Abstract
Context: Anaplastic thyroid carcinoma (ATC) is a fatal disease with a median survival of only 6 months. Novel therapies are needed to improve dismal outcomes.
Objective: A mutated, replication-competent, vaccinia virus (GLV-1h68) has oncolytic effects on human ATC cell lines in vitro. We assessed the utility of GLV-1h68 in treating anaplastic thyroid cancer in vivo.
Design: Athymic nude mice with xenograft flank tumors of human ATCs (8505C and DRO90–1) were treated with a single intratumoral injection of GLV-1h68 at low dose (5 × 105 plaque-forming unit), high dose (5 × 106 plaque-forming unit), or PBS. Virus-mediated marker gene expression (luciferase, green fluorescent protein, and β-galactosidase), viral biodistribution, and flank tumor volumes were measured.
Results: Luciferase expression was detected 2 d after injection. Continuous viral replication within tumors was reflected by increasing luciferase activity to d 9. At d 10, tumor viral recovery was increased more than 50-fold as compared with the injected dose, and minimal virus was recovered from the lung, liver, brain, heart, spleen, and kidneys. High-dose virus directly injected into normal tissues was undetectable at d 10. The mean volume of control 8505C tumors increased 50.8-fold by d 45, in contrast to 10.5-fold (low dose) and 2.1-fold (high dose; P = 0.028) increases for treated tumors. DRO90–1 tumors also showed significant growth inhibition by high-dose virus. No virus-related toxicity was observed throughout the study.
Conclusions: GLV-1h68 efficiently infects, expresses transgenes within, and inhibits the growth of ATC in vivo. These promising findings support future clinical trials for patients with ATC.
Anaplastic thyroid carcinoma (ATC) is a fatal disease with a median survival of 6 months after diagnosis. Conventional treatment with radiation and chemotherapy is considered palliative because of poor response rates and lack of demonstrable survival benefits (1, 2, 3, 4, 5, 6, 7). Novel therapeutic agents are necessary to improve dismal outcomes.
Oncolytic viral therapy is a promising approach to cancer treatment. Vaccinia has favorable characteristics as an oncolytic virus, including a wide range of susceptible hosts, efficient infection and gene expression, and strong lytic activity. An attractive feature of vaccinia is its established safety record historically in humans as a vaccine for smallpox (8, 9). We reported that a recombinant, replication-competent vaccinia virus (GLV-1h68) can lyse human ATC cell lines in vitro (10). In this study we assessed the ability of GLV-1h68 to treat ATC xenografts in vivo.
Materials and Methods
Cell lines
Two human ATC cell lines, 8505C (Japanese Collection of Research Bioresources Cell Bank, Shinjuku, Japan) and DRO90-1 (Guy Juillard, University of California, Los Angeles, CA), and one benign human thyroid follicular cell line Nthy-ori 3-1 (Norman Eberhardt, Mayo Clinic, Rochester, MN) were studied. 8505C was maintained in MEM, DRO90-1 was maintained in RPMI 1640 with nonessential amino acid, glutamine, sodium pyruvate, and sodium bicarbonate, and Nthy-ori 3-1 was maintained in RPMI 1640 with glutamine. African green monkey kidney fibroblasts (CV-1) were maintained in DMEM. All cell lines were grown in 10% fetal calf serum, with penicillin and streptomycin.
Virus
GLV-1h68 is a recombinant, replication-competent vaccinia virus derived from the vaccinia virus LIVP strain (Lister strain from the Institute of Viral Preparations, Moscow, Russia). The construction of GLV-1h68 has been described (11). GLV-1h68 contains four inserted cassettes encoding Renilla luciferase-green fluorescent protein fusion (RUC-GFP cassette), a reverse-inserted human transferrin receptor (rTfr), β-galactosidase, and β-glucuronidase into the F14.5L, J2R (thymidine kinase), and A56R (hemagglutinin) loci of the viral genome, respectively.
Cytotoxicity assays
8505C and Nthy-ori 3-1 cells were seeded in 12-well plates. GLV-1h68 was added at a multiplicity of infection of 0.01, or PBS added as a control. Supernatants were removed daily, and cells were lysed with Triton X-100 (1.35%). Lactate dehydrogenase was quantified with a Cytotox96 kit (Promega Corp., Madison, WI) at 490 nm by spectrophotometry (EL321e; BioTek Instruments, Inc., Winooski, VT). Results are expressed as the surviving percentage of cells, determined by comparing the measured lactate dehydrogenase of each test sample relative to control samples considered 100% viable. All samples were analyzed in triplicate.
Expression of luciferase, green fluorescent protein (GFP), and β-galactosidase
Four to 6-wk-old athymic male nude mice (National Cancer Institute, Bethesda, MD) were anesthetized with inhalational isoflurane (Baxter Corp., Mississauga, Ontario, Canada). Tumors were established by injecting 1 × 106 cells (8505C or DRO90-1) in 100 μl PBS into the sc flanks of the mice. Once 10- to 15-mm in diameter, the tumors were treated with a single intratumoral injection of GLV-1h68 [5 × 106 plaque-forming unit (pfu)]. Nontumor-bearing mice were injected with the same dose of GLV-1h68 in one of the following areas: liver, muscle (quadriceps femoris), thyroid or testis. At varying times, 5 μl coelenterazine (0.5 μg/μl; Biotium, Inc., Hayward, CA) in 95 μl PBS was injected via the retro-orbital sinus. Luciferase activity was detected with a cooled CCD camera (Xenogen IVIS; Xenogen Corp., Caliper Life Sciences, Hopkinton, MA). Emitted photons were measured for 60 sec. Images were analyzed using Living Image software (Xenogen, Caliper Life Sciences).
An 8505C bearing mouse was killed at d 3 and imaged with fluorescence microscopy (Leica MZFL3; Leica Microsystems GmbH, Wetzlar, Germany) for GFP. The tumor was excised, frozen (Tissue Tek, Sakura Finetek USA, Torrance, CA), and sectioned. Slides were fixed with 1% glutaraldehyde, stained with X-Gal (1 mg/ml) in 5 mm K4Fe(CN)6 and 2 mm MgCl2, and counterstained with nuclear fast red. Virus injected, nontumor-bearing mice and a DRO90-1 bearing mouse were killed at d 10, and the injected tissue sites were excised. Tissue staining with X-Gal was performed, and adjacent sections were stained with hematoxylin and eosin.
Viral biodistribution
DRO90-1 flank tumors were established. When tumors reached approximately 10 mm in diameter, mice (n = 3) received a single intratumoral injection of GLV-1h68 (5 × 106 pfu). Mice were killed 10 d later. Tumors and organs were excised, weighed, and washed with PBS. Tissue protein extraction liquid reagent (Pierce, Rockford, IL) was added. Samples were homogenized and sonicated. After centrifugation, supernatants were stored at −80 C. Serial dilutions were made of the samples, and standard plaque assays were performed on confluent CV-1 cells.
Murine flank tumor therapy
Tumors were established in athymic nude mice. When tumor diameter reached approximately 3–5 mm (8505C) or 6–8 mm (DRO90-1), tumors were treated with a single intratumoral injection of GLV-1h68 (5 × 105 or 5 × 106 pfu) or PBS (n = 5 per group). Tumor volumes were calculated by the formula a2 × b × 0.4, where “a” is the smallest diameter, and “b” is the diameter perpendicular to “a.” Animals were monitored for body weight and signs of toxicity.
Results
GLV-1h68 infects and expresses transgenes in ATC xenografts
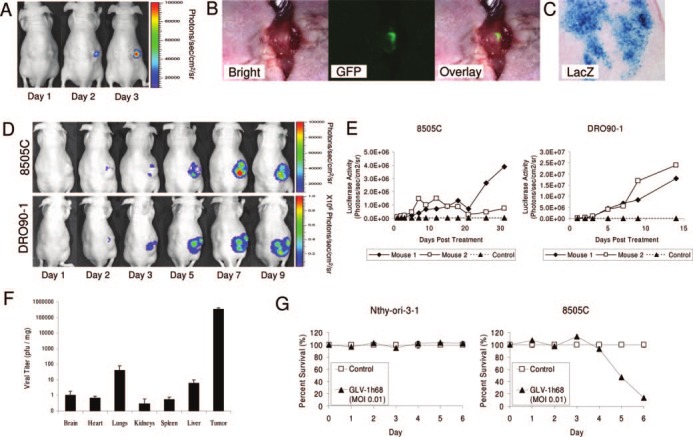
A mouse bearing an 8505C tumor was injected with intratumoral GLV-1h68 (5 × 106 pfu). Luciferase expression was first detected on d 2 and increased in intensity by d 3 (Fig. 1A). This tumor demonstrated GFP expression by fluorescence microscopy (Fig. 1B) as well as lacZ expression on histochemical staining (Fig. 1C).
Fig. 1.
Viral marker gene expression and viral biodistribution in mice with anaplastic thyroid cancer treated with a single intratumoral dose of GLV-1h68 (5 × 106 pfu). A, Luciferase expression measured by bioluminescence imaging in an 8505C tumor was first detected at d 2. GFP expression was detected by fluorescence microscopy (B), and lacZ histochemistry demonstrated robust β-galactosidase expression (magnification, ×50) (C) at d 3. D, Both 8505C and DRO90-1 tumors show a dramatic increase in luciferase activity from d 1–9 after GLV-1h68 injection. Luciferase expression levels were nearly 10-fold higher for DRO90-1 (note different y-axis scales) compared with 8505C. E, A time course of luciferase expression shows increasing luciferase activity and, therefore, continued viral replication for at least 31 (8505C) or 14 (DRO90-1) d. F, Analysis of viral biodistribution 10 d after a single intratumoral GLV-1h68 injection shows high viral recovery in DRO90-1 tumors, and only minimal viral recovery from other mouse organs (P < 0.01, t test). G, Cytotoxicity assays in vitro show sensitivity by 8505C to GLV-1h68 (P < 0.01, t test, d 5 and 6), but resistance by the benign thyroid follicular cell line Nthy-ori-3–1. MOI, Multiplicity of infection.
Mice with 8505C and DRO90-1 tumors were imaged for luciferase activity over a 9-d course after GLV-1h68 treatment. Luciferase expression increased from undetectable at d 1, to being most intense by d 9 (Fig. 1D). The luciferase expression levels were nearly 10-fold higher in DRO90-1 tumors (note different y-axis scales) than 8505C tumors. A longer time course of luciferase expression showed increasing luciferase activity up to d 31 (Fig. 1E). The 8505C tumor with high luciferase activity at d 31 (3.9 × 106 photons per second per cm2 per steradian) had a 2-fold higher volume at viral injection than the one with lower activity (7.6 × 105 photons per second per cm2 per sr). The two DRO90-1 tumors had comparable luciferase activity (1.8 × 107 and 2.4 × 107 photons per second per cm2 per sr) and similar tumor volumes.
GLV-1h68 replicates efficiently and specifically within ATC xenografts
To study viral biodistribution, we collected tumors and organs 10 d after intratumoral injection of GLV-1h68. Tumor demonstrated the highest viral recovery (3.4 × 105 ± 8.1 × 104 pfu/mg tumor tissue), reflecting a more than 50-fold overall titer increase over the initial GLV-1h68 injection dose (P < 0.01, t test). In contrast, the lungs (42 ± 35 pfu/mg), liver (5.9 ± 3.6), brain (1 ± 0.9), heart (0.7 ± 0.3), spleen (0.5 ± 0.3), and kidneys (0.3 ± 0.3) exhibited minimal viral recovery (Fig. 1F). Tumor tissue had more than 8000-fold higher viral pfu as compared with other organs (P < 0.01, t test).
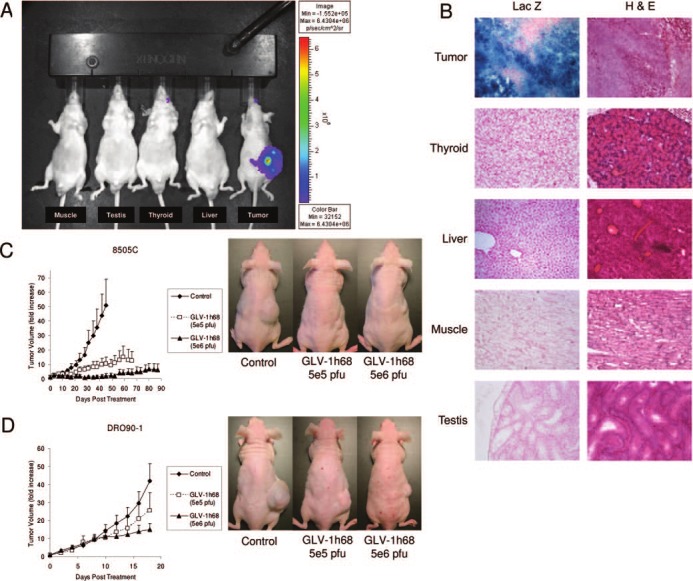
To assess the specificity of GLV-1h68 for ATC, cytotoxicity assays were performed that demonstrated that 8505C is sensitive to GLV-1h68, although a benign thyroid follicular cell line is resistant (Fig. 1G). For in vivo assessment, we directly injected normal tissue sites (thyroid, muscle, testis, liver) of nontumor-bearing mice, or a DRO90-1 tumor, with high-dose virus. There was no luciferase or lacZ expression at d 10 within any of the injected normal tissues, but intense expression by the DRO90-1 tumor (Fig. 2, A and B).
Fig. 2.
In vivo specificity of GLV-1h68 and therapy of anaplastic thyroid cancer tumors with a single intratumoral GLV-1h68 injection. Animals were directly injected with GLV-1h68 (5 × 106 pfu) into normal sites, including muscle (quadriceps femoris), testis, thyroid, liver, and into a DRO90-1 flank tumor. A, After 10 d, luminescence imaging showed no evidence of luciferase expression by the nontumor-bearing animals, although intense expression was identified in the ATC flank tumor. B, Histology of excised normal tissue injection sites and the DRO90-1 flank tumor corroborated the absence of detectable virus in the normal tissues, but intense lacZ expression in the tumor (magnification ×100). Tumor volume progression was significantly impeded for mice treated with GLV-1h68 (5 × 106 pfu) compared with control tumors for both 8505C (P = 0.028 at d 45, t test) (C) and DRO90-1 (P = 0.03 at d 18, t test) (D) tumors. A low GLV-1h68 dose (5 × 105 pfu) retarded the growth of both types of tumors but did not achieve statistical significance. Representative animals of 8505C (C) and DRO90-1 (D) at 42 and 18 d, respectively, after GLV-1h68 treatment demonstrate visible tumor volume differences between treated and untreated control animals. H & E, Hematoxylin and eosin; Max, maximum; Min, minimum; p, photons.
A single intratumoral injection of GLV-1h68 inhibits ATC growth
Treatment of ATC flank tumors with GLV-1h68 was performed to assess effects on tumor growth. Control 8505C tumor sizes increased to a mean 50.8 ± 18.2-fold of their initial starting volumes by d 45. In contrast, treatment of 8505C tumors with a single low dose (5 × 105 pfu) or high dose (5 × 106 pfu) of GLV-1h68 led to only 10.5 ± 4.5-fold and 2.1 ± 1.5-fold increases in tumor volume, respectively (Fig. 2C; P = 0.028 between high dose and control, t test). Control mice were killed at d 45 due to tumor burden, whereas high-dose treated animals demonstrated stable tumor volumes to d 66 (low dose) or 87 (high dose), at the conclusion of the study.
Control DRO90-1 tumors grew to a mean 42.0 ± 9.6-fold of their initial starting volumes by d 18. In contrast, treatment with a single low dose or high dose of GLV-1h68 led to only 25.5 ± 10.1-fold and 15.0 ± 3.5-fold increases in tumor volume, respectively (Fig. 2D; P = 0.03 between high dose and control, t test).
There was no body weight loss in animals bearing 8508C or DRO90-1 tumors treated with high-dose GLV-1h68. There was no morbidity or mortality attributable to viral therapy.
Discussion
ATC is one of the most aggressive human malignancies with a mean survival of 6 months after diagnosis, and conventional treatment is generally palliative in intent. We previously showed that a replication-competent, engineered, vaccinia virus (GLV-1h68) has an ability to infect and lyse a panel of human ATC cell lines in vitro, with a similar efficacy to that of an oncolytic herpes virus (10, 12). Here, we demonstrate the effects of GLV-1h68 on ATC in vivo.
Robust luciferase, GFP, and lacZ expression mediated by the virus was identified in less than 3 d after intratumoral injection with GLV-1h68. Localized luciferase activity increased dramatically in 8505C and DRO90-1 tumors over a 9-d course. The increase in luminescence reflects viral proliferation (13), which was confirmed by our plaque assays. GLV-1h68 is able to replicate efficiently within ATC tumors, and viral replication may be noninvasively monitored by luciferase imaging. The ability to image GLV-1h68 in vivo is an attractive clinical feature in allowing viral localization and activity assessment in patients undergoing therapy.
The inhibition of tumor growth with a single GLV-1h68 injection became apparent 10 d after viral injection. Interestingly, luciferase expression of GLV-1h68 was more robust in DRO90-1 than 8505C, demonstrating that the degree of viral replication may not necessarily reflect its volume response. The dynamic interactions between varying tumor growth rates and GLV-1h68 proliferation might account for this finding. Because DRO90-1 tumors grow much more rapidly than 8505C, the larger DRO90-1 tumors might be able to support higher peak viral replication. However, rapid tumor growth may also diminish the overall viral effect on tumor regression.
The most sensitive (8505C) and most resistant (DRO90-1) ATC cell lines determined from our prior in vitro study were assessed (10). It was encouraging to find that DRO90-1, which showed low sensitivity to GLV-1h68 in vitro, demonstrated significant inhibition of tumor progression with a single dose of GLV-1h68. The growth inhibition of 8505C in response to GLV-1h68 was more impressive, with tumors showing prolonged growth inhibition for 3 months.
GLV-1h68 proliferates efficiently and specifically within tumor tissues, with more than 50-fold higher viral titers recovered from excised tumors at d 10, but minimal viral recovery from other organs. Direct injection of high-dose GLV-1h68 into normal tissues failed to show any signs of viral gene expression at d 10, in contrast to injected tumors. All treated animals remained healthy, without morbidity or mortality attributable to viral therapy. The widespread historical use of vaccinia as a smallpox vaccine and its excellent safety profile are favorable attributes as a potential clinical agent. Vaccinia is estimated to have a 0.1% incidence of side effects, which can be managed with immunoglobulin (14).
In conclusion, these results highlight the ability of GLV-1h68 to express effectively its transgene markers, replicate, and inhibit ATC tumor progression in vivo. No treatment-related morbidity or mortality was identified. Vaccinia use historically was considered safe as a smallpox vaccine, and GLV-1h68 is further attenuated. These findings support the continued study of GLV-1h68 as a novel therapy for patients with ATC.
A recombinant, replication-competent, oncolytic vaccinia virus effectively infects, expresses transgenes within, and impedes tumor growth of a murine model of human anaplastic thyroid cancer.
Footnotes
This work was supported by a Clinical Innovator Award from the Flight Attendant Medical Research Institute (to R.J.W.).
Disclosure Statement: S.-F.L., D.L.P., C.-H.C., P.B., S.L., L.G., Y.F., and R.J.W. have nothing to declare. Q.Z., Y.AY., N.C., and A.A.S. are employed by and have equity interests in Genelux Corporation.
First Published Online August 12, 2008
Abbreviations: ATC, Anaplastic thyroid carcinoma; GFP, green fluorescent protein; pfu, plaque-forming unit.
References
- 1.Shimaoka K, Schoenfeld DA, DeWys WD, Creech RH, DeConti R 1985. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer 56:2155–2160 [DOI] [PubMed] [Google Scholar]
- 2.De Besi P, Busnardo B, Toso S, Girelli ME, Nacamulli D, Simioni N, Casara D, Zorat P, Fiorentino MV 1991. Combined chemotherapy with bleomycin, adriamycin, and platinum in advanced thyroid cancer. J Endocrinol Invest 14:475–480 [DOI] [PubMed] [Google Scholar]
- 3.Ain KB, Egorin MJ, DeSimone PA 2000. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Thyroid 10:587–594 [DOI] [PubMed] [Google Scholar]
- 4.Tennvall J, Lundell G, Wahlberg P, Bergenfelz A, Grimelius L, Akerman M, Hjelm Skog AL, Wallin G 2002. Anaplastic thyroid carcinoma: three protocols combining doxorubicin, hyperfractionated radiotherapy and surgery. Br J Cancer 86:1848–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugino K, Ito K, Mimura T, Nagahama M, Fukunari N, Kubo A, Iwasaki H, Ito K 2002. The important role of operations in the management of anaplastic thyroid carcinoma. Surgery 131:245–248 [DOI] [PubMed] [Google Scholar]
- 6.McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, van Heerden JA, Goellner JR 2001. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery 130:1028–1034 [DOI] [PubMed] [Google Scholar]
- 7.Nilsson O, Lindeberg J, Zedenius J, Ekman E, Tennvall J, Blomgren H, Grimelius L, Lundell G, Wallin G 1998. Anaplastic giant cell carcinoma of the thyroid gland: treatment and survival over a 25-year period. World J Surg 22:725–730 [DOI] [PubMed] [Google Scholar]
- 8.Shen Y, Nemunaitis J 2005. Fighting cancer with vaccinia virus: teaching new tricks to an old dog. Mol Ther 11:180–195 [DOI] [PubMed] [Google Scholar]
- 9.Thorne SH, Kirn DH 2004. Future directions for the field of oncolytic virotherapy: a perspective on the use of vaccinia virus. Expert Opin Biol Ther 4:1307–1321 [DOI] [PubMed] [Google Scholar]
- 10.Lin SF, Yu Z, Riedl C, Woo Y, Zhang Q, Yu YA, Timiryasova T, Chen N, Shah JP, Szalay AA, Fong Y, Wong RJ 2007. Treatment of anaplastic thyroid carcinoma in vitro with a mutant vaccinia virus. Surgery 142:976–983 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Yu YA, Wang E, Chen N, Danner RL, Munson PJ, Marincola FM, Szalay AA 2007. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res 67:10038–10046 [DOI] [PubMed] [Google Scholar]
- 12.Yu Z, Eisenberg DP, Singh B, Shah JP, Fong Y, Wong RJ 2004. Treatment of aggressive thyroid cancer with an oncolytic herpes virus. Int J Cancer 112:525–532 [DOI] [PubMed] [Google Scholar]
- 13.Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, Goebel W, Szalay AA 2004. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol 22:313–320 [DOI] [PubMed] [Google Scholar]
- 14.Sharp JCM, Fletcher W 1973. Experience of anti-vaccinia immunoglobulin in the United Kingdom. Lancet 1:656–659 [DOI] [PubMed] [Google Scholar]