Abstract
Two analogues of glucosamine-6-phosphate (GlcN6P, 1) and five of glucosamine (GlcN, 2) were prepared for evaluation as catalytic cofactor of the glmS ribozyme, a bacterial gene-regulatory RNA that controls cell wall biosynthesis. Glucosamine and allosamine with 3-azido substitutions were prepared by SN2 reactions of the respective 1,2,4,6-protected sugars; final acidic hydrolysis afforded the fully deprotected compounds as their TFA salts. A 6-phospho-2-aminoglucolactam (31) was prepared from glucosamine in a 13-step synthesis, which included a late-stage POCl3-phosphorylation. A simple and widely applicable 2-step procedure with the triethylsilyl (TES) protecting group was developed to selectively expose the 6-OH group in N-protected glucosamine analogs, which provided another route to chemical phosphorylation. Mitsunobu chemistry afforded 6-cyano (35) and 6-azido (36) analogues of GlcN-(Cbz) and the selectivity for the 6-position was confirmed by NMR (COSY, HMBC, HMQC) experiments. Compound 36 was converted to the fully deprotected 6-azido-GlcN (37) and 2,6-diaminoglucose (38) analogs. A 2-hydroxylamino glucose (42) analogue was prepared via an oxaziridine (41). Enzymatic phosphorylation of 42 and chemical phosphorylation of its 6-OH precursor (43) were possible, but 42 and the 6-phospho product (44) were unstable under neutral or basic conditions. Chemical phosphorylation of the previously described 2-guanidinyl-glucose (46) afforded its 6-phospho analogue (49) after final deprotection.
Introduction
The glmS ribozyme is a catalytic RNA domain that resides in the 5′ untranslated region (UTR) of the mRNA encoding the protein enzyme glucosamine-6-phosphate synthetase (GlmS) in most Gram-positive bacteria.1 It has attracted considerable attention because it is the first known example of a natural ribozyme that uses a small molecule coenzyme. (Reviewed in 2,3) The ribozyme employs the amine of GlcN6P (1) as a general acid-base catalyst to catalyze RNA cleavage through internal transesterification.4,5 Binding to the ribozyme lowers the pKa of this functional group by up to 1.6 pH units, improving the catalytic efficiency of the coenzyme.6,7 In Gram-positive bacteria, intact mRNAs have a triphosphate moiety at their 5′ terminus. Ribozyme cleavage of the 5′-UTR of the glmS mRNA exposes a 5′-OH group, which triggers its degradation by RNase J1. Because the GlmS protein is unstable, degradation of its mRNA stops synthesis of 1, thereby allowing negative-feedback regulation.8
Because it controls the synthesis of 1, an essential metabolic precursor to the bacterial cell wall, the glmS ribozyme has been considered a potential drug target.2,9 Established antibiotics such as the beta-lactams and Vancomycin inhibit assembly of the peptidoglycan component of the bacterial cell wall by disrupting the peptide crosslinking. For the glycan portion of the peptidoglycan cell wall biosynthesis, 1 is converted into the [MurNAcβ1-4GlcNAcβ1-4] repeating disaccharide subunit.10 Disrupting the peptidoglycan polysaccharide assembly via misregulation of GlmS is a tantalizing prospect, although it is still unclear whether activation or inhibition of the ribozyme by small molecules would be most effective. The Winkler group demonstrated in the Gram positive bacterium Bacillus subtilis that the glmS ribozyme is indeed essential for peptidoglycan biosynthesis, and that bacteria with a cleavage-defective ribozyme, analogous to an inhibited ribozyme, were unable to sporulate or form biofilms.8
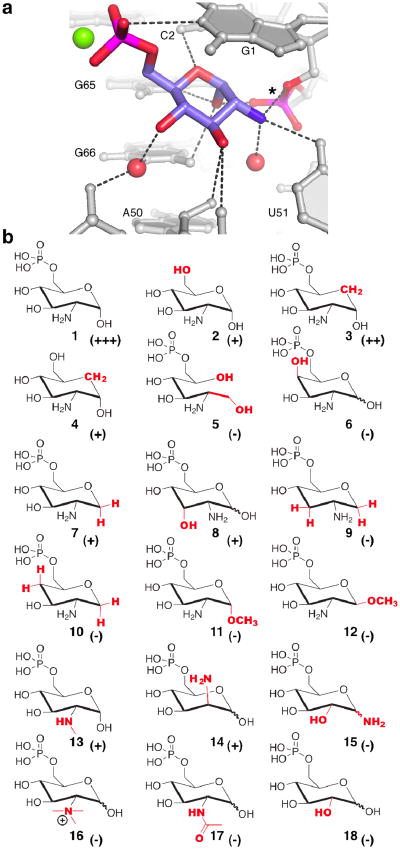
The 1.7Å resolution crystal structure of the glmS ribozyme in complex with 1 (Figure 1a) shows it binding to a shallow, solvent exposed pocket of the RNA in the α-axial anomeric conformation, with the amine in van der Waals contact with the 5′O of G1, the leaving group of the transesterification reaction.11 The functional importance of the amine is borne out by crystal structures of glucose-6-phosphate (18) bound to the ribozyme.4 Even though this compound is an inhibitor, rather than an activator, of the ribozyme,12 18 was found to bind in exactly the same location, and also in the α-axial anomeric conformation.
Figure 1.
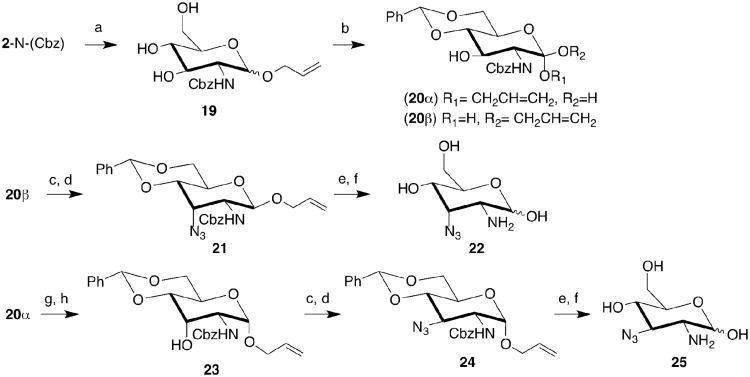
a. Detail of the crystal structure of GlcN6P bound to the glmS riboyzme.11 Green and red spheres represent a magnesium ion and water molecules, respectively. Dotted lines indicate hydrogen bonds. GlcN6P stacks under the nucleobase of RNA residue G1, with its anomeric oxygen participating in hydrogen bonding interactions with the scissile phosphate (*), G65 and G66. The catalytically critical amine of GlcN6P hydrogen bonds with the 5′-O of G1, the base of U51, and a well-ordered water molecule. The hydroxyls at positions 3 and 4 of GlcN6P make direct and water-mediated hydrogen bonds with backbone atoms of A50 and U51. The O4 of GlcN6P receives a hydrogen bond from the nucleobase of C2. Finally, the N1 amide of G1 hydrogen bonds to the phosphate of the small molecule. Figure 1b. Previously described GlcN6P analogues and their effectiveness as coenzymes for the glmS ribozyme. (-) = inactive; (+) = weakly active; (++) modestly active; (+++) = strongly active.
Analog studies aiming to discover potent unnatural coenzymes of the ribozyme have yielded some insight into requirements for coenzyme function (Figure 1b).1,13,14 The phosphate of 1 contributes to its binding to the ribozyme as evidenced by weaker activation by GlcN (2), consistent with the crystal structure. Carba-sugar analogs of 1 and 2, i.e. 3 & 4, are moderate and weak activators, corroborating the importance of O5 and the phosphate for binding. The compound is inactive if the pyranose ring is opened (5). Substituents that distort the chair conformation result in inactive analogs (6). Loss of the anomeric hydroxyl, or inversion in configuration at C3 is tolerated (7, 8), but loss of two hydroxyl groups or introduction of a bulky substituent at C1 results in loss of activity (9 – 12). Although methylation of the amine of GlcN6P (13) or inversion of configuration at C2 (14) is tolerated, activity of the ribozyme requires the amine to be at position 2 (15). Trimethylation of the amine (16) or its acetylation (17) yield compounds as inactive as 18.
With insights from previously published chemistry, biochemistry and X-ray crystallography studies, we sought improved glmS coenzyme candidates (activators) or inhibitors through the synthesis of 5 additional analogues of 2 and 2 additional analogues of 1. The 2-NH2 group's central role in catalysis led us to analogues with modifications to the 2-position (hydroxylamino and guanidinyl) or adjacent positions (3-azido and adjacent amide group in a lactam) that would modify the acid/base properties at the 2-position. Enzymatic phosphorylation was investigated but was of limited utility. A simple, widely applicable procedure for selective exposure of the 6-OH was developed for chemical phosphorylation and functional group transformation at the 6-position. Given the numerous H-bonding opportunities in RNA, changes to the H-bonding between coenzyme candidate and ribozyme active site may change the affinity of the coenzyme candidates relative to 1. In principle, all the analogues described here alter that pattern, but azido groups rarely participate in H-bond interactions.15,16 The azides, however, provide opportunities for future coenzyme candidates through click chemistry, for instance.
Results and Discussion
3-Azido-2-amino Sugars
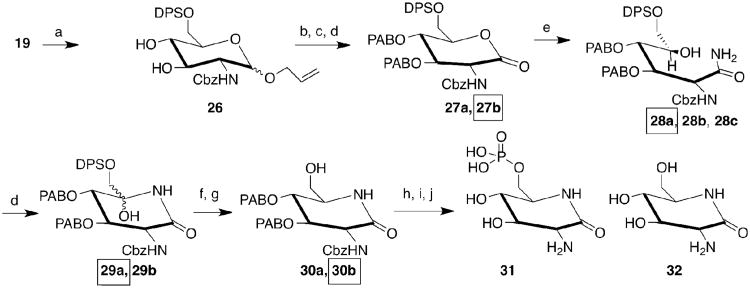
The presence of a 3-azido group allows for altered H-bond interactions and 2-NH2 pKa values compared to GlcN6P. Because allosamine-6-P was the most active, non-gluco configured coenzyme described to date13 we also prepared 3-azidoallosamine (22). GlcN-(Cbz) was protected by Fisher glycosylation and subsequent introduction of the 4,6-benzylidene acetal to produce 19 as a mixture of anomers (Scheme 1). The less soluble β-anomer (20β) was separated from the mixture by fractional crystallization (EtOAc) and cyclohexane trituration from a THF solution. Subsequent recrystallization (EtOAc) and flash chromatography of each anomer afforded 20α and 20β in 22.6 & 27.4 % yield for 2 steps, respectively. Compound 20β was converted to its mesylate, which was then displaced by NaN3 to afford the protected 3-azido-allosamine (21) in 52% yield for 2 steps. Compound 20α was converted to the allo-sugar (23)17 that was then converted to the 23-mesylate (87%). Treatment with NaN3 in DMF led to a mixture of 24 and an elimination side-product that were inseparable by flash chromatography. After deallylation, deallyl-24 (16% for 2 steps) was separated from the side product. Removal of all protecting groups from 21 and 24 was possible with extended heating in 1:1 TFA/H2O; however, purer 22 (38% from 21) and 25 (77% from deallyl-24) were isolated when the anomeric allyl group was removed first. Azido-substituted aminosugars 22, and 25 can be added to the two unprotected hexoses with azido and amino functional groups that have appeared in the literature: 3-azido-mannosamine,18 and 4-azido-galactosamine.19 Aminosugar 25 could be utilized to image glycans that use GlcN6P and GlcN,20, but given that allosamine is a rare aminosugar,21 22 is unlikely to find such utility.
Scheme 1. Preparation of the 3-Azido-2-amino Carbohydrates.
a) CH2=CHCH2OH, H2SO4 (cat.), reflux; b) PhCH(OMe)2, (±)-camphorsulfonic acid (cat), DMF; c) Ms2O, py; d) NaN3, DMF, 120°C; e) SeO2, AcOH, 1,4-dioxane, 120°C; f) 1:1 TFA:H2O, 120°C; g) dmso, Ac2O; h) NaBH4, MeOH.
Chemical and enzymatic phosphorylations
The 6-phosphate of GlcN6P is a key feature that improves coenzyme binding to the glmS ribozyme, facilitating discrimination among many possible carbohydrate ligands. Chemical phosphorylation of several of the remaining coenzyme candidates was accomplished by POCl3-treatment in pyridine of a selectively exposed 6-OH group, which was prepared via orthogonal protection groups (lactam synthesis) or via selective removal of the 6-O-triethylsilyl (TES) group from a tetrasilylated sugar. Compounds 22, 25, 32, 42, and 45 were all investigated as yeast hexokinase (HK) substrates. Only 25 (see Supporting Information) and 42 (see below) were phosphorylated, despite the fact that HK accepts a wide variety of glucose-similar substrates and that changes to the hexose 2- and 3-positions are well tolerated.22
Phosphoamino Lactam Synthesis
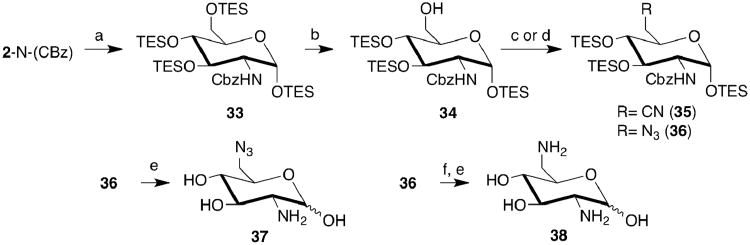
The phosphoamino lactam (31) analogue of GlcN6P was chosen because of its relatively inert nature, which could be an advantage for extended biochemical studies. Lactam 31 lacks an anomeric OH, is unable to undergo mutarotation and would not be a substrate for the next step in peptidoglycan synthesis, namely, conversion of GlcN6P to its 1-phosphate by phosphoglucosamine mutase.23 The 2-amino gluconolactam (32) appeared in the literature,24,25 but not the phosphorylated analogue (31). Thus the synthesis of 31 was similar to related molecules24-26 but also included a late-stage phosphorylation of the primary OH. Protection groups stable to basic (NH3) and acidic conditions (HCOOH) as well as orthogonality in removing the anomeric and 6-OH protecting groups were necessary. In addition, selective, facile removal of the 3,4-O-groups was desired as to provide an N-Cbz-protected phospho-lactam that could be HPLC-purified before the final, rapid Cbz-removal. These criteria appeared to be satisfied by the allyl pyranoside with 6-O-DPS, and 3,4-bis(p-N-(pivaloyl)aminobenzyl) (PAB)27 protecting groups.
Regioselective protection of the 6-OH of 19 using DPS-Cl proceeded smoothly producing 26 (93%); however, introduction of the PAB groups was problematic (Scheme 2). A 7-fold excess of PAB-Cl in the presence of BaO and Ba(OH)2•8 H2O resulted in an intractable mixture of mono- and bis-PAB protected sugars (both anomers) and uncharacterized side products that appeared to originate from the PAB-Cl reagent. The sugar products were partially purified by chromatography and the mixture was treated with a second round of PAB-Cl and base; unfortunately, the yield of bis-PAB product did not improve substantially. Numerous modifications to the benzylation conditions were investigated to improve the yield. The most successful, PAB-I and KOtBu in toluene, was then applied to the carbohydrate mixture. After partial purification by chromatography, the allyl group was removed, followed by oxidation with Dess-Martin reagent. The result was two separable lactone isomers (3-step yield: 27a, 2.5%; 27b, 15.3%); 27a had JH2/H3 = 3.2 Hz and very weak COSY correlation with no scalar coupling between H3 and H4. This suggests a lactone with skewed conformation, which is perhaps unsurprising given the steric demands of the protecting groups, or possibly an inversion of configuration at C2. The 1H-NMR spectra of 27b had too many overlapping peaks to draw any conclusions on the configuration, but it was the major product and the synthesis proceeded with it. Lactone 27b was opened using NH3, producing 28 as 2 separable amides (28a, 80%, and 28b, 6.4%). When mixed with NH3 in MeOH, 27a also produced two separable amides 28a (19%) and 28c (63%). Amide 28a was then oxidized with Dess-Martin reagent, producing two 6-hydroxylactams (29a, 29b); the NMR data (1H, 13C, 1H-COSY) were insufficient to determine configuration of the hydroxylactams. However, these two isomers can be rationalized through epimeric 5-hydroxylactams having the gluco and idoso configurations;26 in addition, steric demands of the protecting groups could result in chromatographically separable hydroxylactams with skewed conformations. Finally, reductive amidation of 29a followed by removal of the DPS group with TBAF afforded the 6-OH glucolactam 30a (9%) and 30b (43%) as the two principle products. NMR spectra of 30b confirmed the gluco configuration and it was phosphorylated with POCl3 in py followed by PAB group removal (DDQ) to afford the N-Cbz phospholactam (31). Final product purification by HPLC was followed the removal of the Cbz group by transfer hydrogenolysis; the solvent was removed by lyophilization affording 31 (10%, 3 steps). Whether other, minor products of 28 and 29 could have been converted to 31 was not investigated. In conclusion, the chosen protecting groups were adequate for the carbohydrate to lactam conversion, but the combined steric demands of the protecting groups on a carbohydrate scaffold likely led to unsatisfactory product yields.
Scheme 2. 2-Amino-glucono-1,5-lactam-6-phosphate Synthesis.
a) DPS-Cl, py; b) PAB-Cl, BaO, Ba(OH)2•8H2O, DMF, then PAB-I, KOtBu, toluene; c) SeO2, AcOH, dioxane, reflux; d) Dess-Martin reagent, py; e) 7N NH3 in MeOH; f) HCOOH, NaBH3CN, CH3CN, reflux; g) TBAF, THF, AcOH; h) POCl3, py, 30m 0°C, then H2O; i) CHCl3, H2O, DDQ, 80°C; j) Pd(OH)2/C, NH4•HCOO, H2O/MeOH, reflux. PAB = p-N-(pivaloyl)aminobenzyl; DPS = tert-Butyldiphenylsilyl. A box indicates the major product component from each step; only the major component was carried through to the next step.
Modifications at the 6-OH of hexopyranoses
Chemical phosphorylation of the 6-OH in the GlcN analogues requires N-protection and discrimination between the primary and secondary hydroxyl groups. Orthogonal protection group schemes, like that used for the phospholactam synthesis, were an option for the remaining coenzyme candidates. During the course of this work, however, a simpler, widely applicable OH-discrimination scheme was developed using the triethylsilyl (TES) protecting group. All hydroxyls of GlcN-(Cbz) were protected using TES-Cl and diisopropylethyamine (DIEA) in DMF (Scheme 3) to produce 33 (62%). Taking into account the slightly more polar, incompletely silylated carbohydrates, 90% of the starting material could be accounted for; these could be re-subjected to silylation to improve the yield. Conveniently, the primary 6-OH was exposed via transilylation (to MeOH) with catalytic PPTS in MeOH/DCM. Once 33 had been consumed (TLC), the reaction mixture was loaded directly on to a silica cartridge and chromatographed; the least polar product was 34 (58%). In general, the deprotection rate varied with the amount of PPTS and was different for every per-silylated substrate. Again, less-silylated, more polar carbohydrates could be recovered and converted again to 33.
Scheme 3. Selective 6-Deprotection and Derivatization.
a) TES-Cl (12×), DMF, DIEA (15×), 60°C; b) DCM, MeOH, PPTS (cat.); c) Ph3P, acetone cyanohydrin, DIAD, THF; d) Ph3P, DPPA, DIAD, THF; e) TFA:H2O (1:1), 110°C; f) Pd(OH)2/C, NH4•HCOO, MeOH, H2O, 60°C.
Selectivity for the 6-position was confirmed and silyl group migration was ruled out with 35 (64% yield) which was prepared by a modified Mitsunobu coupling with acetone cyanohydrin.28 1H, 13C, COSY, and HMBC NMR analyses of 35 showed clear correlation between the C6H (2.9 ppm) and cyano (118 ppm) 13C peaks. The azido group was introduced using diphenylphosphorylazide (DPPA)29 and was used to make coenzyme candidates 37 and 38. Although 36 has not been previously reported, the 27%, 3-step yield from GlcN-(Cbz) is similar to the 23%, 3-step conversion of ManN-(Cbz) to 1,3,4-triacetyl-6-azido-2-N(Cbz)-mannosamine in which N3- displaced a 6-OTs group.30
Treating 36 with 1:1 TFA/H2O afforded 6-azido glucosamine 37 (75%). Although the azide of 37 could be reduced via catalytic hydrogenation over Pd/C or Pd(OH)2/C with NH4•HCOO or HCOOH, 38 could not be isolated cleanly. Instead, transfer hydrogenation both reduced the azide and removed the Cbz group from 36; then the TES groups were hydrolyzed to afford 38 in 68% yield as the (TFA)2 salt.
Modifications to the 2-amino group
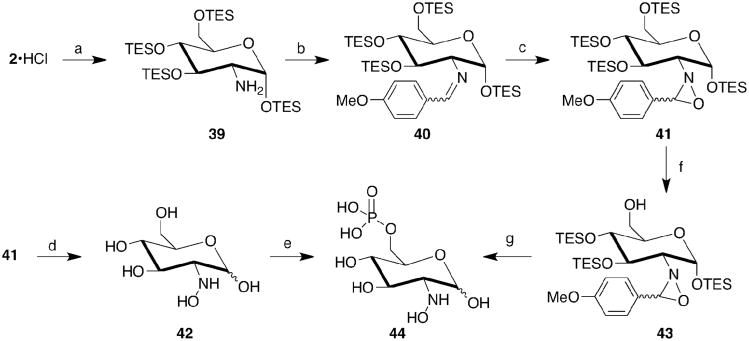
The 2-amino group is critical for the coenzyme function of GlcN6P in activating the glmS ribozyme. Available evidence point to it as both a general acid and general base in the cleavage event.6,7 Thus, modification of the 2-amino group expands the possibilities of finding an activator or inhibitor. To this end, hydroxylamine- and guanidine-substituted gluco-configured carbohydrates were prepared and phosphorylated at their 6-positions.
The hydroxylamino analogue was prepared from GlcN through an oxaziridine intermediate (Scheme 4). The OH groups of GlcN were protected as their TES-ethers, and 39 (61%) was isolated by chromatography exclusively as the alpha-anomer. This was subsequently converted to the imine (40), which was oxidized (mCPBA) to afford the oxaziridine (41) as one major (51%, 2 steps) and one minor isomer (5%, 2 steps). Conversion of 41 to 42 was accomplished with TFA/H2O. The NMR spectra of isolated 42 indicated α-and β-anomers and the presence of a putative p-OMe benzylidene adduct in the β-anomeric configuration in a ratio of 1.6:1.2:1, respectively. Although 42 in this mixture was phosphorylated by HK, a steady loss of 44 by LCMS was apparent under the phosphorylation conditions. A similar loss of product was apparent with 42 alone under slightly basic conditions. Chemical phosphorylation was pursued through the 6-OTES removal under acidified conditions, MeOH with catalytic ±-camphorsulfonic acid, to afford 43 in 47% yield. Treatment with POCl3/lutidine then TFA/H2O afforded 44. Partial purification was achieved by passing through a C18 column (95:5 NH4•HCOO adjusted to pH ∼2.5: MeOH) and 44 eluted at the solvent front, which was subsequently lyophilized. Compound 44 was then purified by “aqueous normal phase” HPLC31 at pH=3. Through each purification step, a steady loss of 44 was apparent (LCMS) even under the mildly acidic conditions. After final lyophilization of the product fractions no 44 was present (LCMS). The decomposition mechanism is unknown but the presence of the hydroxylamine may facilitate rearrangement or elimination reactions, which are common in carbohydrates. These results suggested that 2-hydroxylamino sugars were stable only under strongly acidic conditions and were not pursued further because the glmS ribozyme functions most effectively under slightly basic conditions.
Scheme 4. 2-Hydroxylamino-2-Deoxyglucose Synthesis.
a) TES-Cl (12 equiv), DIEA (15 equiv), DMF, 70°C; b) p-anisaldehyde, PPTS, toluene, reflux; c) mCPBA, aq. NaHCO3, DCM; d) TFA:H2O (4:1); e) yeast hexokinase, ATP, MgCl2, citric acid•H2O, pH 8.0; f) cat. CSA, MeOH; g) POCl3, 2,6-lutidine, then TFA/H2O (4:1).
The guanidinylated glucose (45) was prepared using published procedures.32 Chemical phosphorylation of the di-BOC precursor (46) was accomplished through the TES-protection, affording 47, and 6-OH deprotection described above to afford 48 (Scheme 5). Treatment with POCl3/pyridine followed by acidic TES- and BOC-removal afforded 49, which may adopt more conformations than the bicyclic alpha anomer according to NMR and MS (see supporting information). This locked-in configuration could be beneficial for ribozyme binding given that only the α-anomer of GlcN6P is bound to the ribozyme at the active site.2,6,7
Scheme 5. Preparation of the 2-Guanidinylglucose-6-phosphate Coenzyme Candidate (49).
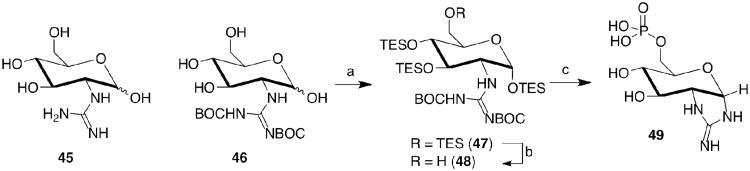
a) TES-Cl, DIEA, DMF, 70°C; b) cat. PPTS, DCM, MeOH; c) POCl3/py, then H2O, DMF/AcOH followed by 1 N HCl.
In conclusion, analogues of GlcN and GlcN6P were prepared that provided altered electronic environments around the 2-amino group, modified amino groups, altered H-bonding patterns for the ribozyme's active site, and the scaffold for additional derivatives via azide derivatization. Further characterization and evaluation of the candidates as coenzymes of the glmS ribozyme will be reported elsewhere.
Experimental Section
2-Propen-1-yl 2-(Benzyloxycarbonyl)amino-2-deoxy-4,6-O-(phenylmethylene)-D-glucopyranoside (20α, 20β)
Allyl alcohol (200 mL), concd H2SO4 (30 μL), and 2-(benzyloxycarbonyl)amino-2-deoxyglucose33 (5.28 g, 16.9 mmol) were combined and then refluxed for 2 h. Then, NaHCO3 (2 g) was added; 1 h later the solution was filtered and the solvent was removed. The residue was recrystallized (EtOAc), then the filtrate's solvent was removed and the residue was chromatographed on silica/EtOAc:CH3CN (3:1). The desired product (two anomers, TLC Rf=0.45-0.5) was collected; product recrystallization and chromatography of the subsequent filtrate was repeated, followed by a final recrystallization to afford a combined 4.40 g (12.4 mmol, 73.7%) of an off-white powder (19). Under an Ar atmosphere, 19 (4.29 g, 12.1 mmol) was dissolved in anhyd DMF (50 mL). The reaction flask was cooled in an ice bath, then ±-camphorsulfonic acid (100 mg) and benzaldehyde dimethyl acetal (5.47 mL, 36.3 mmol) were added and the mixture was stirred overnight while the solution warmed to rt. The solvent was removed and the residue was mixed with DCM (36 mL), H2O (9 mL), and 5% NaHCO3(aq) (3 mL). After vigorous stirring (5 min), MeOH (6 mL) and hexanes (25 mL) were added. The flask was cooled in the freezer for 4 h, and then the precipitate was filtered off (1st crop); reducing the filtrate vol afforded two more product crops. The 1st crop was recrystallized (EtOAc) to afford 1.62 g of 20β, a portion of which was chromatographed, which indicated the 1st crop was 87% pure. The filtrate residue and remaining product crops were combined and dissolved in a minimum vol of THF. The β-anomer was triturated with cyclohexane and filtered off. Both precipitate and filtrate residue were chromatographed, each on a 50 g silica cartridge (3:1 DCM:EtOAc); 20α eluted first. Any fractions with 20α/20β mixtures were combined and subjected to the trituration and chromatography steps. Isolated yields of purified 20α and 20β, each a white powder, were 1.64 g (3.73 mmol, 22.6% for 2 steps) and 1.99 g (4.52 mmol, 27.4% for 2 steps), respectively.
20α. 1H NMR (CDCl3, 500 MHz) δ: 2.7 (s br, 1H), 3.59 (dd, J=9.0, 9.0 Hz, 1H), 3.76 (dd, J=10.0, 10.0 Hz, 1H), 3.85 (ddd, J=9.6, 9.6, 4.7 Hz, 1H), 3.90 to 4.01 (m, 3H), 4.19 (dddd, J=12.8, 5.4, 1.4, 1.4 Hz, 1H), 4.27 (dd, J=10.1, 4.7 Hz, 1H), 4.89 (d, J=3.2 Hz, 1H), 5.1 to 5.16 (m, 3H), 5.21 (ddd, J=10.4, 2.3, 1.1 Hz, 1H), 5.28 (ddd, J=15.7, 2.9, 1.5 Hz, 1H), 5.56 (s, 1H), 5.83 to 5.91 (m, 1H), 7.32 to 7.39 (m, 8H), 7.49 to 7.51 (m, 2H). 13C NMR (CDCl3, 125 MHz) δ: 56.0, 63.0, 67.8, 69.0, 69.4, 70.9, 82.4, 97.7, 102.4, 118.6, 126.7, 128.7, 129.0, 129.7, 133.6, 136.5, 137.5, 157.2. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H28NO7 442.1860; Found 442.1867.
20β. 1H NMR (dmso-d6, 600 MHz) δ: 2.26 to 3.33 (m, 2H, over H2O peak, C2H, C5H), 3.43 (dd, J=9.3, 9.3 Hz, 1H, C4H), 3.57 (m, 1H, C3H), 3.72 (dd, J=10.1, 10.1 Hz, 1H, C6H), 3.99 (dddd, J=13.7, 5.2 Hz, smaller couplings not resolved, 1H, allyl-C1H), 4.18 to 4.23 (m, 2H, allyl-C1H, C6H), 4.45 (d, J=8.4 Hz, 1H, C1H), 4.98 (d, J=12.7 Hz, 1H, Bn-CH), [5.06 (d, J=12.7 Hz, Bn-CH) over 5.07 to 5.15 (m, allyl-C3H), 2H], 5.23 (ddd, J=17.2, 3.7, 1.8 Hz, 1H, allyl-C3H), 5.37 (d, J=5.8 Hz, 1H, C2-NH), 5.59 (s, 1H, acetal-CH), 5.76 to 5.83 (m, 0.2 H, allyl-C2H), 7.27 to 7.38 (m, 8H, aromatic-H), 7.42 to 7.45 (m, 2H, aromatic-H). 13C NMR (dmso-d6, 150 MHz) δ: 57.9, 65.1, 66.0, 67.8, 69.1, 70.3, 81.2, 100.6, 101.4, 116.1, 126.3, 127.6, 127.7, 128.0, 128.3, 128.8, 134.4, 137.3, 137.7, 156.0. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H28NO7 442.1860; Found 442.1867.
2-Propen-1-yl 3-Azido-2-(benzyloxycarbonyl)amino-2,3-dideoxy-4,6-O-(phenylmethylene)-β-D-allopyranoside (21)
Compound 20β (1.1 g, 2.5 mmol) was dissolved in anhyd py and the reaction flask was cooled in an ice bath. Then, Ms2O (1.09 g, 6.25 mmol) was added and the mixture was stirred for 16 h. The solution was added to H2O (40 mL) with stirring; 20 min later, the solution was filtered and the precipitate was air-dried to afford 1.27 g of an off white powder (crude 20β-mesylate), which was used without further purification. NaN3 (45 mg, 0.75 mmol) and 20β-mesylate (126 mg) were dissolved in anhyd DMF (1 mL). The reaction vial was heated (hot plate at 110°C) and mixture was stirred for 48 h. Then, the solvent was removed and the residue was chromatographed on a 12 g silica cartridge (0 to 50% EtOAc in DCM). The isolated yield of 21 (a white powder, TLC Rf=0.75 with 1:1 DCM:EtOAc) was 61 mg (0.13 mmol, 52% for 2 steps). The reaction could be scaled up to 1 g of 21 with similar results. A sample of 21 was recrystallized from DCM/hexanes for NMR analysis.
20β-mesylate. 1H NMR (acetone-d6, 600 MHz) δ: 2.97 (s, 3H, SO2-CH3), 3.56 (ddd, J=10.0, 9.0, 5.0 Hz, 1H, C5H), 3.74 (dd, J=9.8, 9.1, 8.3 Hz, 1H, C2H), 3.88 (dd, J=10.3, 10.3 Hz, 1H, C6H), 3.90 (dd, J=9.5, 9.5 Hz, 1H, C4H), 4.13 (dddd, J=13.3, 5.6, 1.5, 1.5 Hz, 1H, allyl-C1H), 4.31 to 4.35 (m, 2H, C6H, allyl-C1H), 4.91 (d, J=8.3 Hz, 1H, C1H), 4.95 (dd, J=9.8, 9.8 Hz, 1H, C3H), 5.09 to 5.14 (m, 3H, allyl-C3H, Bn-CH2), 5.29 (ddd, J=17.3, 3.5 1.7 Hz, 1H, allyl-C3H), 5.76 (s, 1H, acetal-CH), 6.74 (d, J=9.1 Hz, 1H, C2-NH), 7.29 to 7.40 (m, 7H, aromatic-H), 7.52 to 7.55 (m, 3H, aromatic-H). Allyl-C2H multiplet was too broad to observe, but showed up in COSY. 13C NMR (dmso-d6, 150 MHz) δ: 38.5, 55.8, 65.2, 65.4, 67.5, 69.5, 78.1, 80.0, 100.08, 100.09, 100.7, 116.5, 125.9, 127.1, 127.5, 127.7, 128.1, 128.3, 128.9, 134.1, 137.0, 137.1, 155.8. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C25H30NO9S 520.1636; Found 520.1649.
21. 1H NMR (acetone-d6, 600 MHz) δ: 3.82 (dd, J=10.1, 10.1 Hz, 1H, C6H), 3.87 to 3.93 (m, 2H, C2H, C5H), 4.05 (dd, J=9.2, 2.4 Hz, 1H, C4H), 4.08 (dddd, J=13.5, 5.5 Hz, remaining coupling unresolved, 1H, allyl-C1H), 4.28 to 4.33 (m, 2H, C6H, allyl-C1H), 4.46 (dd, J=3.2, 3.2 Hz, 1H, C3H), 4.71 (d, J=8.5 Hz, 1H, C1H), 5.08 to 5.14 (m, 3H, Bn-CH, allyl-C3H), 5.27 (ddd, J=17.3, 3.5, 1.6 Hz, 1H, allyl-C3H), 5.74 (s, 0.7H, acetal-CH), 5.86 to 5.9 (m, 0.01H, allyl-C2H), 6.63 (d, J=8.0 Hz, 0.5H, C2-NH), 7.33 to 7.41 (m, 8H, aromatic H), 7.52 to 7.54 (m, 2H, aromatic H). 13C NMR (acetone-d6, 150 MHz) δ: 54.6, 63.4, 65.1, 66.8, 69.4, 70.6, 79.0, 100.2, 102.3, 116.7, 127.1, 128.59, 128.61, 128.8, 129.2, 129.7, 135.3, 138.1, 138.7, 156.6. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H27N4O6 467.1925; Found 467.1927.
General deallylation procedure
SeO2 (20 mg, 1.8 mmol) was mixed with AcOH (13.4 μL, 0.24 mmol) and the allyl pyranoside (0.12 mmol) in anhyd 1,4-dioxane (0.5 mL). The reaction vial was set on a hot plate (120°C) and the mixture was stirred for 2.5 h. Then, the vial was allowed to cool and the solution was filtered; the solvent was removed from the filtrate and the residue was chromatographed.
General acid hydrolysis procedure
The hydrolysis substrate was mixed with TFA/H2O (1 mL: 1 mL). The reaction vial was heated (hot plate at 120°C) and the mixture was stirred for 24 h. The heat was removed, then H2O (3 mL) was added and the aq phase was extracted 2× with EtOAc (3 mL). The aq phase was frozen and then lyophilized. The resulting residue was redissolved in H2O (3 mL) and lyophilized a second time.
2-Amino-3-azido-2,3-dideoxyallose (22)
The general conditions for allyl group removal were applied to 21 (55 mg, 0.12 mmol). The residue was chromatographed 2×, each on a 12 g silica cartridge with a 3:1 to 2:3 DCM/EtOAc gradient to afford 24.5 mg of semi-purified product. A portion of this product (20.5 mg) was subjected to the general acid hydrolysis conditions, which afforded 12 mg of 22 as the TFA salt (38 μmol, 38% for 2 steps). Injecting a 1:1 MeOH/H2O solution of 22 on a semipreparative C18 column and eluting with 95:5 H2O:MeOH removed the small amount of residual low polarity material. Compound 22 eluted immediately after the void volume; the sample was reacidified with HCOOH then lyophilized, affording an off white powder. 1H NMR (D2O, 600 MHz) δ: main product β:α ratio = 6:1, 3.17 (dd, J=8.4, 3.4 Hz, 6H, βC2H), 3.60 (dd, J=12.1, 5.6 Hz, 6.5H, βC6H), 3.66 (dd, J=12.8, 4.7 Hz, 2H, αC6H), 3.72 to 3.77 (m, 14H, βC5H in here), 3.87 (dd over m, J=10.6, 3.5 Hz, 8.2H, βC4H/αC4H/αC5H), 4.23 (dd, J=3.8, 3.8 Hz, 1.1H, αC3H), 4.28 (dd, J=3.3, 3.3 Hz, 6H, βC3H), 4.86 (d, J=8.3 Hz, 6H, βC1H), 5.16 (d, J=4.1 Hz, 1H, αC1H). [Signals corresponding to 2 additional minor carbohydrate products were present: 3.04 (dd, J=8.7, 3.5 Hz, <0.1H), 3.78 to 3.81 (m, 0.5H), 4.18 (dd, J=4.5, 4.2 Hz, 0.3H), 4.38 (dd, J=7.8, 3.5 Hz, 0.3H), 5.36 (d, J=3.5 Hz, 0.1H), 5.49 (d, J=4.6 Hz, 0.25H)]. 13C NMR (D2O/dmso-d6; referenced to dmso CD3, 150 MHz) δ: 50.2, 54.0, 61.8, 62.1, 62.2, 63.6, 67.8, 68.6, 75.9, 89.6, 92.3, [118.0 (q, J=291 Hz), 164.3 (q, J=34.7 Hz), TFA], [172.1 from formic acid]. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C6H13N4O4 205.0931; Found 205.0930.
2-Propen-1-yl 3-Azido-2-(benzyloxycarbonyl)amino-2,3-dideoxy-4,6-O-(phenylmethylene)-α-D-glucopyranoside (24)
The conversion of 20α to 23 was carried out as previously described,34 then, 23 (0.62 g, 1.4 mmol) was treated with Ms2O (0.61 g, 3.5 mmol) in anhyd py (5 mL) as described for the production of 21. The residue was chromatographed on a 25 g silica cartridge with 0 to 33% EtOAc in DCM, affording 637 mg of a white powder (23-mesylate, 1.23 mmol, 87.6 % yield). Then, 23-mesylate (0.600 g, 1.16 mmol) was dissolved in anhyd DMF (5 mL). NaN3 (0.228 g, 3.5 mmol at the start; 75 mg, 1.2 mmol at 58 h) was added; the reaction was heated (oil bath, 120°C) and stirred for 70 h. Then, after cooling, H2O (20 mL) was added to the mixture. The precipitate was filtered, washed with H2O (20 mL), and then air-dried. The precipitate was chromatographed on a 40 g silica cartridge with a 0 to 30% EtOAc in DCM gradient. An elimination product consistent with loss of MsOH co-eluted with 24 (TLC Rf=0.9; 10:1 DCM:EtOAc), affording 0.286 g of an off-white solid. A portion of 24 was purified for NMR analysis from the mixture by HPLC on a semipreparative C18 column: A=H2O, B=MeOH, 0 to 1 min (75%B), 1 min to 20 min (gradient to 85%B) at 4 mL/min. The product eluting at Rt=20 min, was 24.
23-mesylate. 1H NMR (acetone-d6, 600 MHz) δ: 3.03 (s, 3H, SO2CH3), 3.82 (dd, J=10.3, 10.3 Hz, 1H, C6H), 4.05 to 4.09 (m, 2H, allyl-C1H, C4H), [4.16 (ddd, J=10.1, 10.0, 5.2 Hz, C5H), 4.20 (ddd, J=9.1, 4.0, 4.0 Hz, C2H) overlapping, 2H], 4.29 (dddd, J=13.4, 4.7, 1.7, 1.7 Hz, 1H, allyl-C1H), 4.33 (dd, J=10.3, 5.2 Hz, C6H), 4.97 (d, J=4.3 Hz, 1H, C1H), 5.10 (d, J=12.5 Hz, 1H, Bn-CH), 5.12 (d, J=12.5 Hz, 1H, Bn-CH), 5.17 (unresolved ddd, one J=1.3 Hz, 1H, allyl-C3H), 5.24 (unresolved dd, one J=2.6 Hz, 1H, C3H), 5.38 (ddd, unresolved ddd, one J=1.7 Hz, 1H, allyl-C3H), 5.77 (s, 1H, acetal-CH), 5.93 to 6.0 (m, 0.3H, allyl-CH), 7.31 to 7.41 (m, 7H, aromatic H), 7.52 to 7.55 (m, 3H, aromatic H) 13C NMR (acetone-d6, 150 MHz) δ: 39.0, 51.3, 58.9, 67.2, 69.2, 69.4, 76.4, 77.4, 96.9, 102.4, 116.8, 127.1, 128.6, 128.9, 129.1, 129.8, 135.0, 137.8, 138.6, 156.2. HRMS (ESI-TOF) m/z: [M + NH4]+ Calcd for C25H33N2O9S 537.1901; Found 537.1900.
24. 1H NMR (acetone-d6, 600 MHz) δ: 3.75 (dd, J=9.4, 9.4 Hz, 1H, C4H), 3.81 to 3.90 (m, 3H, C2H, C5H, C6H), 4.01 (dd, J=10.7, 10.1 Hz, 1H, C3H), 4.06 (dddd, J=13.3, 7.1, 1.4, 1.4 Hz, 1H, allyl-C1H), 4.25 to 4.30 (m, 2H, allyl-C1H, C6H), 4.94 (d, J=3.6 Hz, 1H, C1H), 5.12 (s, 2H, Bn-CH2), 5.16 (ddd, J=10.5, 1.4 Hz, remaining coupling not resolved, 1H, allyl-C3H), 5.37 (ddd, J=17.9, 3.4, 1.7 Hz, 1H, allyl-C3H), 5.76 (s, 1H, acetal-CH), 5.92 to 5.98 (m, 0.3H, allyl-C2H), 6.71 (d, J=9.2 Hz, 0.6H, C2-NH), 7.31 to 7.42 (m, 8H, aromatic H), 7.49 to 7.53 (m, 2H, aromatic H). 13C NMR (acetone-d6, 150 MHz) δ: 55.2, 62.0, 64.0, 66.9, 69.1, 69.3, 81.3, 97.9, 102.1, 117.3, 127.0, 128.6, 128.7, 128.8, 129.2, 129.6, 134.9, 138.1, 138.7, 156.8. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H27N4O6 467.1925; Found 467.1929.
2-Amino-3-azido-2,3-dideoxyglucose (25)
The general deallylation procedure was applied to the mixture of 24 and the side product (100 mg) on a 1.9× scale. Then, the crude mixture was chromatographed on a 12 g silica cartridge with a 10 to 50% EtOAc in DCM gradient to afford 34 mg of semi-purified de-allyl product. Final purification from co-eluting Se-related products on a 4 g silica cartridge afforded 27 mg of a white powder, deallyl-24 (16%, two-step yield from 23-mesylate). Then deallyl-24 (15 mg, 35 μmol) treated with the general acid hydrolysis procedure, affording 25 as the TFA salt (a white solid, 8.6 mg, 27 μmol, 77%). 1H NMR (D2O, 600 MHz) δ: [α:β ratio = 2.5:1] 2.84 (dd, J=10.9, 8.5 Hz, 1H, Hβ2), 3.14 (dd, J=11.2, 3.3 Hz, 2.5H, Hα2), 3.22-3.24 (m, 1 H), 3.48-3.52 (m, 1H), 3.54 to 3.70 (overlapping m's, 9.3H), 3.73 (unresolved dd, J=12.2 Hz, 2.5H), 3.79 (unresolved dd, J=12.2 Hz, 1H), 3.81-3.87 (m, 5H), 4.86 (d, J=8.4 Hz, 1H, Hβ1), 5.29 (d, J=3.1 Hz, 2.5H, Hα1). 13C NMR (D2O, 150 MHz) δ: 55.1, 57.3*, 62.8, 63.0*, 64.5, 66.5*, 72.0, 72.4*, 74.2, 79.5*, 91.4, 95.5*, [119.1 (q, J=290 Hz), 166.0 (q, J=33 Hz) TFA]; *=less intense peak, consistent with β-anomer signals. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C6H13N4O4 205.0931; Found 205.0928.
2-Propen-1-yl 2-(Benzyloxycarbonyl)amino-6-O-(tert-butyldiphenylsilyl)-2-deoxy-D-glucopyranoside (26)
Under an Ar atmosphere, 19 (6.23 g, 17.6 mmol) was dissolved in anhyd py (75 mL). The reaction flask was cooled in an ice bath, then DPS-Cl (5.0 mL, 19.4 mmol) was added. The ice bath was allowed to warm to rt and the reaction progress was monitored by TLC. More DPS-Cl was added at 6 h (0.685 mL, 2.6 mmol) and at 20 h (0.45 mL, 1.7 mmol). After 23 h total, the solvent was removed and the residue was taken up in hexanes (100 mL) and EtOAc (150 mL). The organic phase was extracted 2× with H2O (100 mL) and then dried (Na2SO4). The solvent was removed and the residue was chromatographed on an 80 g silica cartridge with a 1:1 to 0:1 hexanes/EtOAc gradient to afford a mixture of semi-pure anomers (TLC Rf=0.3 and 0.45, 11.02 g, a viscous oil), which was used without further purification. An 85.5 mg sample of the semi-pure mixture was rechromatographed (1:1 to 1:4 hexanes:EtOAc) to afford pure 26 (73.5 mg, 91% reaction yield) as a viscous oil that crystallized over a period of weeks. 1H NMR (CD3OD, 600 MHz) δ: 1.06 (s, 9H, tBu-CH3), 3.45 (m, 2H), 3.65 to 3.74 (m, 2H), 3.89 (ddd, J=10.9, 10.9, 5.8 Hz, 1H), 3.97 to 4.08 (m, 2H), 4.21 (dd, br, J=12.8, 4.8 Hz, 0.5H), 4.30 to 4.34 (m, 0.4H), 4.44 (s, v br, 0.3H, βC1H), 4.89 (s br, 0.5 H, αC1H), 5.07 to 5.16 (m, 3H), 5.23 to 5.31 (m, 1H), 5.88 to 5.95 (m, 0.1H, allyl C2H), 7.25 to 7.45 (m, 11H, aromatic H), 7.65 to 7.75 (m, 4H, aromatic H). 13C NMR (CD3OD, 150 MHz) δ: 20.1, 27.3, 57.2, 59.0, 64.8, 67.4 67.6, 69.2, 70.7, 72.0, 72.2, 73.3, 74.2, 76.1, 78.1, 97.9, 102.1, 117.0, 117.6, 128.6, 128.8, 129.4, 130.7, 134.8, 134.9, 135.4, 135.6, 136.8, 138.2, 138.4, 158.8, 159.0. HRMS (ESI-TOF) m/z: [M + H – allyl alcohol]+ Calcd for C33H42NO7Si 534.2306; Found 534.2304.
2-(Benzyloxycarbonyl)amino-6-O-(tert-butyldiphenylsilyl)-2-deoxy-3,4-bis-O-(4-(pivaloylamino)benzyl)-D-glucono-1,5-lactone (27a, 27b)
Compound 26 (10.8 g, 86% pure, 15.7 mmol) was dissolved in anhyd DMF (100 mL), then BaO (13 g), Ba(OH)2•8H2O (5.1 g) and 4-pivaloylaminobenzyl chloride (PAB-Cl)27 (29 g, 128.5 mmol) were added and the mixture was stirred overnight. LCMS analysis indicated a mixture of mono- and bis-PAB sugars of both anomers as well as numerous lower mol wt products that were consistent with PAB-OH, (PAB)2O and PAB oligomers. The solid was filtered off then washed with DCM. The filtrate volume was reduced and some side products were removed by precipitation in EtOAc. Then filtration of the supernatant through a plug of silica with EtOAc as eluent afforded the semi-purified product mixture. This was subjected to the benzylation conditions with PAB-Cl again 0.5× scale of that above. LCMS indicated the reaction had not progressed much and the purification scheme above was applied again. An additional column purification on a 90 g silica cartridge with a 5:1 to 0:1 DCM:EtOAc gradient before proceeding to the modified benzylation conditions.
PAB-I
PAB-Cl (14.75 g, 65.4 mmol) and NaI (25 g, 167 mmol) were added to acetone (250 mL) and the mixture was refluxed for 1 h. Then, the heat was removed and EtOAc (100 mL) was added. The solution was filtered and the precipitate was washed with more EtOAc (100 mL). After solvent removal the filtrate residue was chromatographed on silica column (5 cm × 25 cm (dia × length) with 1:1 EtOAc:hexanes. Fractions with PAB-I (TLC Rf=0.75) were combined and the solvent was removed to afford 20.1 g (63.3 mmol, 97%) of a light yellow powder.
Modified benzylation conditions
The mixture of mono- and bis-PAB product mixture was dissolved in dry toluene (200 mL). Then (CH3)3COK (7.3 g, 65 mmol) and PAB-I (20 g, 63 mmol) were added. After stirring overnight, AcOH (2 mL) was added and then the solution was filtered. Partial purification was achieved with a silica column (3.5cm × 28cm, dia × length) using 3:1 to 0:1 DCM/EtOAc. After solvent removal, residual PAB-I was removed by adding SiliaMetS® Diamine (Silicycle Inc., 20 g, 1.33 mmol/g) in DCM/MeOH (100 mL:30 mL) and stirring for 24 h.
After filtration and solvent removal, the general deallylation procedure was applied; in dioxane (150 mL), SeO2 and AcOH were added in 2 portions, (2.23 g and 1.1 g) and (1.6 mL and 0.78 mL), respectively, at the start and at 18 h. At 24 h, the heat was removed and the mixture was chromatographed on a 220 g silica cartridge, then a 120 g cartridge with 5:1 to 0:1 DCM:EtOAc gradient. The deallyl product (TLC Rf=0.2 to 0.25; 5:1 DCM:EtOAc) was kept separate from unreacted starting material (TLC Rf=0.6 to Rf=0.75). The latter was subjected again to the general deallylation conditions in dioxane (35 mL) with SeO2 (0.24 g) and AcOH (0.16 mL). The combined deallyl product fractions were dissolved in anhyd py (75 mL) then Dess-Martin periodinane (7.5 g, 17.7 mmol) was added and the mixture was stirred for 3 h. Then the solvent was removed; the residue was taken up in DCM (100 mL) and then filtered. The filtrate was chromatographed 2×, each on a 220 g silica cartridge with a 5:1 to 3:1 DCM:EtOAc gradient to afford semi-purified 27a and 27b. Each was again chromatographed 2× on 120 g silica cartridges with 2:2:1 DCM:hexanes:EtOAc for 27a (TLC Rf=0.4, 378 mg, 0.41 mmol, 2.6% yield for 3 steps) and 1:1:1 DCM:hexanes:EtOAc for 27b (TLC Rf=0.4, 2.33 g, 2.51 mmol, 16.0% yield for 3 steps). Both were stiff white foams in appearance.
27a. An HPLC-purified sample (87.5:12.5 CH3CN:H2O isocratic elution on the semi-prep C18 column), 1H NMR (acetone-d6, 600 MHz) δ: 1.06 (s, 9H, tBu-CH3), 1.29 (s, 9H, tBu-CH3), 1.30 (s, 9H, tBu-CH3), 3.94 (dd, J=11.6, 4.3 Hz, 1H, C6H), 3.99 (dd, J=11.6, 2.3 Hz, 1H, C6H), 4.12 (d, J=7.8 Hz, 1H, C4H with very weak COSY correlation to C3H), 4.24 (d, J=3.0 Hz, 1H, C3H with very weak COSY correlation to C4H), 4.44 (d, J=11.5 Hz, 1H, Bn-CH), 4.53 (m, 1H, C5H), 4.59 (d, J=11.9 Hz, 1H, Bn-CH), 4.62 (d, J=11.6 Hz, 1H, Bn-CH), 4.67 (d, J=11.9 Hz, 1H, Bn-CH), 4.89 (dd, J=7.7, 3.1 Hz, 1H, C2H), 5.12 (d, J=12.7 Hz, Bn-CH), 5.14 (d, J=12.7 Hz, Bn-CH), 6.49 (d, J=7.7 Hz, 0.05 H, C2-NH), 7.22 (d, J=8.2 Hz, 2H, aromatic H), 7.27 (d, J=8.5 Hz, 2H, aromatic H), 7.33 (dd, J=7.2, 7.2 Hz, 1H, aromatic H), 7.39 (dd, J=7.5, 7.5 Hz, 1H, aromatic H), 7.41 to 7.45 (m, 6H, aromatic H), 7.46 to 7.49 (m, 2H, aromatic H), [7.65 (d, J=8.5 Hz) overlapping with 7.68 (d, J=8.5 Hz), 4H, aromatic H], 7.71 to 7.75 (m, 5H, aromatic H), 8.6 (2 s br, 2H, piv-NH). 13C NMR (acetone-d6, 150 MHz) δ: 19.9, 27.3, 27.8, 40.21, 40.23, 53.8, 63.9, 67.1, 72.0, 72.3, 74.4, 78.2, 80.4, 120.81, 120.84, 128.66, 128.70, 128.72, 128.8, 129.25, 129.28, 129.32, 130.8, 133.3, 133.37, 133.83, 134.05, 140.2, 140.3, 156.8, 169.9, 177.3. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C54H66N3O9Si 928.4563; Found 928.4574.
27b. An HPLC-purified sample (85:15 CH3CN:H2O isocratic elution on the semi-prep C18 column), 1H NMR (acetone-d6, 600 MHz) δ: 1.07 (s, 9H, tBu-CH3), 1.29 (s, 9H, tBu-CH3), 1.30 (s, 9H, tBu-CH3), 3.98 to 4.03 (m, 2H), 4.14 to 4.18 (m, 2H), 4.30 to 4.35 (m, 2H), 4.70 (d, J=10.9 Hz, 1H, Bn-CH), 4.74 (d, J=11.2 Hz, 1H, Bn-CH), 4.77 (d, J=11.2 Hz, 1H, Bn-CH), 4.89 (d, J=10.9 Hz, 1H, Bn-CH), 5.10 (d, J=12.5 Hz, 1H, Bn-CH), 5.15 (d, J=12.5 Hz, 1H, Bn-CH), 7.19 (d, J=8.4 Hz, 2H, aromatic H), 7.22 (d, J=8.3 Hz, 1H, aromatic H), 7.25 (d, J=8.5 Hz, 2H, aromatic H), 7.32 (m, 1H, aromatic H), 7.34 to 7.50 (m, 9H, aromatic H), 7.65 to 7.68 (m, 4H, aromatic H), 7.71 to 7.76 (m, 4H, aromatic H), 8.63 (s, 1H, piv-NH), 8.65 (s, 1H, piv-NH). 13C NMR (acetone-d6, 150 MHz) δ: 19.8, 27.2, 27.7, 40.2, 57.4, 63.5, 67.0, 74.8, 76.6, 80.3, 80.5, 120.6, 120.7, 128.7, 128.8, 129.0, 129.2, 130.8, 133.5, 133.8, 133.9, 134.1, 136.4, 136.5, 138.2, 140.0, 140.1, 157.3, 169.2, 177.07, 177.08. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C54H66N3O9Si 928.4563; Found 928.4568.
2-(Benzyloxycarbonyl)amino-6-O-(tert-butyldiphenylsilyl)-2-deoxy-3,4-bis-O-(4-(pivaloylamino)benzyl)-D-gluconamide (28)
Lactone 27b (2.19g, 2.36 mmol) was dissolved in 7N NH3 in MeOH (15 mL) and the mixture was stirred for 30 min. The solvent was removed and the residue was chromatographed on a 120 g silica cartridge (3:1 EtOAc/hexanes) to afford two products, 28a, (TLC Rf=0.4, 1.79g, 1.89 mmol, 80.1%) and 28b (TLC Rf=0.35, 0.143 g, 0.15 mmol, 6.4%); both were white powders.
28a. An HPLC-purified sample on the semi-prep C18 column (78.5:21.5 MeOH:H2O, 0 to 3.5 min, then gradient to 100% MeOH over 8 min, 3.5 mL/min): 1H NMR (CD3OD, 600 MHz) δ: 1.06 (s, 9H, tBu-CH3), 1.27 (s, 9H, tBu-CH3), 1.29 (s, 9H, tBu-CH3), 3.71 (dd, J=6.5, 6.5 Hz, 1H, C4H), 3.75 (dd, J=10.3, 4.9 Hz, 1H, C6H), 3.81-3.84 (m, 1H, C5H), 3.87 (dd, J=10.3, 3.6 Hz, 1H, C6H), 4.39 (dd, J=5.8, 2.6 Hz, 1H, C3H), 4.46 (d, J=10.8 Hz, 1H, Bn-CH), 4.51 (d, J=2.6 Hz, 1H, C2H), [4.55 (d, J=10.9 Hz, Bn-CH), 4.56 (d, J=10.9 Hz, Bn-CH), 2H], 4.66 (d, J=10.8 Hz, 1H, Bn-CH), 5.06 (s, 2H, Bn-CH2), 7.03 (d, J=8.4 Hz, 2H, aromatic H), 7.22 (d, J=8.5 Hz, 2H, aromatic H), 7.24 to 7.45 (m, 15H, aromatic H), 7.68 (d, J=6.8 Hz, 2H, aromatic H), 7.71 (d, J=6.8 Hz, 2H, aromatic H). 13C NMR (d6-acetone, 125 MHz) δ: 19.8, 27.3, 27.7, 40.1, 57.1, 66.3, 67.1, 73.3, 74.4, 75.1, 80.3, 120.5, 120.6, 128.6, 128.9, 129.16, 129.24, 134.2, 134.3, 134.5, 136.4, 136.5, 137.9, 139.6, 139.9, 157.2, 173.7, 177.0. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C54H69N4O9Si 945.4828; Found 945.4826.
28b. 1H NMR (CD3OD, 600 MHz) δ: 1.04 (s, 9H, tBu-CH3), 1.28 (s, 9H, tBu-CH3), 1.30 (s, 9H, tBu-CH3), 3.45 (dd, J=10.9, 4.9 Hz, 1H, C6H), 3.80 (dd, J=10.9, 7.8 Hz, 1H, C6H), 3.91 (dd, J=8.3, 1.7 Hz, 1H, C4H), 4.12 (ddd, J=7.7, 4.9, 1.8 Hz, 1H, C5H), 4.34 (d, J=1.9 Hz, 1H, C2H), 4.38 (dd, J=8.3, 1.8 Hz, 1H, C3H), 4.49 (d, J=10.6 Hz, 1H, Bn-CH), 4.62 (d, J=11.0 Hz, 1H, Bn-CH), 4.65 (d, J=10.6 Hz, 1H, Bn-CH), 4.81 (d, J=11.0 Hz, 1H, Bn-CH), 4.89 (d, J=12.4 Hz, 1H, Bn-CH), 5.03 (d, J=12.04 Hz, 1H, Bn-CH), 7.16 (d, J=8.5 Hz, 2H, aromatic H), 7.24 to 7.48 (m, 17H, aromatic H), 7.66 to 7.71 (m, 4H, aromatic H). 13C NMR (CD3OD, 125 MHz) δ: 20.2, 27.5, 27.7, 27.8, 30.7, 40.5, 57.7, 63.3, 68.1, 68.2, 75.5, 75.9, 76.0, 76.2, 80.9, 85.2, 122.2, 122.39, 122.43, 128.7, 128.9, 129.0, 129.06, 129.11, 129.5, 129.7, 130.9, 131.0, 134.7, 135.0, 135.7, 136.4, 136.8, 136.9, 137.1, 137.9, 139.0, 139.2, 158.4, 175.8, 179.8. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C54H69N4O9Si 945.4828; Found 945.4835.
Preparation of gluconamide 28c
Lactone 27a (0.346 g, 0.37 mmol) was dissolved in 7N NH3 in MeOH (2 mL) and the mixture was stirred for 30 min. The solvent was removed and the residue was chromatographed on a 25 g silica cartridge (3:1 EtOAc/hexanes) to afford 290 mg 0.3 mmol, 82%) of a mixture of gluconamide 28a and 28c (TLC Rf=0.4) in 19 and 63% yields, respectively; both were white solids. 28c: 1H NMR (CD3OD, 600 MHz) δ: 1.06 (s, 9H, tBu-CH3), 1.27 (s, 9H, tBu-CH3), 1.29 (s, 9H, tBu-CH3), 3.79 (dd, J=10.8, 4.3 Hz, 1H, C6H), 3.83 (dd, J=8.0, 1.7 Hz, 1H, C4H), 3.89-3.93 (m, 2H, C5H, C6H), 4.21 (dd, J=8.3, 1.7 Hz, 1H, C3H), 4.38 (d, J=10.5 Hz, 1H, Bn-CH2), 4.44 (d, J=10.5 Hz, 1H, Bn-CH2), 4.55 (s, 2H, Bn-CH2), 4.60 (d, J=8.3 Hz, 1H, C2H), 5.03 (d, J=12.4 Hz, 1H, Bn-CH2), 5.14 (d, J=12.5 Hz, 1H, Bn-CH2), 6.99 (d, J=8.4 Hz, 2H, aromatic H), 7.22-7.28 (m, 5H, aromatic H), 7.31-7.38 (m, 8H, aromatic H), 7.40-7.45 (m, 4H, aromatic H), 7.66-7.69 (m, 4H, aromatic H), 7.97 (s, 0.1H, Piv-NH), 8.54 (s, 0.04H, Piv-NH). 13C NMR (CD3OD, 150 MHz) δ: 20.1, 27.5, 27.77, 27.80 40.49, 40.52, 56.3, 66.6, 67.8, 72.4, 74.7, 74.8, 79.0, 79.4, 122.2, 122.3, 128.8, 128.87, 128.91, 129.0, 129.5, 129.7, 129.8, 130.9, 131.0, 134.4, 134.6, 135.0, 135.4, 136.87, 136.93, 138.1, 139.3, 158.2, 170.3, 175.8, 179.78, 179.81. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C54H69N4O9Si 945.4828; Found 945.4833.
2-(Benzyloxycarbonyl)amino-6-O-(tert-butyldiphenylsilyl)-2-deoxy-3,4-bis-O-(4-(pivaloylamino)benzyl)-D-xylo-hexo-5-ulosonamide (29)
Amide 28a (1.25 g, 1.32 mmol) was combined with Dess-Martin periodinane (0.85g, 2.0 mmol) in anhyd py. The mixture was stirred for 7 h, then diluted with hexanes (40 mL) and EtOAc (40 mL). The solution was filtered, the solvent was removed from the filtrate and then the residue was chromatographed on a 220 g silica cartridge (1:2 to 1:4 hexanes:EtOAc gradient) and then an 80 g silica cartridge (1:1 EtOAc:DCM) to afford 29a (TLC Rf=0.55 with 1:1 EtOAc:DCM, 0.51 g (0.54 mmol, 41%) and 29b (TLC Rf=0.35 with 1:1 EtOAc:DCM, 0.14 g, 0.15 mmol, 11%). Compounds 29a and 29b were white powders.
29a. An HPLC-purified sample on the semi-prep C18 column (80:20 CH3CN:H2O isocratic elution, 4 mL/min): 1H NMR (acetone-d6, 600 MHz) δ: 1.12 (s, 9H, tBu-CH3), 1.30 (s, 9H, tBu-CH3), 1.31 (s, 9H, tBu-CH3), 3.75 (d, J=10.3 Hz, 1H, C6H), 3.92 (d, J=10.3 Hz, 1H, C6H), 4.09 (m, 1H, C4H), 4.25 to 4.30 (m, 2H, C2H and C3H), [4.57 (d, J=11.0 Hz, Bn-CH) over 4.58 (s, OH), 2H], 4.74 (s, 2H, Bn-CH2), 4.90 (d, J=10.9 Hz, 1H, Bn-CH), 5.11 (d, J=12.6 Hz, 1H, Bn-CH), 5.17 (d, J=12.6 Hz, 1H Bn-CH), 6.83 (s br, 0.5 H, NH), 6.94 (s, 1H, NH), 7.10 (d, J=8.5 Hz, 2H, aromatic H), 7.24 (d, J=8.5 Hz, 2H, aromatic H), 7.27 to 7.35 (m, 3H, aromatic H), 7.40 to 7.50 (m, 8H, aromatic H), 7.60 (d, J=8.5 Hz, 2H, aromatic H), 7.63 (d, J=8.5 Hz, 2H, aromatic H), 7.70 to 7.74 (m, 4H, aromatic H), [8.54 (s), 8.55 (s), 2H, piv-NH].13C NMR (acetone-d6, 150 MHz) δ: 19.9, 27.4, 27.8, 40.2, 66.8, 67.3, 75.2, 75.6, 79.1, 79.4, 83.9, 120.6, 120.7, 128.56, 128.60, 128.7, 128.8, 129.0, 129.1, 129.2, 130.81, 130.84, 133.7, 133.9, 134.2, 134.6, 136.5, 136.7, 138.4, 139.8, 139.9, 170.2, 177.1, 177.1. HRMS (ESI-TOF) m/z: [M + NH4]+ Calcd for C54H70N5O9Si 960.4937; Found 960.4939.
29b. An HPLC-purified sample on the semi-prep C18 column (80:20 CH3CN:H2O isocratic elution, 4 mL/min), 1H NMR (acetone-d6, 600 MHz) δ: 1.09 (s, 9H, tBu-CH3), 1.30 (s, 9H, tBu-CH3), 1.31 (s, 9H, tBu-CH3), 3.82 (d, J=9.8 Hz, 1H, C6H), 4.03-4.05 (m, 2H, C6H and C4H), 4.11 (dd, J=8.5, 6.3 Hz, 1H, C3H), 4.61 (dd, J=9.1, 9.1 Hz, 1H, C2H), 4.66 (d, J=11.3 Hz, 1H, Bn-CH), 4.71 (s, 2H, Bn-CH2), 4.74 (d, J=11.3 Hz, 1H, Bn-CH), 5.10 (d, J=12.6 Hz, 1H, Bn-CH), 5.12 (d, J=2.7 Hz, 1H, OH), 5.16 (d, J=12.6 Hz, 1H, Bn-CH), 7.15 (d br, 1H, NH), 7.19 (d, J=8.5 Hz, 2H), 7.24 (d, J=8.5 Hz, 2H, aromatic H), 7.29 to 7.48 (m, 10 H, aromatic H), [7.63 (d, J=8.5 Hz) overlapping 7.64 (d, J=8.5 Hz), 4H, aromatic H], 7.75 to 7.78 (m, 4H, aromatic H), 8.58 (s, 1H, piv-NH), 8.60 (s, 1H, piv-NH).13C NMR (acetone-d6, 150 MHz) δ: 19.8, 27.3, 27.7, 40.16, 40.18, 66.7, 67.9, 73.9, 74.3, 80.1, 83.3, 85.2, 120.64, 120.65, 128.5, 128.6, 128.66, 128.68, 129.0, 129.1, 129.2, 130.67, 130.70, 133.7, 133.8, 134.0, 134.4, 136.49, 136.50, 138.4, 138.8, 140.0, 169.1, 177.05, 177.09. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C54H67N4O9Si 943.4672; Found 943.4665.
5-Amino-2-(benzyloxycarbonyl)amino-2,5-dideoxy-3,4-bis-O-(4-(pivaloylamino)benzyl)-D-glucono-1,5-lactam (30)
Formic acid (0.5 mL), 29a (0.475g, 0.50 mmol) and NaCNBH3 (55 mg, 0.88 mmol) were mixed in anhyd CH3CN (10 mL) and the mixture was refluxed for 3.5 h. The solvent was removed and H2O (50 mL) and hexanes:EtOAc (1:3, 50 mL) were added to the residue. The lactam was then extracted into the organic phase. Two additional washes of hexanes:EtOAc were performed and the combined organic phases were dried (Na2SO4), then the solvent was removed. Then a 1 M solution of TBAF in THF (625 μL) was treated with AcOH (250 μL); the lactam product residue was redissolved in CH3CN (6 mL) and this was added to the TBAF solution. After 36h stirring, the reaction was incomplete (LCMS). More 1M TBAF (625 μL) was mixed with AcOH (250 μL) and the solution was added to the reaction mixture, which was then heated to 80 °C for 3 h. The solvent was removed and then the residue was chromatographed on a 80 g silica cartridge (10:0 to 10:1 EtOAc:MeOH) then fractions with co-eluting products were rechromatographed a 25 g silica cartridge (20:1 to 10:1 EtOAc:MeOH) to afford 30a (TLC Rf=0.7 with 10:1 EtOAc/MeOH, 30 mg, 44 μmol, 8.8%, 2 steps) and 30b (TLC Rf=0.45 with 10:1 EtOAc/MeOH, recrystallized from EtOAc, 147 mg, 0.213 mmol, 43%, 2 steps) and two other minor lactam components that were not characterized. Compounds 30a and 30b were white powders.
30a. 1H NMR (CD3OD, 600 MHz) δ: [1.28 (s), 1.29 (s), 18H, tBu-CH3], 3.74 (dd, J=10.9, 3.9 Hz, 1H, C6H), 3.85 to 3.95 (m, 1H, C5H), 3.98 to 4.03 (m, 1H, C6H), 4.17 to 4.22 (m, 1H, C4H), 4.30 (dd, J=7.2, 4.2 Hz, 1H, C3H), [4.50 (d br, J=10.9 Hz, C2-NH), 4.55 (d br, J=9.8 Hz, Bn-CH) over (s br, Bn-CH), 3H], 4.63 (s br, 2H, C2H, ring NH), [5.11 (d, J=12.7 Hz, 1H), 5.13 (d, J=12.7 Hz, 1H), Bn-CH2), 7.21 (d br, J=7.7 Hz, 2H, aromatic H), 7.26 to 7.36 (m, 7H, aromatic H), [7.48 (d, J=2.8 Hz), 7.49 (d, J=2.8 Hz) 4H, aromatic H]. 13C NMR (dmso-d6, 150 MHz) δ: 27.2, 58.5, 59.5, 62.4, 63.0, 63.6, 66.1, 66.3, 70.7, 70.8, 71.6, 79.9, 80.7, 80.8, 81.5, 119.9, 120.0, 126.9, 127.3, 127.6, 127.8, 128.1, 128.3, 128.4, 132.5, 132.7, 136.7, 138.7, 138.8, 154.2, 154.7, 170.3, 170.6, 176.4. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C38H49N4O8 689.3545; Found 689.3548.
30b. 1H NMR (CD3OD, 600 MHz) δ: [1.28 (s), 1.29 (s) 18H, tBu-CH3], 3.45 (m, 1H, C5H), 3.58 (dd, J=11.2, 5.2 Hz, 1H, C6H), 3.73 (dd, J=11.2, 2.7 Hz, 1H, C6H), 3.78 (dd, J=8.8, 8.8 Hz, 1H, C4H), 3.93 (dd, J=9.3, 9.3 Hz, 1H, C3H), 4.06 (d, J=9.9 Hz, 1H, C2H), [4.62 (d, J=11.1 Hz), 4.64 (d, J=11.0 Hz), 2H, Bn-CH2], 4.68 (d, J=11.1 Hz, 1H, Bn-CH), 4.82 (d, below H2O peak, Bn-CH), 5.04 (d, J=12.3 Hz, 1H, Bn-CH), 5.10 (d, J=12.3 Hz, 1H, Bn-CH), 7.20 (d, J=8.5 Hz, 2H, aromatic H), 7.23-7.36 (m, 7H, aromatic H), 7.46 (d, J=8.5 Hz, 2H, aromatic H), 7.49 (d, J=8.5 Hz, 2H, aromatic H). 13C NMR (d6-dmso, 150 MHz) δ: 27.2, 55.5, 56.0, 60.8, 65.3, 73.1, 73.4, 76.8, 80.1, 119.8, 119.9, 120.0, 127.6, 127.7, 127.8, 128.1, 128.3, 133.0, 133.1, 137.12, 138.7, 138.8, 156.4, 168.7, 176.36, 176.38. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C38H49N4O8 689.3545; Found 689.3559.
2,5-Diamino-2,5-dideoxy-D-glucono-1,5-lactam-6-phosphate (31)
Lactam 30b (66 mg, 96 μmol) was dissolved in anhyd py (1 mL) and the reaction vial was cooled in an ice bath. Then, POCl3 (13.4 μL, 0.14 mmol) was added; 45 min later H2O (0.25 mL) was added. After 30 min, the solvent was removed and the residue was chromatographed on a 40 g SiliaBond®-C18 with a 20:1 to 0:1 (50 mM NH4•COO(aq):MeOH) gradient. Fractions containing monophospho-30b (determined by LCMS) were pooled and the mixture was lyophilized; some unreacted 30b and a bis-phospho adduct of 30b co-eluted with the desired product. Then 40% of the lyophilized residue was mixed with DDQ (41 mg, 0.18 mmol) were combined with CHCl3 (3 mL), H2O (1 mL) and 5% aq NaHCO3 (300 μL). The mixture was heated for 6 h at 70 °C and then stored at -20 °C for 1h. The solution was filtered and then the aq phase was separated and lyophilized. The residue was chromatographed on a 40 g SiliaBond®-C18 with a 20:1 to 0:1 (50 mM NH4•COO(aq):MeOH); product fractions with 31-N-(CBz) (determined by MS) were pooled and then lyophilized. The residue was dissolved in H2O (4 mL) and this was passed through a DEAE-Sepharose® Fast Flow column (1.5 cm dia × 8 cm L). The flow-through was collected to afford 32-N-(CBz) (1.5 mg, 4.8 μmol, 12% after lyophilization), then eluted with 140 mM NH4•HCO3; 31-N-(CBz) eluted in the first column volume of NH4•HCO3 and the DDQ-related compounds were retained on the column. Final purification on the analytical anion exchange column using an identical elution profile afforded 31-N-(CBz)•NH4 (4.0 mg, 10 μmol, 26% for 2 steps) after lyophilization. Then, 31-N-(CBz)•NH4 (4 mg, 10 μmol), ammonium formate (20 mg), and Pd(OH)2/C (5 mg) were combined in H2O (1 mL). The mixture was heated for 20 min at 80 °C, then the solution was filtered and the filtrate was lyophilized 2× to afford 31 (2.6 mg, 10 μmol, 99%), which was a white powder. 1H NMR (D2O, 600 MHz): 3.58 (ddd, J=8.6, 5.3, 2.7 Hz, 1H, C5H), 3.94 (ddd, J=8.4, 8.4, 1.4 Hz, 1H, C4H), 3.96-4.03 (m, 3H, C2H, C3H, C6H), 4.09 (ddd, J=11.1, 5.5, 2.8 Hz, 1H, C6H). 13C NMR: DEPTQ (D2O, 150 MHz) δ: 56.9 (+), 59.2 (+, d, J=7.2 Hz), 66.4 (-, d, J=4.1 Hz), 71.1 (+), 73.0 (+), 170.7 (-). HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C6H14N2O7P 257.0533; Found 257.0528.
General silylation conditions
Under an Ar atmosphere, the silylation substrate (3 mmol), diisopropylethylamine (7.8 mL, 45 mmol, 15 equiv.) were mixed in dry DMF (20 mL). Then, chlorotriethylsilane (6 mL, 36 mmol, 12 equiv.) was added and the reaction flask was heated in an oil bath at 60°C for 4 h. The solvent was removed and the residue was chromatographed.
Triethylsilyl 2-deoxy-2-((phenylmethoxy)carbonyl)amino-3,4,6-tris-O-(triethylsilyl)-D-glucopyranoside (33)
The general silylation conditions were used with GlcN-(CBz) (0.107 g, 0.34 mmol), DMF (2.5 mL), diisopropylethylamine (1.25 mL), and TES-Cl (0.8 mL). Chromatography on a 40 g silica cartridge (1:1 to 0:1 hexanes:DCM then 1:1 to 1:1 DCM:EtOAc) afforded 33 (TLC Rf=0.5; 10:1 hexanes:EtOAc, 0.16 g, 0.21 mmol, 62%). A tris-TES product (incompletely silylated) product (TLC Rf=0.25) was also collected (45 mg) and this was set aside for recovery by re-silylation; additional bis-TES products can also be isolated and recovered, accounting for an additional 20-30% of the starting material. The reaction could be scaled to 3 mmol with similar yields. 1H NMR (acetone-d6, 600 MHz) δ: [anomeric H-signals indicate a 4:1 ratio α:β anomers], 0.62 to 0.72 (m, 18H, Si-CH2), 0.73 to 0.79 (m, 6H, Si-CH2), 0.94 to 1.03 (m, 36H, CH3), 3.25 to 3.30 (m, 0.2H, βC5H), 3.31 to 3.37 (m, 0.2H, βC2H), 3.63 (ddd, J=9.7, 9.7, 3.2 Hz, 0.8 H, αC2H), 3.67 to 3.75 (m, 1.8H), 3.81 to 3.85 (m, 1.2H), 3.88 (dd, J=9.3, 7.7 Hz, 0.8H, αC3H), 3.92 (dd, J=11.2, 3.3 Hz, 1H, C6H), 4.84 (d, J=7.4 Hz, 0.2 H, βC1H), [5.06 (d, J=12.4 Hz), 5.07 (s), 5.11 (d, J=12.4 Hz), 2H, Bn-CH2], 5.16 (d, J=3.2 Hz, 0.8H, αC1H), 5.77 (d, J=10.1 Hz, 0.01H, αC2-NH), 6.31 (d, J=9.5 Hz, 0.03H, βC2-NH), 7.31 to 7.41 (m, 5H, aromatic H). 13C NMR (acetone-d6, 150 MHz) δ: 5.15, 5.19, 5.4, 5.9, 6.01, 7.0, 7.10, 7.2, 7.4, 58.8, 57.8, 60.8, 62.6, 63.3, 66.7, 66.9, 72.8, 72.9, 74.4, 74.9, 76.9, 78.0, 92.9, 96.7, 128.55, 128.63, 128.90, 128.94, 129.03, 129.07, 138.0, 138.1, 155.8, 156.8. HRMS (ESI-TOF) m/z: [M + H – TES-OH]+ Calcd for C32H60NO6Si3 638.3723; Found 638.3731.
Triethylsilyl 2-deoxy-2-((phenylmethoxy)carbonyl)amino-3,4-bis-O-(triethylsilyl)-α-D-glucopyranoside (34)
Compound 33 (0.5 g, 0.65 mmol) was dissolved in a mixture of DCM (7.5 mL) and MeOH (2.5 mL). Then, PPTS (2 mg) was added and the reaction was monitored by TLC (10:1 DCM/EtOAc). After 1 h, no 33 (TLC Rf=0.5) remained and a new spot had appeared (TLC Rf=0.2, 34). The mixture was loaded directly on a 40 g silica cartridge and chromatographed (1:1 to 0:1 hexanes:DCM, then 1:0 to 1:1 DCM:EtOAc gradient). Compound 34 was a colorless oil (0.25 g, 0.38 mmol, 58%); the α-anomer was the sole, isolated product. More polar mono- and bis-silylated products from this reaction can be subjected again to the silylation conditions to reclaim another 30% of the product as 33. 1H NMR (acetone-d6, 600 MHz) δ: 0.65 (q, J=8.0 Hz, 6H, Si-CH2), 0.69 (q, J=8.0 Hz, 6H, Si-CH2), 0.76 (q, J=7.9 Hz, 6H, Si-CH2), 0.96 (overlapping t's, J=8.0 Hz, 18H, CH3), 1.01 (t, J=8.1 Hz, 9H, CH3), 3.56 (dd, J=7.7, 4.7 Hz, 1H, OH), 3.62 (ddd, J=9.8, 9.8, 3.2 Hz, 1H, C2H), 3.69 (ddd, J=8.8, 2.9, 3.1 Hz, 1H, C5H), 3.70 to 3.79 (m, 3H, C4H, C6H, C6H), 3.88 (dd, J=9.5, 7.8 Hz, 1H, C3H), 5.07 (J=12.3 Hz, 1H, Bn-CH), 5.11 (d, J=12.3 Hz, 1H, Bn-CH), 5.14 (d, J=3.2 Hz, 1H, αC1H), 5.76 (d, J=10.3 Hz, 0.04 H, C2-NH), 7.30 to 7.39 (m, 5H, aromatic H). 13C NMR (acetone-d6, 150 MHz) δ: 5.1, 5.9, 6.0, 6.9, 7.4, 57.9, 61.6, 67.0, 72.9, 74.4, 74.8, 93.0, 128.7, 128.9, 129.2, 138.0, 156.9). HRMS (ESI-TOF) m/z: [M + H – TES-OH]+ Calcd for C26H46NO6Si2 524.2858; Found 524.2866.
Triethylsilyl 6-cyano-2,6-dideoxy-2-((phenylmethoxy)carbonyl)amino-3,4-bis-O-(triethylsilyl)-α-D-glucopyranoside (35)
Under an Ar atmosphere, 34 (44 mg, 67 μmol) was dissolved in anhyd THF (2 mL). Then, five additions of Ph3P (39 mg, 0.15 mmol), diisopropyl azodicarboxylate (29.5 μL, 0.15 mmol) and acetone cyanohydrin (9.2 μL, 0.1 mmol) each were made. The first addition was at the reaction start, and the remaining additions were made over the next 36 h. The reaction progress was monitored by LCMS. At 38 h, the solvent was removed and the residue was chromatographed on a 25 g silica cartridge with a 0 to 25% EtOAc in DCM gradient. The fractions with unreacted 34 and those with 35 were identified by MS. Solvent removal from these fractions afforded 34 (8 mg, 18%), and 35 (23.5 mg, 35 μmol; 64% yield on 82% conversion). 1H NMR (acetone-d6, 600 MHz) δ: 0.67 to 0.75 (overlapping q's, J= 7.9, 7.8 Hz, 12H, Si-CH2), 0.78 (q, J=7.8 Hz, 6H, Si-CH2), 0.95 to 0.99 (overlapping t's, J=7.9, 7.8 Hz, 18 H, CH3), 1.03 (t, J=7.9 Hz, 9H, CH3), 2.84 (dd, J=17.0, 6.7 Hz, 1H, C6H), 2.90 (J=17.0, 4.1 Hz, 1H, C6H), 3.58 (dd, J= 8.0, 8.0 Hz, 1H, C4H), 3.70 (ddd, J=10.0, 10.0, 3.2 Hz, 1H, C2H), 3.92 (dd, J=8.9, 7.7 Hz, 1H, C3H), 3.98 (ddd, J=8.0, 6.7, 4.1 Hz, 1H, C5H) 5.09 (d, J=12.3 Hz, 1H, Bn-CH), 5.12 (d, J=12.3 Hz, 1H, Bn-CH), 5.21 (d, J=3.1 Hz, 1H, αC1H), 5.85 (d, J=10.1 Hz, 0.75 H, C2-NH), 7.32 to 7.42 (m, 5H, aromatic H). 13C NMR (acetone-d6, 150 MHz) δ: 5.1, 5.99, 6.02, 6.9, 7.27, 7.32, 21.6, 57.7, 67.1, 70.0, 74.4, 76.1, 92.6, 118.7, 128.8, 129.0, 129.2, 137.9, 156.9. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C33H60N2O6Si3 665.3832; Found 665.3826. See supporting information for HMBC and HMQC data.
Triethylsilyl 6-azido-2,6-dideoxy-2-((phenylmethoxy)carbonyl)amino-3,4-bis-O-(triethylsilyl)-α-D-glucopyranoside (36)
Under an Ar atmosphere, 34 (0.25 g, 0.38 mmol) was dissolved in anhyd THF (11 mL). Then, Ph3P (0.39 g, 1.5 mmol), diisopropyl azodicarboxylate (0.275 mL, 1.4 mmol) and diphenylphosphorylazide (0.3 mL, 1.4 mmol) were added in two equal portions; the first to initiate the reaction and the second was added 60 min later. Stirring was continued for 6 h. Then the solvent was removed and the residue was chromatographed on a 40 g silica cartridge using a 20:1 to 3:1 hexanes:EtOAc gradient. The first product to elute from the column was 36 affording an isolated yield of 0.196 g (a colorless oil, 0.29 mmol, 76%). 1H NMR (acetone-d6, 600 MHz) δ: 0.66 to 0.73 (m, 12H, Si-CH2), 0.75 (q, J=7.9 Hz, 6H, Si-CH2), 0.95 (t, J=7.9 Hz, 9H, CH3), 0.98 (t, J=8.0 Hz, 9H, CH3), 1.01 (t, J=8.0 Hz, 9H, CH3), 3.47 (dd, J=13.1, 5.5 Hz, 1H, C6H), 3.58 (dd, J=13.1, 2.7 Hz, 1H, C6H), 3.63 (dd, J=8.6, 8.3 Hz, 1H, C4H), 3.68 (ddd, J=9.9, 9.9, 3.2 Hz, 1H, C2H), 3.87 to 3.90 (m, 2H, C2H, C5H), 5.08 (d, J=12.4 Hz, 1H, Bn-CH), 5.12 (d, J=12.4 Hz, 1H, Bn-CH), 5.20 (d, J=3.2 Hz, 1H, αC1H), 7.33 to 7.39 (m, 5H, aromatic H). 13C NMR (acetone-d6, 150 MHz) δ: 5.1, 5.97, 6.02, 6.9, 7.3, 7.4, 52.4, 57.9, 67.2, 73.2, 74.4, 74.6, 92.9, 128.8, 129.0, 129.2, 137.9, 156.9. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C32H60NO6Si3 638.3723; Found 638.3727.
2-Amino-6-azido-2,6-dideoxy-D-glucose (37)
Compound 36 (30 mg, 44 μmol) was treated with the standard acid hydrolysis conditions on ¼-scale and heating and stirring were applied for only 3 h; the yield of 37, a white powder, was 10.5 mg (33 μmol, 75 %) as the TFA salt. 1H NMR (D2O, 600 MHz) δ: [anomeric H-signals indicate a 2.5:1 ratio α:β anomers and less prominent peaks for other forms of this sugar, e.g. furanoses], 2.91 (dd, J=10.6, 8.5 Hz, 1H, βC2H), 3.21 (dd, J=10.6, 3.6 Hz, 2.6H, αC2H), 3.39 (dd, J=9.5, 9.5 Hz, 3.6 H, αC2H), [overlapping dd's: 3.45 (J=13.4, 5.8 Hz), 3.47 (J=13.6, 5.5 Hz) 3.6H], 3.50 to 3.61 (m, 6H), 3.77 (dd, J=10.6, 9.2 Hz, 2.4 H, αC3H), 3.97 to 4.08 (m, 0.75 H), 4.21 (d, J=2.6 Hz, 0.24 H), 4.29 (d, J=5.4 Hz, 0.24 H), 4.84 (d, J=8.5 Hz, 1H, βC1H), 5.33 (d, J=3.6 Hz, 2.1 H, αC1H), [6.16 (d, J=5.4 Hz), 6.18 (d, J=5.5 Hz) 0.2H]. 13C NMR (D2O/dmso-d6, 150 MHz; referenced to dmso) δ: 52.2, 55.6, 55.7, 58.2, 65.6, 68.4, 70.9, 71.85, 71.91, 72.0, 73.3, 74.7, 76.2, 82.1, 90.7, 94.3, 105.3, [117.9 (q, J=292.4 Hz), 164.0 (q, J=35.5 Hz), TFA]. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C6H13N4O4 205.0931; Found 205.0932.
2,6-Diamino-2,6-dideoxy-D-glucose (38)
Compound 36 (50 mg, 73 μmol) was mixed with ammonium formate (26 mg) and Pd(OH)2/C (10 mg) in MeOH (1 mL). The mixture was heated (60 °C) and stirred for 1h, then the Pd(OH)2 was filtered off and washed with MeOH (0.5 mL). Then, H2O (5 mL) and TFA (1 mL) was added to the filtrate. The mixture was stirred for 16h, then it was extracted with EtOAc (2 × 2 mL). The aqueous phase was lyophilized 2×, affording a viscous resin, 38•(TFA)2 with (NH4•O2C2F3)6•TFA0.5•H2O0.5 (MW=1258.56 g/mol; 50 μmol, 68 %) based on NMR and elemental analyses. 1H NMR (D2O, 600 MHz) δ: [2:1 ratio of α:β anomers] 3.16 (dd, J=10.6, 8.5 Hz, 0.5H, Hβ2), 3.22 to 3.30 (m, 1.5H), 3.45 (dd, J=10.6, 3.6 Hz, 1H, Hα2), 3.49 to 3.62 (m, 3H), 3.78 to 3.83 (m, 1H), 4.01 (dd, J=10.4, 9.2 Hz, 1H, Hα3), 4.18 (ddd, J=9.5, 9.5, 2.7 Hz, 1H, Hα5), 5.08 (d, J=8.5 Hz, 0.5 H, Hβ1), 5.57 (d, J=3.5 Hz, 1H, Hα1). 13C NMR (D2O, 150 MHz) δ: 43.2, 57.0, 59.4, 70.3, 72.2, 74.3, 74.5, 74.8, 92.0, 95.6, [119.2 (q, J=290.0 Hz), 165.7 (q, J=35.0 Hz), from TFA]. Anal. Calcd for C23H41.5F25.5N8O21.5: C, 21.95; H, 3.32; N, 8.90; F, 38.49. Found: C, 21.76; H, 3.17; N, 8.89; F, 38.55. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C6H15N2O4 179.1026; Found 179.1028.
Triethylsilyl 2-amino-2-deoxy-3,4,6-tris-O-(triethylsilyl)-α-D-glucopyranoside (39)
The general silylation conditions were applied to glucosamine•HCl (2× scale, 1.29 g, 6 mmol); a second addition of N,N-diisopropylethylamine (5.2 mL, 30 mmol) and chlorotriethylsilane (4 mL, 24 mmol) was made at 90 min. After another 60 min at 60°C, solvent removal followed by chromatography on an 80 g cartridge (10:1 to 0:1 of hexanes:EtOAc gradient) afforded 39 (TLC Rf=0.45 with 5:1 hexanes:EtOAc, a colorless oil, 2.33 g, 3.66 mmol, 61%), exclusively as it's α-anomer. 1H NMR (C6D6, 500 MHz) δ: 0.60 (q, J=7.8 Hz, 6H), 0.715 (overlapping q's, J=7.9 Hz, 6H), 0.83 to 0.94 (m, 12H), 0.98 (t, J=8.0 Hz, 9H), 1.07 to 1.05 (overlapping t's, J=8.0 Hz, 27H), 2.55 (dd, J=8.6, 3.2 Hz, 1H), 3.86 to 3.92 (m, 4H), 4.08 (dd, J=11.1, 2.8 Hz, 1H), 4.99 (d, J=3.3 Hz, 1H). 13C NMR (CD3CN, 125 MHz) δ: 5.0, 5.1, 5.8, 6.4, 6.9, 7.1, 7.4, 7.5, 58.1, 62.5, 72.5, 74.0, 78.0, 95.4. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C30H70NO5Si4 636.4326; Found 636.4318.
Triethylsilyl 2-Deoxy-2-(4-methoxyphenylmethylidene)-amino-3,4,6-tris-O-(triethylsilyl)-α-D-glucopyranoside (40)
Compound 39 (2.33 g, 3.66 mmol) was mixed with p-anisaldehyde (0.67 mL, 5.5 mmol) and PPTS (15 mg) in toluene (40 mL). The mixture was refluxed 2 h; water was removed using a Dean-Stark trap. Then, the solvent was removed and the residue was chromatographed on a 50 g silica cartridge (10:1 to 5:1 hexanes:EtOAc gradient). The first compound to elute (TLC Rf = 0.4; 10:1 hexanes:EtOAc, some hydrolysis of the imine occurred on the TLC plate) was 40 (a colorless oil, 2.52 g, 3.34 mmol, 91.3 %). 1H NMR (acetone-d6, 500 MHz) δ: 0.58 to 0.76 (m, 18H), 0.81 to 0.86 (m, 6H), 0.93 (t, J=7.9 Hz, 9H), 1.01 to 1.22 (m, 27H), 3.16 (dd, J=9.4, 3.1 Hz, 1H), 3.75 (dd, J=9.5, 8.5 Hz, 1H), [3.94 (s) overlapping 3.85 to 3.96 (m), 6H], 4.44 (dd, J=9.4, 8.0 Hz, 1H), [α-anomeric H: 5.02 (d, J=3.2 Hz); β-anomeric H: 4.82 (d, J=7.3 Hz); α:β ratio = 17:1, 1H], 7.08 (d, J=8.8 Hz, 2H), 7.83 (d, J=8.8 Hz, 2H), 8.34 (s, 1H). 13C NMR (CD3CN, 125 MHz) δ: 5.17, 5.21, 5.99, 6.00, 6.88, 6.95, 7.0, 7.2, 7.4, 56.0, 63.1, 73.2, 74.1, 75.9, 77.9, 96.0, 114.7, 130.4, 130.9, 162.6, 163.2. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C38H76NO6Si4 754.4744; Found 754.4739.
Triethylsilyl 2-Deoxy-2-((4-methoxyphenyl)-oxaziridin-2-yl)-3,4,6-tris-O-(triethylsilyl)-α-D-glucopyranoside (41)
Imine 40 (2.52 g, 3.34 mmol) was dissolved in DCM (20 mL) then saturated aq NaHCO3 (3.3 mL) and m-CPBA (70%, 1.14 g) were added. The solution was stirred vigorously for 2 h; the organic and aqueous layers were separated and the latter was extracted with DCM (20 mL). The combined organic layers were dried (Na2SO4); after solvent removal the residue was chromatographed on a 50 g silica cartridge (1:1 to 1:0 DCM:hexanes gradient). A minor product of the reaction with the same MS as 41 eluted first (Rf=0.65, typically 5% yield) was discarded. The yield of the main product of 41 was 1.44 g (a viscous oil, TLC Rf=0.5 with 2:1 DCM:hexanes, 1.86 mmol, 56%). 1H NMR (acetone-d6, 500 MHz) δ: 0.38 to 0.54 (m, 6H, Si-CH2), 0.65 (q, J=7.9 Hz, 6H, Si-CH2), 0.77 to 0.84 (m, 15H, Si-CH2, SiCH2CH3), 0.91 (t, J=7.7 Hz, 6H, Si-CH2), 1.00 (t, J=7.9 Hz, 9H, SiCH2CH3), 1.04 (t, J=7.9 Hz, 9H, SiCH2CH3), 1.10 (t, J=7.8 Hz, 9H, SiCH2CH3), 2.17 (dd, J=9.4, 3.1 Hz, 1H, C2H), 3.65 (dd, J=9.2, 8.3 Hz, 1H, C4H), 3.72 (ddd, J=9.3, 3.7, 1.9 Hz, 1H, C5H), 3.80 (dd, J=11.4, 1.9 Hz, 1H, C6H), 3.84 (s, 3H, OCH3), 3.88 (dd, J=11.4, 3.8 Hz, 1H, C6H), 4.42 (dd, J=9.2, 8.4 Hz, 1H, C3H), 4.89 (s, 1H, oxaziridine CH), 5.42 (d, J=3.1 Hz, 1H, αC1H), 6.97 (d, J=8.7 Hz, 2H, aromatic H), 7.48 (d, J=8.7Hz, 2H, aromatic H); 13C NMR (acetone-d6, 125 MHz) δ: 5.1, 5.2, 5.9, 6.1, 6.8, 7.1, 7.4, 7.6, 56.6, 62.7, 73.0, 74.9, 75.0, 76.7, 76.8, 91.1, 114.4, 128.3, 130.4, 162.1. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C38H76NO7Si4 770.4693; Found 770.4691.
Triethylsilyl 2-Deoxy-2-((4-methoxyphenyl)-oxaziridin-2-yl)-3,4-bis-O-(triethylsilyl)-α-D-glucopyranoside (43)
Oxaziridine 41 (100 mg, 0.13 mmol) was dissolved in MeOH (2.5 mL). Then DL-10-camphorsulfonic acid (100 μL of a 1 mg/mL solution in MeOH) was added and the mixture was stirred for 10 min. The solvent volume was reduced to 0.5 mL then the mixture was loaded directly on to a 25 g silica cartridge and chromatographed (DCM:EtOAc gradient: 100:0 to 9:1 in 2.5 column volumes then to 0:1 in 4 column volumes). The first compound to elute was unreacted 41 (4.5 mg, 4.5%; TLC Rf=0.95 with 20:1 DCM:EtOAc), which was followed by 43 (a viscous oil, TLC Rf = 0.85, 38 mg, 58 μmol, 45%). Another band (TLC Rf = 0.45, 20 mg) was consistent (MS) with bis-O-(triethylsilyl) sugar(s). Subsequent elution with 4:1 EtOAc:MeOH eluted the final product(s) from the column that were consistent (MS) with mono-O-(triethylsilyl) sugar(s) (10 mg, 23 μmol, 18%). 43: 1H NMR (acetone-d6, 600 MHz) δ: 0.37 to 0.52 (m, 6H, Si-CH2), 0.76 to 0.83 (m, 15H, SiCH2CH3 and Si-CH2), 0.87 to 0.92 (m, 6H, SiCH2), 1.03 (t, J=7.9 Hz, 9H, SiCH2CH3), 1.10 (t, J=7.9 Hz, 9H, SiCH2CH3), 2.16 (dd, J=9.4, 3.0 Hz, 1H, C2H), 3.41 (dd, J=6.7, 5.2 Hz, 1H, C6H), 3.66 (dd, J=8.6, 8.6 Hz, 1H, C4H), 3.69 to 3.74 (m, 3H, OH, C6H, C5H), 3.83 (s, 3H, O-CH3), 4.43 (dd, J=9.2, 8.3 Hz, 1H, C3H), 4.84 (s, 1H, oxaziridine CH), 5.38 (d, J=2.9 Hz, 1H, αC1H), 6.97 (d, J=8.7 Hz, 2H, aromatic H), 7.46 (d, J=8.7 Hz, 2H, aromatic H). 13C NMR (acetone-d6, 125 MHz) δ: 5.1, 5.9, 6.1, 6.8, 7.4, 7.6, 29.6, 55.6, 61.6, 73.0, 74.86, 74.91, 76.7, 76.8, 93.2, 114.4, 128.3, 130.4, 162.1. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C32H62NO7Si3 656.3829; Found 656.3830.
2-Deoxy-2-(N-hydroxyl-amino)-D-glucose (42)
Oxaziridine 41 (125 mg, 0.162 mmol) was dissolved in TFA:H2O (2 mL: 0.5 mL). The mixture was stirred for 5 h, then another 2.5 mL H2O was added and stirring was continued overnight. Then DCM (5 mL) was added and the mixture was stirred 2 h. The organic phase was discarded and the aq phase was lyophilized (2×) to afford 30 mg of a mixture of 42 and a benzylidene acetal of 42; the mixture was an off-white powder. NMR analysis showed one α-anomeric and two β-anomeric sugars. 1H NMR (D2O, 600 MHz) δ: The C1H, C2H, and C3H signals, respectively, inside [ ]: α-anomer [5.69 (d, J=3.5 Hz, 0.8H), 3.61 (dd, J=10.8, 3.5 Hz, 0.8H)], β-anomer no. 1 [5.33 (d, J=7.9 Hz, 0.5 H), 3.85 (in m), 4.29 (dd, J=10.0, 8.8 Hz, 0.5H)], β-anomer no. 2 [5.22 (d, J=8.2 Hz, 0.6H), 3.25 (dd, J=10.7, 8.2 Hz, 0.6H), 4.1 (dd, J=10.7, 8.7 Hz, 0.6H)], 7.92 (s, 0.5 H). Aromatic Hs: 7.19 (d, J=9.1 Hz, 1H), 8.33 (d, J=9.1 Hz, 1H). Based on integration values, β-anomer no. 1 corresponds with the putative p-OMe benzylidene acetal of 42, which is in a 1:3 ratio with 42. The remaining signals [3.56-3.65 (m, 4.4H), 3.81-3.88 (m, 3H), 3.91-4.06 (m, 6.5H)] overlapped and no further interpretation was possible. 13C NMR (D2O, 150 MHz) δ: 58.2, 63.0, 63.2, 63.3, 66.4, 67.6 70.3, 70.5, 72.4, 72.6, 72.8, 73.3, 74.1, 78.66, 78.73, 82.0, 90.2, 93.2, 95.3, 117.1, 118.1, 120.0, 124.3, 135.3, 145.5. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C6H14NO6 196.0816; Found 196.0813. Putative p-OMe benzylidene acetal: [M + H]+ Calcd for C14H20NO7 314.1234; Found 314.1232.
Triethylsilyl 2-[[bis[[(1,1-dimethylethoxy)carbonyl]amino]methylene]amino]-2-deoxy-3,4,6-tris-O-(triethylsilyl)-α-D-Glucopyranoside (47)
The general silylation conditions were applied to 4532 (0.505g, 1.2 mmol, 0.6× scale) with the addition of DMAP (50 mg). After solvent removal, the mixture was chromatographed 3× on 90 g silica cartridges (100:7.5 to 10:1 hexanes:EtOAc) to separate the main product of 47 (TLC Rf=0.5 100:7.5 hexanes:EtOAc) exclusively as the α-anomer from two minor products of 47 (TLC Rf=0.55, 27 mg; Rf=0.45, 45 mg). The isolated yield of the main 47 isomer was 675 mg (a viscous oil, 0.77 mmol, 64%); the minor products were discarded. 1H NMR (acetone-d6, 500 MHz) δ: 0.62 to 0.71 (m, 18H, Si-CH2), 0.74 to 0.79 (m, 6H, Si-CH2), 0.94 to 1.02 (m, 36H, SiCH2CH3), 1.42 (s, 9H, tBu-CH3), 1.55 (s, 9H, tBu-CH3), 3.70 to 3.75 (m, 2H), 3.84 (dd, J=11.4, 2.0 Hz, 1H), 3.91 to 3.96 (m, 2H), 4.22 (ddd, J=9.4, 9.4, 3.2 Hz, 1H, C2H), 5.19 (d, J=3.2 Hz, 1H, αC1H), 8.56 overlapping d's, J=9.4 Hz, 1H, C2-NH), 11.80 (s, 0.2 H, guanidine NH(BOC)). 13C NMR (acetone-d6, 125 MHz) δ: 5.1, 5.8, 5.9, 6.9, 7.1, 7.3, 7.4, 28.0, 28.4, 56.5, 62.5, 72.5, 74.8, 74.9, 79.0, 84.0, 92.2, 153.8, 157.1, 164.6. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C41H88N3O9Si4 878.5592; Found 878.5591.
Triethylsilyl 2-[[bis[[(1,1-dimethylethoxy)carbonyl]amino]methylene]amino]-2-deoxy-3,4-bis-O-(triethylsilyl)-α-D-Glucopyranoside (48)
Compound 47 (0.65 g, 0.74 mmol) was dissolved in a mixture of CH2Cl2 (10 mL) and MeOH (20 mL). Then PPTS was added (20 mg) and the mixture was stirred for 12 h. The solvent was removed and the residue was chromatographed on a 90 g silica cartridge (10:1 to 5:1 hexanes:EtOAc). Unreacted 47 (TLC 0.182 g, 28%, Rf=0.85 with 10:1 hexanes:EtOAc) and 48 (a viscous oil, 0.25 g, 0.33 mmol, Rf=0.25, 45%, or 61% on 72% conversion) were isolated. 1H NMR (C6D6, 500 MHz) δ: [NMR data suggest the presence of two species, both α-anomers, consistent with 2 conformers and in a 1:0.16 ratio] 0.55 (q, J=7.9 Hz, 4H, Si-CH2), 0.63 to 0.75 (m, 2H, Si-CH2), 0.79 to 0.88 (m, 12H, Si-CH2), 0.94 (t, J=8.0 Hz, 6H, SiCH2CH3), [1.02 to 1.08 (m) overlapping with 1.11 (t, J=8.0 Hz), 21H, SiCH2CH3], [1.20 (s) and 1.24 (s), 9H, tBu-CH3], [1.44 and 1.45 (s), 9H, tBu-CH3], [3.74 to 3.80 (m) and 3.83 to 3.88 (m) and 3.91 to 3.95 (m), 4H], [4.04 (dd, J=10.3, 5.6 Hz), 4.11 (dd, J=10.2, 5.2 Hz), 4.16 (dd, J=9.9, 8.1 Hz) and 4.26 (m), 2H], [4.59 (ddd, J=8.5, 6.5, 2.7 Hz, and 4.71 (ddd, J=9.7, 9.8 and 3.4 Hz), 1H, C2H], [5.30 (d, J=3.4 Hz) over 5.31 (d, J=2.7 Hz), 1H, αC1H], [8.75 (d, J=9.7 Hz) and 9.06 (d, J=8.5 Hz) 1H, C2-NH], [12.27 (s) and 12.51 (s), 1H]. 13C NMR (C6D6, 125 MHz) δ: 4.8, 4.9*, 5.3*, 5.4*, 5.7, 6.7, 7.1*, 7.2*, 7.3, 7.4, 27.6, 27.7*, 28.3, 31.9, 55.4*, 56.4, 61.8, 62.5*, 70.9*, 72.5, 73.3, 73.8*, 74.6, 77.3*, 78.7*, 78.8, 82.4*, 82.6, 90.7*, 92.3, 153.0*, 153.6, 156.8, 157.3*, 164.2*, 164.7. *=smaller peaks adjacent to larger peaks, supporting the presence of a conformer. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C35H74N3O9Si3 764.4727; Found 764.4729.
2-[(Aminoiminomethyl)amino]-2-deoxy- D-glucose-6-phosphate (49)
Compound 48 (0.23 g, 0.30 mmol) was dissolved in anhyd py (3 mL). Then POCl3 (35 μL, 0.375 mmol) was added and the mixture was stirred for 40 min, at which point H2O (0.5 mL) was added. After 5 min, the solvent was removed; then the residue was mixed with DMF:H2O:AcOH (3.2 mL: 1.6 mL: 0.8 mL) and the mixture was stirred for 12 h. The solvent was removed and the mixture was taken up in H2O (5 mL) and EtOAc (5 mL); the pH of the aq phase was adjusted to 7 with 1N NaOH. The biphasic mixture was shaken and after separation, the organic phase was discarded; the aq phase was washed 2× more with EtOAc (5 mL ea). Then 49, as the mono-BOC adduct (49-BOC) was isolated by anion exchange chromatography on a DEAE-Sepharose® Fast Flow column (1.5 cm dia × 8 cm L) with 6 injections of 2 mL injections per run and a gradient of 0-200 mM NH4•HCO3. The fractions with 49-BOC were lyophilized 2× to afford 0.251 g of an off-white powder. Then 49-BOC (125 mg) was dissolved in 0.25M HCl (5 mL), the reaction vial was heated to 95°C for 1 h and then the mixture was allowed to cool. The solution was neutralized with saturated NaHCO3; TLC analysis (5:4:1 nPrOH:concd NH4OH:H2O) indicated the conversion from 49-BOC (Rf = 0.75) to 49 (Rf=0.25). The sample was lyophilized and then purified by “Aqueous Normal Phase” HPLC31 on a semi-preparative Cogent Diamond Hydride™column (Musolv, 1 × 25 cm, 4 μm; A=50 mM aq NH4•HCO3, B=CH3CN; 0 to 3 min (95% B), 3 to 13 min (gradient to 30% B)). The CH3CN was removed from product containing fractions on a rotary evaporator with a water bath at 50 °C and then the remaining solution was lyophilized to afford purified 49 (a white powder, 13.1 mg, 46 μmol, 30% from 48). 1H NMR (D2O, 600 MHz) δ: 3.70 (m, 0.2H), 3.91 (dd, J=8.4, 2.5 Hz, 1H), 3.93 to 4.05 (m, 1.6 H), 4.06 (dd, J=8.0, 4.3 Hz, 0.2 H), 4.08 to 4.20 (m, 2.8H), 4.25 to 4.28 (m, 0.2H), 5.02 (d, J=4.3 Hz, 0.2 H), 5.06 (d, J=2.9 Hz, 0.8 H), 5.07 (d, J= 2.3 Hz, 0.2H), 6.87 (s, 0.8H), 6.90 (s, 0.2H). 13C NMR: DEPTQ (D2O, 150 MHz) δ: (+) 66.8, (+) 66.9, (+) 68.3, (-) 69.0 (d, J=5.0 Hz), (-) 69.1 (d, J=5.3 Hz), (+) 72.6 (d, J=7.5 Hz), (+) 73.7 (d, J=7.6 Hz), (+) 74.3 (d, J=6.3 Hz), (+) 74.9, (+) 75.2, (+) 77.8 (d, J=5.7 Hz), (-) 113.1, (-) 130.1, (-) 150.7. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C7H14N3O7P 284.0642; Found 284.0637
Supplementary Material
Acknowledgments
We would like to thank N. Tjandra and his group (NHLBI) for assistance with NMR experiments. D.-Y. Lee of the NHLBI Biochemistry Core Facility, R. Levine (NHLBI), P. Gafken (Proteomics Core at Fred Hutchinson Cancer Research Center, FHCRC) and D. Shen's group (Univ. of Washington) assisted with MS and LCMS experiments. We also thank M. Roth and J. Simon (both FHCRC) for the generous loan of equipment and reagents. This research was supported in part by the Howard Hughes Medical Institute, the Bill and Melinda Gates Foundation and the Intramural Research Program of the NIH, National Heart, Lung and Blood Institute.
Footnotes
Supporting Information: General experimental information, copies of 1D and 2D NMR spectra, MS/HRMS and HPLC analyses can be found in the supporting information. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Nature. 2004;428:281–286. doi: 10.1038/nature02362. [DOI] [PubMed] [Google Scholar]
- 2.Ferré-D'amaré AR. Quart Rev Biophys. 2010;43:423–447. doi: 10.1017/S0033583510000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferre-D'amare AR, Scott WG. Cold Spring Harbor Perspectives in Biology. 2010;2:a003574–a003574. doi: 10.1101/cshperspect.a003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein DJ, Ferré-D'amaré AR. Science. 2006;313:1752–1756. doi: 10.1126/science.1129666. [DOI] [PubMed] [Google Scholar]
- 5.Viladoms J, Fedor M. Journal of the American Chemical Society. 2012;134:19043–19049. doi: 10.1021/ja307021f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis JH, Dunican BF, Strobel SA. Biochemistry. 2011;50:7236–7242. doi: 10.1021/bi200471c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong B, Klein DJ, Ferré-D'amaré AR, Carey PR. J Am Chem Soc. 2011;133:14188–14191. doi: 10.1021/ja205185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins JA, Irnov I, Baker S, Winkler WC. Genes & Development. 2007;21:3356–3368. doi: 10.1101/gad.1605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deigan KE, Ferré-D'amaré AR. Acc Chem Res. 2011;44:1329–1338. doi: 10.1021/ar200039b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esko JD, D TL, Raetz CRH. In: Essentials of Glycobiology. 2nd. Varki A, c RD, Esko JD, et al., editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2009. [PubMed] [Google Scholar]
- 11.Klein DJ, Wilkinson SR, Been MD, Ferré-D'amaré AR. J Mol Biol. 2007;373:178–89. doi: 10.1016/j.jmb.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mccarthy TJ, Plog MA, Floy SA, Jansen JA, Soukup JK, Soukup GA. Chem Biol. 2005;12:1221–1226. doi: 10.1016/j.chembiol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Lim J, Grove BC, Roth A, Breaker RR. Angew Chem Int Ed Engl. 2006;45:6689–6893. doi: 10.1002/anie.200602534. [DOI] [PubMed] [Google Scholar]
- 14.Lünse CE, Schmidt MS, Wittmann V, Mayer G. ACS Chem Biol. 2011;6:675–678. doi: 10.1021/cb200016d. [DOI] [PubMed] [Google Scholar]
- 15.Tanrikulu IC, Schmitt E, Mechulam Y, Goddard WA, Tirrell DA. Proceedings of the National Academy of Sciences. 2009;106:15285–15290. doi: 10.1073/pnas.0905735106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tchertanov L. Acta Crystallographica Section B: Structural Science. 1999;55:807–809. doi: 10.1107/s0108768199003936. [DOI] [PubMed] [Google Scholar]
- 17.Hanessian S, Massé R, Nakagaw T. Canadian Journal of Chemistry. 1978;56:1509–1517. [Google Scholar]
- 18.Kok GB, Campbell M, Mackey BL, Von Itzstein M. Carbohydrate Research. 2001;332:133–139. doi: 10.1016/s0008-6215(01)00091-x. [DOI] [PubMed] [Google Scholar]
- 19.Paulsen H, Grage U. Chemische Berichte. 1974;107:2016–2026. [Google Scholar]
- 20.Laughlin ST, Bertozzi CR. Proceedings of the National Academy of Sciences. 2009;106:12–17. doi: 10.1073/pnas.0811481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen OA, Dixon MJ, Eggleston IM, Van Aalten DMF. Nat Prod Rep. 2005;22:563–579. doi: 10.1039/b416660b. [DOI] [PubMed] [Google Scholar]
- 22.Chenault HK, Mandes RF, Hornberger KR. J Org Chem. 1997;62:331–336. doi: 10.1021/jo961715g. [DOI] [PubMed] [Google Scholar]
- 23.Barreteau H, Kovaä A, Boniface A, Sova M, Gobec S, Blanot D. FEMS Microbiology Reviews. 2008;32:168–207. doi: 10.1111/j.1574-6976.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 24.Panday N, Meyyappan M, Vasella A. Helvetica Chimica Acta. 2000;83:513–538. [Google Scholar]
- 25.Terinek M, Vasella A. Tetrahedron: Asymmetry. 2005;16:449–469. [Google Scholar]
- 26.Overkleeft HS, van Wiltenburg J, Pandit UK. Tetrahedron. 1994;50:4215–4224. [Google Scholar]
- 27.Fukase K, Yoshimura T, Hashida M, Kusumoto S. Tetrahedron letters. 1991;32:4019–4022. [Google Scholar]
- 28.Tsunoda T, Uemoto K, Nagino C, Kawamura M, Kaku H, Itô S. Tetrahedron Letters. 1999;40:7355–7358. [Google Scholar]
- 29.Thompson AS, Humphrey GR, DeMarco AM, Mathre DJ, Grabowski EJJ. J Org Chem. 1993;58:5886–5888. [Google Scholar]
- 30.Murakami M, Ikeda K, Achiwa K. Carbohydrate Research. 1996;280:101–110. doi: 10.1016/0008-6215(95)00295-2. [DOI] [PubMed] [Google Scholar]
- 31.Matyska MT, Pesek JJ, Duley J, Zamzami M, Fischer SM. J Sep Sci. 2010;33:930–938. doi: 10.1002/jssc.200900648. [DOI] [PubMed] [Google Scholar]
- 32.Baker TJ, Luedtke NW, Tor Y, Goodman M. J Org Chem. 2000;65:9054–9058. doi: 10.1021/jo001142e. [DOI] [PubMed] [Google Scholar]
- 33.Kamst E, Zegelaar-Jaarsveld K, van der Marel GA, van Boom JH, Lugtenberg BJJ, Spaink HP. Carbohydrate Research. 1999;321:176–189. [Google Scholar]
- 34.Sugawara T, Narisada M. Carbohydrate Research. 1989;194:125–138. doi: 10.1016/0008-6215(89)85012-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.