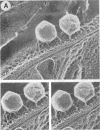
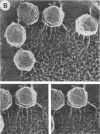
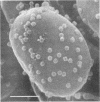
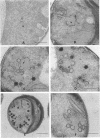
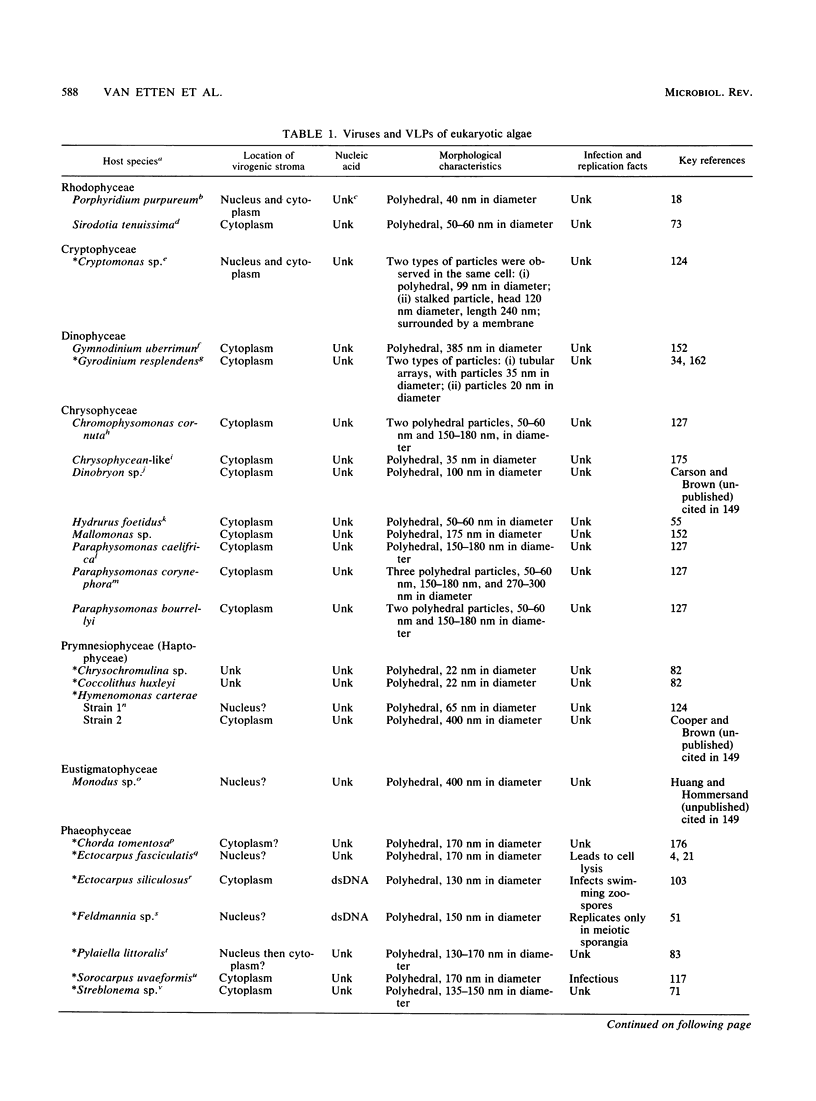
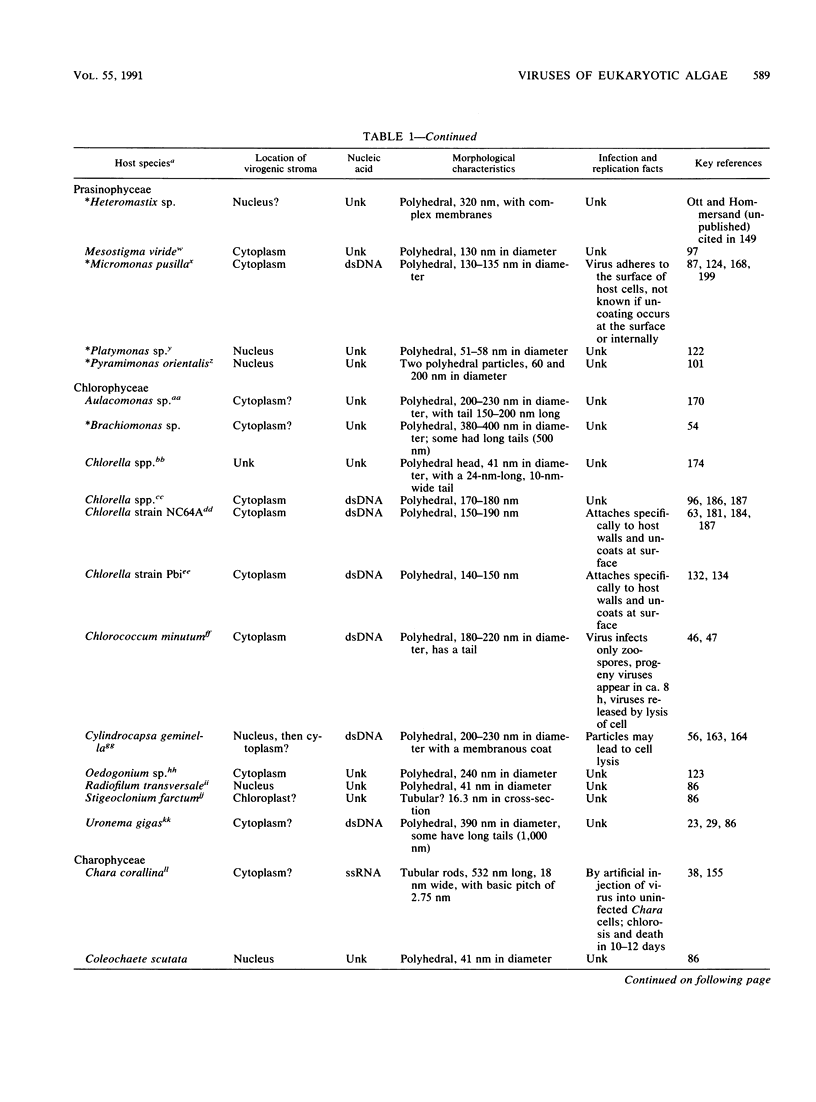
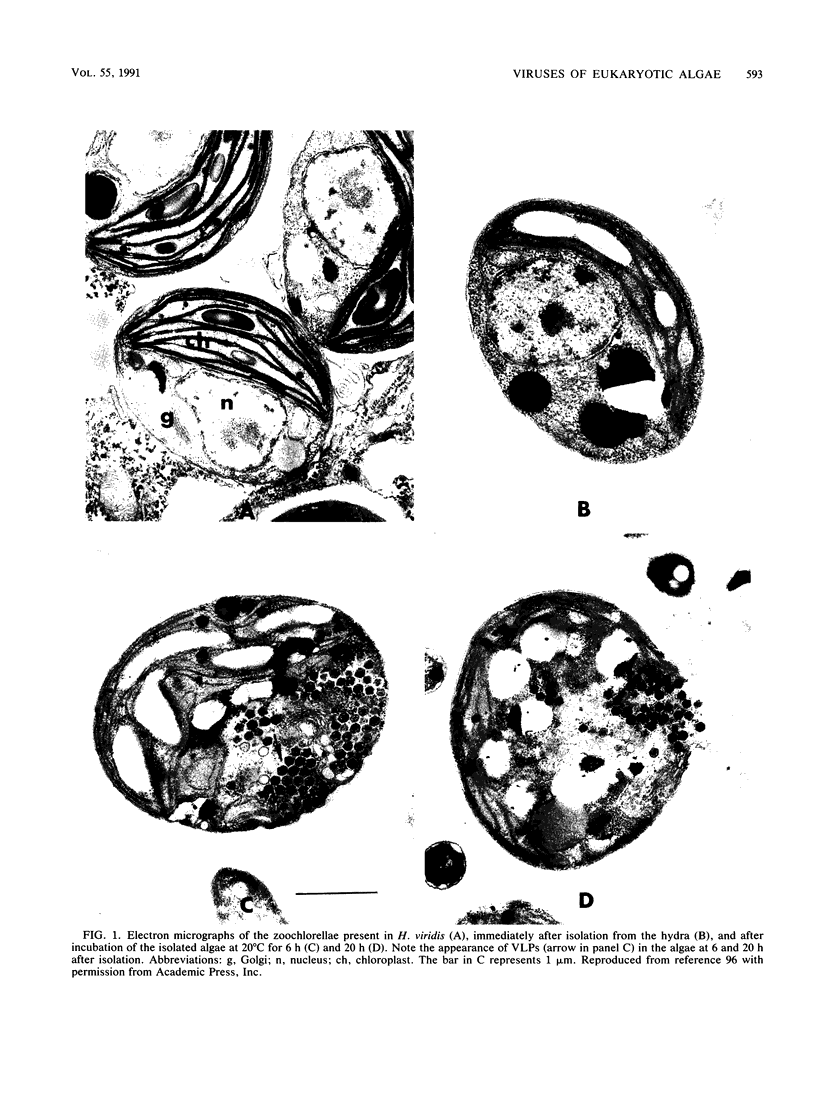
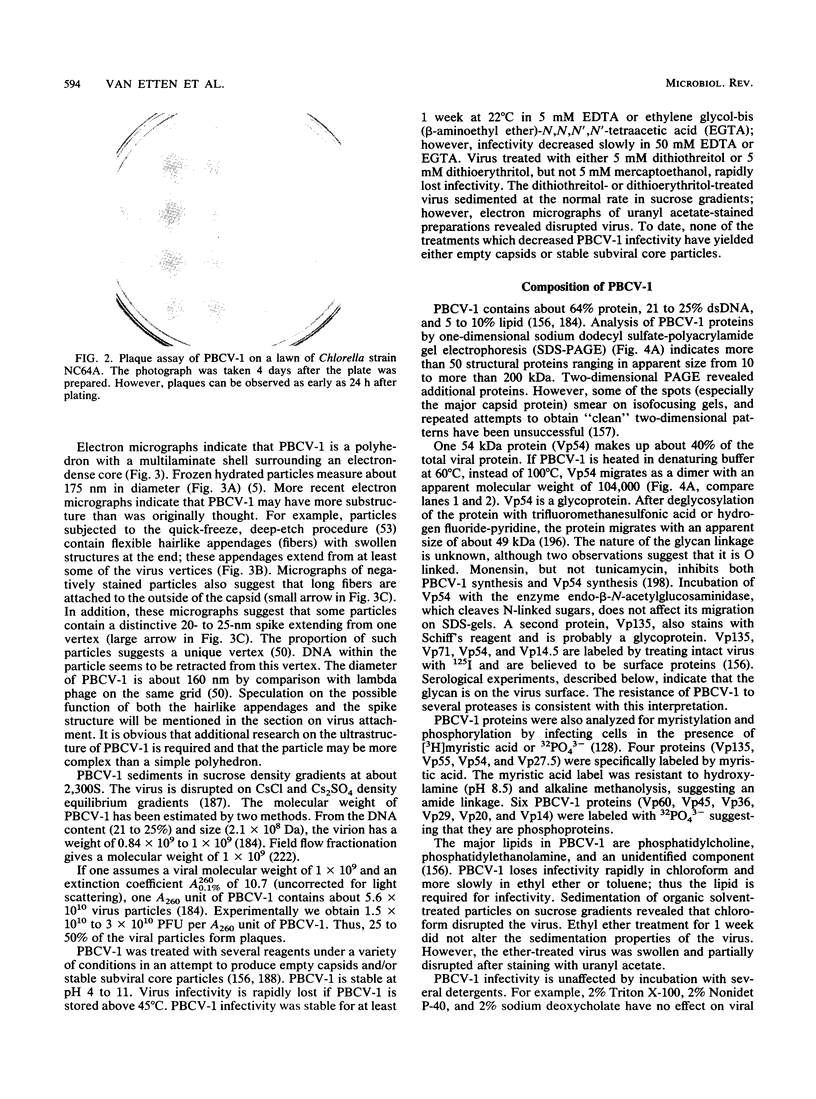
Abstract
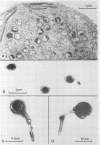
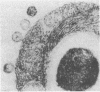
Until recently there was little interest or information on viruses and viruslike particles of eukaryotic algae. However, this situation is changing. In the past decade many large double-stranded DNA-containing viruses that infect two culturable, unicellular, eukaryotic green algae have been discovered. These viruses can be produced in large quantities, assayed by plaque formation, and analyzed by standard bacteriophage techniques. The viruses are structurally similar to animal iridoviruses, their genomes are similar to but larger (greater than 300 kbp) than that of poxviruses, and their infection process resembles that of bacteriophages. Some of the viruses have DNAs with low levels of methylated bases, whereas others have DNAs with high concentrations of 5-methylcytosine and N6-methyladenine. Virus-encoded DNA methyltransferases are associated with the methylation and are accompanied by virus-encoded DNA site-specific (restriction) endonucleases. Some of these enzymes have sequence specificities identical to those of known bacterial enzymes, and others have previously unrecognized specificities. A separate rod-shaped RNA-containing algal virus has structural and nucleotide sequence affinities to higher plant viruses. Quite recently, viruses have been associated with rapid changes in marine algal populations. In the next decade we envision the discovery of new algal viruses, clarification of their role in various ecosystems, discovery of commercially useful genes in these viruses, and exploitation of algal virus genetic elements in plant and algal biotechnology.
Full text
PDF




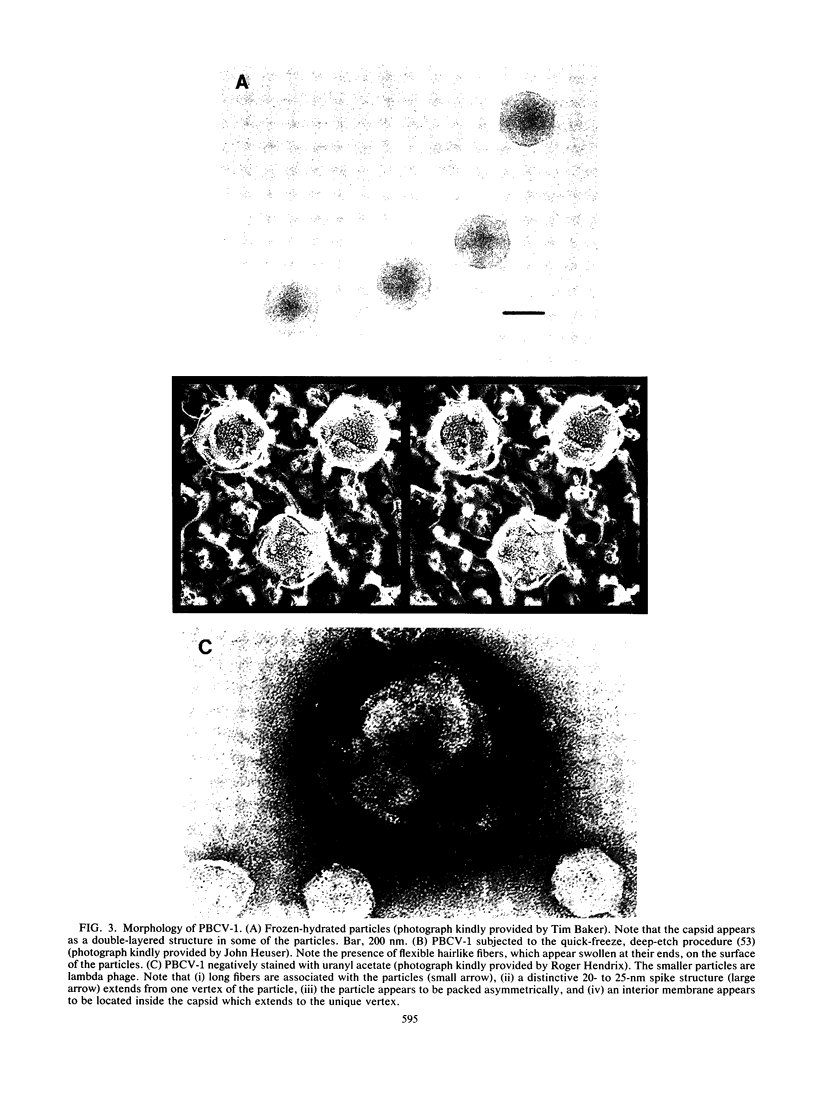
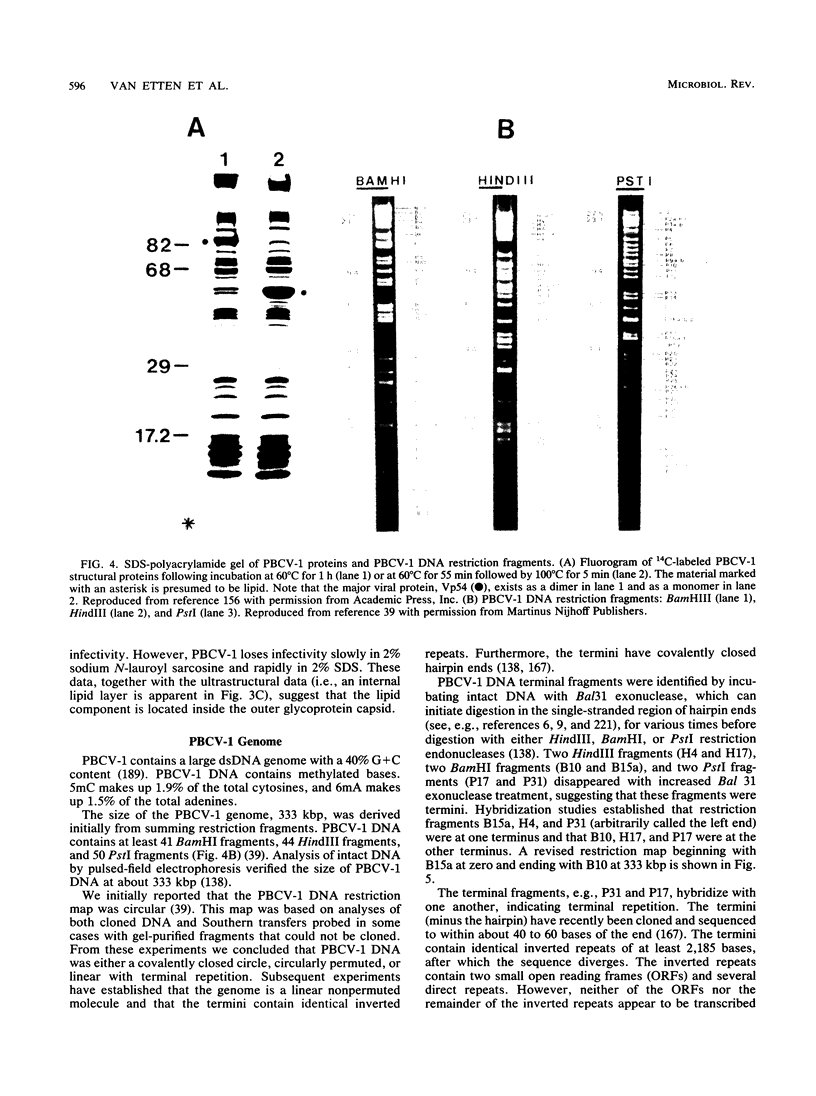


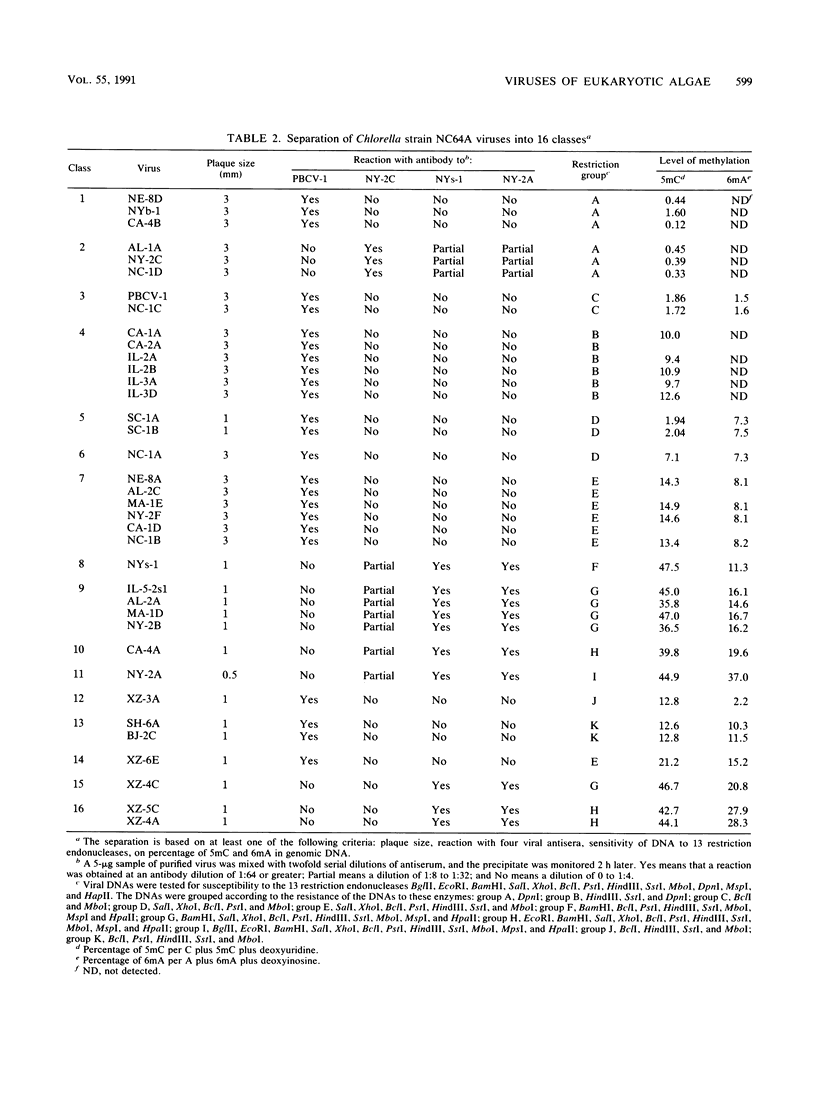
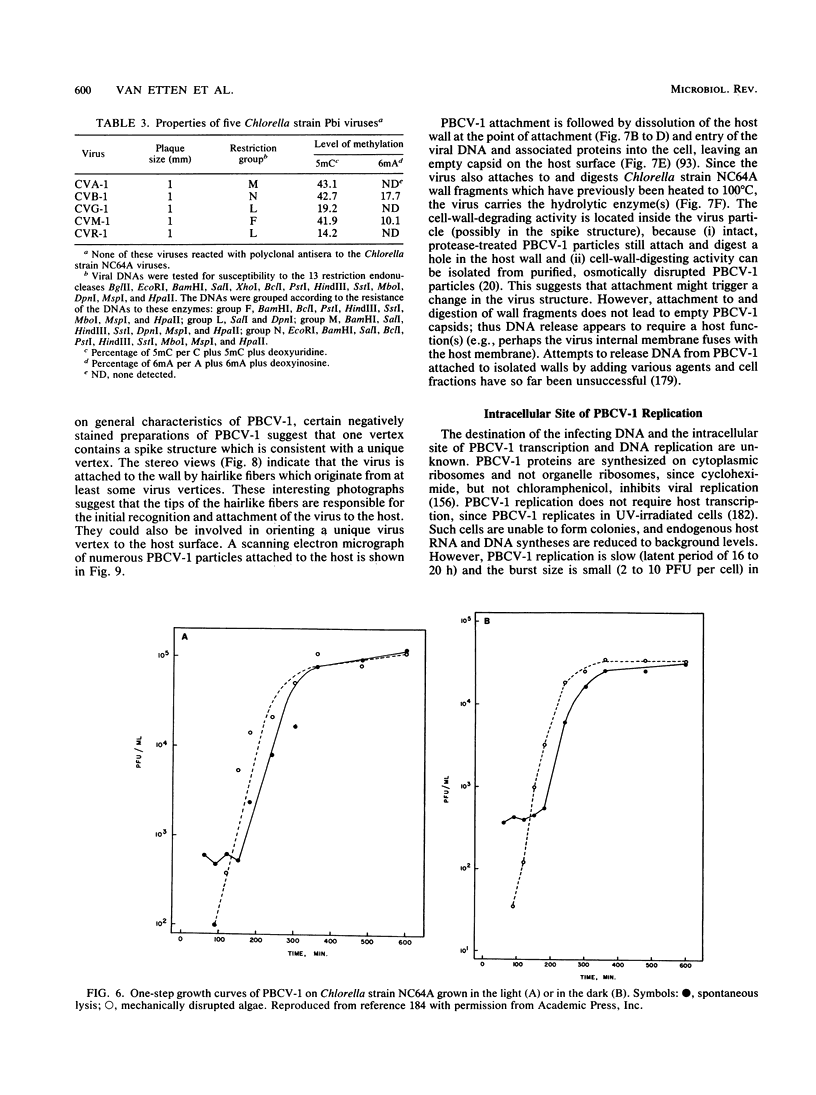





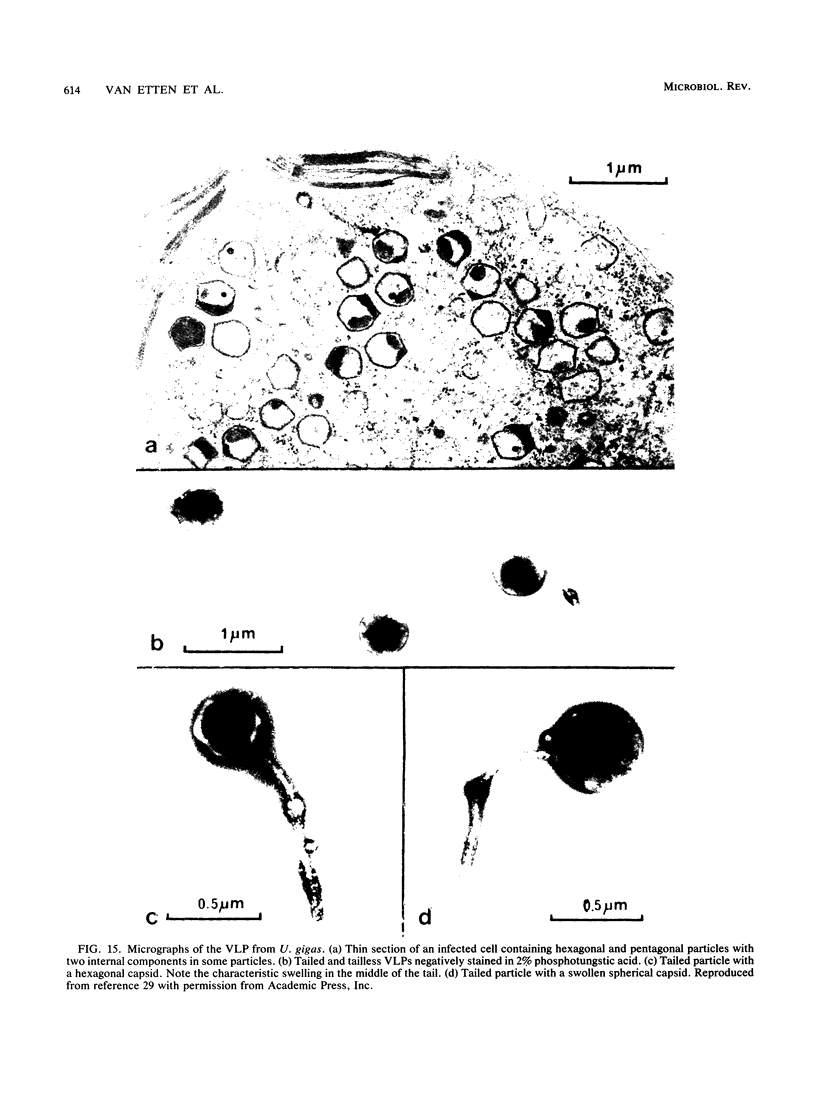
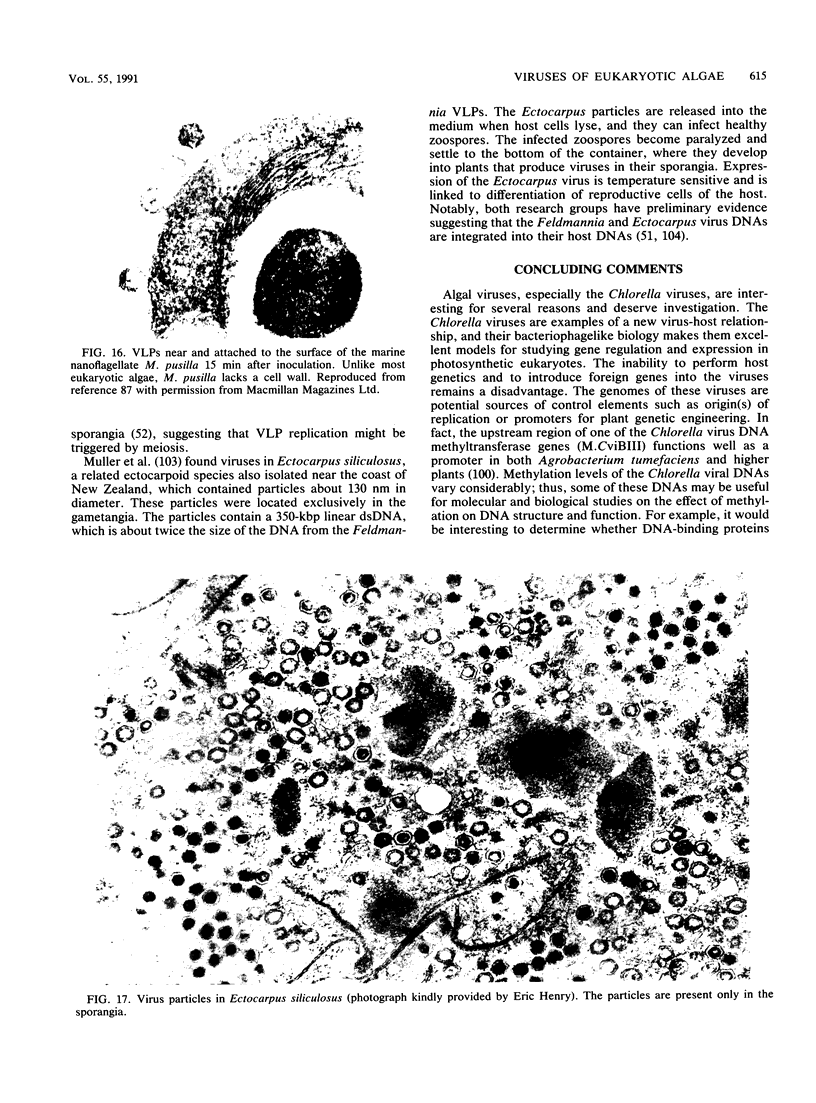





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Bestor T., Laudano A., Mattaliano R., Ingram V. Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. The carboxyl-terminal domain of the mammalian enzymes is related to bacterial restriction methyltransferases. J Mol Biol. 1988 Oct 20;203(4):971–983. doi: 10.1016/0022-2836(88)90122-2. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H., Challoner P. B. Identification of a telomeric DNA sequence in Trypanosoma brucei. Cell. 1984 Feb;36(2):447–457. doi: 10.1016/0092-8674(84)90238-1. [DOI] [PubMed] [Google Scholar]
- Blasco R., de la Vega I., Almazán F., Agüero M., Viñuela E. Genetic variation of African swine fever virus: variable regions near the ends of the viral DNA. Virology. 1989 Nov;173(1):251–257. doi: 10.1016/0042-6822(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Bonnefoy A. M., Kolenkine X., Vago C. Particules d'allure virale chez les hydres. C R Acad Sci Hebd Seances Acad Sci D. 1972 Nov 6;275(19):2163–2165. [PubMed] [Google Scholar]
- Brunt A. A., Richards K. E. Biology and molecular biology of furoviruses. Adv Virus Res. 1989;36:1–32. doi: 10.1016/s0065-3527(08)60581-3. [DOI] [PubMed] [Google Scholar]
- Burbank D. E., Shields S. L., Schuster A. M., Van Etten J. L. 5-Azacytidine-resistant mutants of Chlorella virus IL-3A. Virology. 1990 May;176(1):311–315. doi: 10.1016/0042-6822(90)90261-o. [DOI] [PubMed] [Google Scholar]
- Chase T. E., Nelson J. A., Burbank D. E., Van Etten J. L. Mutual exclusion occurs in a Chlorella-like green alga inoculated with two viruses. J Gen Virol. 1989 Jul;70(Pt 7):1829–1836. doi: 10.1099/0022-1317-70-7-1829. [DOI] [PubMed] [Google Scholar]
- Classification and nomenclature of viruses. Fourth report of the International Committee on Taxonomy of Viruses. Intervirology. 1982;17(1-3):1–199. doi: 10.1159/000149278. [DOI] [PubMed] [Google Scholar]
- Clitheroe S. B., Evans L. V. Viruslike particles in the brown alga Ectocarpus. J Ultrastruct Res. 1974 Nov;49(2):211–217. doi: 10.1016/s0022-5320(74)80032-8. [DOI] [PubMed] [Google Scholar]
- DaSilva E. J., Gyllenberg H. G. A taxonomic treatment of the genus Chlorella by the technique of continuous classification. Arch Mikrobiol. 1972;87(2):99–117. doi: 10.1007/BF00424992. [DOI] [PubMed] [Google Scholar]
- Deom C. M., Schulze I. T. Oligosaccharide composition of an influenza virus hemagglutinin with host-determined binding properties. J Biol Chem. 1985 Nov 25;260(27):14771–14774. [PubMed] [Google Scholar]
- Garon C. F., Barbosa E., Moss B. Visualization of an inverted terminal repetition in vaccinia virus DNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4863–4867. doi: 10.1073/pnas.75.10.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geshelin P., Berns K. I. Characterization and localization of the naturally occurring cross-links in vaccinia virus DNA. J Mol Biol. 1974 Oct 5;88(4):785–796. doi: 10.1016/0022-2836(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Gibbs A., Skotnicki A. H., Gardiner J. E., Walker E. S., Hollings M. A tobamovirus of a green alga. Virology. 1975 Apr;64(2):571–574. doi: 10.1016/0042-6822(75)90136-1. [DOI] [PubMed] [Google Scholar]
- González A., Talavera A., Almendral J. M., Viñuela E. Hairpin loop structure of African swine fever virus DNA. Nucleic Acids Res. 1986 Sep 11;14(17):6835–6844. doi: 10.1093/nar/14.17.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R., Murti K. G. The genome of frog virus 3, an animal DNA virus, is circularly permuted and terminally redundant. Proc Natl Acad Sci U S A. 1982 Jan;79(2):248–252. doi: 10.1073/pnas.79.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R., Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VI. Frog virus 3 replication is dependent on the cell nucleus. J Virol. 1977 Feb;21(2):802–805. doi: 10.1128/jvi.21.2.802-805.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann V., Kessler E. Physiologische und biochemische Beiträge zur Taxonomie der Gattung Chlorella. 8. Die Basenzusammensetzung der DNS. Arch Mikrobiol. 1974 Feb 13;95(4):311–318. [PubMed] [Google Scholar]
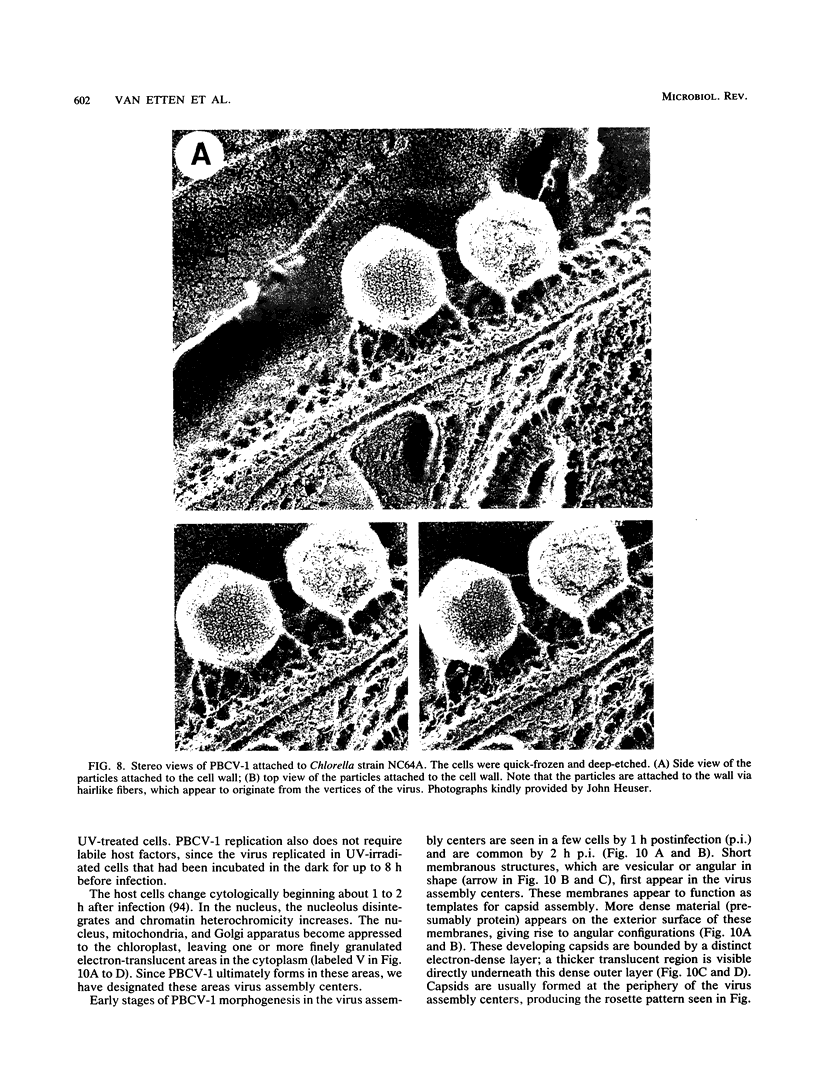
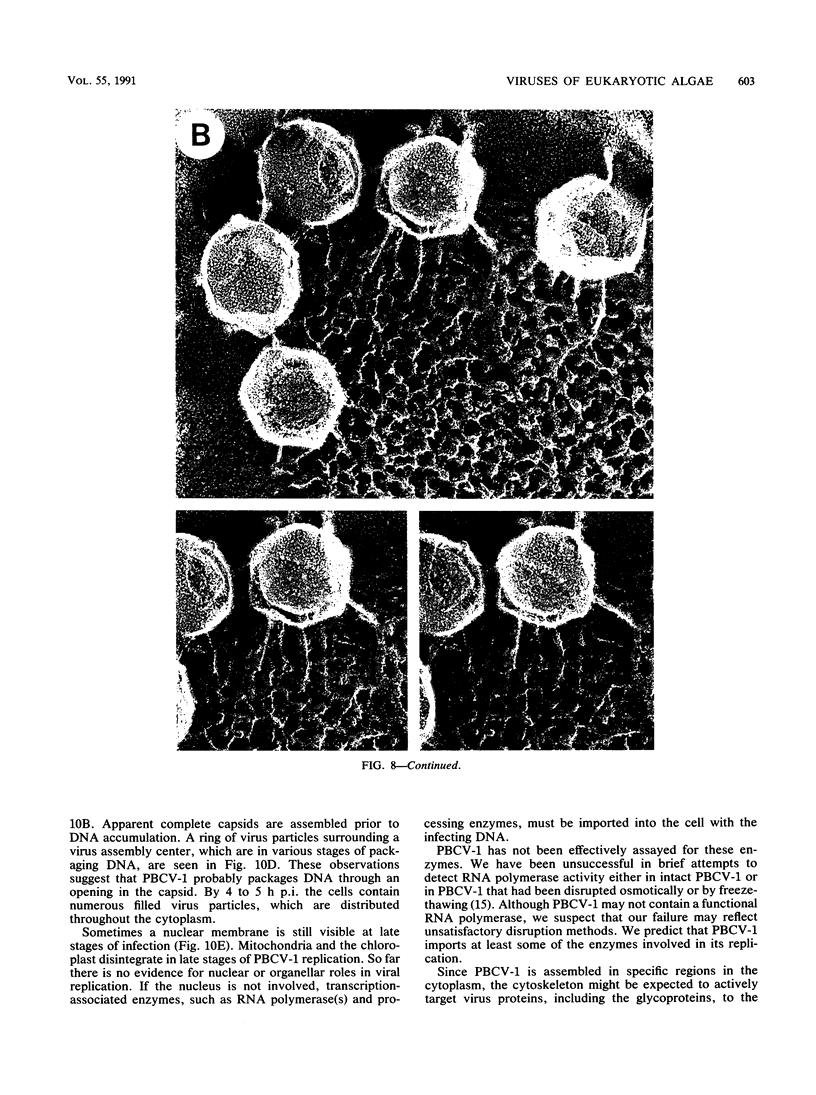
- Heuser J. Three-dimensional visualization of coated vesicle formation in fibroblasts. J Cell Biol. 1980 Mar;84(3):560–583. doi: 10.1083/jcb.84.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houts G. E., Gravell M., Granoff A. Electron microscopic observations on early events of frog virus 3 replication. Virology. 1974 Apr;58(2):589–594. doi: 10.1016/0042-6822(74)90093-2. [DOI] [PubMed] [Google Scholar]
- Karakashian M. W. Symbiosis in Paramecium Bursaria. Symp Soc Exp Biol. 1975;(29):145–173. [PubMed] [Google Scholar]
- Kelly D. C. Frog virus 3 replication: electron microscope observations on the sequence of infection in chick embryo fibroblasts. J Gen Virol. 1975 Jan;26(1):71–86. doi: 10.1099/0022-1317-26-1-71. [DOI] [PubMed] [Google Scholar]
- Klimasauskas S., Timinskas A., Menkevicius S., Butkienè D., Butkus V., Janulaitis A. Sequence motifs characteristic of DNA[cytosine-N4]methyltransferases: similarity to adenine and cytosine-C5 DNA-methylases. Nucleic Acids Res. 1989 Dec 11;17(23):9823–9832. doi: 10.1093/nar/17.23.9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauster R. Evolution of type II DNA methyltransferases. A gene duplication model. J Mol Biol. 1989 Mar 20;206(2):313–321. doi: 10.1016/0022-2836(89)90481-6. [DOI] [PubMed] [Google Scholar]
- Lauster R., Trautner T. A., Noyer-Weidner M. Cytosine-specific type II DNA methyltransferases. A conserved enzyme core with variable target-recognizing domains. J Mol Biol. 1989 Mar 20;206(2):305–312. doi: 10.1016/0022-2836(89)90480-4. [DOI] [PubMed] [Google Scholar]
- Lee R. E. Systemic viral material in the cells of the freshwater red alga Sirodotia tenuissima (Holden) skuja. J Cell Sci. 1971 May;8(3):623–631. doi: 10.1242/jcs.8.3.623. [DOI] [PubMed] [Google Scholar]
- Lemke P. A. Viruses of eucaryotic microorganisms. Annu Rev Microbiol. 1976;30:105–145. doi: 10.1146/annurev.mi.30.100176.000541. [DOI] [PubMed] [Google Scholar]
- Lundström M., Jeansson S., Olofsson S. Host cell-induced differences in the O-glycosylation of herpes simplex virus gC-1. II. Demonstration of cell-specific galactosyltransferase essential for formation of O-linked oligosaccharides. Virology. 1987 Dec;161(2):395–402. doi: 10.1016/0042-6822(87)90132-2. [DOI] [PubMed] [Google Scholar]
- Lundström M., Olofsson S., Jeansson S., Lycke E., Datema R., Månsson J. E. Host cell-induced differences in O-glycosylation of herpes simplex virus gC-1. I. Structures of nonsialylated HPA- and PNA-binding carbohydrates. Virology. 1987 Dec;161(2):385–394. doi: 10.1016/0042-6822(87)90131-0. [DOI] [PubMed] [Google Scholar]
- Luo L. Z., Li Y., Snyder R. M., Wagner R. R. Spontaneous mutations leading to antigenic variations in the glycoproteins of vesicular stomatitis virus field isolates. Virology. 1990 Jan;174(1):70–78. doi: 10.1016/0042-6822(90)90055-v. [DOI] [PubMed] [Google Scholar]
- Mann M. B., Rao R. N., Smith H. O. Cloning of restriction and modification genes in E. coli: the HbaII system from Haemophilus haemolyticus. Gene. 1978 Apr;3(2):97–112. doi: 10.1016/0378-1119(78)90054-9. [DOI] [PubMed] [Google Scholar]
- Markey D. R. A possible virus infection in the brown alga Pylaiella littoralis. Protoplasma. 1974;80(1):223–232. doi: 10.1007/BF01666361. [DOI] [PubMed] [Google Scholar]
- Mattox K. R., Stewart K. D. Probably virus infections in four genera of green algae. Can J Microbiol. 1972 Oct;18(10):1620–1621. doi: 10.1139/m72-249. [DOI] [PubMed] [Google Scholar]
- McAuley P. J., Muscatine L. The cell cycle of symbiotic Chlorella. IV. DNA content of algae slowly increases during host starvation of green hydra. J Cell Sci. 1986 Sep;85:73–84. doi: 10.1242/jcs.85.1.73. [DOI] [PubMed] [Google Scholar]
- Meints R. H., Burbank D. E., Van Etten J. L., Lamport D. T. Properties of the Chlorella receptor for the virus PBCV-1. Virology. 1988 May;164(1):15–21. doi: 10.1016/0042-6822(88)90614-9. [DOI] [PubMed] [Google Scholar]
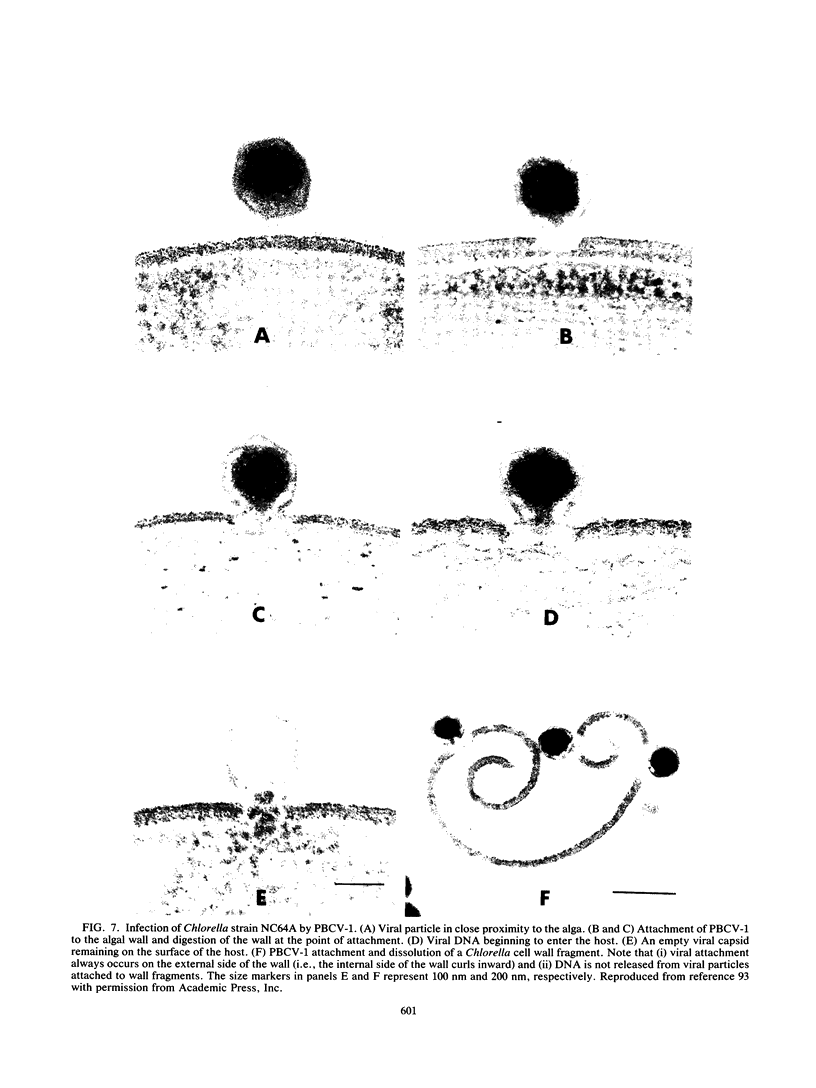
- Meints R. H., Lee K., Burbank D. E., Van Etten J. L. Infection of a Chlorella-like alga with the virus, PBCV-1: ultrastructural studies. Virology. 1984 Oct 30;138(2):341–346. doi: 10.1016/0042-6822(84)90358-1. [DOI] [PubMed] [Google Scholar]
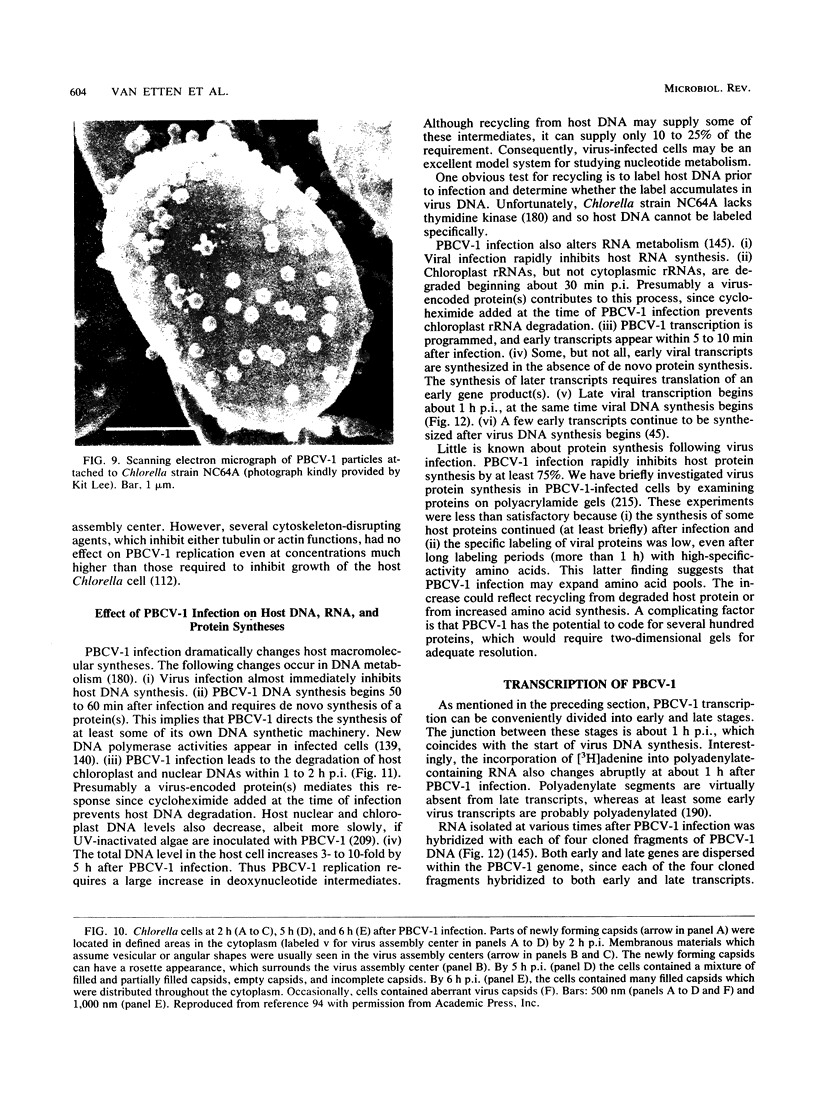
- Meints R. H., Lee K., Van Etten J. L. Assembly site of the virus PBCV-1 in a Chlorella-like green alga: ultrastructural studies. Virology. 1986 Oct 15;154(1):240–245. doi: 10.1016/0042-6822(86)90448-4. [DOI] [PubMed] [Google Scholar]
- Meints R. H., Pardy R. L. Quantitative demonstration of cell surface involvement in a plant-animal symbiosis: lectin inhibition of reassociation. J Cell Sci. 1980 Jun;43:239–251. doi: 10.1242/jcs.43.1.239. [DOI] [PubMed] [Google Scholar]
- Moss B. Regulation of vaccinia virus transcription. Annu Rev Biochem. 1990;59:661–688. doi: 10.1146/annurev.bi.59.070190.003305. [DOI] [PubMed] [Google Scholar]
- Muscatine L., Cook C. B., Pardy R. L., Pool R. R. Uptake, recognition and maintenance of symbiotic Chlorella by Hydra viridis. Symp Soc Exp Biol. 1975;(29):175–203. [PubMed] [Google Scholar]
- NORTHCOTE D. H., GOULDING K. J., HORNE R. W. The chemical composition and structure of the cell wall of Chlorella pyrenoidosa. Biochem J. 1958 Nov;70(3):391–397. doi: 10.1042/bj0700391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Host cell- and virus strain-dependent differences in oligosaccharides of hemagglutinin glycoproteins of influenza A viruses. Virology. 1979 May;95(1):8–23. doi: 10.1016/0042-6822(79)90397-0. [DOI] [PubMed] [Google Scholar]
- Narva K. E., Van Etten J. L., Slatko B. E., Benner J. S. The amino acid sequence of the eukaryotic DNA [N6-adenine]methyltransferase, M.CviBIII, has regions of similarity with the prokaryotic isoschizomer M.TaqI and other DNA [N6-adenine] methyltransferases. Gene. 1988 Dec 25;74(1):253–259. doi: 10.1016/0378-1119(88)90298-3. [DOI] [PubMed] [Google Scholar]
- Narva K. E., Wendell D. L., Skrdla M. P., Van Etten J. L. Molecular cloning and characterization of the gene encoding the DNA methyltransferase, M.CviBIII, from Chlorella virus NC-1A. Nucleic Acids Res. 1987 Dec 10;15(23):9807–9823. doi: 10.1093/nar/15.23.9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortín J., Enjuanes L., Viñuela E. Cross-links in African swine fever virus DNA. J Virol. 1979 Sep;31(3):579–583. doi: 10.1128/jvi.31.3.579-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardy R. L. Some factors affecting the growth and distribution of the algal endosymbionts of Hydraviridis. Biol Bull. 1974 Aug;147(1):105–118. doi: 10.2307/1540572. [DOI] [PubMed] [Google Scholar]
- Pool R. R., Jr The role of algal antigenic determinants in the recognition of potential algal symbionts by cells of Chlorohydra. J Cell Sci. 1979 Feb;35:367–379. doi: 10.1242/jcs.35.1.367. [DOI] [PubMed] [Google Scholar]
- Pósfai J., Bhagwat A. S., Pósfai G., Roberts R. J. Predictive motifs derived from cytosine methyltransferases. Nucleic Acids Res. 1989 Apr 11;17(7):2421–2435. doi: 10.1093/nar/17.7.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisser W., Burbank D. E., Meints S. M., Meints R. H., Becker B., Van Etten J. L. A comparison of viruses infecting two different Chlorella-like green algae. Virology. 1988 Nov;167(1):143–149. doi: 10.1016/0042-6822(88)90063-3. [DOI] [PubMed] [Google Scholar]
- Rohozinski J., Girton L. E., Van Etten J. L. Chlorella viruses contain linear nonpermuted double-stranded DNA genomes with covalently closed hairpin ends. Virology. 1989 Feb;168(2):363–369. doi: 10.1016/0042-6822(89)90277-8. [DOI] [PubMed] [Google Scholar]
- Rohozinski J., Van Etten J. L. Characterization of DNA polymerases in an uninfected and virus PBCV-1-infected green alga--Chlorella strain NC64A. Intervirology. 1989;30(3):156–162. doi: 10.1159/000150087. [DOI] [PubMed] [Google Scholar]
- SIEGEL R. W. Hereditary endosymbiosis in Paramecium bursaria. Exp Cell Res. 1960 Mar;19:239–252. doi: 10.1016/0014-4827(60)90005-7. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Domains of virus glycoproteins. Adv Virus Res. 1987;33:1–44. doi: 10.1016/S0065-3527(08)60315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnepf E., Soeder C. J., Hegewald E. Polyhedral viruslike particles lysing the aquatic phycomycete Aphelidium sp., a parasite of the green alga Scenedesmus armatus. Virology. 1970 Oct;42(2):482–487. doi: 10.1016/0042-6822(70)90291-6. [DOI] [PubMed] [Google Scholar]
- Schuster A. M., Burbank D. E., Meister B., Skrdla M. P., Meints R. H., Hattman S., Swinton D., Van Etten J. L. Characterization of viruses infecting a eukaryotic Chlorella-like green alga. Virology. 1986 Apr 15;150(1):170–177. doi: 10.1016/0042-6822(86)90276-x. [DOI] [PubMed] [Google Scholar]
- Schuster A. M., Girton L., Burbank D. E., Van Etten J. L. Infection of a Chlorella-like alga with the virus PBCV-1: transcriptional studies. Virology. 1986 Jan 15;148(1):181–189. doi: 10.1016/0042-6822(86)90413-7. [DOI] [PubMed] [Google Scholar]
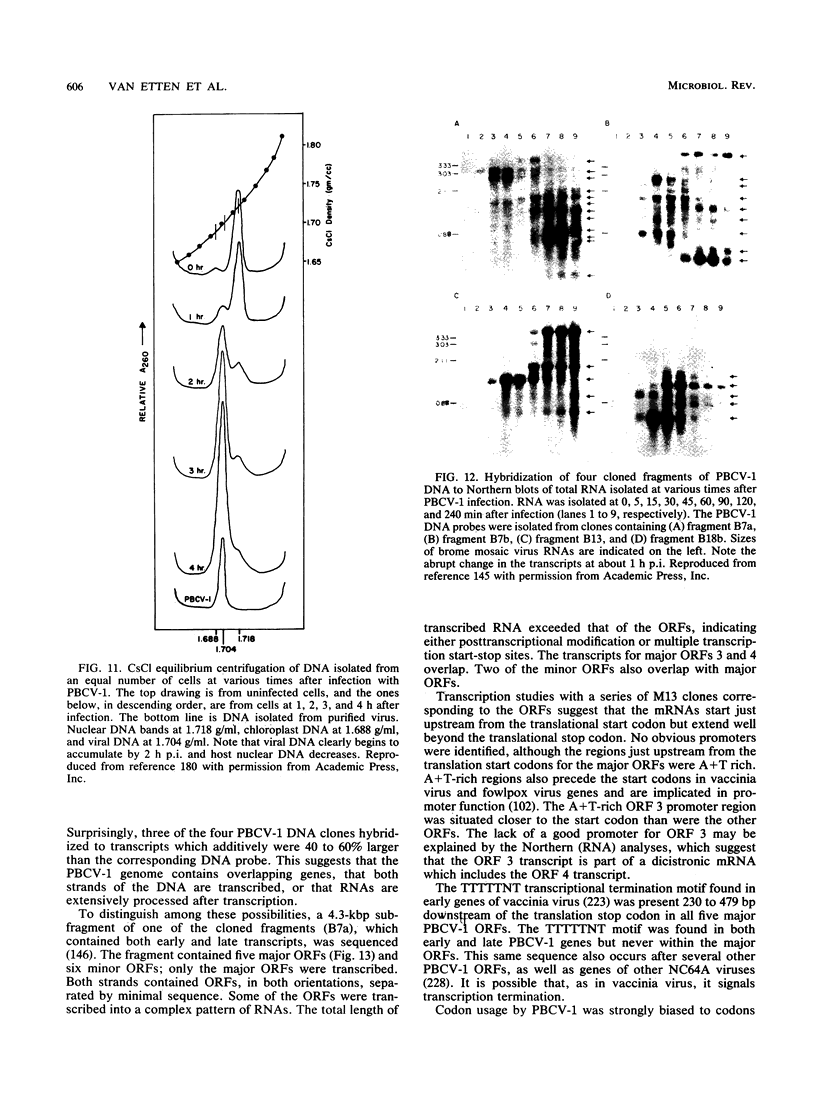
- Schuster A. M., Graves M., Korth K., Ziegelbein M., Brumbaugh J., Grone D., Meints R. H. Transcription and sequence studies of a 4.3-kbp fragment from a ds-DNA eukaryotic algal virus. Virology. 1990 Jun;176(2):515–523. doi: 10.1016/0042-6822(90)90021-i. [DOI] [PubMed] [Google Scholar]
- Schuster A. M., Waddle J. A., Korth K., Meints R. H. The chloroplast genome of an exsymbiotic Chlorella-like green alga. Plant Mol Biol. 1990 May;14(5):859–862. doi: 10.1007/BF00016519. [DOI] [PubMed] [Google Scholar]
- Shields S. L., Burbank D. E., Grabherr R., van Etten J. L. Cloning and sequencing the cytosine methyltransferase gene M. CviJI from Chlorella virus IL-3A. Virology. 1990 May;176(1):16–24. doi: 10.1016/0042-6822(90)90225-g. [DOI] [PubMed] [Google Scholar]
- Skotnicki A., Gibbs A., Wrigley N. G. Further studies on Chara corallina virus. Virology. 1976 Dec;75(2):457–468. doi: 10.1016/0042-6822(76)90043-x. [DOI] [PubMed] [Google Scholar]
- Skrdla M. P., Burbank D. E., Xia Y., Meints R. H., Van Etten J. L. Structural proteins and lipids in a virus, PBCV-1, which replicates in a Chlorella-like alga. Virology. 1984 Jun;135(2):308–315. doi: 10.1016/0042-6822(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Slatko B. E., Benner J. S., Jager-Quinton T., Moran L. S., Simcox T. G., Van Cott E. M., Wilson G. G. Cloning, sequencing and expression of the Taq I restriction-modification system. Nucleic Acids Res. 1987 Dec 10;15(23):9781–9796. doi: 10.1093/nar/15.23.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Almendral J. M., Talavera A., Viñuela E. Terminal and internal inverted repetitions in African swine fever virus DNA. Virology. 1984 Mar;133(2):271–275. doi: 10.1016/0042-6822(84)90394-5. [DOI] [PubMed] [Google Scholar]
- Stefan C., Xia Y. N., Van Etten J. L. Molecular cloning and characterization of the gene encoding the adenine methyltransferase M.CviRI from Chlorella virus XZ-6E. Nucleic Acids Res. 1991 Jan 25;19(2):307–311. doi: 10.1093/nar/19.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser P., Zhang Y. P., Rohozinski J., Van Etten J. L. The termini of the chlorella virus PBCV-1 genome are identical 2.2-kbp inverted repeats. Virology. 1991 Feb;180(2):763–769. doi: 10.1016/0042-6822(91)90089-t. [DOI] [PubMed] [Google Scholar]
- Swale E. M., Belcher J. H. A light and electron microscope study of the colourless flagellate aulacomonas skuja. Arch Mikrobiol. 1973;92(2):91–103. doi: 10.1007/BF00425007. [DOI] [PubMed] [Google Scholar]
- Tikhonenko A. S., Zavarzina N. B. Morfologiia liticheskogo agenta Chlorella pyrenoidosa. Mikrobiologiia. 1966 Sep-Oct;35(5):850–852. [PubMed] [Google Scholar]
- VAN Etten J. L., Burbank D. E., Kuczmarski D., Meints R. H. Virus infection of culturable chlorella-like algae and dlevelopment of a plaque assay. Science. 1983 Feb 25;219(4587):994–996. doi: 10.1126/science.219.4587.994. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Burbank D. E., Schuster A. M., Meints R. H. Lytic viruses infecting a Chlorella-like alga. Virology. 1985 Jan 15;140(1):135–143. doi: 10.1016/0042-6822(85)90452-0. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Meints R. H., Kuczmarski D., Burbank D. E., Lee K. Viruses of symbiotic Chlorella-like algae isolated from Paramecium bursaria and Hydra viridis. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3867–3871. doi: 10.1073/pnas.79.12.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Schuster A. M., Girton L., Burbank D. E., Swinton D., Hattman S. DNA methylation of viruses infecting a eukaryotic Chlorella-like green alga. Nucleic Acids Res. 1985 May 24;13(10):3471–3478. doi: 10.1093/nar/13.10.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Van Etten C. H., Johnson J. K., Burbank D. E. A survey for viruses from fresh water that infect a eucaryotic chlorella-like green alga. Appl Environ Microbiol. 1985 May;49(5):1326–1328. doi: 10.1128/aem.49.5.1326-1328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddle J. A., Schuster A. M., Lee K. W., Meints R. H. The mitochondrial genome of an exsymbiotic Chlorella-like green alga. Plant Mol Biol. 1990 Feb;14(2):187–195. doi: 10.1007/BF00018559. [DOI] [PubMed] [Google Scholar]
- Weis D. S. Synchronous development of symbiotic chlorellae within Paramecium bursaria. Trans Am Microsc Soc. 1977 Jan;96(1):82–86. [PubMed] [Google Scholar]
- Wiggins B. A., Alexander M. Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl Environ Microbiol. 1985 Jan;49(1):19–23. doi: 10.1128/aem.49.1.19-23.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Granoff A. DNA methyltransferase induced by frog virus 3. J Virol. 1984 Jan;49(1):86–91. doi: 10.1128/jvi.49.1.86-91.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Frog virus 3 DNA is heavily methylated at CpG sequences. Virology. 1980 Nov;107(1):250–257. doi: 10.1016/0042-6822(80)90290-1. [DOI] [PubMed] [Google Scholar]
- Wilson G. G. Type II restriction--modification systems. Trends Genet. 1988 Nov;4(11):314–318. doi: 10.1016/0168-9525(88)90109-6. [DOI] [PubMed] [Google Scholar]
- Wittek R., Menna A., Müller H. K., Schümperli D., Boseley P. G., Wyler R. Inverted terminal repeats in rabbit poxvirus and vaccinia virus DNA. J Virol. 1978 Oct;28(1):171–181. doi: 10.1128/jvi.28.1.171-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Moss B. Tandem repeats within the inverted terminal repetition of vaccinia virus DNA. Cell. 1980 Aug;21(1):277–284. doi: 10.1016/0092-8674(80)90135-x. [DOI] [PubMed] [Google Scholar]
- Xia Y. N., Burbank D. E., Uher L., Rabussay D., Van Etten J. L. IL-3A virus infection of a Chlorella-like green alga induces a DNA restriction endonuclease with novel sequence specificity. Nucleic Acids Res. 1987 Aug 11;15(15):6075–6090. doi: 10.1093/nar/15.15.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. N., Burbank D. E., Uher L., Rabussay D., Van Etten J. L. Restriction endonuclease activity induced by PBCV-1 virus infection of a Chlorella-like green alga. Mol Cell Biol. 1986 May;6(5):1430–1439. doi: 10.1128/mcb.6.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. N., Morgan R., Schildkraut I., Van Etten J. L. A site-specific single strand endonuclease activity induced by NYs-1 virus infection of a Chlorella-like green alga. Nucleic Acids Res. 1988 Oct 25;16(20):9477–9487. doi: 10.1093/nar/16.20.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. N., Narva K. E., Van Etten J. L. The cleavage site of the RsaI isoschizomer, CviII, is G decreases TAC. Nucleic Acids Res. 1987 Dec 10;15(23):10063–10063. doi: 10.1093/nar/15.23.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. N., Van Etten J. L. DNA methyltransferase induced by PBCV-1 virus infection of a Chlorella-like green alga. Mol Cell Biol. 1986 May;6(5):1440–1445. doi: 10.1128/mcb.6.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Burbank D. E., Van Etten J. L. Restriction endonuclease activity induced by NC-1A virus infection of a Chlorella-like green alga. Nucleic Acids Res. 1986 Aug 11;14(15):6017–6030. doi: 10.1093/nar/14.15.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T. Isolation and Characterization of Chloroplast DNA from Chlorella ellipsoidea. Plant Physiol. 1982 Jul;70(1):92–96. doi: 10.1104/pp.70.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Repeated hexanucleotide C-C-C-C-A-A is present near free ends of macronuclear DNA of Tetrahymena. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7436–7439. doi: 10.1073/pnas.78.12.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonker C. R., Caldwell K. D., Giddings J. C., Van Etten J. L. Physical characterization of PBCV virus by sedimentation field flow fractionation. J Virol Methods. 1985 Jun;11(2):145–160. doi: 10.1016/0166-0934(85)90038-2. [DOI] [PubMed] [Google Scholar]
- Yuen L., Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. P., Burbank D. E., Van Etten J. L. Chlorella viruses isolated in China. Appl Environ Microbiol. 1988 Sep;54(9):2170–2173. doi: 10.1128/aem.54.9.2170-2173.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]