Abstract
Silver nitrate staining of decalcified bone sections is known to reveal osteocyte canaliculi and cement lines. Nucleolar Organising Regions (NOR) are part of the nucleolus, containing argyrophilic proteins (nucleoclin/C23, nucleophosmin/B23) that can be identified by silver staining at low pH. The aim of this study was to clarify the mechanism explaining why AgNOR staining also reveals osteocyte canaliculi. Human bone and kidney sections were processed for silver staining at light and electron microscopy with a modified method used to identify AgNOR. Sections were processed in parallel for immunohistochemistry with an antibody direct against osteopontin. Protein extraction was done in the renal cortex and decalcified bone and the proteins were separated by western blotting. Purified hOPN was also used as a control. Proteins were electro-transferred on polyvinylidene membranes and stained for AgNOR proteins. In bone, Ag staining identified AgNOR in cell nuclei, as well as in osteocyte canaliculi, cement and resting lines. In the distal convoluted tubules of the kidney, silver deposits were also observed in cytoplasmic granules on the apical side of the cells. Immunolocalization of osteopontin closely matched with all these locations in bone and kidney. Ag staining of membranes at low pH revealed bands for NOR proteins and 56 KDa (kidney), 60KDa (purified hOPN) and 75 KDa (bone) bands that corresponded to osteopontin. NOR proteins and osteopontin are proteins containing aspartic acid rich regions that can bind Ag. Staining protocols using silver nitrate at low pH can identify these proteins on histological sections or membranes.
Keywords: Animals; Antigens, Nuclear; metabolism; Biopsy; Blotting, Western; Bone and Bones; cytology; embryology; metabolism; surgery; ultrastructure; Cattle; Embryo, Mammalian; Histocytochemistry; methods; Humans; Immunohistochemistry; Kidney Cortex; chemistry; metabolism; Kidney Glomerulus; cytology; metabolism; Nuclear Proteins; metabolism; Osteocytes; metabolism; ultrastructure; Osteopontin; isolation & purification; metabolism; Silver Staining
Keywords: Osteopontin, AgNOR Proteins, Silver Staining, Histochemistry, Non Collagenous Proteins.
INTRODUCTION
The histochemical demonstration of osteocyte canaliculi in bone sections was reported to be possible with silver impregnation methods several years ago (Christie, 1977; Kusuzaki et al., 1995). However, the exact mechanisms underlying these techniques have not been clearly elucidated. We reported that a staining method used for the demonstration of Nucleolar Organiser Regions (NORs) could be adapted to bone at light and electron microscopic levels (Chappard et al., 1996; Chappard et al., 1998). NORs are loops of DNA that contain the genes coding for ribosomal RNA, which are involved with the synthesis of ribosomes. Active NORs are associated with a subset of specific nonhistone proteins and some of them can be detected by cytochemical reactions first described in the 1970s (Goodpasture and Bloom, 1975; Howell et al., 1975). These cytochemical methods are based on the specific argyrophilic (Ag) affinity at low pH of the NOR-associated proteins, which are located in the fibrillar centers of the nucleoli. Pathological and embryological studies have shown that AgNOR number is correlated with the proliferative activity of cell populations, whether normal or malignant (Ploton et al., 1984; Derenzini et al., 1989; Ruschoff et al., 1989; Derenzini and Trere, 1994). On western blots of nucleolus proteins, an adaptation of the AgNOR histological technique has identified nucleolin and protein B23 as the main argyrophilic proteins associated with the ribosomal genes (Hozak et al., 1992; Roussel et al., 1992; Derenzini and Trere, 1994).
On histological bone sections, we found that AgNOR staining detected not only NORs located in the nucleoli, but also revealed argyrophilic materials in the bone matrix that corresponded to osteocyte canaliculi and cement lines. The mapping of silver deposits obtained after AgNOR staining seems closely related with the immunocytochemical localization of osteopontin (OPN), an acidic glyco and phosphoprotein of the bone matrix (Pinero et al., 1995; Sodek et al., 2000). OPN is a member of the SIBLING (Small Integrin-Binding LIgand, N-linked Glycoprotein) family (Fisher et al., 2001). It is produced by both osteoblasts and osteoclasts, and is localized in the osteoid (especially enriched in the cement lines and the arrest lines at the bone surfaces). It is also highly expressed in the vicinity of osteocytes (in the periphery of the osteocyte lacuna and in the canaliculi) (McKee and Nanci, 1996). Although the exact roles of OPN are not fully understood, some studies have shown that it is implicated in the calcification process by its ability to regulate the growth of hydroxyapatite crystals (Shiraga et al., 1992; Boskey et al., 1993; Hunter et al., 1994). OPN is also expressed in a variety of other cell types, including macrophages, angiogenic cells and epithelial cells of the distal convoluted tubule in the kidney (Lopez et al., 1993). The functions of OPN in the kidney have been recently reviewed, and the major action seems to be the inhibition of calcium oxalate deposition (Shiraga et al., 1992; Hwang et al., 1994; Rittling and Denhardt, 1999; Mazzali et al., 2002).
The aim of the present study was to verify if the AgNOR staining method could be used as a reliable cytochemical technique for the detection of OPN, explaining the silver labelling of osteocyte canaliculi. Immunohistochemistry and AgNOR staining were used to localize and compare OPN distribution and silver deposits on human bone and kidney sections, since OPN is essentially expressed in these two tissues. Western blot analysis and AgNOR staining adapted to membranes were performed in parallel and compared to see whether OPN is an argyrophilic protein detected by the AgNOR staining method.
MATERIAL AND METHODS
Tissue samples preparation
Human renal cortex was obtained from the normal renal parenchyma of a patient with cancer of the upper pole. Three femoral heads were obtained from patients with hip fractures or arthritis, and subjected to hip prosthesis. Tissue samples of both kidney and bone were received unfixed soon after surgical removal. They were immediately sampled with a scalpel or a banding saw. Some specimens were fixed for immunohistochemistry analysis, while others were frozen at −80°C for further biochemical analysis.
For histochemistry, samples of renal cortex and bone were immersed in freshly prepared 4% paraformaldehyde in sodium phosphate buffer (PBS), pH 7.4, for 24h at +4°C. The fixed tissues were decalcified in 0.5M EDTA pH 7.5 at room temperature (RT) for 2weeks, extensively rinsed in PBS, dehydrated in ethanol and embedded in paraffin wax at 56°C. The blocks were stored at +4°C until use. Sections (5μm thick) were prepared and mounted on silanized glass slides.
Histological analysis
AgNOR staining on kidney and bone tissue sections
Paraffin sections were dewaxed in xylene, rehydrated through a series of graded ethanol and stained with a modification of the Ploton’s technique (Ploton et al., 1986). Briefly, sections were incubated for 55min at RT in the dark, in a staining solution prepared by combining silver nitrate (2 volumes of a 50% aqueous solution) with formic acid (1 volume of a 1% solution containing 2% gelatin). After staining, sections were thoroughly washed in distilled water (DW) and transferred for 10min to a 5% aqueous sodium thiosulfate solution prepared extemporaneously. Sections were then rinsed with DW, mounted and observed by light microscopy.
AgNOR staining on bone thin sections for transmission electron microscopy (TEM)
Undecalcified human bone biopsies were cut into small cubes (1mm3) and immediately transferred to paraformaldehyde 4% in a pH 7.4 phosphate buffer. Fixation was carried out at 4°C for 90 min. Samples were washed in PBS and dehydrated in a series of graded ethanols. Embedding was performed in the LR-White resin (London Resin Company Ltd, Hampshire, UK). Infiltration was done at low temperature (−18°C) with a series of graded concentrations of LR-White in absolute ethanol. LR-White was polymerized at −18°C by UV irradiation. Ultrathin sections (60 nm thick) of undecalcified bone were cut with a diamond knife on a Reichert Ultracut S and transferred to gold grids coated with a thin film of collodion. AgNOR staining was done as previously described for TEM (Chappard et al., 1996). Grids were air-dried and contrasted with uranyl acetate (saturated solution in 50° ethanol). After a final rinse in 50° ethanol, sections were viewed in a JEOL 100 CX electron microscope at 80 kV.
Immunohistochemistry of kidney and bone tissue sections
For antigen unmasking, deparaffinized sections were incubated with 0.05% pronase in PBS for 20min at +37°C. The enzymatic reaction was stopped after several washes in cold PBS. Saturation of non-specific binding sites was done with 1% bovine serum albumin (BSA) in PBS for 15min. Sections were then incubated overnight at +4°C with a 1:50 rabbit anti human osteopontin peptide (75-90) primary polyclonal antibody recognizing the PSKSNESHDHMDDMDD sequence (Immundiagnostik AG, Bensheim, Germany). After rinsing in PBS, immunoreaction was revealed with a 1:100 diluted goat anti-rabbit IgG secondary antibody coupled with Alexa Fluor 488 (Molecular Probes, Eugen, Oregon, USA) for 1h at room temperature. After incubation, sections were extensively rinsed in PBS and mounted in glycerol-PBS (9:1). The immunohistochemical labeling was then visualized by epifluorescence. The specificity of each antibody has been documented by immunocytochemical controls, including incubation of sections with goat anti-rabbit fluorochrome conjugate.
Biochemical analysis
Protein extraction from tissue samples
Samples of renal cortex frozen at −80°C were minced using a clean blade, and immediately thawed at +4°C in lysis buffer (50mM Tris-HCl pH 7.5, 100mM NaCl, 50mM sodium fluoride, 3mM sodium orthovanadate, 0.1% SDS, 1% NP-40) containing proteinase inhibitors (PI) (1mM phenylmethylsulfonyl fluoride, 5μg/ml pepstatin, 5μg/ml leupeptin, 5μg/ml aprotinin). We used 3ml of ice cold lysis buffer + PI per gram of tissue. Tissue was then mixed and homogenized with an Ultra-Turrax T25 device for 3min at 24000rpm, maintaining temperature at +4°C throughout all procedures. The cell lysate was incubated overnight at +4°C with a continuous agitation, followed by centrifugation at 15620G for 15min at +4°C. The supernatant was removed and centrifuged again to obtain a clarified lysate. Protein extract was finally aliquoted and stored at −80°C until ready to use.
Biochemical analysis was also performed to characterize proteins extracted from a bovine fetal bone sample containing the target OPN protein (a gift from L. Malaval INSERM, U-403, Lyon, France). This purified and freezedried protein sample was resuspended in 0.1M Tris-HCl buffer, pH 7.5, overnight at +4°C with continuous agitation.
In parallel, purified hOPN was purchased from R&D Systems (Lille, France) and was used as a control.
Silver staining of bone and kidney total proteins in gel
Extracted proteins from kidney (16μg), bone (10μg), and hOPN control (6.25ng) were resolved on a 12% SDS-PolyAcrylamide gel electrophoresis using a Protean II cell (Bio-Rad Laboratories, Richmond, CA, USA) according to the method of Laemmli (Laemmli, 1970). Low molecular-mass protein standards (Sigma, St Louis, MO, USA) were used to estimate the molecular-weight values of migrated proteins. On the one hand, the gel was stained using the silver staining method as described by Shevchenko et al., in order to visualize total proteins in polyacrylamide gels (Shevchenko et al., 1996). On the other hand, following electrophoresis, proteins in the gel were electrotransferred for 2h at 50mA onto PolyVinylidene DiFluoride (PVDF) membrane (Millipore, Bedford, MA, USA) using a Multiphor II Electrophoresis System (Amersham Pharmacia Biotech, Uppsala, Sweden). The membrane was then stained by a specific AgNOR method (Hozak et al., 1992).
OPN detection by western blot analysis
Extracted proteins from kidney was immunoprecipitated overnight at +4°C in the presence of an anti human OPN peptide (75-90) polyclonal antibody (Immundiagnostik AG, Bensheim, Germany) (4μg/ml). Immunoprecipitated proteins were then isolated with beads coupled to Protein A/G PLUS-Agarose for 1h at +4°C. Immunoprecipitated complexes (10μl) and hOPN control (6.25ng) were submitted to 12% SDS-PAGE and proteins in the gel were electrophoretically transferred for 2h at 50mA onto PVDF membrane. The membrane was further blocked in 5% nonfat dry milk in 1X Tris Buffer Saline (TBS) (50mM Tris-HCl, pH 7.6, 200mM NaCl) overnight at +4°C. The membrane was washed 3 × 5min with TBS and then incubated with anti human OPN peptide (75-90) primary antibody diluted at 1:30000 in TBS with 1% nonfat dry milk for 4–5h at RT. Following extensive rinsing, horseradish-peroxidase (HRP) labeled goat anti rabbit secondary antibody (Santa Cruz Biotechnology, Inc., Heidelberg, Germany) used at 1:20000 was added and incubated with membrane for 45min. Immunoreactive bands were visualized using an ECL western-blotting reagent according to the manufacturer protocol (Santa Cruz Biotechnology).
AgNOR staining on membrane
After immunoblotting, the membrane was stripped in 0.1M glycine-HCl, 0.1M pH 2.5 overnight at +4°C and neutralized in Tris-HCl 1M, pH 7.6. The membrane was washed twice for 5min each with TBS, then extensively rinsed with DW, and stained by a specific AgNOR method adapted to western blots (Hozak et al., 1992). Briefly, the membrane was stained in plastic dishes, all solutions being prepared in freshly deionized water. The AgNOR staining method with gelatin colloidal developer was used with a 2:1 (V/V) ratio of staining solutions A (0.5g/ml AgNO3 in water) and B (1g gelatin in 100ml of water containing 1% formic acid). The membrane was stained for 20min at RT in the dark and under continuous stirring. The staining was stopped by thorough rinsing in water and the membrane was then incubated for 2min at RT in 5% sodium thiosulfate in water (prepared extemporaneously). After extensive washing in water, the membrane was dried at +40°C for 15min.
RESULTS
Histological analysis
AgNOR staining on tissue sections
AgNORs appeared as black dots in the nucleoli of all cells. The reaction was clearly evidenced in marrow and bone cells and also on the renal tubular cells. On the bone sections, dense stripes of argyrophilic material were found around the osteocytes and canaliculi and at the periphery of their lacunae (Figure 1A). The delicate inter-osteocyte relationships were clearly evidenced. Linear deposits were also observed in the matrix and corresponded to the cement and resting lines.
Figure 1.
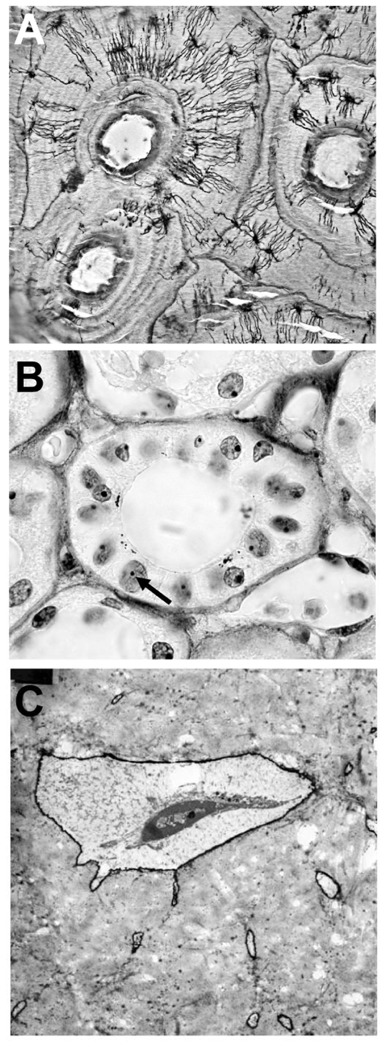
AgNOR staining on human tissues. A) cortical bone: note the intense staining in the osteocyte canaliculi and lacunar walls. Staining is also evident on resting and arrest lines. Original magnification: ×100. B) renal cortex: a distal convoluted tubule is observed in cross section. Note the NORs in a tubular nucleus (arrow) and the granules at the apical side of the cells. Original magnification: ×400. C) TEM of a bone osteocyte and lacuna stained by the AgNOR method. The staining is restricted to the lacuna’s wall and the periphery of the canaliculi. Original magnification ×9,000.
On kidney sections, silver precipitates were seen as small black dots in a significant number of cells of the cortex. AgNOR staining identified intracytoplasmic granules only in cells of the distal tubule and the ascending limb of the loop of Henle. The black granules were seen in the supra-nuclear area of the cells, toward the lumen of the renal tubules (Figure 1B).
On thin sections processed for TEM, dense stripes of argyrophylic material were found around the osteocytic canaliculi and at the periphery of their lacunae. Linear deposits were also observed in the matrix and corresponded to the cement lines observed with light microscopy. In the cells, components of the nucleoli and nuclei were identified by the light contrast provided by uranyl acetate, although nonspecific, minute silver deposits were randomly distributed (Figure 1C).
Immunolocalization of OPN
Immunohistochemistry revealed a strong and specific labeling for osteopontin in the extracellular matrix of bone. Positive labeling was localized at the border of mineralized areas (arrest and cement lines) and at the bone surface. In addition, OPN was associated with lining cells, osteocyte canaliculi and in the wall of osteocyte’s lacunae (Figure 2). Immunofluorescence showed that the distribution for osteopontin was only in calcified bone and not in the osteoid. In human kidney, osteopontin was strongly expressed in the epithelial cells lining distal renal tubules (Figure 3A). We observed that OPN was preferentially localized to the distal convulated tubules and to the thin loop of Henle. A layer of intense staining was seen at the apical cell side, with lesser fluorescence at the baso-lateral sides (Figure 3B).
Figure 2.
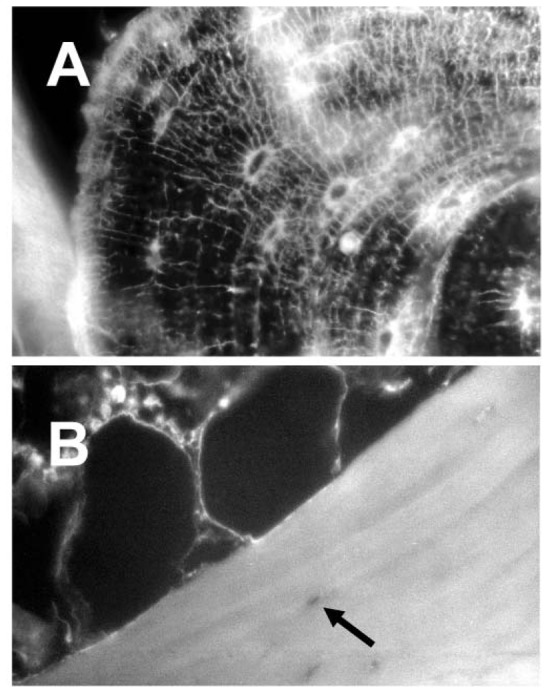
Immunohistochemical identification of osteopontin in human bone. A) trabecular bone: note the intense positivity in the osteocyte canaliculi, lacunar walls and arrest lines. Original magnification: ×100. B) negative control without the 1st antibody, note the negativity of the osteocyte lacuna (arrow). Original magnification: × 200.
Figure 3.
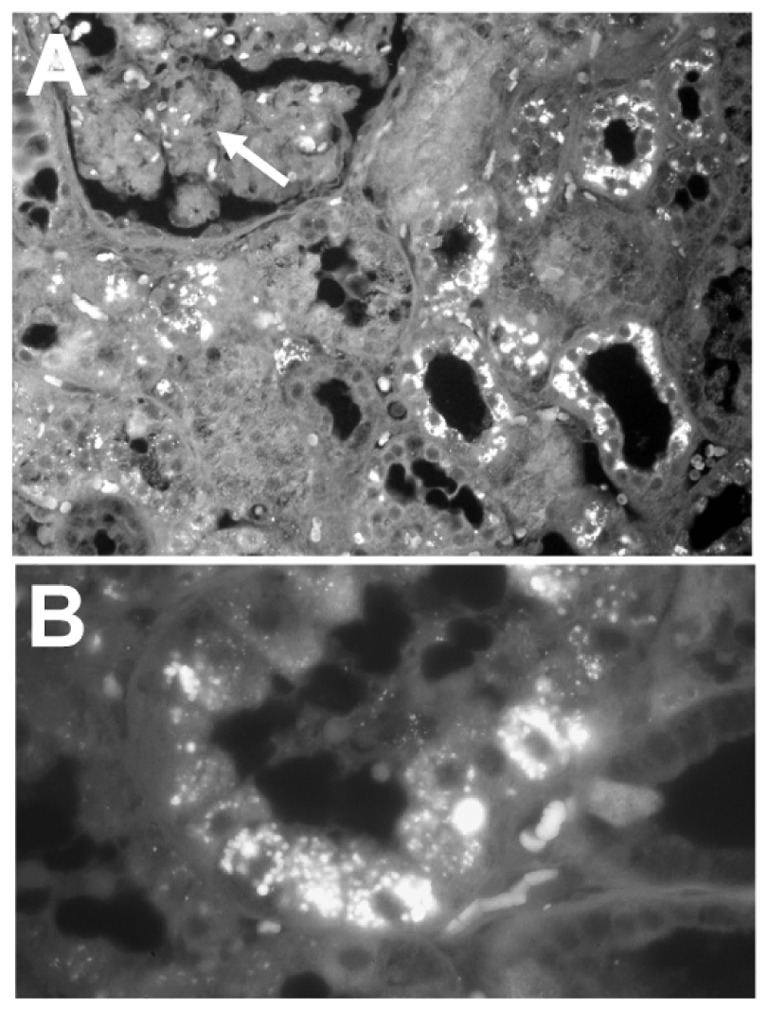
Immunohistochemical identification of osteopontin in human kidney. A) Part of a glomerulus is identified (arrow). Note the intense positivity in the convoluted tubules on the right. Original magnification: ×50. B) Higher magnification of a section of a convoluted tubule, the OPN granules are identified within the cell cytoplasms. Original magnification: ×200.
Similar localization of OPN and silver staining sites
Comparing the two staining methods, AgNOR staining was closely correlated with immunofluorescence for OPN in both renal cortex and bone tissues. The resulting OPN immunolabeling reproduced the staining pattern of NOR-silver staining. The relationship between these staining methods was clearly evidenced, since fluorescence and silver precipitate distributions demonstrated a similar localization of OPN expression and argyrophilic sites in human kidney and bone.
Biochemical analysis
Argyrophilic protein detection in kidney and bone tissues
For visualization of all proteins, the nonspecific silver staining of polyacrylamide gels exhibited a complex pattern with many bands of different intensity in human kidney and bovine fetal bone extracts (Figure 4). In contrast to these complex spectra, the AgNOR staining revealed only a subset of them. Positive bands appeared brown on the PVDF membrane (Figure 5). This approach facilitated the individual characterization and identification of these specific argyrophilic proteins. The AgNOR banding pattern of the human renal cortex and bovine fetal bone extracts were almost similar. A small number of positive bands were revealed, with the major bands at 70, 65, 56, 40, 38 and 29 kDa for human kidney tissue, and 75, 50, 39 and 29 kDa for bovine bone extract. Purified hOPN protein was positively AgNOR stained and could be associated with an argyrophilic protein in human kidney (56 kDa). Additional positive bands were observed in bone and kidney tissue but they were not further characterized.
Figure 4.
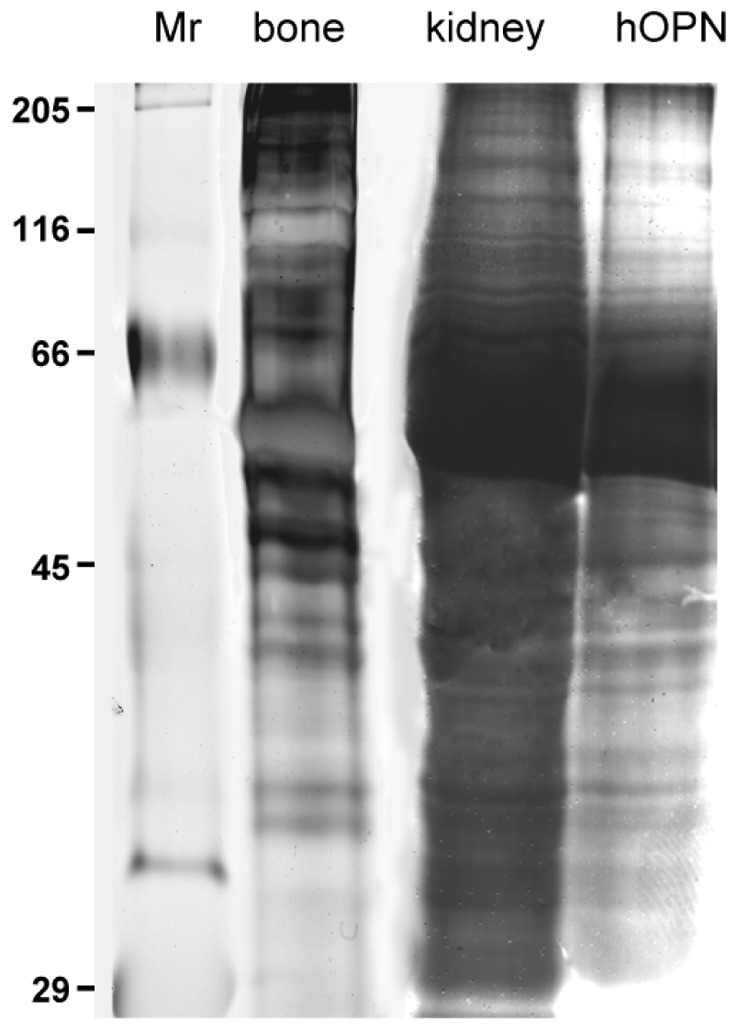
Analysis of protein extracts by 12% SDS-PAGE. Silver staining on gel. Bovine bone proteins: 10μg, human kidney proteins: 16μg, purified hOPN: 2.5ng stained by a nonspecific silver staining exhibiting a very complex pattern with many positive bands. Protein standards (Mr) indicate the position of the molecular weight markers
Figure 5.
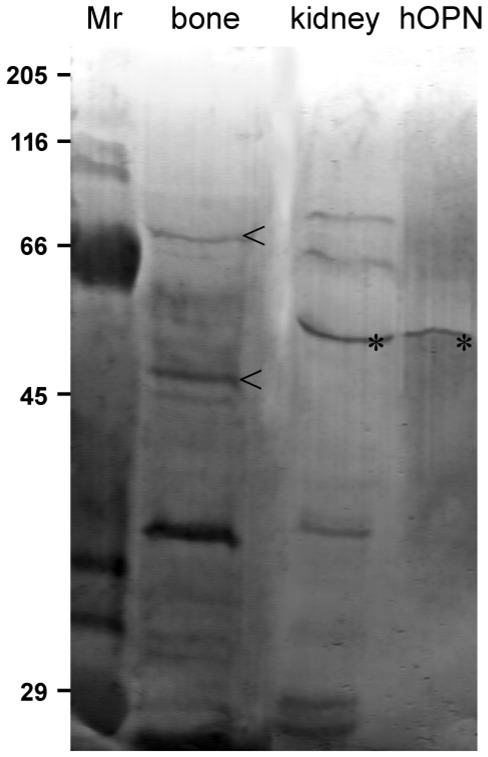
Analysis of protein extracts by 12% SDS-PAGE. AgNOR staining on membrane. The same amounts of proteins as in figure 4. Only a few specific bands of proteins are stained. Note the positive OPN bands at 56kDa for the purified hOPN that can be associated to a similar argyrophilic protein in human renal cortex (*). Bovine bone tissue exhibit AgNOR protein bands at 75 and 50kDa (<). Protein standards (Mr) indicate the position of the molecular weight markers.
Co-localization of OPN in western blot analysis and AgNOR staining on membrane
Western blot analysis was then performed to verify if the 56 kDa argyrophilic protein detected in kidney extract was osteopontin. On the blot, the immunoprecipitated complex revealed an intense 56 kDa osteopontin band in the kidney tissue, and the purified hOPN reported a 60 kDa protein (Figure 6A). The AgNOR banding pattern of these identical protein samples (Figure 6B) showed a co-localization with OPN immunoblotting pattern. This result confirmed that 56 kDa argyrophilic protein detected in human kidney tissue was OPN protein. The reactivity of human OPN peptide (75-90) antibody was also performed on bone extract and on kidney extract without immunoprecipitation stage (10μg and 20μg total protein respectively were loaded). However, bone and kidney OPN was not detected, probably because the level of OPN transferred to the membrane was below the level of detection of this assay.
Figure 6.
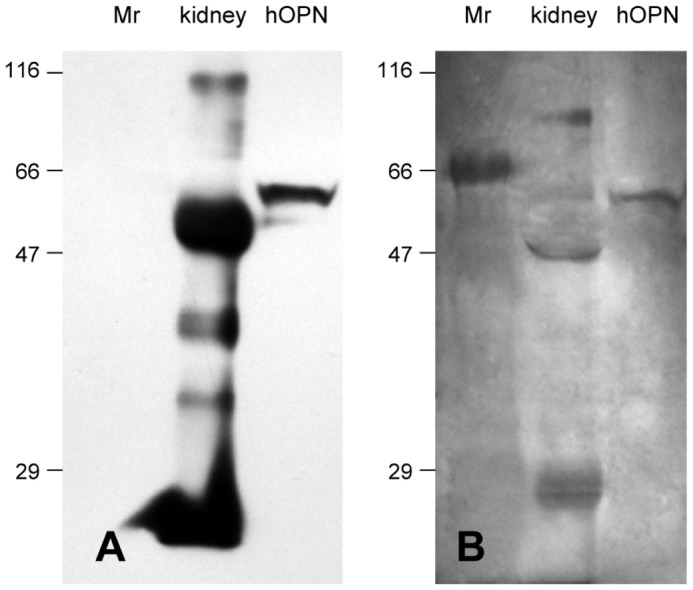
Human OPN detection in kidney tissue by western blotting (A) and AgNOR staining on the same membrane (B). Kidney cell lysate was immunoprecipitated with anti human OPN peptide (75-90) antibody. Immunoprecipitated complexes and 6.25ng of hOPN control were subjected to 12% SDS-PAGE and western blot analysis was performed with anti-hOPN. The blot reveals an intense 56kDa OPN protein in kidney tissue and a 60kDa purified hOPN control. After immunoblotting, membrane stained by AgNOR exhibited a similar pattern with a 56kDa OPN band in human kidney extract and a 60kDa purified hOPN protein. Protein standards (Mr) indicate the position of the molecular weight markers.
DISCUSSION
AgNOR proteins represent a good marker for ribosomal genes in metaphase chromosomes and interphase nuclei. During interphase, the NORs are present in the fibrillar components of the nucleolus (Hernandez-Verdun and Derenzini, 1983). There is evidence that nucleolar changes can be effectively evaluated by considering the number and size of interphase NORs. In the present study, at the light microscopy level, NORs appeared as well-defined black dots distributed within the cell nuclei, as previously reported by many authors (Ploton et al., 1984; Moreno et al., 1985; Derenzini et al., 1989; Derenzini and Trere, 1994). Nucleolin, protein B23, RNA polymerase I represent the main argyrophilic NORs proteins observed in cell nucleoli (Lischwe et al., 1979; Ochs and Busch, 1984; Spector et al., 1984; Roussel et al., 1992; Derenzini and Trere, 1994; Sirri et al., 1997)
AgNOR staining also identified osteocyte canaliculi, cement and resting lines of the bone matrix and granules in the apical cytoplasm of the renal tubular epithelium. In the present study, OPN immunolocalization in bone and kidney was similar to the distribution of silver stained structures in both tissues considered here. OPN antibodies never recognized the NOR in the nuclei of bone and kidney cells. Immunohistochemistry indicated that OPN was confined to the calcified bone matrix (especially concentrated at cement lines and around osteocyte canaliculi) and not in the osteoid. TEM analysis confirmed that AgNOR was localized in the osteocyte canaliculi and in the wall of its lacuna. OPN is one of the most abundant non-collagenous proteins in bone. Numerous biochemical and immunocytochemical reports have largely documented OPN expression in bone and kidney in various species (Gerstenfeld et al., 1990; Grynpas et al., 1994; McKee et al., 1995; Devoll et al., 1997; Giachelli and Steitz, 2000; Sodek et al., 2000). The exact roles of OPN in bone are not fully understood. In vitro studies by several groups have established that OPN is an inhibitor of calcification (Boskey et al., 1993; Hunter et al., 1994), except when crosslinked at high concentrations to agarose beads (Ito et al., 2004). In vivo, calcification of vascular smooth muscle cells is potently inhibited by OPN (Wada et al., 1999). Bones from OPN-knockout mice have a greater mineral content and contain larger hydroxyapatite crystals than wild type animals (Boskey et al., 2002). Phosphorylation of serine sequences, together with acidic amino acids (aspartic residues) of the OPN molecule have been implicated as factors having an inhibiting potential for hydroxyapatite crystallization (Singh et al., 1993; Pampena et al., 2004). It is probable that osteocytes elaborate OPN to inhibit the growth of hydroxyapatite in the wall of their lacuna and in the canaliculi to avoid “suffocation” due to secondary mineralization. OPN is also produced and localized in the kidney, and more particularly in epithelial cells of the convoluted distal tubule, a region with a high propensity for spontaneous precipitation of calcium salts. That OPN is often enriched in biological fluids having elevated levels of calcium salts is also indicative of a role in preventing spontaneous precipitation of calcium salts (Wesson et al., 2003).
In the present study, OPN was characterized by its high affinity for silver in the AgNOR reaction done on histological sections, TEM thin sections and membranes, but the chemical structure(s) responsible for specific argyrophilia is questionable. The histochemical mechanisms of the argyrophilia are quite well understood and probably occur in two stages, namely, an initial attachment of silver to a reaction site on a protein, followed by nucleation of further silver on the original bound metal, giving a black appearance (Horobin, 1988; Crocker, 1996). The argyrophilic nature of nucleolin is related to its N-terminal domain which is particularly enriched with acidic amino acids (aspartic residues). No correlation has been found between the level of phosphorylation of nucleolin and its silver stainability. Using CHO-purified nucleolin and different nucleolin fragments generated by cleavage, it was demonstrated that the N-terminal domain of nucleolin and not the carboxy-part of the protein, was involved in the AgNOR staining (Roussel et al., 1992). The specificity of AgNOR staining to acidic stretches in other proteins was confirmed for protein B23. The aspartic residues seem responsible for the AgNOR staining of the protein B23 (Valdez et al., 1995), similar findings have been described for SSP29, a leucin-rich repeat protein containing Asp residues (Zhu et al., 1997). OPN structure is highly correlated with those of nucleolin and protein B23: it is a negatively-charged glyco and phosphoprotein of approximately 300 amino acid residues containing a RGD cell binding sequence, a calcium and hydroxyapatite binding site, two heparin binding domains and a thrombin cleavage site. In addition to these structural elements, OPN is aspartic acid-rich and may be highly phosphorylated on serines and threonines, depending on the tissue, endowing the protein with a highly acidic character (pI = 5) (Giachelli and Steitz, 2000; Pampena et al., 2004). The technical acid conditions of the AgNOR staining are responsible for its specificity. The acidity of the gelatin-silver bath appears to be a critical step which confers specificity to AgNOR binding (Lindner, 1993). Furthermore, the final rinse in sodium thiosulfate avoids the many fine granules due to unreacted reagents (Horobin, 1988). Other silver techniques that do not use such washing may produce fine positive granules, especially when an autometallographic step including gold chloride is used to increase staining intensity (Kusuzaki et al., 1995; Kusuzaki et al., 2000). In contrast, standard silver staining uses various oxidizing or reducing agents before silver-staining, resulting in positive or negative bands for all proteins. The comparison of total protein staining on gel and the NOR-silver staining on membrane shows that most proteins, even when abundant, are not revealed by NOR-silver staining. So, only proteins containing a high amount of Asp acidic amino acid residues, such as OPN, B23 and nucleolin, are candidates for argyrophilia after AgNOR staining. Aspartic acid has also be found, by Raman spectroscopy, to bind silver due to its two carboxylic groups and the possibility to form the HOOC-CH2-CH(NH3 +)-COO− species at a low pH (Arenas et al., 2001).
AgNOR staining on membrane detected several bands corresponding to OPN, consistent with previous reports (Devoll et al., 1997). The 56 kDa band observed in the kidney cortex exactly matched the purified protein in the hOPN lane stained on AgNOR membrane. In most species, OPN is a protein whose biochemical properties, including high negativity, lead to highly anomalous behavior on SDS-PAGE gels, so molecular weights between 44 and 85kDa have been reported in various tissues and species. OPN exists in several molecular forms, including intact (Prince et al., 1987), cleaved, (Senger et al., 1989) and multimeric isoforms (Beninati et al., 1994), which can explain suspected multi-band patterns of OPN observed on membranes in bone and kidney tissues. Monomeric OPN is a single gene product, but resolves into several bands during SDS-PAGE as a result of variable post-translational modification of the molecule (Sodek et al., 1995) or alternate mRNA splicing (Kiefer et al., 1989). Nevertheless, OPN cDNA exhibits a high degree of sequence homology from various mammalian species; three regions have conserved motifs between species: RGD sequence, thrombin cleavage site and the polyaspartic acid sequence (Giachelli and Steitz, 2000; Sodek et al., 2000).
The AgNOR staining method appears to possess specificity for proteins associated with the ribosomal genes containing aspartic acidic repeats in their N-terminal part. OPN is a SIBLING protein containing a polyaspartic sequence. It was detectable in bone and kidney by AgNOR staining and immunohistochemistry at the same locations. The AgNOR staining of membrane also identified OPN in tissue extract and on the purified protein. The staining also works on bone made free of osteocytes (bone allografts cleaned by industrial processes) and clearly identified AgNOR materials as a matrix protein (Dumas et al., 2006).
Acknowledgments
Authors wish to thank Mrs Nadine Gaborit-Retailleau for technical assistance. This work was supported by funds from “Pays de la Loire” – Axe Biomatériaux Bioregos and INSERM.
References
- Arenas JF, Castro JL, Otero JC, Marcos JI. Study of interaction between aspartic acid and silver by surface-enhanced Raman scattering on H(2)O and D(2)O sols. Biopolymers. 2001;62:241–248. doi: 10.1002/bip.1019. [DOI] [PubMed] [Google Scholar]
- Beninati S, Senger DR, Cordella-Miele E, Mukherjee AB, Chackalaparampil I, Shanmugam V, Singh K, Mukherjee BB. Osteopontin: its transglutaminase-catalyzed posttranslational modifications and cross-linking to fibronectin. J Biochem (Tokyo) 1994;115:675–682. doi: 10.1093/oxfordjournals.jbchem.a124395. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Maresca M, Ullrich W, Doty SB, Butler WT, Prince CW. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993;22:147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- Chappard D, Retailleau-Gaborit N, Filmon R, Audran M, Baslé MF. Increased nucleolar organizer regions in osteoclast nuclei of Paget’s bone disease. Bone. 1998;22:45–49. doi: 10.1016/s8756-3282(97)00226-3. [DOI] [PubMed] [Google Scholar]
- Chappard D, Retailleau N, Filmon R, Baslé MF, Rebel A. Nuclear organiser regions (AgNORs) staining on undecalcified bone embedded in resin: Light and TEM methodologies. J Histotechnol. 1996;19:27–32. [Google Scholar]
- Christie KN. The demonstration of canaliculi in sections of decalcified bone by a silver impregnation method. Stain Technol. 1977;52:301–302. doi: 10.3109/10520297709116801. [DOI] [PubMed] [Google Scholar]
- Crocker J. Molecular and biochemical aspects of interphase nucleolar organiser regions. J Clin Pathol: Mol Pathol. 1996;49:M8–M11. doi: 10.1136/mp.49.1.m8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derenzini M, Nardi F, Farabegoli F, Ottinetti A, Roncaroli F, Bussolati G. Distribution of silver-stained interphase nucleolar organizer regions as a parameter to distinguish neoplastic from nonneoplastic reactive cells in human effusions. Acta Cytol. 1989;33:491–498. [PubMed] [Google Scholar]
- Derenzini M, Trere D. AgNOR proteins as a parameter of the rapidity of cell proliferation. Zentralbl Pathol. 1994;140:7–10. [PubMed] [Google Scholar]
- Devoll RE, Pinero GJ, Appelbaum ER, Dul E, Troncoso P, Butler WT, Farach-Carson MC. Improved immunohistochemical staining of osteopontin (OPN) in paraffin- embedded archival bone specimens following antigen retrieval: anti- human OPN antibody recognizes multiple molecular forms. Calcif Tissue Int. 1997;60:380–386. doi: 10.1007/s002239900247. [DOI] [PubMed] [Google Scholar]
- Dumas A, Gaudin-Audrain C, Mabilleau G, Massin P, Hubert L, Baslé MF, Chappard D. The influence of processes for the purification of human bone allografts on the matrix surface and cytocompatibility. Biomaterials. 2006;27:4204–4211. doi: 10.1016/j.biomaterials.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Gotoh Y, McKee MD, Nanci A, Landis WJ, Glimcher MJ. Expression and ultrastructural immunolocalization of a major 66 kDa phosphoprotein synthesized by chicken osteoblasts during mineralization in vitro. Anat Rec. 1990;228:93–103. doi: 10.1002/ar.1092280113. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615–622. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- Goodpasture C, Bloom SE. Visualization of nucleolar organizer regions im mammalian chromosomes using silver staining. Chromosoma. 1975;53:37–50. doi: 10.1007/BF00329389. [DOI] [PubMed] [Google Scholar]
- Grynpas MD, Tupy JH, Sodek J. The distribution of soluble, mineral-bound, and matrix-bound proteins in osteoporotic and normal bones. Bone. 1994;15:505–513. doi: 10.1016/8756-3282(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Hernandez-Verdun D, Derenzini M. Non-nucleosomal configuration of chromatin in nucleolar organizer regions of metaphase chromosomes in situ. Eur J Cell Biol. 1983;31:360–365. [PubMed] [Google Scholar]
- Horobin R. Understanding Histochemistry: Selection, evaluation and design of biological stains. Chichester, England: Ellis Horwood Ltd; 1988. [Google Scholar]
- Howell WM, Denton TE, Diamond JR. Differential staining of the satellite regions of human acrocentric chromosomes. Experientia. 1975;31:260–262. doi: 10.1007/BF01990741. [DOI] [PubMed] [Google Scholar]
- Hozak P, Roussel P, Hernandez-Verdun D. Procedures for specific detection of silver-stained nucleolar proteins on western blots. J Histochem Cytochem. 1992;40:1089–1096. doi: 10.1177/40.8.1619275. [DOI] [PubMed] [Google Scholar]
- Hunter GK, Kyle CL, Goldberg HA. Modulation of crystal formation by bone phosphoproteins: structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem J. 1994;300:723–728. doi: 10.1042/bj3000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SM, Lopez CA, Heck DE, Gardner CR, Laskin DL, Laskin JD, Denhardt DT. Osteopontin inhibits induction of nitric oxide synthase gene expression by inflammatory mediators in mouse kidney epithelial cells. J Biol Chem. 1994;269:711–715. [PubMed] [Google Scholar]
- Ito S, Saito T, Amano K. In vitro apatite induction by osteopontin: interfacial energy for hydroxyapatite nucleation on osteopontin. J Biomed Mater Res A. 2004;69:11–16. doi: 10.1002/jbm.a.20066. [DOI] [PubMed] [Google Scholar]
- Kiefer MC, Bauer DM, Barr PJ. The cDNA and derived amino acid sequence for human osteopontin. Nucleic Acids Res. 1989;17:3306. doi: 10.1093/nar/17.8.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuzaki K, Kageyama N, Shinjo H, Maruta H, Takeshita H, Ashihara T, Hirasawa Y. A staining method for bone canaliculi. Acta Orthop Scand. 1995;66:166–168. doi: 10.3109/17453679508995514. [DOI] [PubMed] [Google Scholar]
- Kusuzaki K, Kageyama N, Shinjo H, Takeshita H, Murata H, Hashiguchi S, Ashihara T, Hirasawa Y. Development of bone canaliculi during bone repair. Bone. 2000;27:655–659. doi: 10.1016/s8756-3282(00)00383-5. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindner LE. Improvements in the silver-staining technique for nucleolar organizer regions (AgNOR) J Histochem Cytochem. 1993;41:439–445. doi: 10.1177/41.3.8429207. [DOI] [PubMed] [Google Scholar]
- Lischwe MA, Smetana K, Olson MO, Busch H. Proteins C23 and B23 are the major nucleolar silver staining proteins. Life Sci. 1979;25:701–708. doi: 10.1016/0024-3205(79)90512-5. [DOI] [PubMed] [Google Scholar]
- Lopez CA, Hoyer JR, Wilson PD, Waterhouse P, Denhardt DT. Heterogeneity of osteopontin expression among nephrons in mouse kidneys and enhanced expression in sclerotic glomeruli. Lab Invest. 1993;69:355–363. [PubMed] [Google Scholar]
- Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J. Osteopontin--a molecule for all seasons. QJMed. 2002;95:3–13. doi: 10.1093/qjmed/95.1.3. [DOI] [PubMed] [Google Scholar]
- McKee MD, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech. 1996;33:141–164. doi: 10.1002/(SICI)1097-0029(19960201)33:2<141::AID-JEMT5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- McKee MD, Nanci A, Khan SR. Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi. J Bone Miner Res. 1995;10:1913–1929. doi: 10.1002/jbmr.5650101211. [DOI] [PubMed] [Google Scholar]
- Moreno FJ, Hernandez-Verdun D, Masson C, Bouteille M. Silver staining of the nucleolar organizer regions (NORs) on Lowicryl and cryo-ultrathin sections. J Histochem Cytochem. 1985;33:389–399. doi: 10.1177/33.5.2580879. [DOI] [PubMed] [Google Scholar]
- Ochs RL, Busch H. Further evidence that phosphoprotein C23 (110 kD/pI 5.1) is the nucleolar silver staining protein. Exp Cell Res. 1984;152:260–265. doi: 10.1016/0014-4827(84)90251-9. [DOI] [PubMed] [Google Scholar]
- Pampena DA, Robertson KA, Litvinova O, Lajoie G, Goldberg HA, Hunter GK. Inhibition of hydroxyapatite formation by osteopontin phosphopeptides. Biochem J. 2004;378:1083–1087. doi: 10.1042/BJ20031150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinero GJ, Farach-Carson MC, Devoll RE, Aubin JE, Brunn JC, Butler WT. Bone matrix proteins in osteogenesis and remodelling in the neonatal rat mandible as studied by immunolocalization of osteopontin, bone sialoprotein, alpha 2HS-glycoprotein and alkaline phosphatase. Arch Oral Biol. 1995;40:145–155. doi: 10.1016/0003-9969(94)00144-z. [DOI] [PubMed] [Google Scholar]
- Ploton D, Menager M, Adnet JJ. Simultaneous high resolution localization of Ag-NOR proteins and nucleoproteins in interphasic and mitotic nuclei. Histochem J. 1984;16:897–906. doi: 10.1007/BF01002794. [DOI] [PubMed] [Google Scholar]
- Ploton D, Menager M, Jeannesson P, Himber G, Pigeon F, Adnet JJ. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region at the optical level. Histochem J. 1986;18:5–14. doi: 10.1007/BF01676192. [DOI] [PubMed] [Google Scholar]
- Prince CW, Oosawa T, Butler WT, Tomana M, Bhown AS, Bhown M, Schrohenloher RE. Isolation, characterization, and biosynthesis of a phosphorylated glycoprotein from rat bone. J Biol Chem. 1987;262:2900–2907. [PubMed] [Google Scholar]
- Rittling SR, Denhardt DT. Osteopontin function in pathology: lessons from osteopontin-deficient mice. Exp Nephrol. 1999;7:103–113. doi: 10.1159/000020591. [DOI] [PubMed] [Google Scholar]
- Roussel P, Belenguer P, Amalric F, Hernandez-Verdun D. Nucleolin is an Ag-NOR protein; this property is determined by its amino-terminal domain independently of its phosphorylation state. Exp Cell Res. 1992;203:259–269. doi: 10.1016/0014-4827(92)90063-e. [DOI] [PubMed] [Google Scholar]
- Ruschoff J, Plate K, Bittinger A, Thomas C. Nucleolar organizer regions (NORs). Basic concepts and practical application in tumor pathology. Pathol Res Pract. 1989;185:878–885. doi: 10.1016/S0344-0338(89)80290-0. [DOI] [PubMed] [Google Scholar]
- Senger DR, Perruzzi CA, Papadopoulos A, Tenen DG. Purification of a human milk protein closely similar to tumor-secreted phosphoproteins and osteopontin. Biochim Biophys Acta. 1989;996:43–48. doi: 10.1016/0167-4838(89)90092-7. [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Shiraga H, Min W, VanDusen WJ, Clayman MD, Miner D, Terrell CH, Sherbotie JR, Foreman JW, Przysiecki C, Neilson EG, et al. Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci U S A. 1992;89:426–430. doi: 10.1073/pnas.89.1.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Deonarine D, Shanmugam V, Senger DR, Mukherjee AB, Chang PL, Prince CW, Mukherjee BB. Calcium-binding properties of osteopontin derived from non-osteogenic sources. J Biochem (Tokyo) 1993;114:702–707. doi: 10.1093/oxfordjournals.jbchem.a124240. [DOI] [PubMed] [Google Scholar]
- Sirri V, Roussel P, Gendron MC, Hernandez-Verdun D. Amount of the two major Ag-NOR proteins, nucleolin, and protein B23 is cell-cycle dependent. Cytometry. 1997;28:147–156. [PubMed] [Google Scholar]
- Sodek J, Chen J, Nagata T, Kasugai S, Todescan R, Jr, Li IW, Kim RH. Regulation of osteopontin expression in osteoblasts. Ann N Y Acad Sci. 1995;760:223–241. doi: 10.1111/j.1749-6632.1995.tb44633.x. [DOI] [PubMed] [Google Scholar]
- Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- Spector DL, Ochs RL, Busch H. Silver staining, immunofluorescence, and immunoelectron microscopic localization of nucleolar phosphoproteins B23 and C23. Chromosoma. 1984;90:139–148. doi: 10.1007/BF00292451. [DOI] [PubMed] [Google Scholar]
- Valdez BC, Henning D, Le TV, Busch H. Specific aspartic acid-rich sequences are responsible for silver staining of nucleolar proteins. Biochem Biophys Res Commun. 1995;207:485–491. doi: 10.1006/bbrc.1995.1214. [DOI] [PubMed] [Google Scholar]
- Wada T, McKee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ Res. 1999;84:166–178. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- Wesson JA, Johnson RJ, Mazzali M, Beshensky AM, Stietz S, Giachelli C, Liaw L, Alpers CE, Couser WG, Kleinman JG, Hughes J. Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol. 2003;14:139–147. doi: 10.1097/01.asn.0000040593.93815.9d. [DOI] [PubMed] [Google Scholar]
- Zhu L, Perlaky L, Henning D, Valdez BC. Cloning and characterization of a new silver-stainable protein SSP29, a member of the LRR family. Biochem Mol Biol Int. 1997;42:927–935. doi: 10.1080/15216549700203371. [DOI] [PubMed] [Google Scholar]


