Abstract
Temporal lobe epilepsy (TLE) is the most common type of medically intractable epilepsy in adults and children, and mesial temporal sclerosis is the most common underlying cause of TLE. Unlike in the case of adults, TLE in infants and young children often has etiologies other than mesial temporal sclerosis, such as tumors, cortical dysplasia, trauma, and vascular malformations. Differences in seizure semiology have also been reported. Motor manifestations are prominent in infants and young children, but they become less obvious with increasing age. Further, automatisms tend to become increasingly complex with age. However, in childhood and especially in adolescence, the clinical manifestations are similar to those of the adult population. Selective amygdalohippocampectomy can lead to excellent postoperative seizure outcome in adults, but favorable results have been seen in children as well. Anterior temporal lobectomy may prove to be a more successful surgery than amygdalohippocampectomy in children with intractable TLE. The presence of a focal brain lesion on magnetic resonance imaging is one of the most reliable independent predictors of a good postoperative seizure outcome. Seizure-free status is the most important predictor of improved psychosocial outcome with advanced quality of life and a lower proportion of disability among adults and children. Since the brain is more plastic during infancy and early childhood, recovery is promoted. In contrast, long epilepsy duration is an important risk factor for surgically refractory seizures. Therefore, patients with medically intractable TLE should undergo surgery as early as possible.
Keywords: Temporal lobe epilepsy, Child, Adult, Temporal lobectomy
Introduction
While temporal lobe epilepsy (TLE) is the most common type of medically intractable epilepsy in adults, only about 20% of pediatric patients have epilepsy of temporal lobe origin. TLE is known to be remediable by surgery. Further, mesial temporal sclerosis (MTS, also called hippocampal sclerosis) is the most common underlying cause of TLE, observed in about 81% of all cases1).
TLE associated with MTS frequently manifests between 6 and 10 years of age, although cases presenting in infancy and up to age 32 years have also been reported2). Clinically, MTS appears to be a progressive disorder, and although seizures can initially be controlled with antiepileptic drugs (AEDs), they reemerge and become medically intractable in 60-90% of cases2).
Previous studies have reported favorable surgical outcomes following temporal lobectomy in children and adolescents with intractable seizures3,4). These results appear to favor surgery as a possible treatment measure for TLE. Nonetheless, presurgical evaluations are much more difficult to conduct for young children than for older patients. Additionally, studies on the postoperative cognitive and/or memory outcomes in children are relatively rare, because of measurement scale limitations, low frequencies of complaints about memory problems, and the relatively recent development of early epilepsy surgery4).
For these reasons, a comparison of clinical characteristics including histopathology, seizure semiology, electroencephalogram (EEG) findings, and postoperative seizure and neuropsychological outcomes between children and adults undergoing temporal lobe surgery may yield important information on the appropriateness of surgical treatment for TLE.
Etiologies
Although there are many similarities between children and adults with temporal seizures, there are also significant differences, because of the unique characteristics of the pediatric brain. MTS is relatively less frequent in infants and children. Specifically, while only 20% of children with TLE have MTS, 60% of adults with TLE have MTS5). Several pediatric patients show other etiologies, including tumors, malformation of cortical development (MCD), trauma or nonspecific gliosis, and vascular malformations6,7), and it is often difficult to distinguish diverse pathologies using neuroimaging5).
On pathologic examination, MTS shows neuronal loss in the hippocampus, predominantly involving the hilar region, CA1, CA3, CA4, and the dentate gyrus. Magnetic resonance imaging (MRI) findings correlate with pathologic results, showing hippocampal atrophy and abnormal T2 signal intensity in the hippocampus, which are best seen on thin (2 mm) coronal sections through the hippocampus8). MRI abnormalities in both lobes are observed in about 20% of patients2).
Clinical features
Complex partial seizures (CPSs) are the most common manifestation of mesial TLE2). About one-third of all patients have secondarily generalized tonic-clonic seizures in addition to CPSs or as the primary seizure type. Distinct characteristics of mesial TLE seizures include the following:
1) An aura or a simple partial seizure with sensory symptoms occurs in most patients, including a rising epigastric sensation and psychic phenomena such as deja vu, jamais vu, or fear2). Auras of taste and smell are less common but relatively specific for TLE.
2) A CPS usually manifests with behavioral arrest and staring that lasts between 30 and 120 seconds. The patients are usually unaware and unresponsive during this period. Occasionally, more detailed observation reveals the presence of olfactory hallucinations or ictal automatisms9).
3) Automatisms are common, occurring in about 60% of CPSs related to MTS-associated TLE2). These are repetitive, stereotyped, purposeless movements that are typically mild, involving the hands (picking, fidgeting, or fumbling) and mouth (chewing or lip smacking).
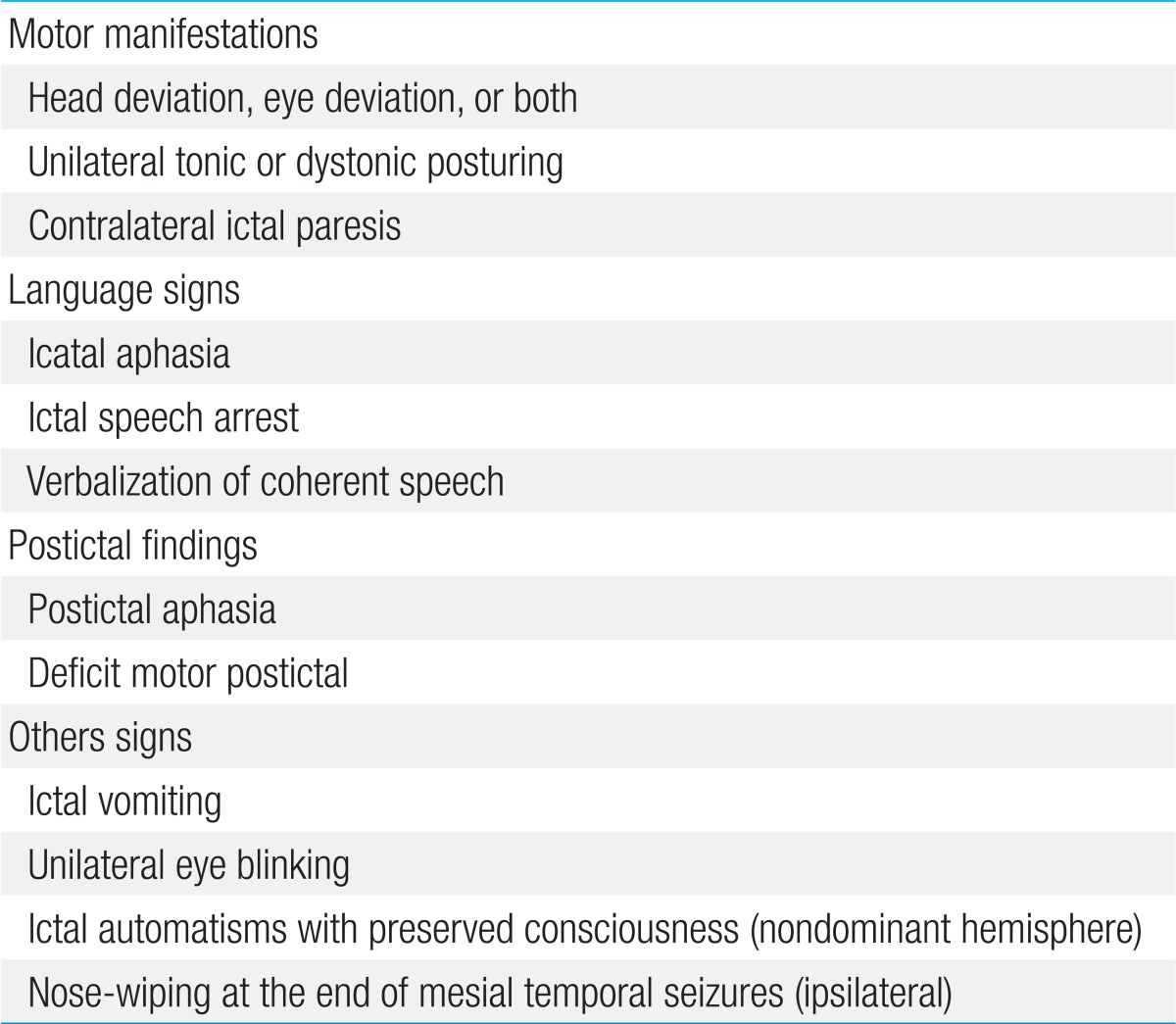
4) Lateralizing features can occur during or after a partial or secondary generalized seizure2). Unilateral automatisms are usually ipsilateral to the seizure focus, while dystonic posturing almost always occurs on the contralateral side. Head deviation at onset is usually ipsilateral to the seizure; however, when it occurs later as a more forceful appearance (so-called "versive"), it is contralateral10). Contralateral ictal paresis has been documented as a reliable lateralizing sign. Ictal vomiting has been connected to right-sided onset, and unilateral eye blinking usually occurs on the ipsilateral side. Both ictal aphasia and ictal speech arrest have been regarded as seizure onset in the language-dominant lobe, whereas verbalization is related to seizure onset in the nondominant lobe11). Table 1 presents the lateralizing or localizing signs in MTS.
Table 1.
Lateralizing or localizing signs in temporal lobe epilepsy
5) Less common behaviors include ictal speech, affective behaviors (laughing, crying, or showing fear), hypermotor behaviors usually associated with frontal lobe seizures, and so-called "leaving behavior" (walking or running away)12).
6) Postictal confusion usually lasts for minutes. Postictal hemiparesis can occur contralateral to the seizure focus, and postictal aphasia can occur with a seizure from the dominant hemisphere. Nose-wiping is usually performed by the hand ipsilateral to the ictal focus13).
Children with TLE have diverse ictal semiology11). Prominent motor signs are the usual manifestations in early life6), and children less than 6 years of age show ictal semiology more similar to frontal lobe seizures, such as bilateral tonic posture and head drop. Convulsive motor signs tend to decrease with increasing age. These differences in semiology are strongly related to brain maturation and neuropsychological development. Auras are rarely observed in very young children, probably because they cannot describe their subjective feelings.
Brockhaus and Elger14) studied 29 children with TLE, aged 18 months to 16 years. They reported that seizure semiology in children >6 years of age was similar to that in adults and included auras, staring or behavioral arrest, automatisms, and versive and dystonic posturing. However, children <6 years of age showed different semiology, such as symmetric motor signs of the limbs (up to 80%), which are less common (about 40%) in older children. The frequency of complex automatisms increases with age. While simple automatisms (e.g., oroalimentary or gestural) have been noted in preschool children, more complex automatisms such as clapping, beating hands, or shuffling have only been observed in children older than 8. A summary of the characteristics seen in these subtypes of TLE is shown in Table 2.
Table 2.
Characteristics of temporal lobe epilepsy in different age groups
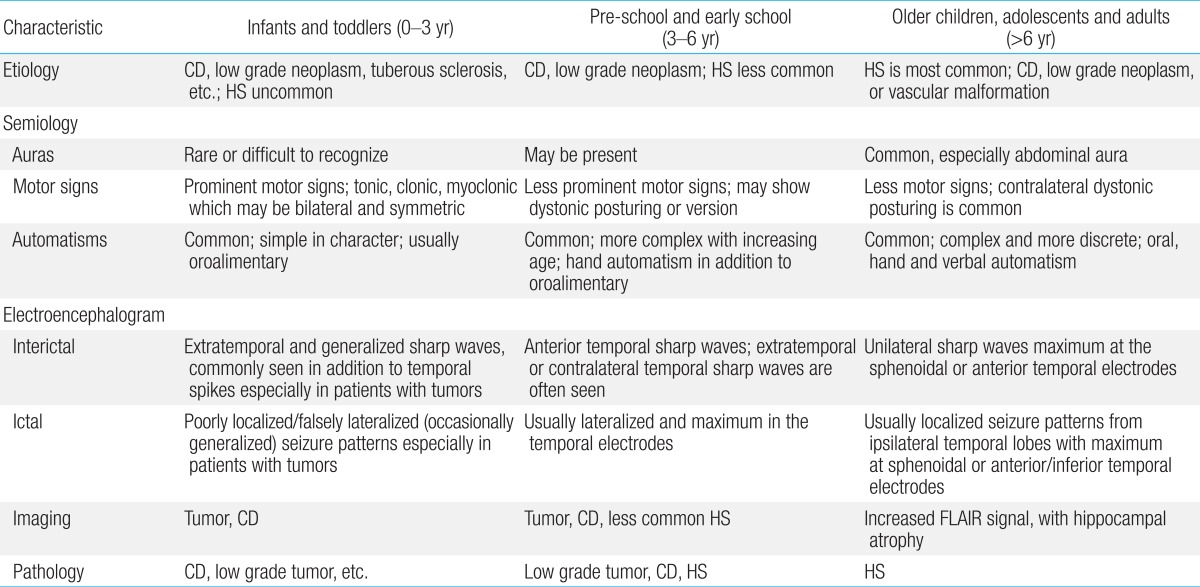
CD, cortical dysplasia; HS, hippocampal sclerosis; FLAIR, fluid attenuated inversion recovery.
Reproduced from Ray and Wyllie. Expert Rev Neurother 2005;5:785-801, with permission of Expert Reviews Ltd.11).
EEG findings
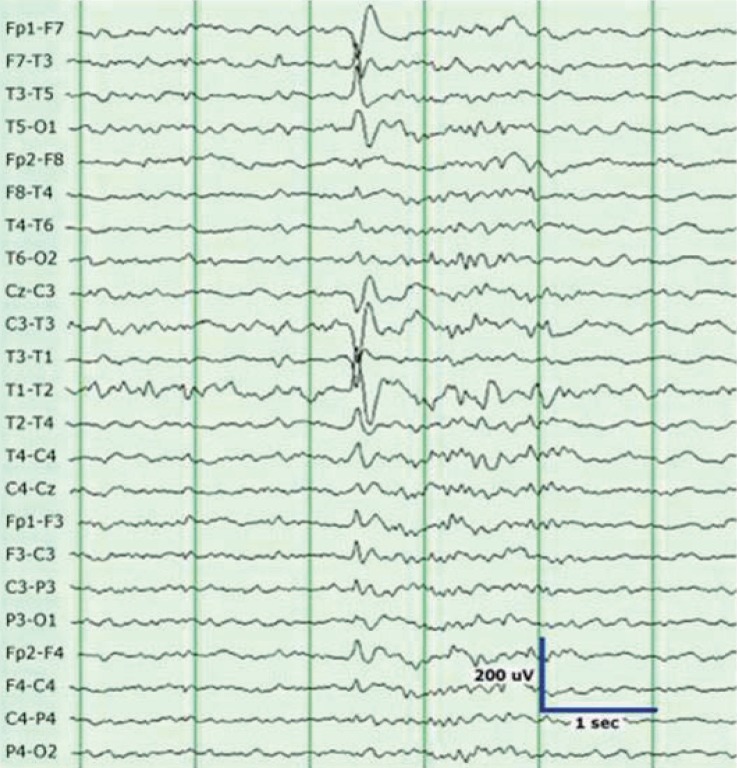
Interictal EEG typically shows temporal sharp waves, and the maximum values are observed at the anterior or midtemporal electrodes (F7/F8, T1/T2, T3/T4) (Fig. 1). Bitemporal independent discharges are observed in 30-40% of patients with mesial TLE1). The ictal EEG shows well-defined rhythmic and evolving discharges with a buildup, and lateralized postictal slowing is often observed. Sphenoidal electrodes sometimes detect inferiorly directed mesial temporal discharges not seen with scalp electrodes, and they may provide valuable localization findings for patients with TLE1).
Fig. 1.
Sharp waves in the left temporal region are seen in a patient with left temporal lobe epilepsy. These waves clearly stand out from the background activity.
Epileptiform abnormalities in younger children with TLE may have limited localizing value because of their widespread distribution. Interictal and ictal EEG findings in younger children with TLE usually show more diffuse and irregular discharges and suggest further difficulties in the determination of temporal lobe seizure onset3). Specifically, children with neoplasms frequently have unusual EEG features, including multifocal epileptiform discharges and poorly localized or lateralized ictal activities11).
Decision for temporal lobe surgery
Most epileptologists view MTS as a progressive disorder characterized by medically intractable seizures, memory loss, and variable behavioral changes. Because temporal lobectomy in such cases is a considerably better treatment option than continued AED therapy, there is no reason to defer presurgical evaluation once medical intractability has been established. Additionally, because children with intractable seizures show intellectual decline over time, it is thought that early surgery could reduce the severity of cognitive impairment15).
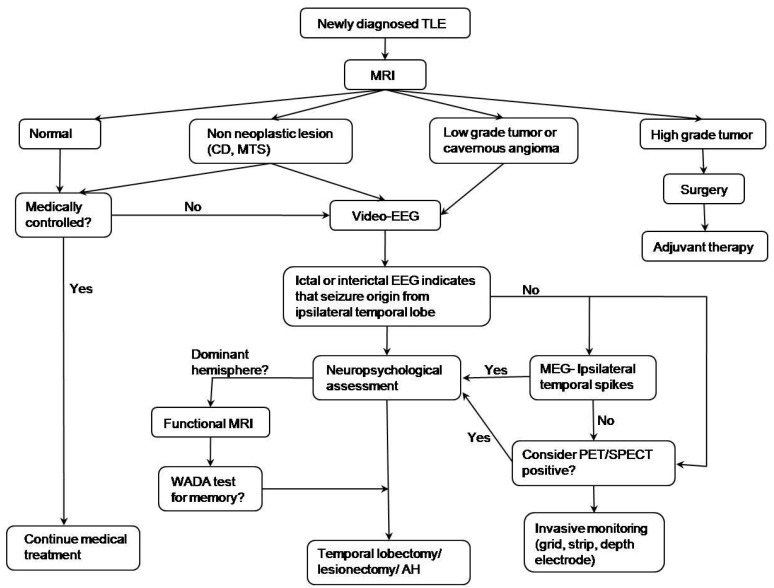
The algorithm16) for decision making regarding epileptic surgery in patients with TLE is given in Fig. 2. Optimal selection of surgical candidates requires a comprehensive evaluation of clinical, electrophysiological, neuroimaging, and neuropsychological data by a multidisciplinary team that includes epileptologists, neuroradiologists, neurosurgeons, and neuropsychologists. If a focal lesion in a unilateral temporal lobe is suspected on MRI, the patients are subjected to further evaluations, such as video/EEG monitoring, neuropsychological testing, and functional MRI for language and memory hemisphere dominance. Language localization studies (Wada test) and other functional studies are considered in the case of cooperative patients. Invasive evaluation is performed when the findings are discordant among non-invasive tests. The surgical approach is selected according to the preoperative diagnosis, EEG findings, and anatomic characteristics on MRI. Multimodal imaging techniques have recently been shown to yield greatly improved surgical outcomes in temporal lobectomies (Fig. 3).
Fig. 2.
Algorithm of epileptic surgery in a patient with temporal lobe epilepsy (TLE). MRI, magnetic resonance imaging; CD, cortical dysplasia; MTS, mesial temporal sclerosis; EEG, electroencephalogram; MEG, magnetoencephalography; PET/SPECT, positron emission tomography/single photon emission computed tomography; AH, amygdalohippocampectomy. Reprinted from Benifla, et al. Neurosurgery 2006;59:1203-13, with permission of Lippincott Williams & Wilkins16).
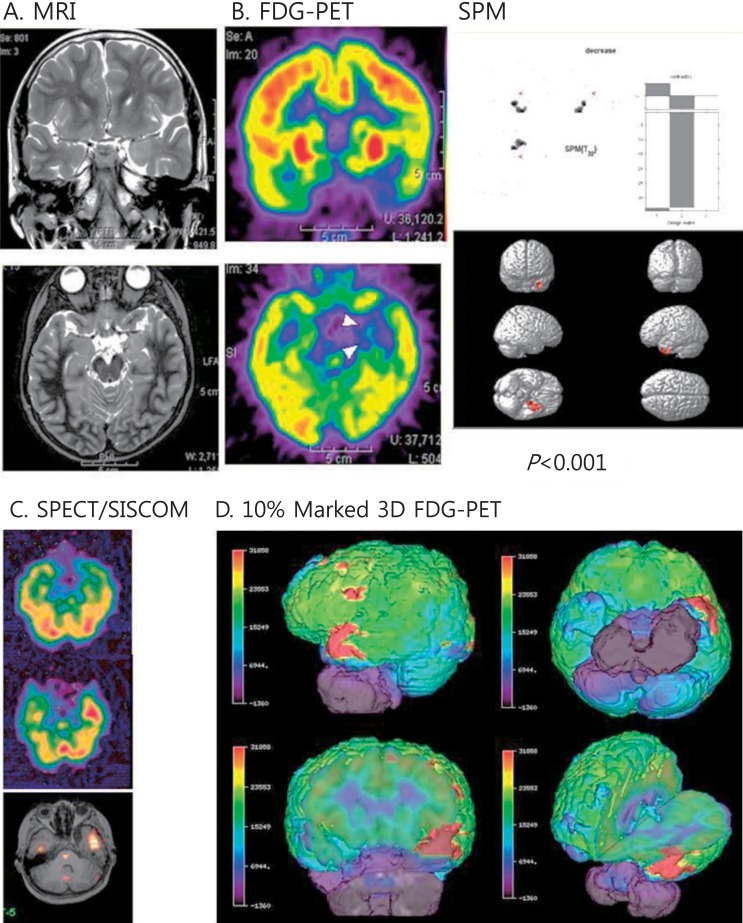
Fig. 3.
Multimodal neuroimages of a patient with left temporal lobe epilepsy due to ganglioglioma. (A) Magnetic resonance imaging (MRI) showed increased signals on the left uncus and hippocampus. (B) Fluorodeoxyglucose-positron emission tomography (FDG-PET) showed decreased glucose metabolism in the same areas. Statistical parametric mapping (SPM) analysis also indicated decreased glucose metabolism in the same areas compared to normal controls (P<0.001). (C) Ictal single photon emission computed tomography (SPECT) and SISCOM (subtraction ictal SPECT coregistered to the MRI) demonstrated a significant increased ictal uptake on the left temporal region. (D) Coregistered surface marked FDG-PET images with a 10% asymmetric threshold to the MRI showed red zones on the left temporal areas in various views.
1. Seizure-related outcomes
Temporal lobe surgery for intractable seizures has been demonstrated to be safe and effective for both adults and children17-19). In a randomized controlled trial, Wiebe et al.17) confirmed the effectiveness of anterior temporal lobectomy (ATL) for adults with intractable TLE seizures. They found that 58% of patients in the surgical group became seizure free, compared to only 8% in the medical group. Duchowny et al.6) reported that 75% of children were seizure-free after temporal lobectomy conducted before 12 years of age. The Cleveland Clinic Foundation revealed similar results, with 74% of preadolescents and 80% of adolescents becoming seizure-free after temporal lobe surgery15).
A systematic review and meta-analysis were performed to determine the net benefit of surgery20). The results suggested that in the case of intractable epilepsy, surgery was 4 times as likely as medication alone to result in a seizure-free outcome. Nevertheless, a seizure-free outcome was achieved by AED alone in 12% of similar patients who did not undergo surgery.
Selective amygdalohippocampectomy (SAH) is a preferred technique in many centers, because it spares tissue by preserving the lateral temporal neocortex and has a similar seizure outcome as ATL. A previous study showed that patients who underwent SAH achieved the same degree of seizure control and a more favorable neuropsychological profile as those who underwent ATL21). Similarities and differences are observed between children and adults with intractable TLE following SAH. A retrospective study involving 9 children and 14 adults with intractable TLE18) revealed that SAH could yield excellent postoperative seizure outcomes in adults, while less favorable results were obtained in children. Most of the adults had MTS, whereas the children had a wide range of pathologies. Accordingly, ATL may prove to be a more successful operation than SAH for children with intractable TLE.
In one study, the postoperative outcomes of 52 children and adults with medically intractable TLE were compared19). Seizure-free outcomes were achieved in 63.2% (12/19) of the children and 72.7% (24/33) of the adults. Further, the mean extent of surgical resection was significantly broader in children than in adults (5.0 cm vs. 4.1 cm). Additionally, MTS was the most common pathologic finding in both groups (57.9% in children, 78.8% in adults), while MCD and dual pathology were significantly more frequent in children than in adults.
Long-term follow-up studies in adults and children demonstrated a decline in seizure-free rates as the postoperative time increased22,23). In a large retrospective review of 325 adults and pediatric patients, the seizure-free rate was found to have decreased from 61% at 1 year to 41% at 10 years after temporal lobectomy22). Among children with temporal lobe surgery, 67% were seizure free during the 10- to 20-year period of the follow-up study23). Further, patients with tumors or cavernous angioma had better outcomes than those with other histopathologies.
2. Cognitive and memory outcomes
There are some risks to cognitive functioning following temporal lobectomy. Left-sided resections have been associated with a decrease in verbal memory, while spatial memory and learning may be affected by right-sided surgery. Approximately 30-60% of patients who undergo left-sided (speech dominant) resection experience a substantial decline in verbal memory following surgery24). In contrast, patients who have undergone right-sided surgery generally show postoperative improvement in verbal memory, although some do exhibit a decline. Patients with higher presurgical abilities are at greater risk for postoperative memory decline24).
One study evaluating children after temporal lobe surgery reported stable verbal memory scores 6 months after left-sided surgery and improved scores after right-sided resection7). Children appear to recover lost cognitive function more quickly and completely than adults25). Decrease in postoperative verbal memory was found 3 months after surgery in children, but recoveries were obvious just 1 year after surgery25). On the other hand, the left-resected adults had acutely worse level of verbal memory one year after surgery than those of their preoperative examination25). Another study with a follow-up duration of less than 2 years found no improvements in intellectual functioning26), suggesting that a long period was required for cognitive recovery.
Skirrow et al.27) reported the results of the long-term follow-up of 42 children who had undergone temporal lobe surgery after an average postoperative period of 9 years. A significant increase in intelligence quotient (IQ) was found in the surgical group after a follow-up period of >5 years, and this was not observed in the nonsurgical control group. The surgical group also reported better psychosocial outcomes, including quality of life. Additionally, better postsurgical cognitive improvements in children with lower preoperative IQs have been noted in previous studies27,28).
Preoperative predictors of surgical outcomes
The presence of a focal brain lesion on MRI is one of the most reliable independent predictors of a good outcome following temporal lobe surgery22). McIntosh et al.22) indicated that the lack of an obvious abnormality or the presence of diffuse pathology is a risk factor for recurrence after surgery. Neoplastic lesions may be completely removed using intraoperative navigation with MRI in addition to lesionectomy. Conversely, the entire epileptogenic zone of MTS and MCDs is not always revealed on MRI, and this may hamper effective surgical resection.
A long duration of epilepsy is an important risk factor for surgically refractory seizures, and patients with medically intractable TLE should be surgically treated as early as possible15). The effect of low IQ has been confirmed to be associated with poor postoperative seizure outcomes as it implies bilateral and potentially diffuse rather than focal brain pathology29). Early febrile seizures, early postoperative seizures, AED withdrawal, and duration of epilepsy have also been reported to contribute to late relapses22).
Previous studies have reported various predictors related to postoperative memory outcome, including the side of resection, preoperative memory level, duration of epilepsy, extent of MTS, and Wada test results for language lateralization and memory deficits28,30). Binder et al.30) used a multivariate approach to predict the variables of postoperative verbal memory outcome. Good preoperative performance, late age at onset of epilepsy, and left dominance on the Wada test were all predictive factors of memory decline.
Conclusions
In sum, patients who undergo temporal lobectomy in childhood have distinctly different clinical features and interictal EEG and pathologic findings from those who undergo the procedure in adulthood; however, both groups show favorable surgical outcomes. Although there are many similarities between children and adults with TLE, there are significant differences resulting from features unique to the pediatric brain. Another reason for this marked difference is probably the difference in pathology between the groups.
Temporal lobe resection for intractable epilepsy in children is a safe and effective procedure that has a favorable impact on seizure control. The success in children may be attributed to the shorter epilepsy duration and greater plasticity of the brain, compared to adults. It should be noted that there is a risk of language deficits when the procedure is performed in the dominant hemisphere. Further, the disruptive effects of frequent seizures and AEDs on the developing brain in children can result in brain damage and progressive mental handicap. Therefore, early diagnosis of intractable epilepsy and referral for surgical treatment are critical in order to improve long-term prognoses.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Williamson PD, French JA, Thadani VM, Kim JH, Novelly RA, Spencer SS, et al. Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol. 1993;34:781–787. doi: 10.1002/ana.410340605. [DOI] [PubMed] [Google Scholar]
- 2.Sadler RM. The syndrome of mesial temporal lobe epilepsy with hippocampal sclerosis: clinical features and differential diagnosis. Adv Neurol. 2006;97:27–37. [PubMed] [Google Scholar]
- 3.Mohamed A, Wyllie E, Ruggieri P, Kotagal P, Babb T, Hilbig A, et al. Temporal lobe epilepsy due to hippocampal sclerosis in pediatric candidates for epilepsy surgery. Neurology. 2001;56:1643–1649. doi: 10.1212/wnl.56.12.1643. [DOI] [PubMed] [Google Scholar]
- 4.Clusmann H, Kral T, Gleissner U, Sassen R, Urbach H, Blumcke I, et al. Analysis of different types of resection for pediatric patients with temporal lobe epilepsy. Neurosurgery. 2004;54:847–859. doi: 10.1227/01.neu.0000114141.37640.37. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair DB, Wheatley M, Aronyk K, Hao C, Snyder T, Colmers W, et al. Pathology and neuroimaging in pediatric temporal lobectomy for intractable epilepsy. Pediatr Neurosurg. 2001;35:239–246. doi: 10.1159/000050429. [DOI] [PubMed] [Google Scholar]
- 6.Duchowny M, Levin B, Jayakar P, Resnick T, Alvarez L, Morrison G, et al. Temporal lobectomy in early childhood. Epilepsia. 1992;33:298–303. doi: 10.1111/j.1528-1157.1992.tb02319.x. [DOI] [PubMed] [Google Scholar]
- 7.Robinson S, Park TS, Blackburn LB, Bourgeois BF, Arnold ST, Dodson WE. Transparahippocampal selective amygdalohippocampectomy in children and adolescents: efficacy of the procedure and cognitive morbidity in patients. J Neurosurg. 2000;93:402–409. doi: 10.3171/jns.2000.93.3.0402. [DOI] [PubMed] [Google Scholar]
- 8.Bonilha L, Halford JJ, Rorden C, Roberts DR, Rumboldt Z, Eckert MA. Automated MRI analysis for identification of hippocampal atrophy in temporal lobe epilepsy. Epilepsia. 2009;50:228–233. doi: 10.1111/j.1528-1167.2008.01768.x. [DOI] [PubMed] [Google Scholar]
- 9.Butler CR, Graham KS, Hodges JR, Kapur N, Wardlaw JM, Zeman AZ. The syndrome of transient epileptic amnesia. Ann Neurol. 2007;61:587–598. doi: 10.1002/ana.21111. [DOI] [PubMed] [Google Scholar]
- 10.Remi J, Wagner P, ODwyer R, Silva Cunha JP, Vollmar C, Krotofil I, et al. Ictal head turning in frontal and temporal lobe epilepsy. Epilepsia. 2011;52:1447–1451. doi: 10.1111/j.1528-1167.2011.03076.x. [DOI] [PubMed] [Google Scholar]
- 11.Ray A, Wyllie E. Treatment options and paradigms in childhood temporal lobe epilepsy. Expert Rev Neurother. 2005;5:785–801. doi: 10.1586/14737175.5.6.785. [DOI] [PubMed] [Google Scholar]
- 12.Staack AM, Bilic S, Wendling AS, Scholly J, Kraus U, Strobl K, et al. Hyperkinetic seizures in patients with temporal lobe epilepsy: clinical features and outcome after temporal lobe resection. Epilepsia. 2011;52:1439–1446. doi: 10.1111/j.1528-1167.2011.03100.x. [DOI] [PubMed] [Google Scholar]
- 13.Lineweaver TT, Morris HH, Naugle RI, Najm IM, Diehl B, Bingaman W. Evaluating the contributions of state-of-the-art assessment techniques to predicting memory outcome after unilateral anterior temporal lobectomy. Epilepsia. 2006;47:1895–1903. doi: 10.1111/j.1528-1167.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 14.Brockhaus A, Elger CE. Complex partial seizures of temporal lobe origin in children of different age groups. Epilepsia. 1995;36:1173–1181. doi: 10.1111/j.1528-1157.1995.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 15.Wyllie E, Chee M, Granstrom ML, DelGiudice E, Estes M, Comair Y, et al. Temporal lobe epilepsy in early childhood. Epilepsia. 1993;34:859–868. doi: 10.1111/j.1528-1157.1993.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 16.Benifla M, Otsubo H, Ochi A, Weiss SK, Donner EJ, Shroff M, et al. Temporal lobe surgery for intractable epilepsy in children: an analysis of outcomes in 126 children. Neurosurgery. 2006;59:1203–1213. doi: 10.1227/01.NEU.0000245615.32226.83. [DOI] [PubMed] [Google Scholar]
- 17.Wiebe S, Blume WT, Girvin JP, Eliasziw M Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 18.Datta A, Sinclair DB, Wheatley M, Jurasek L, Snyder T, Quigley D, et al. Selective amygdalohippocampectomy: surgical outcome in children versus adults. Can J Neurol Sci. 2009;36:187–191. [PubMed] [Google Scholar]
- 19.Lee YJ, Kang HC, Bae SJ, Kim HD, Kim JT, Lee BI, et al. Comparison of temporal lobectomies of children and adults with intractable temporal lobe epilepsy. Childs Nerv Syst. 2010;26:177–183. doi: 10.1007/s00381-009-1015-3. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt D, Stavem K. Long-term seizure outcome of surgery versus no surgery for drug-resistant partial epilepsy: a review of controlled studies. Epilepsia. 2009;50:1301–1309. doi: 10.1111/j.1528-1167.2008.01997.x. [DOI] [PubMed] [Google Scholar]
- 21.Wieser HG, Ortega M, Friedman A, Yonekawa Y. Long-term seizure outcomes following amygdalohippocampectomy. J Neurosurg. 2003;98:751–763. doi: 10.3171/jns.2003.98.4.0751. [DOI] [PubMed] [Google Scholar]
- 22.McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127(Pt 9):2018–2030. doi: 10.1093/brain/awh221. [DOI] [PubMed] [Google Scholar]
- 23.Benifla M, Rutka JT, Otsubo H, Lamberti-Pasculli M, Elliott I, Sell E, et al. Long-term seizure and social outcomes following temporal lobe surgery for intractable epilepsy during childhood. Epilepsy Res. 2008;82:133–138. doi: 10.1016/j.eplepsyres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Dulay MF, Levin HS, York MK, Mizrahi EM, Verma A, Goldsmith I, et al. Predictors of individual visual memory decline after unilateral anterior temporal lobe resection. Neurology. 2009;72:1837–1842. doi: 10.1212/WNL.0b013e3181a71132. [DOI] [PubMed] [Google Scholar]
- 25.Gleissner U, Sassen R, Schramm J, Elger CE, Helmstaedter C. Greater functional recovery after temporal lobe epilepsy surgery in children. Brain. 2005;128(Pt 12):2822–2829. doi: 10.1093/brain/awh597. [DOI] [PubMed] [Google Scholar]
- 26.Jambaqué I, Dellatolas G, Fohlen M, Bulteau C, Watier L, Dorfmuller G, et al. Memory functions following surgery for temporal lobe epilepsy in children. Neuropsychologia. 2007;45:2850–2862. doi: 10.1016/j.neuropsychologia.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Skirrow C, Cross JH, Cormack F, Harkness W, Vargha-Khadem F, Baldeweg T. Long-term intellectual outcome after temporal lobe surgery in childhood. Neurology. 2011;76:1330–1337. doi: 10.1212/WNL.0b013e31821527f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freitag H, Tuxhorn I. Cognitive function in preschool children after epilepsy surgery: rationale for early intervention. Epilepsia. 2005;46:561–567. doi: 10.1111/j.0013-9580.2005.03504.x. [DOI] [PubMed] [Google Scholar]
- 29.Malmgren K, Olsson I, Engman E, Flink R, Rydenhag B. Seizure outcome after resective epilepsy surgery in patients with low IQ. Brain. 2008;131(Pt 2):535–542. doi: 10.1093/brain/awm296. [DOI] [PubMed] [Google Scholar]
- 30.Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008;49:1377–1394. doi: 10.1111/j.1528-1167.2008.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]