:H7 ATCC 700728 and EC4045 with the lpfA primers was 100 CFU/ml or 5000 CFU per plant or leaf (between 100 to 1000 CFU/g depending on the plant weight) (Fig. S2B, supporting information). Therefore, the lpfA primers were used in further studies for quantifying E. coli O157:H7 ATCC 700728 and EC4045 recovered from plants.
Interference of dead cells during viable cell quantification
Previous studies have shown that PMA combined with real-time PCR is most accurate for viable cell enumeration when viable cells greatly outnumber dead cells [28]–[30]. In our field studies, E. coli O157:H7 ATCC 700728 populations recovered on laboratory culture media declined drastically shortly after inoculation of 106 cells per lettuce head [14], establishing the potential for high numbers of dead cells that may interfere with quantification of the viable cells remaining on the plants. Indeed, suspensions of 106 dead E. coli 0157:H7 ATCC 700728 cells were partially detected by PMA real-time PCR (estimated log 3 CFU; Fig. 2). In a mixture of 106 dead and 104 viable E. coli O157:H7, the PMA real-time PCR assay accurately estimated viable cell amounts (Fig. 2). However, the assay over-estimated the numbers of E. coli O157:H7 when 103 viable cells were measured in the presence of 106 dead cells of the same strain (estimated log 3.5 CFU; Fig. 2).
Figure 2. Interference of dead cells in the enumeration of live E. coli O157:H7 by PMA real-time PCR.
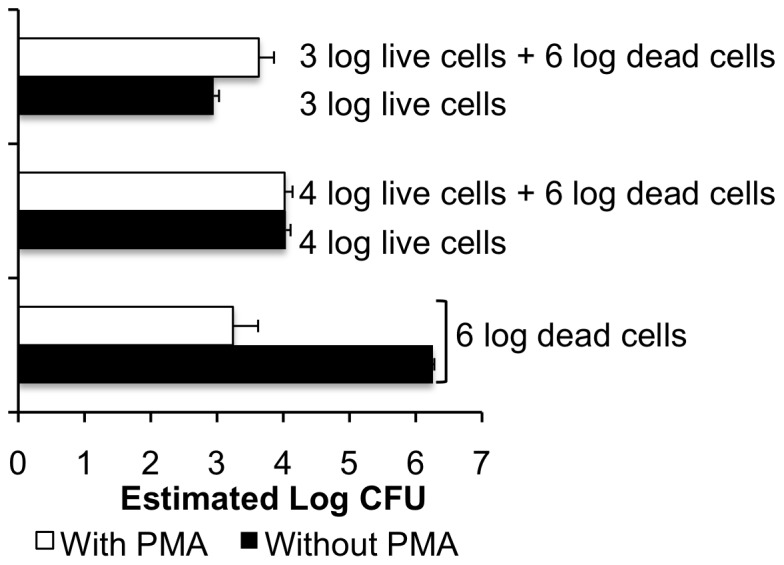
Cell amounts were quantified by real-time PCR (total; black bars) or PMA real-time PCR (viable; open bars) in mixtures of 103 or 104 live cells mixed with 106 isopropanol-inactivated dead cells. Samples were treated with 40 µM PMA for 30 min. Estimates of E coli O157:H7 cell amounts were based on comparisons to standard curves constructed using E. coli O157:H7 ATCC 700728 genomic DNA. Each bar represents the mean ± stdev for three independent replicates.
Quantification of Viable Cells of E. coli O157:H7 on Lettuce in the Growth Chamber
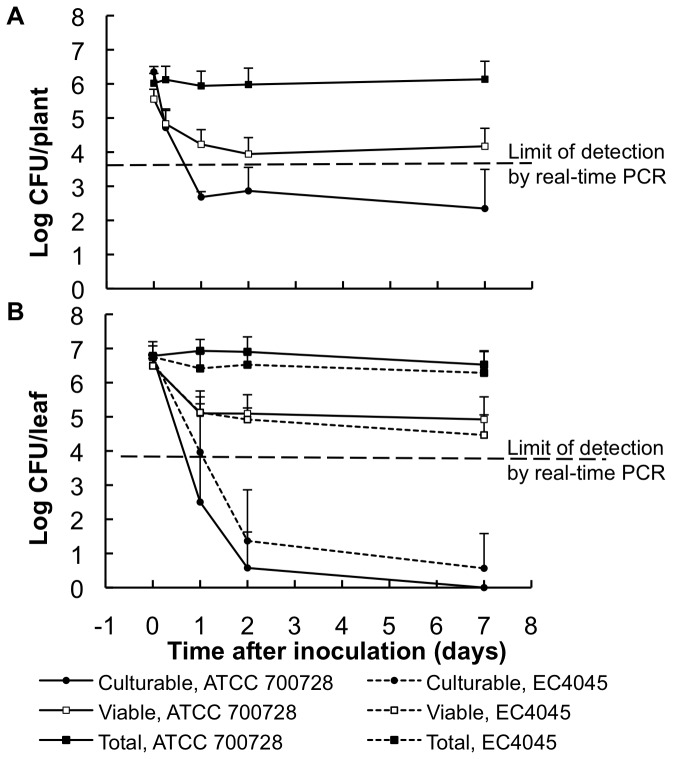
E. coli O157:H7 ATCC 700728 was applied to lettuce plants in the growth chamber either by spray or application of 2-µl drops directly onto the leaves. The latter was used specifically to prevent the hazards associated with aerosolization of the virulent strain EC4045 that can occur during spray application. The inoculum level was adjusted to deliver approximately 106 CFU per plant by spray or per leaf by drop, and after inoculation, the plants were immediately incubated at low (30%) relative humidity and moderate daytime/nighttime temperatures of 22 and 18°C. With both inoculation methods, culturable E. coli O157:H7 ATCC 700728 cell populations declined to an average of 2.6±0.2 log CFU per leaf or plant within the first 24 h after inoculation (Fig. 3). For plants inoculated by the drop method, the number of culturable cells declined by 4 to 5 log CFU/leaf by day 2 to an average of 10 CFU/leaf (Fig. 3B). After inoculation by spray, the average number of culturable E. coli O157:H7 ATCC 700728 cells remained higher and ranged from 100 to 1000 CFU/plant, even on day 7 of the study (Fig. 3A). The cell numbers of strain EC4045 also declined rapidly after inoculation onto lettuce (Fig. 3B). Although there were significantly higher numbers of culturable EC4045 cells than strain ATCC 700728 cells at 24 h after inoculation, the culturable amounts of both strains declined to equivalently low levels within 48 h on lettuce (Fig. 3B).
Figure 3. Survival of E. coli O157:H7 ATCC 700728 and EC4045 on inoculated lettuce in a growth chamber.
E. coli O157:H7 cell amounts were measured by plate count (culturable), real-time PCR (total), and PMA real-time PCR (viable) methods. Plants were inoculated with a spray bottle (A) or by drop (B) and incubated at a relative humidity of 30%. The mean ± stdev of 15 lettuce plants or leaves at each sampling time for three replicate experiments is shown.
Real-time PCR was used to quantify total E. coli O157:H7 cells on the lettuce. The number of E. coli O157:H7 cells remained equivalent to the inoculum cell density for 7 days, regardless of the strain or the inoculation method used (Fig. 3). The addition of PMA prior to real-time PCR amplification revealed that viable cell quantities were equal to the numbers of total and culturable cells at the time of inoculation but then declined by an average of 100-fold within the first day. For both ATCC 700278 and EC4045 strains, the number of viable cells detected using PMA real-time PCR remained above the detection limit and was significantly higher than estimated by the standard plate count method (Fig. 3).
Quantification of Viable and Total E. coli on Lettuce in the Field
Lettuce plants in the field were spray inoculated with E. coli O157:H7 ATCC 700278 at a density of 106 CFU/plant. E. coli O157:H7 was not detected on uninoculated (control) lettuce plants either by culture or by real-time PCR with or without PMA. Within 2 h after application onto the lettuce, culturable cell amounts declined by 2 and 4 log for plants inoculated in the spring and summer trials, respectively (Fig. 4). Two days after inoculation, the amounts of culturable E. coli ATCC 700278 were reduced to the lower limit of detection by plating for most plants (less than 10 CFU per plant). At that time, E. coli O157:H7 was not retrieved by plating or enrichment for 3% and 13% of the spring and summer plants, respectively.
Figure 4. E. coli O157:H7 ATCC 700728 survival on lettuce in the field.
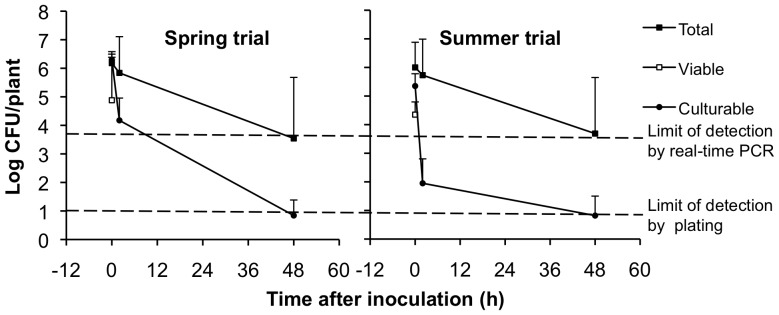
E. coli O157:H7 amounts were measured by plate count (culturable), real-time PCR (total), and PMA real-time PCR (viable). Viable amounts were below the detection limit (3.7 log CFU/plant or leaf) at 2 and 48 h. The mean ± stdev of 12 lettuce plants at each sampling time is shown.
In contrast to colony enumerations of E. coli O157:H7, total (viable and dead) cell numbers determined by real-time PCR quantification were equivalent to the inoculum levels 2 h after application to lettuce plants. Two days after application the numbers of E. coli O157:H7 estimated by real-time PCR decreased, on average, by 2 log CFU/plant and were below the limit of detection (3.7 log CFU/plant) for six out of 24 plants tested for both field trials.
PMA real-time PCR (viable cells) underestimated E. coli O157:H7 numbers by 1 log for plants collected at the time of E. coli O157:H7 ATCC 700278 inoculation. The average numbers of viable E. coli O157:H7 cells determined by this method were below the limit of detection (3.7 log CFU/plant) 2 h after inoculation. Hence, in contrast to the growth chamber studies, the utility of PMA real-time PCR was limited on field-grown plants. The finding was supported upon examination of lettuce heads collected from the field and spiked with known quantities of viable E. coli O157:H7 cells in the laboratory. PMA-qPCR underestimated the number of viable E. coli O157:H7 cells compared with CFU enumeration by approximately five-fold (data not shown).
Discussion
Accurate measurements of pathogen amounts and viability are important for risk assessments and control mechanisms aimed at preventing outbreaks of foodborne illness. This study was the first to apply a culture-independent method to quantify the number of E. coli O157:H7 cells on plants inoculated with the (attenuated) pathogen in the field. We also compared the utility of the method for quantifying virulent and avirulent pathogen survival on laboratory-grown lettuce. The first step in the development of culture-independent methods for bacterial enumeration is to ensure that the technique is optimized for the specific organism and environmental conditions of interest [31]. While numerous assessment methods are available [25], [27], we selected PMA combined with real-time PCR because this technique offered the possibility of rapid enumeration of viable cells in a variety of environments. For E. coli O157:H7 ATCC 700728, we first identified appropriate real-time PCR primers, PMA concentrations, and PMA incubation times with a goal of selective and sensitive detection of viable E. coli O157:H7 cells in the presence of high levels of dead E. coli O157:H7 cells. Among the results was the confirmation that PMA-mediated PCR-inhibition is incomplete when the product size is less than 200 bp [28]–[30], [32]. Although our tests on PMA real-time PCR revealed some potential short-comings with the method, most notably the interference of high quantities of inactivated cells and the relatively high numbers of cells required for detection, these parameters were within the range found in other PCR-based studies [28]–[30], [33]–[35].
E. coli O157:H7 strains ATCC 700728 and EC4045 were inoculated onto leaves of potted lettuce plants and incubated in a growth chamber under temperature and low humidity conditions that favored the decline of cell numbers in trends similar to those recorded in field trials performed in the Salinas Valley. This approach differs from most other laboratory studies that examined plants exposed to high moisture and temperatures supportive of pathogen growth [4], [11], [36], [37]. Within 2 days after inoculation on lettuce plants, the number of E. coli O157:H7 ATCC 700728 cells able to form a colony on TSA declined 1000-fold when the pathogen was initially applied at a level of 6 log CFU per plant or per leaf using an aerosolized spray and declined >105-fold when either strain was applied directly in 2-µl drops. The numbers of culturable cells remained similar for the subsequent 5 days, suggesting that the initial events after contact with the plant are crucial for determining the survival of the organism on lettuce.
In contrast with plate counts, real-time PCR targeting of the E. coli O157:H7 ATCC 700278 and EC4045 inoculants showed that the genomic DNA of the organisms remained on the lettuce in quantities nearly equivalent to the inoculum concentrations over the duration of the 7-day experiment. This finding confirmed that both strains adhered to the leaf surfaces and the recovery method was sufficient to remove the majority of the cell inoculants from the plant. Moreover, the results indicated the presence of high numbers of intact E coli O157:H7 cells on lettuce even when very few were able to form colonies on TSA.
Exposure of E. coli O157:H7 suspensions to PMA prior to real-time PCR quantification facilitated the detection of cells with an intact cell membrane, and these cells were likely viable. Application of this method to E. coli ATCC 700728 and EC4045 recovered from growth chamber lettuce indicated that a significant fraction of the pathogen inoculants were viable, even though colony-based assessments indicated otherwise. This finding was particularly evident for E. coli O157:H7 inoculated onto leaves by direct drop inoculation. While an average of 5 log CFU per leaf were viable as measured by PMA real-time PCR, less than 1 log CFU per leaf were enumerated by culturing. These results are supported by previous studies reporting that E. coli O157:H7 enters into a viable but not culturable (VBNC) state on lettuce leaves incubated at cold (4 to 16°C) temperatures [27]. Similar outcomes were reported for other E. coli including E. coli O104:H4 in water or after exposure to toxic concentrations of copper [38], [39]. Once the stress condition(s) was removed, a fraction of the cells recovered the capacity to grow in laboratory media, thereby indicating a potential of the E. coli O1O4:H4 cells to cause human disease [38]. The formation of VBNC bacterial cells on plants also was previously described for Listeria monocytogenes on parsley [25]. The number of viable L. monocytogenes cells was 1 to 2 log higher than the culturable cells recovered from parsley grown in greenhouses at 20°C under low relative humidity (between 47 to 69% RH); growth of the VBNC cells was not restored on the plants when the RH was increased to 100% [25]. Although we did not examine for E. coli O157:H7 recovery from the VBNC-like state, future efforts might investigate whether those cells can recover and resume growth either under growth-conducive conditions on lettuce or after removal of the cells from the plants and prior to or after plating for viable cell enumeration.
Overall, the toxigenic strain EC4045 survived in similar quantities as ATCC 700728 on lettuce. A recent study showed that certain lineages of E. coli are more commonly associated with plants and presumably have evolved the capacity to tolerate plant-associated environments better than E. coli isolated from other sites [40]. Because we only compared two strains, subsequent investigations should examine multiple attenuated and virulent O157:H7 strains isolated from different sites (e.g. plants and cattle) for their capacity to colonize and persist on lettuce under field-relevant conditions.
Survival of E. coli O157:H7 on lettuce also was measured in two field studies. Culturable amounts of strain ATCC 700728 declined shortly after inoculation onto plants in the field, as we reported previously [14]. Rates of cell decline were similar to E coli O157:H7 on lettuce in the growth chamber directly inoculated in drops with a pipette. Real-time PCR estimates of E. coli O157:H7 ATCC 700728 in lettuce leaf washes showed that this strain was present on the plants immediately after inoculation and 2 h later in quantities equivalent to the inoculum levels. Importantly, the rapid decline in culturable E. coli during the first hours after application onto plants in the field was not due to an inability to remove the organism from the lettuce or from dispersal and lack of strain attachment. Rather, it appears that the majority of the E. coli cells in the inoculum either died shortly after application or entered a VBNC state.
In contrast to the growth chamber experiment results, the numbers of E. coli O157:H7 cells were below detection by real-time PCR within 2 days after inoculation onto field lettuce. These findings suggest that the E. coli O157:H7 cells and genomic DNA were degraded rapidly. Environmental parameters such as solar radiation, heat, and water stress could be responsible for the differences in the stability of E. coli O157:H7 DNA in the field compared with the laboratory. Alternatively, cell maintenance might depend on other microorganisms on the leaves [41]. Also, it is notable that different lettuce cultivars were used in the field and growth chamber studies, which may have impacted survival. The potential for cultivar-dependent effects was shown for E. coli O157:H7 on lettuce cultivars grown under axenic conditions in the laboratory [42].
Because the E. coli O157:H7 ATCC 700728 DNA was degraded on field-grown plants within 2 days after inoculation, it is unlikely that the organism developed a VBNC state, particularly over longer time scales (days and weeks). However, this possibility could not be directly addressed using the PMA real-time PCR assay in the field trials. Viable cell amounts measured by culturing and PMA real-time PCR were in agreement immediately after application of ATCC 700728 onto laboratory-grown lettuce, but PMA-mediated detection was impaired on plants from the field. For those plants, the viable cell number estimates for strain ATCC 700278 were 10-fold lower as measured by PMA real-time PCR than by colony enumeration and total cell numbers estimated by real-time PCR. These differences might have been due to the higher turbidity or opacity of the lettuce plant washes from the field samples, thereby preventing light from penetrating the suspension during the PMA photoinactivation step. This interference would prevent the inactivation of free (unbound) PMA, resulting in sufficient quantities of the compound to bind genomic DNA released from viable cells during the subsequent DNA extraction and amplification steps. In addition, the PMA real-time PCR assay was unable to detect low numbers of cells. Attempts to detect the ATCC 700278 strain after concentrating the leaf washes were unsuccessful (data not shown). Similarly, PMA real-time PCR was found to be more reliable for viable cell detection in diluted wastewater than in pooled and concentrated wastewater samples [32]. Such factors strongly limit the overall usefulness of this approach for field-grown plants. However, this method is informative for examination of E. coli O157:H7 on “cleaner” plants grown in the growth chamber and not exposed to the (a)biotic conditions that are common outdoors.
In conclusion, this study illustrates the similarities and differences between controlled studies of human pathogens on plants in a growth chamber and experiments examining the population dynamics of pathogens on plants under production-like conditions in the field. By applying relevant environmental conditions (moderate temperature and low RH) and droplet inoculation in the growth chamber, we were able to more closely mimic the rapid decline in E. coli O157:H7 culturability that was observed after inoculation of this organism onto lettuce plants in the Salinas Valley [14]. Culture-independent assessments confirmed that the pathogen remains on the plant long after application. However, field studies showed that at least for the majority of E. coli O157:H7 cell inoculants, the loss in culturability was most likely due to cell death rather than an inability to form colonies on standard laboratory media. Hence, this work confirmed our observations that low numbers of E. coli O157:H7 persist on lettuce grown in the Salinas Valley and variations in pathogen survival among individual plants are dependent on other unknown factors (e.g. location on the leaf, composition of the indigenous microbiota, and plant position in the field).
Supporting Information
Effect of increasing PMA concentration on detection of live and dead cells by real-time PCR. Live or isopropanol-inactivated dead cells of E. coli O157:H7 ATCC 700728 (5×106 CFU) were incubated for 30 min in 0, 50, or 100 µM PMA prior to real-time PCR with the lpfA primer set. Each bar represents the mean ± stdev for three independent replicates.
(DOCX)
Standard curves for detection of E. coli O157:H7 with hlyA primers (A) and lpfA primers (B). DNA was extracted by boiling as described in the Materials and Methods from a stationary phase culture of E. coli O157:H7 diluted to a cell density of 106 CFU/ml. Real-time PCR was performed for each dilution in triplicate. The limit of detection for E. coli O157:H7 cells with the lpfA and hlyA primers was 100 CFU/ml and 1000 CFU/ml, respectively. The amplification efficiency was 95% for EC4045 and 100% for ATCC700728 with the lpfA primers and 84% for ATCC700728 with the hlyA primers.
(DOCX)
Primers used in this study.
(DOCX)
Ct values for real-time PCR measurements performed using different annealing temperatures and gene targets of E. coli O157:H7 ATCC700728 and E. coli K12.
(DOCX)
Acknowledgments
We thank Tyann Blessington, Neha Dhawan, Anuja Ganpule, Raymond Li, Thomas Williams, and Irene Zhao from UC Davis, and Michael D. Cahn and Steven T. Koike from the University of California Cooperative Extension Monterey County for their technical assistance in completing this research.
Funding Statement
This project was supported by Agriculture and Food Research Initiative Grant 2010-65201-20572 from the USDA National Institute of Food and Agriculture. The field trials were supported by funds from the California Leafy Greens Research Board and the U.S. Food and Drug Administration (Project No. U01-003-572). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Painter JA, Hoekstra RM, Ayers T, Tauxe RV, Braden CR, et al. (2013) Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg Infect Dis 19: 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. (CDC) Centers for Disease Control and Prevention (2006) Ongoing multistate outbreak of Escherichia coli serotype O157:H7 infections associated with consumption of fresh spinach–United States, September 2006. MMWR Morb Mortal Wkly Rep 55: 1045–1046. [PubMed] [Google Scholar]
- 3. (CDC) Centers for Disease Control and Prevention (2008) Outbreak of Salmonella serotype Saintpaul infections associated with multiple raw produce items–United States, 2008. MMWR Morb Mortal Wkly Rep 57: 929–934. [PubMed] [Google Scholar]
- 4. Jablasone J, Warriner K, Griffiths M (2005) Interactions of Escherichia coli O157:H7, Salmonella typhimurium and Listeria monocytogenes plants cultivated in a gnotobiotic system. Int J Food Microbiol 99: 7–18. [DOI] [PubMed] [Google Scholar]
- 5. Aruscavage D, Lee K, Miller S, LeJeune JT (2006) Interactions affecting the proliferation and control of human pathogens on edible plants. J Food Sci 71: R89–R99. [Google Scholar]
- 6. Brandl MT (2006) Fitness of human enteric pathogens on plants and implications for food safety. Annu Rev Phytopathol 44: 367–392. [DOI] [PubMed] [Google Scholar]
- 7. Delaquis P, Bach S, Dinu LD (2007) Behavior of Escherichia coli O157:H7 in leafy vegetables. J Food Prot 70: 1966–1974. [DOI] [PubMed] [Google Scholar]
- 8. Franz E, van Bruggen AHC (2008) Ecology of E. coli O157:H7 and Salmonella enterica in the primary vegetable production chain. Crit Rev Microbiol 34: 143–161. [DOI] [PubMed] [Google Scholar]
- 9. Tyler HL, Triplett EW (2008) Plants as a habitat for beneficial and/or human pathogenic bacteria. Annu Rev Phytopathol 46: 53–73. [DOI] [PubMed] [Google Scholar]
- 10. Hirneisen KA, Sharma M, Kniel KE (2012) Human enteric pathogen internalization by root uptake into food crops. Foodborne Pathog Dis 9: 396–405. [DOI] [PubMed] [Google Scholar]
- 11. Brandl MT, Amundson R (2008) Leaf age as a risk factor in contamination of lettuce with Escherichia coli O157:H7 and Salmonella enterica . Appl Environ Microbiol 74: 2298–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erickson MC, Webb CC, Diaz-Perez JC, Phatak SC, Silvoy JJ, et al. (2010) Surface and internalized Escherichia coli O157:H7 on field-grown spinach and lettuce treated with spray-contaminated irrigation water. J Food Prot 73: 1023–1029. [DOI] [PubMed] [Google Scholar]
- 13. Bezanson G, Delaquis P, Bach S, McKellar R, Topp E, et al. (2012) Comparative Examination of Escherichia coli O157:H7 survival on romaine lettuce and in soil at two independent experimental sites. J Food Prot 75: 480–487. [DOI] [PubMed] [Google Scholar]
- 14. Moyne AL, Sudarshana MR, Blessington T, Koike ST, Cahn MD, et al. (2011) Fate of Escherichia coli O157:H7 in field-inoculated lettuce. Food Microbiol 28: 1417–1425. [DOI] [PubMed] [Google Scholar]
- 15. Islam M, Doyle MP, Phatak SC, Millner P, Jiang X (2004) Persistence of enterohemorrhagic Escherichia coli O157:H7 in soil and on leaf lettuce and parsley grown in fields treated with contaminated manure composts or irrigation water. J Food Prot 67: 1365–1370. [DOI] [PubMed] [Google Scholar]
- 16. Lindow SE, Brandl MT (2003) Microbiology of the phyllosphere. Appl Environ Microbiol 69: 1875–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nocker A, Camper AK (2009) Novel approaches toward preferential detection of viable cells using nucleic acid amplification techniques. FEMS Microbiol Lett 291: 137–142. [DOI] [PubMed] [Google Scholar]
- 18. Kotewicz ML, Mammel MK, LeClerc JE, Cebula TA, et al. (2008) Optical mapping and 454 sequencing of Escherichia coli O157:H7 isolates linked to the US 2006 spinach-associated outbreak. Microbiology 154: 3518–3528. [DOI] [PubMed] [Google Scholar]
- 19. Bettelheim KA (1998) Reliability of CHROMagar O157 for the detection of enterohaemorrhagic Escherichia coli (EHEC) O157 but not EHEC belonging to other serogroups. J Appl Microbiol 85: 425–428. [DOI] [PubMed] [Google Scholar]
- 20. Sharma VK, Bearson SMD, Bearson BL (2010) Evaluation of the effects of sdiA, a luxR homologue, on adherence and motility of Escherichia coli O157:H7. Microbiology 156: 1303–1312. [DOI] [PubMed] [Google Scholar]
- 21. Wang G, Clark CG, Rodgers FG (2002) Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157:H7 serotype, and components of the type 2 Shiga toxin family by multiplex PCR. J Clin Microbiol 40: 3613–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang B, Taguri T, Sugiyama K, Amemura-Maekawa J, Kura F, et al. (2010) Comparison of ethidium monoazide and propidium monoazide for the selective detection of viable Legionella cells. Jpn J Infec Dis 63: 119–123. [PubMed] [Google Scholar]
- 23. Varma M, Field R, Stinson M, Rukovets B, Wymer L, et al. (2009) Quantitative real-time PCR analysis of total and propidium monoazide-resistant fecal indicator bacteria in wastewater. Water Research 43: 4790–4801. [DOI] [PubMed] [Google Scholar]
- 24. Van Frankenhuyzen JK, Trevors JT, Lee H, Flemming CA, Habash MB (2011) Molecular pathogen detection in biosolids with a focus on quantitative PCR using propidium monoazide for viable cell enumeration. J Microbiol Meth 87: 263–272. [DOI] [PubMed] [Google Scholar]
- 25. Dreux N, Albagnac C, Federighi M, Carlin F, Morris CE, et al. (2007) Viable but non-culturable Listeria monocytogenes on parsley leaves and absence of recovery to a culturable state. J Appl Microbiol 103: 1272–1281. [DOI] [PubMed] [Google Scholar]
- 26. Liang N, Dong J, Luo L, Li Y (2011) Detection of viable Salmonella in lettuce by propidium monoazide real-time PCR. J Food Sci 76: M234–M237. [DOI] [PubMed] [Google Scholar]
- 27. Dinu LD, Bach S (2011) Induction of viable but non-culturable Escherichia coli O157:H7 in the phyllosphere of lettuce: a food safety risk factor. Appl Environ Microbiol 77: 8295–8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pan Y, Breidt F (2007) Enumeration of viable Listeria monocytogenes cells by real-time PCR with propidium monoazide and ethidium monoazide in the presence of dead cells. Appl Environ Microbiol 73: 8028–8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Løvdal T, Hovda MB, Björkblom B, Møller SG (2011) Propidium monoazide combined with real-time quantitative PCR underestimates heat-killed Listeria innocua . J Microbiol Meth 85: 164–169. [DOI] [PubMed] [Google Scholar]
- 30. Contreras PJ, Urrutia H, Sossa K, Nocker A (2011) Effect of PCR amplicon length on suppressing signals from membrane-compromised cells by propidium monoazide treatment. J Microbiol Meth 87: 89–95. [DOI] [PubMed] [Google Scholar]
- 31. Nocker A, Cheung CY, Camper AK (2006) Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J Microbiol Meth 67: 310–320. [DOI] [PubMed] [Google Scholar]
- 32. Luo JF, Lin WT, Guo Y (2010) Method to detect only viable cells in microbial ecology. Appl Microbiol Biotechnol 86: 377–384. [DOI] [PubMed] [Google Scholar]
- 33. Sánchez G, Elizaquível P, Aznar R (2012) A single method for recovery and concentration of enteric viruses and bacteria from fresh-cut vegetables. Int J Food Microbiol 152: 9–13. [DOI] [PubMed] [Google Scholar]
- 34. Elizaquível P, Aznar R (2008) A multiplex RTi-PCR reaction for simultaneous detection of Escherichia coli O157:H7, Salmonella spp. and Staphylococcus aureus on fresh, minimally processed vegetables. Food Microbiol 25: 705–713. [DOI] [PubMed] [Google Scholar]
- 35.Maurer JJ (2006) The mythology of PCR: A warning to the wise. In: Maurer JJ, editor. PCR methods in foods. New York: Springer US. 27–40.
- 36. Brandl MT (2008) Plant lesions promote the rapid multiplication of Escherichia coli O157:H7 on postharvest lettuce. Appl Environ Microbiol 74: 5285–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cooley MB, Chao D, Mandrell RE (2006) Escherichia coli O157:H7 survival and growth on lettuce is altered by the presence of epiphytic bacteria. J Food Prot 69: 2329–2335. [DOI] [PubMed] [Google Scholar]
- 38. Aurass P, Prager R, Flieger A (2011) EHEC/EAEC O104:H4 strain linked with the 2011 German outbreak of haemolytic uremic syndrome enters into the viable but non-culturable state in response to various stresses and resuscitates upon stress relief. Environ Microbiol 13: 3139–3148. [DOI] [PubMed] [Google Scholar]
- 39. Grey B, Steck TR (2001) Concentrations of copper thought to be toxic to Escherichia coli can induce the viable but nonculturable condition. Appl Environ Microbiol 67: 5325–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Méric G, Kemsley EK, Falush D, Saggers EJ, Lucchini S (2013) Phylogenetic distribution of traits associated with plant colonization in Escherichia coli . Environ Microbiol 15: 487–501. [DOI] [PubMed] [Google Scholar]
- 41. Gourabathini P, Brandl MT, Redding KS, Gunderson JH, Berk SG (2008) Interactions between food-borne pathogens and protozoa isolated from lettuce and spinach. Appl Environ Microbiol 74: 2518–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quilliam RS, Williams AP, Jones DL (2012) Lettuce cultivar mediates both phyllosphere and rhizosphere activity of Escherichia coli O157:H7. PLOS ONE 7: e33842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of increasing PMA concentration on detection of live and dead cells by real-time PCR. Live or isopropanol-inactivated dead cells of E. coli O157:H7 ATCC 700728 (5×106 CFU) were incubated for 30 min in 0, 50, or 100 µM PMA prior to real-time PCR with the lpfA primer set. Each bar represents the mean ± stdev for three independent replicates.
(DOCX)
Standard curves for detection of E. coli O157:H7 with hlyA primers (A) and lpfA primers (B). DNA was extracted by boiling as described in the Materials and Methods from a stationary phase culture of E. coli O157:H7 diluted to a cell density of 106 CFU/ml. Real-time PCR was performed for each dilution in triplicate. The limit of detection for E. coli O157:H7 cells with the lpfA and hlyA primers was 100 CFU/ml and 1000 CFU/ml, respectively. The amplification efficiency was 95% for EC4045 and 100% for ATCC700728 with the lpfA primers and 84% for ATCC700728 with the hlyA primers.
(DOCX)
Primers used in this study.
(DOCX)
Ct values for real-time PCR measurements performed using different annealing temperatures and gene targets of E. coli O157:H7 ATCC700728 and E. coli K12.
(DOCX)