Abstract
The reconstitution of ion channels into chemically defined lipid membranes for electrophysiological recording has been a powerful technique to identify and explore the function of these important proteins. However, classical preparations, such as planar bilayers, limit the manipulations and experiments that can be performed on the reconstituted channel and its membrane environment. The more cell-like structure of giant liposomes permits traditional patch-clamp experiments without sacrificing control of the lipid environment.
Electroformation is an efficient mean to produce giant liposomes >10 μm in diameter which relies on the application of alternating voltage to a thin, ordered lipid film deposited on an electrode surface. However, since the classical protocol calls for the lipids to be deposited from organic solvents, it is not compatible with less robust membrane proteins like ion channels and must be modified. Recently, protocols have been developed to electroform giant liposomes from partially dehydrated small liposomes, which we have adapted to protein-containing liposomes in our laboratory.
We present here the background, equipment, techniques, and pitfalls of electroformation of giant liposomes from small liposome dispersions. We begin with the classic protocol, which should be mastered first before attempting the more challenging protocols that follow. We demonstrate the process of controlled partial dehydration of small liposomes using vapor equilibrium with saturated salt solutions. Finally, we demonstrate the process of electroformation itself. We will describe simple, inexpensive equipment that can be made in-house to produce high-quality liposomes, and describe visual inspection of the preparation at each stage to ensure the best results.
Keywords: Physiology, Issue 76, Biophysics, Molecular Biology, Biochemistry, Genetics, Cellular Biology, Proteins, Membranes, Artificial, Lipid Bilayers, Liposomes, Phospholipids, biochemistry, Lipids, Giant Unilamellar Vesicles, liposome, electrophysiology, electroformation, reconstitution, patch clamp
Introduction
Giant liposomes (often called giant unilamellar vesicles, or GUVs) have primarily been used to study the physics and physical chemistry of lipid bilayers, including studies of bilayer deformation, lateral phase coexistence ("rafts"), membrane fusion, etc1-4. They have a grossly cell-like structure: spherical shell of membrane surrounding an aqueous interior which can easily be made different than the surrounding aqueous buffer. They are, by definition, ≈1-100 μm in diameter, so they can be imaged using a variety of light microscopy approaches. They can be made taut using osmotic gradients or mechanically applied tension, so that while generally soft, their properties can be manipulated for easy handling. In particular, controlling the "stiffness" of the liposome makes it straightforward to form "liposome-attached" or excised patches for electrophysiology. In the past, ion channel reconstitution was largely performed in planar lipid bilayers. Now, the ability to form patches from giant liposomes and use the considerable quiver of tools developed for conventional electrophysiology (fluorescence microscopy, micropipette aspiration, rapid perfusion and temperature control, etc.) makes giant liposomes increasingly attractive for reconstitution studies5,6.
Giant liposomes have been made by many strategies. In fact, giant liposomes form spontaneously by a swelling process when a dried lipid film is rehydrated4,7,8. The desire to more rapidly prepare larger, more uniform liposomes led researchers to other approaches, chief among them electroformation1,9. Electroformation also relies on hydration of a dried lipid film, but speeds the process through the application of an oscillating electric field across the lipid film. The field is applied through two electrodes, either platinum wires or Indium-Tin-Oxide (ITO) coated glass slides, separated by water or buffer and onto which the lipids are deposited. By speeding the swelling of liposomes, one achieves a higher yield of larger liposomes. Thus, electroformation has become the default method to produce giant liposomes4.
The mechanism of electroformation is not fully understood, and most of the protocols are developed empirically (e.g. 10,11). Nonetheless, we can learn a little about what to expect by considering the theory and some empirical results. It is widely believed that electroformation occurs by driving electro-osmotic flow of buffer between individual lipid bilayers stacked in the deposited lipid film10,11. Electrostatic coupling to thermal fluctuations of the lipid bilayers is probably also involved12. These hypotheses qualitatively predict upper limits for the electric field frequency and strength that can be used10,12. In particular, it is predicted that high conductivity solutions (i.e. physiological salt solutions) reduce the electrohydrodynamic forces that may initiate the liposome electroformation12. Electroosmotic flow rates generally decrease with increasing salt concentration and are frequently peaked at some electric field oscillation frequency (e.g. albeit in a different geometry, Green et al.13). Thus, higher field strengths and higher frequencies are reasonable for high conductivity solutions, within limits10.
However, membrane proteins are likely to be incompatible with the usual method of depositing lipids onto electrodes for the electroswelling procedure, namely in organic solvents which are then evaporated off to leave a thin lipid film. There are two principal paths around this difficulty: to incorporate proteins after giant liposome formation, or to adapt how the lipids are deposited. Our approach builds on others5,11 to deposit the lipids and reconstituted membrane protein together from a suspension of small or large "proteoliposomes". We describe the lengthy and more challenging process of producing proteoliposomes from purified protein and lipids elsewhere (Collins and Gordon, in review). Here we describe the protocol in the absence of any protein, but it is the same when protein is incorporated; we include results showing that proteoliposomes containing the ion channel TRPV1 can be transformed into GUVs and used for patch-clamp electrophysiology. In any electroformation approach, visual inspection of the lipid sample during the lipid deposition process is critical to success.
Our approach may be relevant beyond the specialized application to ion channel reconstitution. In the time since we first developed this protocol and now, it has also been shown how the way in which lipids are deposited on electrodes for electroformation affects the compositional heterogeneity of the resulting GUVs. Baykal-Caglar et al.14 showed that GUVs formed from carefully dehydrated liposomes had a 2.5 times smaller variation in the miscibility transition temperature of GUVs formed from mixtures of various phospholipids and cholesterol. Their work indicates that lipids, and especially cholesterol, may precipitate from the lipid mixture when deposited from organic solvents, resulting in large spatial variation in composition of the deposited lipid film. This is especially important for studies of lipid membrane phase behavior, but may also be critical to quantitative experiments on ion channel function. Baykal-Caglar et al.'s protocol is similar but not identical to our own, and readers are encouraged to study it as well.
This protocol (see overview, Figure 1) is one of many that could be used. In principle electroformation success depends on the lipid mixture, hydration, temperature, other solutes (especially ions), and of course the voltage and frequency used in formation. As electroformation becomes better understood, we expect to refine our protocol more.
Finally, there is often a steep learning curve in electroforming giant liposomes. We suggest mastering the conventional protocol (Sections 1 and 4, and, if necessary, Section 5) before learning to deposit lipids from liposomal suspensions (Sections 2-5).
Protocol
1. Deposition of Lipids from Organic Solvents: Classical Protocol
Remove lipids from storage at -20 °C or -80 °C; warm to RT. Caution: lipids are extremely hygroscopic, and many are sensitive to oxygen. Cover lipids in dry Argon or Nitrogen gas and in all steps minimize exposure to air.
If necessary, suspend the lipids in chloroform or cyclohexane at 1-10 mg/ml; note that manufacturer's stated concentrations are typically nominal only. CAUTION: Wear appropriate gloves and other personal protective equipment when using organic solvents. Remove the PPE quickly if solvent is spilled on them, as most materials are still permeable to these solvents and they only provide temporary protection.
Clean both sides of two 25 x 37.5 mm ITO coated glass slides with ethanol
Mix the lipids to achieve the desired molar ratios. For each 1 cm2 of area of ITO slides to be coated with lipid, mix ~15-20 μg of lipids. For example, if both 25 x 37.5 mm slides were to be fully coated, we would typically use 30 μl of 10 mg/ml lipid mixture. Note: add 0.1 mol% Texas Red-DPPE for fluorescent imaging, and see below for cautions about fluorescent dyes.
Verify that the ITO coated side of the glass slides is facing up by measuring the surface resistance with an ohmmeter or multimeter.
Aspirate the lipids into a solvent-resistant syringe. Caution: No glues or plastics, other than PTFE, should contact the organic solvent.
With the syringe needle not quite touching the ITO surface, slowly apply the lipid moving the needle back and forth across the slide. Cover the surface as evenly, looking for a "rainbow sheen" on the glass surface.
Place the slides quickly under < 1 Torr (1 mmHg) vacuum for 0.5-1 hr to remove any trace solvent. Release vacuum with inert gas.
Apply a silicone gasket, with a thin layer of silicone vacuum grease on both sides. Leave at least 5 mm of uncovered slide exposed at one end of the slide.
Proceed immediately to Section 4.
2. Small Liposome Preparation for Controlled Dehydration
Prepare the lipid mixture as in Steps 1.1 to 1.4.
Dry the lipids using a stream of Argon or Nitrogen gas in a 10-15 mm diameter culture tube. For small quantities of lipids, ≤0.5 mg, the resulting film can be hydrated directly after placing it under vacuum for 0.5-1 hr to remove residual solvent; proceed to step 2.5. Larger amounts of lipid tend to trap organic solvents in a thick gel, even under vacuum, and are better prepared by lyophilization; see steps 2.3-4.
Suspend the dried lipid film in cyclohexane, cover with Argon and seal with a stopper, place the tube in a cold block and freeze at -80 °C for 1 hr minimum.
Place the cold block and lipid sample in a vacuum system capable of reaching 100 mTorr. Apply vacuum; it will "stall" at about 1 Torr as solvent sublimes off. Once solvent is completely removed (~1 hr) vacuum will drop below this level. Release vacuum with inert gas and seal the tube or hydrate immediately.
While the lipids are under vacuum, degas hydration buffer using vacuum and sparging15-17.
Hydrate the lipids using degassed buffer at final concentration 1-10 mg/ml. Allow the lipids to hydrate slowly. After 30 min-1 hr, vortex to break up remaining clumps. Wait an additional 0.5-1 hr to allow for complete hydration. Use an osmoticant, such as sorbitol or sucrose up to 200 mM, to prevent complete dehydration in steps below.
Prepare 100-200 nm diameter liposomes by extrusion. See manufacturer's extrusion protocol for details. Note that the buffer must have less than ~25 mM ionic strength, or special electroformation voltage protocols will be needed in Section 4.
3. Deposition of Lipids by Controlled Dehydration of Small Liposomes
Caution: Our protocol requires lipids to be placed in vapor equilibrium with saturated salt solutions for many hours. We strongly recommend performing the protocol in an inert gas atmosphere, using a glove box or similar enclosure.
Caution: If preparing samples containing proteins, avoid complete dehydration. Hydrate the atmosphere in any enclosure with a beaker of warm water, or a sonicator based humidifier. Use a hygrometer to ensure there is at least 30% relative humidity. Use sorbitol or sucrose in the small liposome buffer as an additional precaution to prevent total dehydration.
Prepare a saturated salt solution of appropriate relative humidity. Target humidity depends on the osmolarity of the vesicle preparation. For preparations with physiological osmolarity, use 30-45% RH, while low osmolarity vesicle preparations can be adequately dehydrated in equilibrium with 75-90% RH (See also Table 1).
Put the saturated salt solution and excess salt in a tightly sealable container with an interior shelf. Commercial food containers for yogurt and granola work very well. Replace the shelf after filling, and make sure fluid is 5-10 mm below the shelf.
Clean ITO coated slides as in Section 1. Use the multimeter to determine which side is conductive, and label the non-conductive side with the sample name.
Lightly grease both sides of the silicone (USP Grade VI) gasket(s) with one or multiple holes.
Place the slides conductive side up on the bench, and apply the silicone gasket(s) to each slide to which lipid will be applied, making sure there is at least 5 mm of exposed slide at one end to connect to the electroformation apparatus. Smooth the gasket to ensure a good seal.
Dilute the vesicle preparation in a low-salt isosmotic buffer to ~1-2 mg/ml. We find that higher concentrations produce poor results.
Apply lipid to the slide in 1-10 μl drops. Smaller drops generally produce better results.
Place the slide on the interior shelf above the saturated salt solution and seal the container tightly. Leave at RT for 3 hr to O/N. The container can be placed at 4 °C, but note that for some salts, the RH depends strongly on temperature, and that cold will increase equilibration time.
The resulting film may have a slight rainbow sheen to it, but in any case should appear nearly desiccated. Highly osmotic starting solutions may be impossible to dry completely to a lipid film; this will not adversely affect the results.
4. Electroformation of Giant Liposomes
Lipid films, especially those formed from dehydrated liposomes, are easily dislodged. Carefully hydrate each well by placing a 27 G syringe needle at the edge of the gasket and slowly applying buffer. Buffers can include water, ≤200 mM sucrose, ≤1 M sorbitol and ≤5 mM HEPES. More than 10 mM salt solution may require alternate electroformation voltage protocols. Overfill each gasket well by ~10%.
Note: once the lipids are hydrated, proceed quickly through the remaining steps, since lipid films begin to delaminate immediately. Yield and size are maximized if electroformation begins promptly.
In one smooth motion, apply a second ITO slide, conductive face in, to the top of the gasket. Make sure to have at least 5 mm of overhang outside the gasket area and opposite the first overhang. Press gently to ensure a good seal.
Clean the two overhangs using ethanol and verify that sides facing the gasket are conductive with your multimeter.
The chamber can be further secured with parafilm or light tension spring clips if desired.
Connect the electroformation chamber to a voltage source by securing aluminum or copper bars, "EMI gasket foam", or conductive-adhesive tape to the conductive surfaces of the two slides. We use EMI gasket foam. Plans for a jig and clamp are shown in Figure 5.
Verify that there is no electrical short between the contacts, and that the contacts connect properly to the ITO surfaces, using the multimeter.
Heat the chamber 10 °C above the highest melting temperature of any of the lipids present; e.g. for DPPC, heat to 52 °C minimum, 10 °C above the chain melting temperature of 42 °C.
For low-salt buffers, apply a 10 Hz sine wave, ~0.7 Vrms for each millimeter gap between the two ITO coated surfaces, for 60-90 min. For high salt buffers, other voltage protocols should be used (see references).
5. Imaging and Troubleshooting
Image the liposome using an inverted microscope equipped with a filter cube for Rhodamine or Texas Red dyes. The liposome should be spherical, predominantly unilamellar by eye, and free of defects such as "strings" hanging off the liposome.
If the liposomes are too small, use less lipid.
If there are many defects or few liposomes, this may be due to gel-phase lipids. Consider raising the electroformation temperature.
If few or no giant liposomes formed at all from dehydrated small liposomes, reduce the osmotic strength of the liposome buffer, or increase the osmotic strength of the electroformation buffer. An inrush of buffer into the concentrated interstitial buffer solution can cause delamination of the deposited lipid film.
If vesicle yield, quality, or size is poor, and you included salts or pH buffers in the electroformation or liposome buffers, consider alternative electroformation voltage protocols. For instance, Pott, et al.11, recommend a three step protocol using a 500 Hz sinewave, raising the voltage from 50-1,300 Vpp/m over 30 min, holding for 90 min, then decreasing the frequency to 50 Hz over 30-60 min. Platinum or titanium electrodes may be needed in this case, but this will not substantially alter the protocol.
Representative Results
In our examples, we prepare liposomes from a mixture of approximately 55 mol% POPC (1-palmitoyl-2-oleoyl-sn-glycero-phosphocholine), 15 mol% POPS (1-palmitoyl-2-oleoyl-sn-glycero-phosphoserine, 30 mol% cholesterol, and 0.1 mol% Texas Red-labeled 1,2-dipalmitoyl-sn-phosphoethanolamine (TxR-DPPE). This composition was chosen as approximately representative of dorsal root ganglion lipids18. We note that 15 mol% charged lipid (here POPS) is near the limit of what can be used in electroformation (e.g. Walde et al.4).
Lipid breakdown by hydrolysis and peroxidation is an especially important issue for many lipid mixtures and many sensitive experiments (see Discussion). For clarity alone, we do not use inert gas atmospheres in the video presentation. As is evident from the figures below, this does not noticeably impact the quality of the liposomes, but for sensitive studies or when using highly unsaturated lipids it is important to prevent lipid breakdown.
Since the major hurdle in electroformation is to form a good lipid film on the Indium-Tin Oxide (ITO) substrate, knowing what the lipid film should look like when dried is crucial. However, when electroformation fails, it often does so completely, resulting in no appreciable giant liposome production. Frequently, substantial detritus will be observed; this typically indicates that too much lipid has been used. Our figures show what one expects to see upon success.
Figure 2 shows two different patches of liposomes electroformed from lipids deposited out of chloroform. In the left panel, closely examine the patches indicated by arrows 2 and 3. While arrow 2 indicates a patch with poorly distinguishable liposomes, no liposomes can be brought into focus in the area indicated by arrow 3. Together, these findings indicate a generally poor preparation, probably due to there being too much lipid deposited in this area. In the right panel, such "fuzzy" regions are less common, indicating a generally better preparation. We find that it is rare to completely eliminate such imperfections.
Figure 3 shows liposomes successfully electroformed from lipids deposited by dehydration of small liposomes, at two magnifications, imaged using Texas Red epifluorescence. Liposomes are sparse, but there are several good specimens. Because size varies, several are out of focus. The arrows indicate three good quality liposomes ranging in size from ~5-20 μm, two of which appear in the 40x magnification image. Note the clear "ring" at the edge: this indicates a unilamellar or nearly unilamellar liposome. While some spots of lipid appear to have collapsed or never formed lipid, this is a good quality result.
Finally, our purpose in developing and publishing this protocol is to prepare GUVs with intact, functional mammalian ion channels that are suitable for conventional patch-clamp electrophysiology. In Figure 4 we demonstrate capsaicin-activated TRPV1 ionic currents recorded in lipid membrane patches excised from GUVs formed using this protocol. The current can be blocked by Ruthenium Red, and is not activated by the capsaicin vehicle (0.1% ethanol, 138 mM NaCl, 3 mM HEPES pH 7.4) alone. The preparation of the proteoliposomes for electrophysiology experiments is far beyond the scope of the present protocol, but is the subject of a submitted article.
| Salt | Molality @ 20 °C and saturation | %RH @ 10 °C | %RH @ 20 °C | %RH @ 30 °C |
| Magnesium Chloride Hexahydrate | 5.8 | 33 | 33 | 32 |
| Potassium Carbonate Dihydrate | 8.0 | 47 | 44 | 42 |
| Sodium Bromide | 4.6 | 58 | 57 | 57 |
| Cupric Chloride | 5.6 | 68 | 68 | 67 |
| Sodium Chloride | 6.13 | 75 | 75 | 75 |
| Potassium Chloride | 4.61 | 87 | 86 | 84 |
Table 1. Relative humidity (%RH) of several common salts. Relative humidity data from Greenspan19 and Rockland20; saturation data from the International Critical Tables21.
 Figure 1. Schematic of giant liposome electroformation from small liposomes. (Top left) Small liposomes are deposited in an array of small droplets, < 5 μl. (Top center) The liposomes are dehydrated under controlled relative humidity by placing them in a sealed container above a saturated salt solution. A hygrometer is optional. (Top right) Once dehydrated to a sticky film of lipid and (possibly) osmoticant such as sorbitol, the lipids are rehydrated in a hyperosmotic buffer (see protocol section 5). (Lower left) the electroformation chamber is sealed with a second ITO slide on top. (Lower right) Finally, the chamber is heated above the lipid chain melting temperature, and connected to a signal source providing an oscillating electric field across the two ITO slides.
Figure 1. Schematic of giant liposome electroformation from small liposomes. (Top left) Small liposomes are deposited in an array of small droplets, < 5 μl. (Top center) The liposomes are dehydrated under controlled relative humidity by placing them in a sealed container above a saturated salt solution. A hygrometer is optional. (Top right) Once dehydrated to a sticky film of lipid and (possibly) osmoticant such as sorbitol, the lipids are rehydrated in a hyperosmotic buffer (see protocol section 5). (Lower left) the electroformation chamber is sealed with a second ITO slide on top. (Lower right) Finally, the chamber is heated above the lipid chain melting temperature, and connected to a signal source providing an oscillating electric field across the two ITO slides.
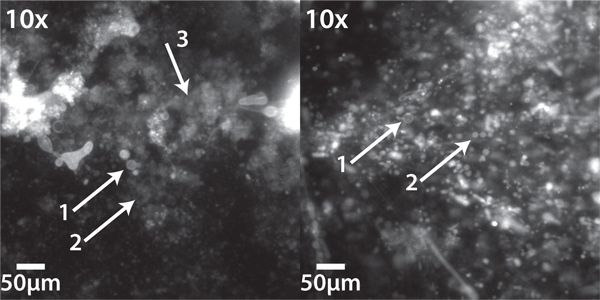 Figure 2. Electroformed giant liposomes formed from solvent-deposited lipids. (Left) Poor preparations have a few good quality liposomes (1) many more regions containing small, barely discernible liposomes, and many "fuzzy" regions with no discernible liposome formation. (2). (Right) While good preparations will still contain some regions of poorly discernible liposomes or "fuzz", there are many large liposomes (1 and 2). Imaged using an inverted epifluorescence microscope equipped with a Chroma 41004 Texas Red filter cube and Stanford Photonics XR/MEGA-10 S30 intensified CCD camera.
Figure 2. Electroformed giant liposomes formed from solvent-deposited lipids. (Left) Poor preparations have a few good quality liposomes (1) many more regions containing small, barely discernible liposomes, and many "fuzzy" regions with no discernible liposome formation. (2). (Right) While good preparations will still contain some regions of poorly discernible liposomes or "fuzz", there are many large liposomes (1 and 2). Imaged using an inverted epifluorescence microscope equipped with a Chroma 41004 Texas Red filter cube and Stanford Photonics XR/MEGA-10 S30 intensified CCD camera.
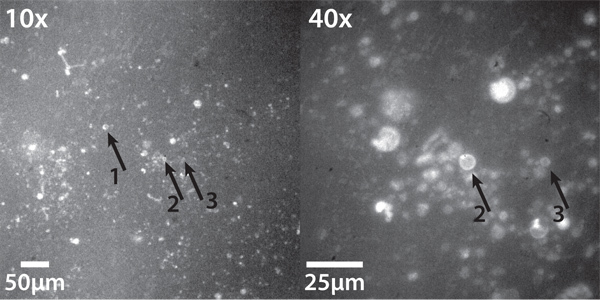 Figure 3. Electroformed giant liposomes formed from dehydration-deposited lipids. (Left) At 10x magnification, several liposomes are visible. Three liposomes are labeled. (right) The same view as at left, at 40x magnification, with the same liposomes (2 and 3) labeled. The number of these liposomes is typically much smaller than when electroforming from solvent-deposited lipid, but is entirely adequate for many purposes. Imaged using an inverted epifluorescence microscope equipped with a Chroma 41004 Texas Red filter cube and Stanford Photonics XR/MEGA-10 S30 intensified CCD camera.
Figure 3. Electroformed giant liposomes formed from dehydration-deposited lipids. (Left) At 10x magnification, several liposomes are visible. Three liposomes are labeled. (right) The same view as at left, at 40x magnification, with the same liposomes (2 and 3) labeled. The number of these liposomes is typically much smaller than when electroforming from solvent-deposited lipid, but is entirely adequate for many purposes. Imaged using an inverted epifluorescence microscope equipped with a Chroma 41004 Texas Red filter cube and Stanford Photonics XR/MEGA-10 S30 intensified CCD camera.
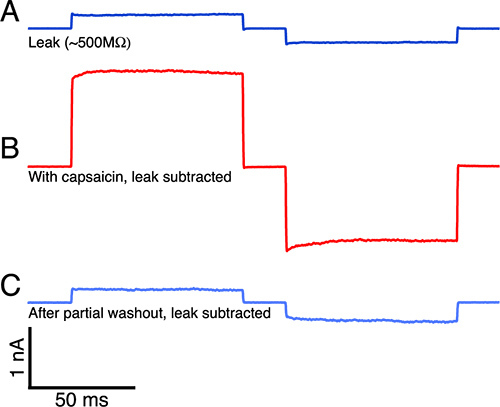 Figure 4. Capsaicin activated TRPV1 current recorded in a membrane patch excised from a GUV formed using this protocol.A. The leak current indicated a 500 MΩ seal resistance before capsaicin was added. B. Saturating capsaicin (>20 μM, in 0.1% ethanol) activates a large TRPV1 current; the current is blocked by Ruthenium Red, and is not activated by vehicle (0.1% ethanol in buffer) alone (data not shown). C. The current returns to near baseline as capsaicin is washed out of the patch.
Figure 4. Capsaicin activated TRPV1 current recorded in a membrane patch excised from a GUV formed using this protocol.A. The leak current indicated a 500 MΩ seal resistance before capsaicin was added. B. Saturating capsaicin (>20 μM, in 0.1% ethanol) activates a large TRPV1 current; the current is blocked by Ruthenium Red, and is not activated by vehicle (0.1% ethanol in buffer) alone (data not shown). C. The current returns to near baseline as capsaicin is washed out of the patch.
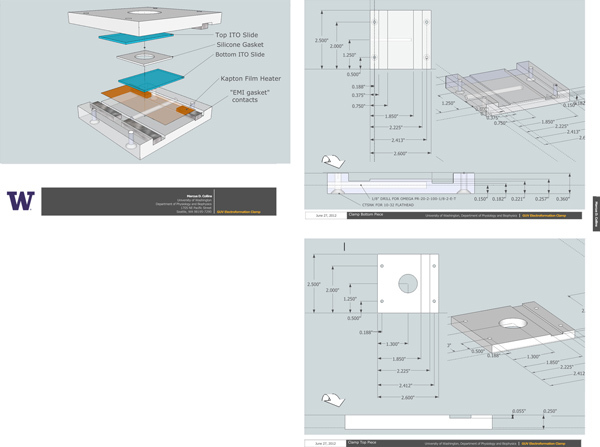 Figure 5. Plans for electroformation chamber. Included is an overview, showing the complete assembly, and dimensioned schematics for the top and bottom plastic pieces. Use acrylic or another easily machined clear plastic. The thin Kapton film heater should be connected to the temperature controller (see Table of specific reagents and equipment), and the EMI gasket foam should be connected to the function generator, so that the sine wave signal is applied across the two ITO coated slides. A hole is provided for a temperature probe. Flathead 10-32 screws are placed in the countersunk holes in the bottom piece, and pass through the upper piece. 10-32 nuts are used to secure the assembly. When fully assembled, the top and bottom plastic pieces should be flush with each other on two sides (the sides parallel to the EMI gasket.) Click here to view larger figure.
Figure 5. Plans for electroformation chamber. Included is an overview, showing the complete assembly, and dimensioned schematics for the top and bottom plastic pieces. Use acrylic or another easily machined clear plastic. The thin Kapton film heater should be connected to the temperature controller (see Table of specific reagents and equipment), and the EMI gasket foam should be connected to the function generator, so that the sine wave signal is applied across the two ITO coated slides. A hole is provided for a temperature probe. Flathead 10-32 screws are placed in the countersunk holes in the bottom piece, and pass through the upper piece. 10-32 nuts are used to secure the assembly. When fully assembled, the top and bottom plastic pieces should be flush with each other on two sides (the sides parallel to the EMI gasket.) Click here to view larger figure.
Discussion
Electroformation of giant liposomes has developed into a flexible technique compatible with diverse lipids, preparations, and buffers. Careful control of the lipid deposition process is most critical to success. We have presented simple tools to make controlled deposition of lipids from small liposome preparations a straightforward process. The relative humidity is critical to proper dehydration of the initial liposomes, and the optimum value will vary with the initial concentration of solutes in the liposome suspension. Lower relative humidity is required to dehydrate more concentrated samples to an adequate level.
The exact hydration protocol and electric field used for electroformation remains a point of discussion1,4,7,10,11,22. The simplest protocols should be mastered first before attempting liposome electroformation in the presence of high salt concentrations, charged lipids, or very high concentrations of high melting temperature lipids. Once those skills are mastered, more advanced protocols typically divide the electroformation process into three parts: initial swelling, growth, and detachment. Initial swelling seems to be favored by slowly increasing the applied electric field11. A second, optional stage at fixed field strength can help control the final size of the liposomes. Finally, decreasing the frequency of the oscillating field may help to detach liposomes from the ITO surface. Higher frequencies are needed to electroform liposomes in physiological salt conditions11, but liposomes can be formed over an extremely wide range of field frequencies and amplitudes10.
The observation that giant liposomes will form spontaneously upon rehydration of dehydrated liposomes with a hypo-osmotic buffer7 indicates several keys to success. First, the liposome swelling process begins immediately, so that for electroformation, some groups have even found it useful to apply the AC electric field before rehydrating the lipid film10. Second, it is likely that fluid flow drives the liposomal swelling process, and that electroformation occurs at least in part because of electro-osmotically driven flow. This places limits on the useful frequency and amplitude range that can be used in electroformation10.
A major point of discussion in the last several years has been the potential for lipid hydrolysis and peroxidation23,24. The first defense against lipid degradation of any kind is to remove oxygen from all materials used in the GUV preparation: since peroxidation depends on molecular oxygen23, this should slow the process considerably. A combination of vacuum and sparging with inert gas should be used to remove as much oxygen as possible15-17. Sonication and light vacuum are ineffective. We use an oxygen test kit (Chemetrics K7501) to verify that we have removed oxygen as thoroughly as possible. Hydrolysis is more pernicious, but is reduced by maintaining neutral pH25, and by reducing the voltage used in electroformation24. Some authors advocate the use of titanium electrodes in place of ITO slides23,24,26,27. We prefer to avoid the possibility of contaminating our samples with titanium (or any metal), since it has in the past been known to interfere with liposome experiments28,29, but it might help to prevent some peroxidation or hydrolysis effects. It goes without saying that prolonged exposure to high temperatures accelerates lipid breakdown30. Finally, thin-layer chromatography24,31, colorimetric assays23, and other methods31 exist to quantify lipid degradation.
A similar issue relates to the fluorescent lipid dyes used to image GUVs, especially for studies of lateral phase separation30,32. In sensitive experiments, dyes should be chosen carefully30,33, and used at minimal concentration.
We are not aware of any consensus on whether the ITO electrode slides can be reused. Many groups do reuse their ITO slides, while some do not. Recent work indicates that the slides do degrade over time, but can be annealed to regain high performance34; this degradation had only a small effect for lipid mixtures including zwitterionic or anionic lipids, but was critical for mixtures containing cationic lipids. We reuse our slides without annealing.
Although there is great variability in the protocols used to electroform giant liposomes, the theoretical understanding and practical experience with the technique is constantly improving. Already liposomes can be formed with large amounts of charged lipids, or in high salt buffers. The key remains efficient deposition of the lipids on the electrode surface, which is the core of our protocol.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
We thank Bryan Venema and Eric Martinson for constructing the electroformation apparatus. This work was funded by grants from the National Institutes of General Medical Sciences of the National Institutes of Health (R01GM100718 to SEG) and the National Eye Institute of the National Institutes of Health (R01EY017564 to SEG).
References
- Dimova R, Aranda S, Bezlyepkina N, Nikolov V, Riske KA, Lipowsky R. A practical guide to giant vesicles. Probing the membrane nanoregime via optical microscopy. Journal of Physics-Condensed Matter. 2006;18(28):S1151–S1176. doi: 10.1088/0953-8984/18/28/S04. [DOI] [PubMed] [Google Scholar]
- Luisi PL, Walde P, editors. Giant Vesicles. John Wiley & Sons Ltd; 2000. [Google Scholar]
- Riquelme G, Lopez E, Garcia-Segura LM, Ferragut JA, Gonzalez-Ros JM. Giant liposomes: a model system in which to obtain patch-clamp recordings of ionic channels. Biochemistry. 1990;29(51):11215–11222. doi: 10.1021/bi00503a009. [DOI] [PubMed] [Google Scholar]
- Walde P, Cosentino K, Engel H, Stano P. Giant vesicles: preparations and applications. Chembiochem. 2010;11(7):848–865. doi: 10.1002/cbic.201000010. [DOI] [PubMed] [Google Scholar]
- Aimon S, Manzi J, Schmidt D, Poveda Larrosa JA, Bassereau P, Toombes GES. Functional reconstitution of a voltage-gated potassium channel in giant unilamellar vesicles. PLoS. One. 2011;6(10):e25529. doi: 10.1371/journal.pone.0025529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard P, Pecreaux J, Lenoir G, Falson P, Rigaud JL, Bassereau P. A new method for the reconstitution of membrane proteins into giant unilamellar vesicles. Biophys. J. 2004;87(1):419–429. doi: 10.1529/biophysj.104.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley S, Gordon VD. Making giant unilamellar vesicles via hydration of a lipid film. Curr. Protoc. Cell. Biol. 2008;24:1–13. doi: 10.1002/0471143030.cb2403s40. [DOI] [PubMed] [Google Scholar]
- Rodriguez N, Pincet F, Cribier S. Giant vesicles formed by gentle hydration and electroformation: a comparison by fluorescence microscopy. Colloids. Surf. B. Biointerfaces. 2005;42(2):125–130. doi: 10.1016/j.colsurfb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Angelova MI, Soleau S, Meleard P, Faucon JF, Bothorel P. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. Progressin Colloid & Polymer Science. 1992;89:127–131. [Google Scholar]
- Politano TJ, Froude VE, Jing B, Zhu Y. AC-electric field dependent electroformation of giant lipid vesicles. Colloids. Surf. B. Biointerfaces. 2010;79(1):75–82. doi: 10.1016/j.colsurfb.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Pott T, Bouvrais H, Méléard P. Giant unilamellar vesicle formation under physiologically relevant conditions. Chem. Phys. Lip. 2008;154(2):115–119. doi: 10.1016/j.chemphyslip.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Sens P, Isambert H. Undulation Instability of Lipid Membranes under an Electric Field. Phys. Rev. Lett. 2002;88(12) doi: 10.1103/PhysRevLett.88.128102. [DOI] [PubMed] [Google Scholar]
- Green NG, Ramos A, González A, Morgan H, Castellanos A. Fluid flow induced by nonuniform ac electric fields in electrolytes on microelectrodes. I. Experimental measurements. Phys. Rev. E. 2000;61(4):4011–4018. doi: 10.1103/physreve.61.4011. [DOI] [PubMed] [Google Scholar]
- Baykal-Caglar E, Hassan-Zadeh E, Saremi B, Huang J. Preparation of giant unilamellar vesicles from damp lipid film for better lipid compositional uniformity. Biochim. Biophys. Acta. 2012;1818(11):2598–2604. doi: 10.1016/j.bbamem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Bakalyar SR, Bradley MPT, Honganen R. The role of dissolved gases in high -performance liquid chromatography. J. Chromatogr. A. 1978;158(0):277–293. [Google Scholar]
- Brown JN, Hewins M, Van Der Linden JHM, Lynch RJ. Solvent degassing and other factors affecting liquid chromatographic detector stability. J. Chromatogr. A. 1981;204:115–122. [Google Scholar]
- Dolan JW. Mobile Phase Degassing-Why, When, and How. LC-GC. 1999;17(10):909–912. [Google Scholar]
- Cheng H, Jiang X, Han X. Alterations in lipid homeostasis of mouse dorsal root ganglia induced by apolipoprotein E deficiency: a shotgun lipidomics study. J. Neurochem. 2007;101(1):57–76. doi: 10.1111/j.1471-4159.2006.04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. 1977;81(1):89–96. [Google Scholar]
- Rockland LB. Saturated Salt Solutions for Static Control of Relative Humidity between 5 ° and 40 °C. Anal. Chem. 1960;32(10):1375–1376. [Google Scholar]
- Washburn EW. International Critical Tables of Numerical Data, Physics, Chemistry and Technology (1st Electronic Edition) 2003. pp. 216–249.
- Estes DJ, Mayer M. Giant liposomes in physiological buffer using electroformation in a flow chamber. Biochim. Biophys. Acta. 2005;1712(2):152–160. doi: 10.1016/j.bbamem.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Ayuyan AG, Cohen FS. Lipid Peroxides Promote Large Rafts: Effects of Excitation of Probes in Fluorescence Microscopy and Electrochemical Reactions during Vesicle Formation. Biophys. J. 2006;91(6):2172–2183. doi: 10.1529/biophysj.106.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Penningston NF, Wu J, et al. GUV preparation and imaging: minimizing artifacts. Biochim. Biophys. Acta. 2010;1798(7):1324–1332. doi: 10.1016/j.bbamem.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grit M, de Smidt JH, Struijke A, Crommelin DJ. Hydrolysis of phosphatidylcholine in aqueous liposome dispersions. Int. J. Pharm. 1989;50(1):1–6. [Google Scholar]
- Zhou Y, Berry CK, Storer PA, Raphael RM. Peroxidation of polyunsaturated phosphatidyl-choline lipids during electroformation. Biomaterials. 2007;28(6):1298–1306. doi: 10.1016/j.biomaterials.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Farkas ER, Webb WW. Multiphoton polarization imaging of steady-state molecular order in ternary lipid vesicles for the purpose of lipid phase assignment. J. Phys. Chem. B. 2010;114(47):15512–15522. doi: 10.1021/jp107025h. [DOI] [PubMed] [Google Scholar]
- Hauser HO. The effect of ultrasonic irradiation on the chemical structure of egg lecithin. Biochem. Biophys. Res. Commun. 1971;45(4):1049–1055. doi: 10.1016/0006-291x(71)90443-8. [DOI] [PubMed] [Google Scholar]
- Hauser H. Phospholipid vesicles. In: Cevc G, editor. Phospholipids Handbook. New York, New York: Marcel Dekker, Inc; 1993. [Google Scholar]
- Veatch SL. Electro-formation and fluorescence microscopy of giant vesicles with coexisting liquid phases. Meth. Mol. Biol. 2007;398:59–72. doi: 10.1007/978-1-59745-513-8_6. [DOI] [PubMed] [Google Scholar]
- Kim RS, LaBella FS. Comparison of analytical methods for monitoring autoxidation profiles of authentic lipids. J. Lipid. Res. 1987;28(9):1110–1117. [PubMed] [Google Scholar]
- Veatch SL, Leung SSW, Hancock REW, Thewalt JL. Fluorescent probes alter miscibility phase boundaries in ternary vesicles. J. Phys. Chem. B. 2007;111(3):502–504. doi: 10.1021/jp067636i. [DOI] [PubMed] [Google Scholar]
- Juhasz J, Davis JH, Sharom FJ. Fluorescent probe partitioning in GUVs of binary phospholipid mixtures: implications for interpreting phase behavior. Biochim. Biophys. Acta. 2012;1818(1):19–26. doi: 10.1016/j.bbamem.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Herold C, Chwastek G, Schwille P, Petrov EP. Efficient electroformation of supergiant unilamellar vesicles containing cationic lipids on ITO-coated electrodes. Langmuir. 2012;28(13):5518–5521. doi: 10.1021/la3005807. [DOI] [PubMed] [Google Scholar]


