Abstract
Purpose
The purpose of this study was to evaluate the efficacy of multiple platelet-rich plasma (PRP) injections on the healing of chronic refractory patellar tendinopathy, and report the quality and duration of the clinical improvement up to a medium-term follow-up.
Methods
Forty-three patients (mean age, 30.6 years; mean BMI, 24.7; 42 men, one woman) affected by chronic patellar proximal tendinopathy were enrolled in this trial. Eleven patients were affected by bilateral tendinopathy. They underwent three ultrasound guided intra-tendinous injections of five millilitres PRP, two weeks apart from each other. Patients were prospectively evaluated initially, then after two, six, and up to mean 48.6 ± 8.1 months of follow-up (minimum evaluation at 36 months). The following evaluation tools were used: Blanzina, VISA-P, EQ-VAS for general health, and Tegner scores. Patients’ overall satisfaction and time to return to sport were also reported.
Results
Good and stable results were documented over time, with the VISA-P score increasing from 44.1 ± 15.6 at baseline to 61.4 ± 22.2 at two months, 76.6 ± 25.4 at six months, and 84.3 ± 21.6 at four years’ follow-up. The same trend was confirmed by the other scores used, and 80 % of the patients were satisfied and returned to previous sports activities. Significantly poorer results were obtained in patients with a longer history of symptoms, and poor results were also observed in bilateral lesions. No correlation between ultrasonographic and clinical findings could be found.
Conclusions
Multiple injections of PRP provided a good clinical outcome for the treatment of chronic recalcitrant patellar tendinopathy with stable results up to medium-term follow-up. Patients affected by bilateral pathology and presenting a long history of pain obtained significantly poorer results.
Keywords: Platelet rich plasma, Patellar tendinopathy, Ultrasound guidance, Intra tendon injections
Introduction
Patellar tendinopathy, also known as jumper’s knee, is a painful and often chronic condition that affects athletes in many sports, particularly elite athletes engaged in explosive jumping activities, but it is also common in the sedentary population and the workplace [1, 2]. It most commonly occurs near the inferior pole of the patella, where it appears as a failed/slow healing response to overuse injury—the healing response is impeded by recurring microtrauma, thus resulting in a degenerated tissue with compromised functional properties and disabling symptoms [3, 4]. The average duration of substantial pain problems and reduced function is nearly three years and at 15 years’ follow-up 53 % of the subjects reported quitting their sports career due to the damaged knee, with symptoms persisting also after that [2, 5].
Various therapeutic approaches have been adopted for the treatment of patellar tendinopathy including physical training, in particular eccentric protocols, which is supported by evidence in the literature, thus appearing to be the treatment of choice. However, other treatment approaches are also independently or concurrently applied to favour healing of this challenging pathological condition, such as electrotherapy, massage, taping, anti-inflammatory medication or corticosteroid injections, extra-corporeal shock-wave therapy, sclerosing injections, and in difficult cases even surgery can be considered [5–9]. Nevertheless, results are often not satisfactory and recovery is slow and sometimes incomplete, thus explaining the interest in novel treatment strategies.
Modulation of bioactive factors in the diseased tendon may increase the potential for tissue regeneration [10, 11], and many of the growth factors contained in the platelet alpha granules have been shown to play a role in tendon healing [12, 13]. Thus, platelet-rich plasma (PRP) has been developed in recent years with the aim of concentrating these bioactive molecules in physiological proportions, and its use as minimally invasive injection treatment is growing rapidly. However, the increasing focus on this biological treatment approach is not motivated by sound evidence, and the current literature only provides a few low-level reports on its application for patellar tendinopathy [14]. Promising preliminary results still need to be supported and documented in the literature for a better understanding of the safety and potential of PRP.
The purpose of this study was to evaluate the therapeutic effects of multiple PRP injections to favour the healing of chronic patellar tendinopathy, by showing the quality and duration of the clinical improvement up to a medium-term follow-up in a large cohort of patients. A secondary aim was to evaluate the changes in neovascularisation level induced by PRP injections and its correlation with the clinical findings.
Materials and methods
Patient selection
Clinical experimentation was approved by the Hospital Ethics Committee and informed consent of all patients was obtained. Forty-three patients (mean age, 30.6 ± 11.7; mean BMI, 24.7 ± 3.0; 42 men, one woman) affected by chronic patellar proximal tendinopathy were enrolled in this trial. Inclusion criteria were: history (more than three months) of exercise-associated pain, pain or tenderness on palpation and imaging findings (MRI or US scans) of degenerative changes in the patellar tendon. Eleven patients were affected by bilateral tendinopathy, therefore 54 tendons in total were treated. The Blanzina score evaluation was 3a or 3b for every patient included in the study. All patients had previously undergone unsuccessful conservative or surgical management.
PRP preparation technique and treatment protocol
The procedure consisted of two centrifugations (the first at 1,480 rpm for six minutes to separate the erythrocytes, and the second at 3,400 rpm for 15 minutes to concentrate the platelets) of a 150-ml venous blood sample to produce four small units of five millilitres PRP each. One unit was sent to the laboratory for quality control, one unit was used for the first injection within two hours, and the other two units were stored at −30 °C. The treatment consisted of three intra-tendinous injections of five millilitres PRP, two weeks apart from each other. For the second and third treatments the samples were thawed in a dry thermostat at 37 °C for 30 minutes just before application. Before each injection, 10 % Ca-chloride (Ca++ = 0.22 mEq x dose) was added to the PRP concentrate to activate the platelets. The patients were placed in the supine position with their knee slightly bent. After skin preparation, a single skin injection with a 22-g needle was performed under US control (Siemens Acuson Antares™ ultrasound system, Premium Edition; CH6-2 Convex Array Probe), injecting PRP directly into the lesion site with multiple penetrations of the tendon. After the second injection the patients were instructed to start a rehabilitation program based on eccentric exercises to be carried out for about 12 weeks. Then, a gradual return to sporting activity was started, encouraging patients to perform stretching and eccentric exercises on a constant basis as a part of their training to prevent relapse.
Follow-up evaluation
Patients were prospectively evaluated initially, then after two, six, and up to a mean 48.6 ± 8.1 months of follow-up (minimum evaluation at 36 months). The following evaluation tools were used: Blanzina [15], VISA-P, EQ-VAS for general health, and Tegner scores. Patients’ overall satisfaction and time to return to sport were also reported. Patients who underwent surgical treatment were considered as failures and the last scores before the operation were used for the analysis at the subsequent follow-ups.
Furthermore, 26 of these tendons also underwent ultrasonographic (US) evaluation to assess changes in both tendon thickness and vascularisation over time; due to failure and subsequent surgical treatment in four patients (two bilateral), only 20 tendons were available for the complete US evaluation over time. US measurements were performed by a radiologist trained in musculoskeletal US who employed the same instrument both for injections and follow-up visits (the evaluation was performed before the first injection, before the third injection, at six months and at a last mean follow-up of 25.2 ± 7.8 months). Tendon thickness was measured at the site of maximum thickness in the proximal third of the patellar tendon viewed in the sagittal plane. Vascularisation was measured according to the method described by Cook et al. [16].
Statistical methods
All continuous data are expressed in terms of mean ± standard deviation (SD), categorical variables are expressed as proportions or percentages. The Kolmogorov Smirnov test was performed to test normality of continuous variables. Repeated measures GLM with post hoc Sidak correction for multiple comparisons was performed to compare normally distributed scores at the different follow-up times. The Friedman non parametric test with Wilcoxon post hoc test with Holm correction for multiple comparisons was performed to compare not normally distributed scores at the different follow-up times. The ANOVA test was performed to assess the between groups differences of continuous and normally distributed and homoscedastic data, and the Mann Whitney test was used otherwise. The Spearman rank correlation was used to assess correlation between continuous and rank data.
Fisher's chi-square test was performed to investigate the relationships between grouping variables. For all tests p < 0.05 was considered significant.
Results
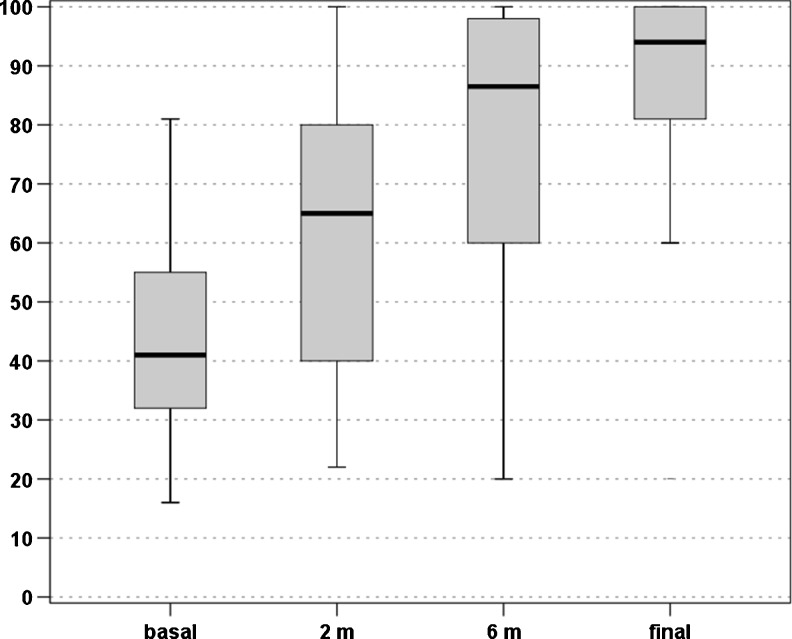
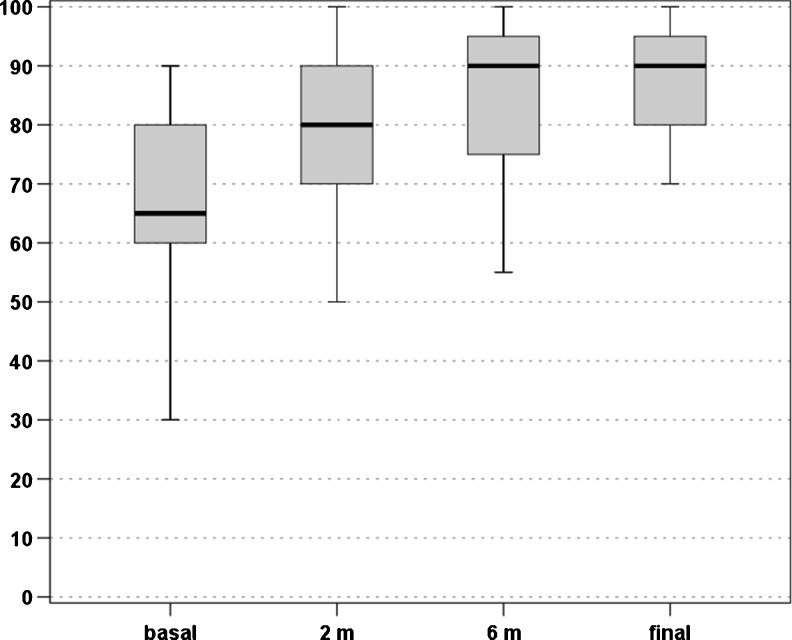
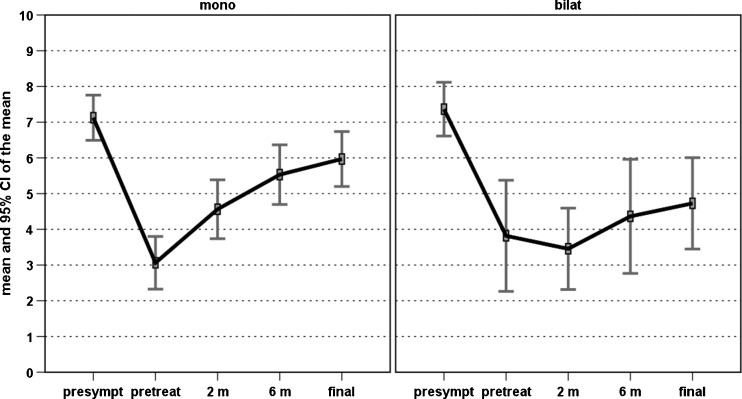
An overall improvement of the scores evaluated was found over time. In particular, the VISA-P score increased from 44.1 ± 15.6 at baseline to 61.4 ± 22.2 at two months, 76.6 ± 25.4 at six months, and 84.3 ± 21.6 at four years, with a significant improvement at every follow-up (p < 0.0005, p < 0.0005, and p = 0.028, respectively) (Fig. 1). The EQ-VAS score increased from 67.8 ± 14.7 at baseline to 78.3 ± 15.1 at two months, 83.5 ± 15.2 at six months, and 85.2 ± 13.7 at four years, with a significant improvement over time (p < 0.0005 at two and six months, then stable results up to four years) (Fig. 2). Patients were classified before the treatment as Blanzina 3a and 3b in 16 and 27 cases, respectively, 15 grade 0, 14 grade 1, three grade 2, four grade 3a, seven grade 3b at six months, and 30 grade 0, three grade 1, three grade 2, one grade 3a, six grade 3b at four years, with a significant improvement at every follow-up (p < 0.0005 and p = 0.003, respectively). The evaluation of the activity level confirmed these findings, showing a significant improvement in the Tegner score over time, i.e. 7.2 ± 6.7 before the onset of symptoms, 3.3 ± 2.6 before treatment, 4.3 ± 3.6 at two months, 5.2 ± 4.5 at six months, and 5.7 ± 5.0 at four years’ follow-up (p < 0.012 at two months, p < 0.0005 at six months, p < 0.0005 at four years with respect to the pre-treatment level, but without achieving the pre-onset of symptoms level, p<0.0005). Moreover, 81.4 % of the patients returned to sport and 79.1 % of the patients were satisfied and would repeat the treatment if needed. Finally, four patients (two bilateral) failed and underwent surgical treatment.
Fig. 1.
VISA-P scores recorded at baseline level, two months, six months, and at the mean final evaluation of four years’ follow-up
Fig. 2.
EQ-VAS scores recorded at baseline level, two months, six months, and at the mean final evaluation of four years’ follow-up
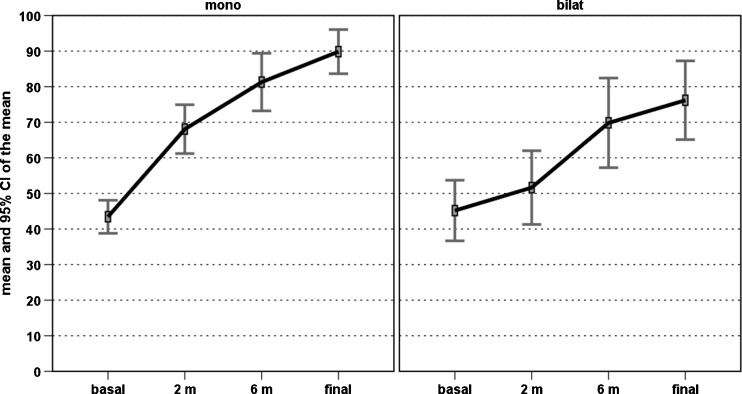
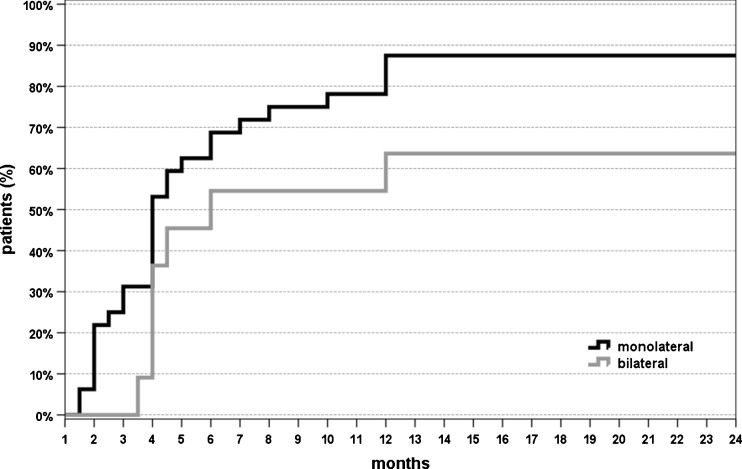
Further analysis was performed to determine if the patients’ characteristics might influence the treatment outcome. Among these, age, BMI, previous conservative or surgical treatment did not influence the results in this series (patients with previously failed surgical treatment also benefited from the injection cycle, and only one of these patients did not return to sport after PRP treatment). Longer duration of symptoms before treatment was correlated with lower VISA-P scores at both six months (rho = −0.271, p = 0.047) and four years’ follow-up (rho = −0.342, p = 0.011). Significantly poorer results were obtained in patients affected by bilateral lesions, i.e. lower VISA-P improvement at two, six months, and four years (p = 0.002, p = 0.019, p < 0.0005, respectively) (Fig. 3); lower Tegner improvement trend over time (η2 = 0.286, p = 0.01) (Fig. 4), lower patient satisfaction (54.5 % vs 87.5 %, p = 0.034) and a higher number of patients not able to return to previous sports activity (36.4 % vs 12.5 %, p = 0.079) (Fig. 5).
Fig. 3.
Comparison of the VISA-P scores over time in patients affected by monolateral or bilateral patellar tendinopathy
Fig. 4.
Comparison of the Tegner scores over time in patients affected by monolateral or bilateral patellar tendinopathy
Fig. 5.
Percentage of patients affected by monolateral or bilateral tendinopathy resuming sports after treatment at different follow-up points
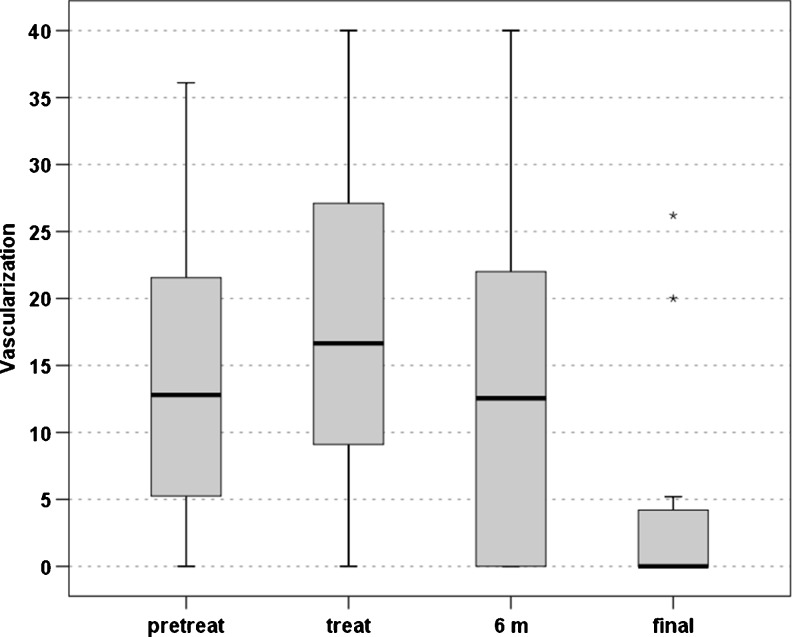
The US evaluation showed an increase in the vascularisation level after the first two injections (20.0 ± 14.3 vs 13.8 ± 10.5, p = 0.08), followed by a gradual slow decrease over time leading to scores similar to baseline level at six months (14.3 ± 17.7) and a lower vascularisation level at the last evaluation (5.5 ± 11.9, p = 0.049) (Fig. 6). Finally, a similar trend (p = 0.022) was also seen in the tendon thickness, with an increase after the injections and a slow gradual decrease over time (8.1 ± 1.7 before the treatment, 9.1 ± 1.7 after two injections, 8.9 ± 2.0 at six months and 8.5 ± 2.0 at the last evaluation).
Fig. 6.
Level of neovascularization tested by US before the treatment, after two PRP injections, at six months and at the final evaluation
Discussion
The main finding of our study was that overall good results were achieved treating patients with PRP injections for patellar tendinopathy, and that the clinical improvement was stable up to a medium-term follow-up. A significantly lower clinical improvement was found in cases of bilateral pathology and also in patients with longer symptom duration. Finally, the analysis of neovascularisation level showed an initial increase followed by a slow decrease, but a correlation with patient symptoms or function could not be found.
The good results observed with this procedure may be explained by different hypothetical mechanisms of action. PRP injections combine a needling stimulus, which may induce internal haemorrhage and consequently an inflammatory response and repair process, with the biological stimulus of the injected platelet growth factors. PRP releasate has been seen to activate circulation-derived cells [17], which play a crucial role in the tissue healing process, and stimulate gene expression of the matrix molecules, collagen production and tendon cell proliferation [18]. Animal studies confirm the potential of this biological treatment, with an increased tendon callus strength and stiffness after PRP application [19, 20]. Apart from the increased mechanical properties, histological examination also showed a superior healing process, and the study of angiogenesis showed an increased neovascularisation during the first phase, thus suggesting an increased metabolic activity and an enhanced and accelerated tendon healing process [21–24].
Despite the promising preclinical premises, the literature on the clinical application of PRP for tendinopathies presents controversial findings. In particular, a randomised trial performed on chronic Achilles tendinopathy [25] comparing patients treated with one PRP injection to those treated with saline injections found no difference in improvement in pain and activity, or in the tendon structure evaluated by ultrasound [26]. However, despite the robust scientific study design, these results do not clearly demonstrate the uselessness of PRP application in tendons for two reasons: some limits in the study design [12] and the nature of PRP itself. In fact, PRP is not a single product, since the exact definition of PRP has not been clearly established and includes a huge number of formulations, whose differences may justify the contradictory findings obtained by heterogeneous products or application modalities. The concentrations provided by most procedures range widely and the number of platelets supplied to the lesion site is an important factor [12] since GFs are potent molecules, and small variations in their concentrations can produce very different effects [27]. Moreover, the appropriate dose, timing and length of intervention are important areas that require further investigation to coordinate the temporal presence of cells and signalling molecules appropriately [28]. Other controversial aspects regard cellularity, since not only platelets but also leukocytes, monocytes, macrophages, and mast cells might be contained in platelet concentrates and play a role in the effects obtained [11–13]. The activation method may also influence the results. In fact, the amount and speed of GFs release and activation may differ, and the molecules used may also influence the tissue by other mechanisms [29]. Thus, it appears clear that different PRPs present different properties and whereas some might produce good results others might be ineffective or even detrimental [30].
Studying PRP efficacy in tendon pathologies is even more complicated, since other factors may influence the results, i.e. the mechanical stimulus, due to normal tissue function or associated physical therapies, may modify the microenvironment by influencing cellular differentiation and tissue repair independently of the presence of stimulatory GFs [31], and may act synergically with the biological treatment in the healing process of musculoskeletal tissues [19]. In this context, the analysis of the mechanism of action and efficacy of PRP appears to be complicated by numerous factors, and it is of primary importance to determine the safety and results of the different PRP procedures before introducing it indiscriminately into clinical practice. Unfortunately, few reports document preliminary results on patellar tendinopathy.
A case series by Van Ark et al. [32] on five patients treated by a single injection of PRP followed by a rehabilitation programme revealed a significant increase in VISA-P score up to 26 weeks. Gosens et al. [33] treated 36 patients with a single intra-tendinous injection of PRP and evaluated them by VISA-P score and VAS for pain up to a mean of 18 months’ follow-up. The results were statistically significant and a good rate of return to pain-free sports activity was found. Furthermore, the authors reported a lower clinical outcome in patients previously treated by corticosteroids, ethoxysclerol injections or surgical management. Mautner et al. [34] evaluated 27 patients affected by patellar tendinopathy at a mean of 15 months’ follow-up considering subjective symptoms improvement on a Likert-scale, and 59 % of patients reported moderate-to-complete resolution of the symptoms ( > 50 % of improvement). A recent randomised trial on 46 patients by Vetrano et al. [35] compared two PRP injections versus three sessions of shock waves: at six and 12 months’ follow-up PRP offered a superior clinical outcome according to VISA-P and VAS pain scores. Finally, treating patellar tendinopathy with PRP multiple injections, we found safety and good clinical results in the short-term when this technique was combined with a concomitant rehabilitative treatment to apply a synergic biological and mechanical stimulation [36]. A positive outcome was also observed in recalcitrant cases where previous non-surgical or surgical treatments had failed [37]. Altogether, the studies available in the literature report promising preliminary results in the short term in small cohorts of patients.
This study shows that in a higher number of tendons treated with multiple PRP injections the results obtained were stable over time, and the good outcome was maintained for up to four years’ follow-up. Interestingly, the results were not homogeneous, i.e. a satisfactory improvement was observed only in monolateral cases, whereas most of the patients affected by bilateral lesions did not benefit from this treatment. It might be postulated that the management of both limbs is more complicated, but it is also likely that monolateral and bilateral tendinopathies are different conditions [38], and their different aetiology might explain the marked difference observed in the response to treatment. Another finding of our study is the poorer results found in patients with a longer history of symptoms and limited function. Finally, the US measurements showed that the injection cycle elicits an increase in both thickness and neovascularisation, which then gradually decrease over time. Unfortunately, probably due to the limited number of patients tested with US, no correlation was found between the level of vascularisation and clinical outcome. However, these findings are in line with those of the literature, both concerning the trend of neovascularisation changes after treatment and the lack of correlation with symptoms and function [26, 39].
The study has some major limitations, such as the lack of a randomised control group and the imaging evaluation performed only in some of the patients at the different follow-ups. However, even though these data cannot show the real potential of PRP, which would require level I studies, they demonstrate that the benefit observed in the short term in patellar tendons treated with PRP is confirmed at medium-term follow-up, with stable results for up to four years. Moreover, the changes of the neovascularisation component over time are also shown, along with its lack of correlation with the symptomatic and functional findings. Therefore, there is a need to evaluate it carefully when managing a tendinopathic condition.
Conclusions
The study shows that multiple injections of PRP offer good overall results for the treatment of chronic recalcitrant patellar tendinopathy, and that the clinical improvement was stable up to a medium-term follow-up. Patients affected by bilateral pathology and presenting a long history of pain and limited function obtained significantly poorer results.
Finally, the analysis of tendon thickness and neovascularisation level showed an initial increase after the injection cycle followed by a slow decrease, but no correlation with patient symptoms or function was found.
Acknowledgments
A. Montaperto, S. Bassini: Biomechanics and Technology Innovation Laboratory, Rizzoli Orthopedic Institute, Bologna, Italy.
P. M. Fornasari, A. Cenacchi, M. Vaccari: Immunohematology and Transfusion Medicine Service, Rizzoli Orthopedic Institute, Bologna, Italy.
E. Pignotti, K. Smith: Task Force, Rizzoli Orthopedic Institute, Bologna, Italy.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Giuseppe Filardo, Phone: +39-051-6366567, FAX: +39-051-583789, Email: g.filardo@biomec.ior.it.
Elizaveta Kon, Email: e.kon@biomec.ior.it.
Berardo Di Matteo, Email: berardo.dimatteo@gmail.com.
Patrizia Pelotti, Email: patrizia.pelotti@ior.it.
Alessandro Di Martino, Email: a.dimartino@biomec.ior.it.
Maurilio Marcacci, Email: m.marcacci@biomec.ior.it.
References
- 1.Cook JL, Khan KM, Harcourt PR, Grant M, Young DA, Bonar SF. A cross sectional study of 100 athletes with jumper's knee managed conservatively and surgically. The Victorian Institute of Sport Tendon Study Group. Br J Sports Med. 1997;31(4):332–336. doi: 10.1136/bjsm.31.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sport Med. 2005;33:561–567. doi: 10.1177/0363546504270454. [DOI] [PubMed] [Google Scholar]
- 3.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 2004;43(2):131–142. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- 4.Samiric T, Parkinson J, Ilic MZ, Cook J, Feller JA, Handley CJ. Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol. 2009;28(4):230–236. doi: 10.1016/j.matbio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Kettunen JA, Kvist M, Alanen E, et al. Long term prognosis for jumper’s knee in male athletes. A prospective follow-up study. Am J Sport Med. 2002;30(5):689–692. doi: 10.1177/03635465020300051001. [DOI] [PubMed] [Google Scholar]
- 6.Peers KH, Lysens RJ. Patellar tendinopathy in athletes: current diagnostic and therapeutic recommendations. Sports Med. 2005;35:71–87. doi: 10.2165/00007256-200535010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Young MA, Cook JL, Purdam CR, et al. Eccentric decline squat protocol offers superior results at 12 months compared with traditional eccentric protocol for patellar tendinopathy in volleyball players. Br J Sports Med. 2005;39(2):102–105. doi: 10.1136/bjsm.2003.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peers KH, Lysens RJ, Brys P, Bellemans J. Cross-sectional outcome analysis of athletes with chronic patellar tendinopathy treated surgically and by extracorporeal shock wave therapy. Clin J Sport Med. 2003;13(2):79–83. doi: 10.1097/00042752-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Alfredson H, Ohberg L. Neovascularisation in chronic painful patellar tendinosis. Promising results after sclerosing neovessels outside the tendon challenge the need for surgery. Knee Surg Sports Traumatol Arthrosc. 2005;13(2):74–80. doi: 10.1007/s00167-004-0549-x. [DOI] [PubMed] [Google Scholar]
- 10.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33(5):381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 11.Cole BJ, Seroyer ST, Filardo G, Bajaj S, Fortier LA. Platelet-rich plasma: where are we now and where are we going? Sports Health. 2010;2(3):203–10. doi: 10.1177/1941738110366385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kon E, Filardo G, Di Martino A, Marcacci M. Platelet-rich plasma (PRP) to treat sports injuries: evidence to support its use. Knee Surg Sports Traumatol Arthrosc. 2011;19(4):516–527. doi: 10.1007/s00167-010-1306-y. [DOI] [PubMed] [Google Scholar]
- 13.Tschon M, Fini M, Giardino R, Filardo G, Dallari D, Torricelli P, Martini L, Giavaresi G, Kon E, Maltarello MC, Nicolini A, Carpi A. Lights and shadows concerning platelet products for musculoskeletal regeneration. Front Biosci (Elite Ed) 2011;1(3):96–107. doi: 10.2741/e224. [DOI] [PubMed] [Google Scholar]
- 14.Filardo G, Kon E. PRP: more words than facts. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1655–1656. doi: 10.1007/s00167-012-2136-x. [DOI] [PubMed] [Google Scholar]
- 15.Lian O, Holen KJ, Engebretsen L, et al. Relationship between symptoms of jumper’s knee and the ultrasound characteristics of the patella tendon among high level male volleyball players. Scand J Med Sci Sports. 1996;6:291–296. doi: 10.1111/j.1600-0838.1996.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 16.Cook JL, Ptazsnik R, Kiss ZS, Malliaras P, Morris ME, De Luca J. High reproducibility of patellar tendon vascularity assessed by colour Doppler ultrasonography: a reliable measurement tool for quantifying tendon pathology. Br J Sports Med. 2005;39:700–703. doi: 10.1136/bjsm.2004.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kajikawa Y, Morihara T, Sakamoto H, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol. 2008;215(3):837–845. doi: 10.1002/jcp.21368. [DOI] [PubMed] [Google Scholar]
- 18.De Mos M, Van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36(6):1171–1178. doi: 10.1177/0363546508314430. [DOI] [PubMed] [Google Scholar]
- 19.Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop. 2006;77(5):806–812. doi: 10.1080/17453670610013033. [DOI] [PubMed] [Google Scholar]
- 20.Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75:93–99. doi: 10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- 21.Bosch G, van Schie HT, de Groot MW, Cadby JA, van de Lest CH, Barneveld A, van Weeren PR. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: A placebo-controlled experimental study. J Orthop Res. 2010;28(2):211–217. doi: 10.1002/jor.20980. [DOI] [PubMed] [Google Scholar]
- 22.Lyras DN, Kazakos K, Verettas D, Botaitis S, Agrogiannis G, Kokka A, Pitiakoudis M, Kotzakaris A. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Arch Orthop Trauma Surg. 2009;129(11):1577–1582. doi: 10.1007/s00402-009-0935-4. [DOI] [PubMed] [Google Scholar]
- 23.Lyras D, Kazakos K, Verettas D, Polychronidis A, Simopoulos C, Botaitis S, Agrogiannis G, Kokka A, Patsouris E. Immunohistochemical study of angiogenesis after local administration of platelet-rich plasma in a patellar tendon defect. Int Orthop. 2010;34(1):143–148. doi: 10.1007/s00264-009-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyras DN, Kazakos K, Verettas D, Polychronidis A, Tryfonidis M, Botaitis S, Agrogiannis G, Simopoulos C, Kokka A, Patsouris E. The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int. 2009;30(11):1101–1106. doi: 10.3113/FAI.2009.1101. [DOI] [PubMed] [Google Scholar]
- 25.De Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, Tol JL. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144–149. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 26.de Jonge S, de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, Tol JL. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. Am J Sports Med. 2011;39(8):1623–1629. doi: 10.1177/0363546511404877. [DOI] [PubMed] [Google Scholar]
- 27.Torricelli P, Fini M, Filardo G, Tschon M, Pischedda M, Pacorini A, Kon E, Giardino R. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35(10):1569–1576. doi: 10.1007/s00264-011-1237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batten ML, Hansen JC, Dahners LE. Influence of dosage and timing of application of platelet-derived growth factor on early healing of the rat medial collateral ligament. J Orthop Res. 1996;14(5):736–741. doi: 10.1002/jor.1100140509. [DOI] [PubMed] [Google Scholar]
- 29.Borzini P, Mazzucco L. Tissue regeneration and in loco administration of platelet derivatives: clinical outcome, heterogeneous products, and heterogeneity of the effector mechanisms. Transfusion. 2005;45(11):1759–1767. doi: 10.1111/j.1537-2995.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 30.Kon E, Filardo G, Delcogliano M, Fini M, Salamanna F, Giavaresi G, Martin I, Marcacci M. Platelet autologous growth factors decrease the osteochondral regeneration capability of a collagen-hydroxyapatite scaffold in a sheep model. BMC Musculoskelet Disord. 2010;27(11):220. doi: 10.1186/1471-2474-11-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Moes M, Koevoet JLM, Jahr H, Verstegen MMA, Heijboer MP, Kops N, van Leeuwen JPMT, Weinans H, Verhaar JAN, van Osch GJVM. Intrinsic differentiation potential of adolescent human tendon tissue: an in vitro cell differentiation study. BMC Musculoskelet Disord. 2007;8:16–27. doi: 10.1186/1471-2474-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Ark M, van den Akker-Scheek I, Meijer LT, Zwerver J (2013) An exercise-based physical therapy program for patients with patellar tendinopathy after platelet-rich plasma injection. Phys Ther Sport 14(2):124–130. doi:10.1016/j.ptsp.2012.05.002 [DOI] [PubMed]
- 33.Gosens T, Den Oudsten BL, Fievez E, van't Spijker P, Fievez A. Pain and activity levels before and after platelet-rich plasma injection treatment of patellar tendinopathy: a prospective cohort study and the influence of previous treatments. Int Orthop. 2012;36(9):1941–1946. doi: 10.1007/s00264-012-1540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mautner K, Colberg RE, Malanga G, Borg-Stein JP, Harmon KG, Dharamsi AS, Chu S, Homer P (2013) Outcomes after ultrasound-guided platelet-rich plasma injections for chronic tendinopathy: a multicenter, retrospective review. PM R 5(3):169–175 doi:10.1016/j.pmrj.2012.12.010 [DOI] [PubMed]
- 35.Vetrano M, Castorina A, Vulpiani MC, Baldini R, Pavan A, Ferretti A (2013) Platelet-rich plasma versus focused shock waves in the treatment of jumper's knee in athletes. Am J Sports Med 41(4):795–803. doi: 10.1177/0363546513475345. [DOI] [PubMed]
- 36.Kon E, Filardo G, Delcogliano M, Presti ML, Russo A, Bondi A, Di Martino A, Cenacchi A, Fornasari PM, Marcacci M. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper's knee. Injury. 2009;40(6):598–603. doi: 10.1016/j.injury.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 37.Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper's knee. Int Orthop. 2010;34(6):909–915. doi: 10.1007/s00264-009-0845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaida JE, Cook JL, Bass SL, Austen S, Kiss ZS. Are unilateral and bilateral patellar tendinopathy distinguished by differences in anthropometry, body composition, or muscle strength in elite female basketball players? Br J Sports Med. 2004;38(5):581–585. doi: 10.1136/bjsm.2003.006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tol JL, Spiezia F, Maffulli N. Neovascularization in Achilles tendinopathy: have we been chasing a red herring? Knee Surg Sports Traumatol Arthrosc. 2012;20(10):1891–1894. doi: 10.1007/s00167-012-2172-6. [DOI] [PubMed] [Google Scholar]