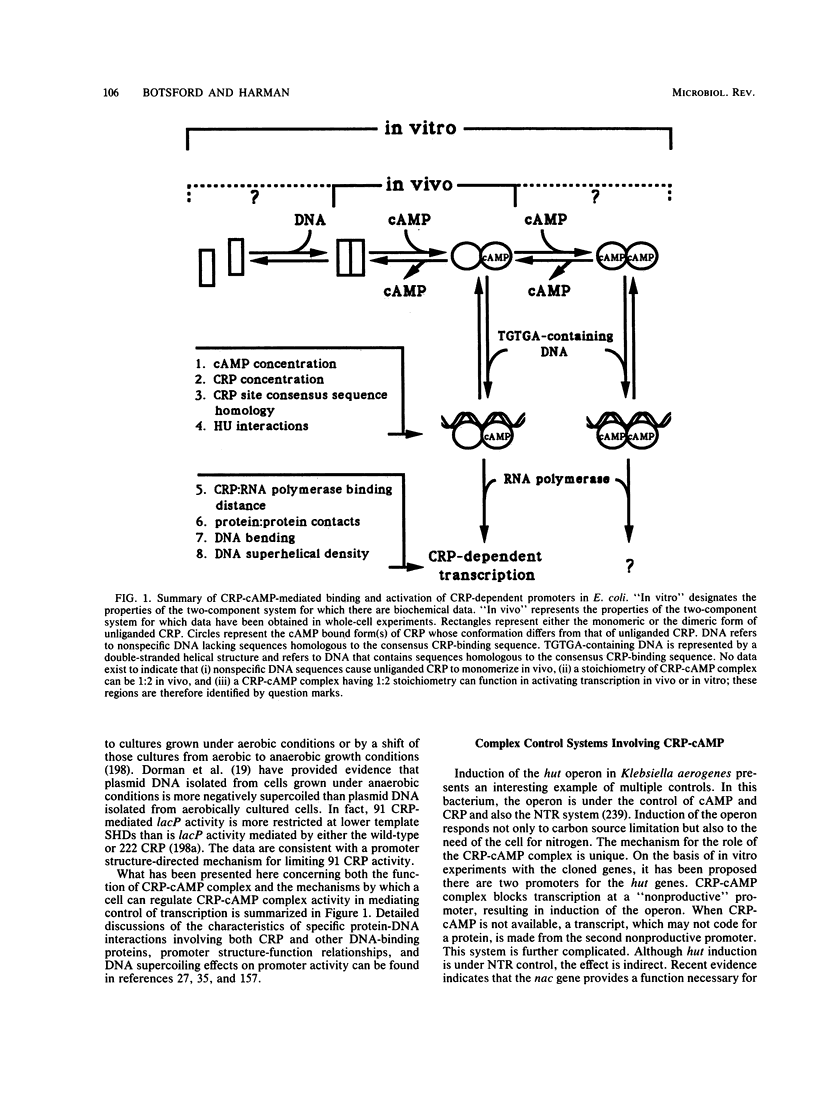
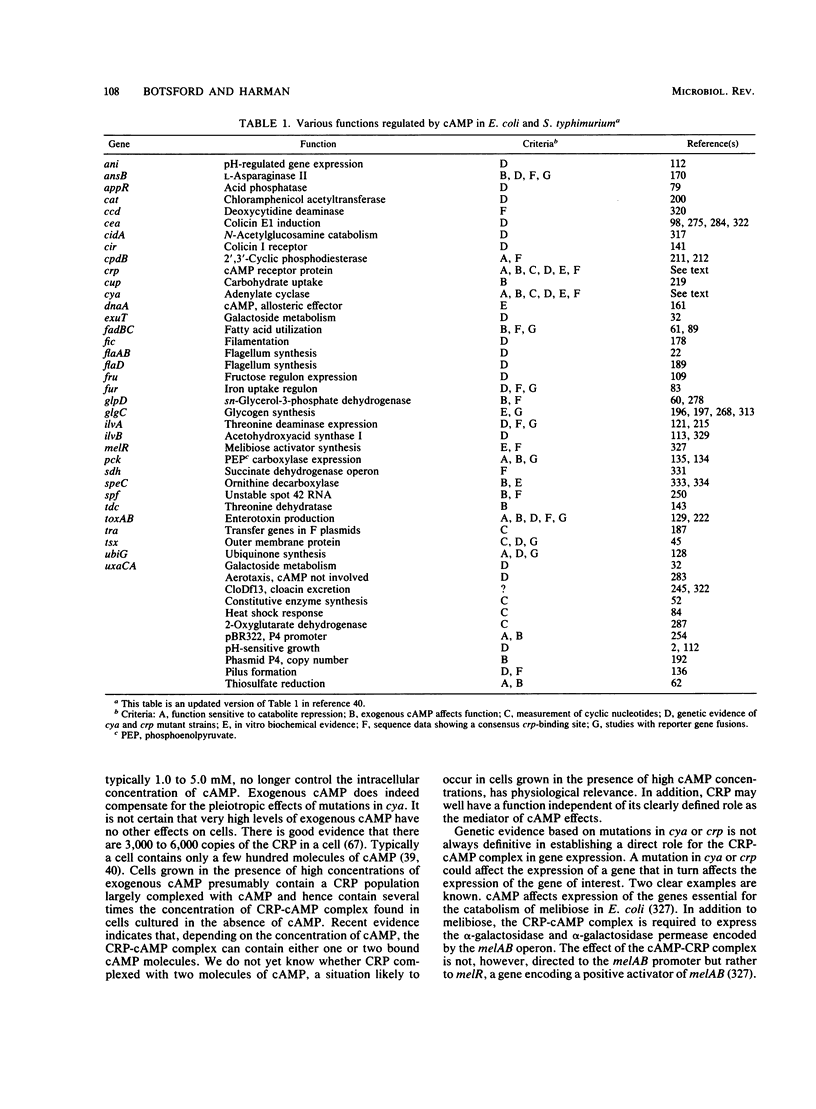
Abstract
Cyclic AMP (cAMP) is found in a variety of prokaryotes including both eubacteria and archaebacteria. cAMP plays a role in regulating gene expression, not only for the classic inducible catabolic operons, but also for other categories. In the enteric coliforms, the effects of cAMP on gene expression are mediated through its interaction with and allosteric modification of a cAMP-binding protein (CRP). The CRP-cAMP complex subsequently binds specific DNA sequences and either activates or inhibits transcription depending upon the positioning of the complex relative to the promoter. Enteric coliforms have provided a model to explore the mechanisms involved in controlling adenylate cyclase activity, in regulating adenylate cyclase synthesis, and in performing detailed examinations of CRP-cAMP complex-regulated gene expression. This review summarizes recent work focused on elucidating the molecular mechanisms of CRP-cAMP complex-mediated processes. For other bacteria, less detail is known. cAMP has been implicated in regulating antibiotic production, phototrophic growth, and pathogenesis. A role for cAMP has been suggested in nitrogen fixation. Often the only data that support cAMP involvement in these processes includes cAMP measurement, detection of the enzymes involved in cAMP metabolism, or observed effects of high concentrations of the nucleotide on cell growth.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Garges S. Positive control. J Biol Chem. 1990 Jul 5;265(19):10797–10800. [PubMed] [Google Scholar]
- Ahmad D., Newman E. B. A deficiency in cyclic AMP results in pH-sensitive growth of Escherichia coli K-12. J Bacteriol. 1988 Aug;170(8):3443–3447. doi: 10.1128/jb.170.8.3443-3447.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H. Autoregulation of the Escherichia coli crp gene: CRP is a transcriptional repressor for its own gene. Cell. 1983 Jan;32(1):141–149. doi: 10.1016/0092-8674(83)90504-4. [DOI] [PubMed] [Google Scholar]
- Aiba H., Fujimoto S., Ozaki N. Molecular cloning and nucleotide sequencing of the gene for E. coli cAMP receptor protein. Nucleic Acids Res. 1982 Feb 25;10(4):1345–1361. doi: 10.1093/nar/10.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H., Hanamura A., Yamano H. Transcriptional terminator is a positive regulatory element in the expression of the Escherichia coli crp gene. J Biol Chem. 1991 Jan 25;266(3):1721–1727. [PubMed] [Google Scholar]
- Aiba H., Mori K., Tanaka M., Ooi T., Roy A., Danchin A. The complete nucleotide sequence of the adenylate cyclase gene of Escherichia coli. Nucleic Acids Res. 1984 Dec 21;12(24):9427–9440. doi: 10.1093/nar/12.24.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H., Nakamura T., Mitani H., Mori H. Mutations that alter the allosteric nature of cAMP receptor protein of Escherichia coli. EMBO J. 1985 Dec 1;4(12):3329–3332. doi: 10.1002/j.1460-2075.1985.tb04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H. Transcription of the Escherichia coli adenylate cyclase gene is negatively regulated by cAMP-cAMP receptor protein. J Biol Chem. 1985 Mar 10;260(5):3063–3070. [PubMed] [Google Scholar]
- Amikam D., Benziman M. Cyclic diguanylic acid and cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol. 1989 Dec;171(12):6649–6655. doi: 10.1128/jb.171.12.6649-6655.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammerman J. W., Azam F. Characteristics of Cyclic AMP Transport by Marine Bacteria. Appl Environ Microbiol. 1987 Dec;53(12):2963–2966. doi: 10.1128/aem.53.12.2963-2966.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammerman J. W., Azam F. Uptake of Cyclic AMP by Natural Populations of Marine Bacteria. Appl Environ Microbiol. 1982 Apr;43(4):869–876. doi: 10.1128/aem.43.4.869-876.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricò B., Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987 Jun;169(6):2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au D. C., Masure H. R., Storm D. R. Site-directed mutagenesis of lysine 58 in a putative ATP-binding domain of the calmodulin-sensitive adenylate cyclase from Bordetella pertussis abolishes catalytic activity. Biochemistry. 1989 Apr 4;28(7):2772–2776. doi: 10.1021/bi00433a005. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke V. L., Gralla J. D. Changes in the linking number of supercoiled DNA accompany growth transitions in Escherichia coli. J Bacteriol. 1987 Oct;169(10):4499–4506. doi: 10.1128/jb.169.10.4499-4506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis V. A., Bassford P. J., Jr Regulation of adenylate cyclase synthesis in Escherichia coli: studies with cya-lac operon and protein fusion strains. J Bacteriol. 1982 Sep;151(3):1346–1357. doi: 10.1128/jb.151.3.1346-1357.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier C. S., Short S. A. Studies on deo operon regulation in Escherichia coli: cloning and expression of the cytR structural gene. Gene. 1985;36(1-2):37–44. doi: 10.1016/0378-1119(85)90067-8. [DOI] [PubMed] [Google Scholar]
- Barry E. M., Weiss A. A., Ehrmann I. E., Gray M. C., Hewlett E. L., Goodwin M. S. Bordetella pertussis adenylate cyclase toxin and hemolytic activities require a second gene, cyaC, for activation. J Bacteriol. 1991 Jan;173(2):720–726. doi: 10.1128/jb.173.2.720-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D. H., Frantz B. B., Matsumura P. Flagellar transcriptional activators FlbB and FlaI: gene sequences and 5' consensus sequences of operons under FlbB and FlaI control. J Bacteriol. 1988 Apr;170(4):1575–1581. doi: 10.1128/jb.170.4.1575-1581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton J. W., Melton T. Generation of deletions in the 3'-flanking sequences of the Escherichia coli crp gene that induce cyclic AMP suppressor functions. J Bacteriol. 1987 Feb;169(2):654–659. doi: 10.1128/jb.169.2.654-659.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batut J., Daveran-Mingot M. L., David M., Jacobs J., Garnerone A. M., Kahn D. fixK, a gene homologous with fnr and crp from Escherichia coli, regulates nitrogen fixation genes both positively and negatively in Rhizobium meliloti. EMBO J. 1989 Apr;8(4):1279–1286. doi: 10.1002/j.1460-2075.1989.tb03502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A., Gaston K., Williams R., Chapman K., Kolb A., Buc H., Minchin S., Williams J., Busby S. Mutations that alter the ability of the Escherichia coli cyclic AMP receptor protein to activate transcription. Nucleic Acids Res. 1990 Dec 25;18(24):7243–7250. doi: 10.1093/nar/18.24.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg O. G., von Hippel P. H. Selection of DNA binding sites by regulatory proteins. II. The binding specificity of cyclic AMP receptor protein to recognition sites. J Mol Biol. 1988 Apr 20;200(4):709–723. doi: 10.1016/0022-2836(88)90482-2. [DOI] [PubMed] [Google Scholar]
- Beuve A., Boesten B., Crasnier M., Danchin A., O'Gara F. Rhizobium meliloti adenylate cyclase is related to eucaryotic adenylate and guanylate cyclases. J Bacteriol. 1990 May;172(5):2614–2621. doi: 10.1128/jb.172.5.2614-2621.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini G. M., Pastini A. C., Muschietti J. P., Téllez-Iñn M. T., Martinetto H. E., Torres H. N., Flawiá M. M. Adenylate cyclase activity in cyanobacteria: activation by Ca(2+)-calmodulin and a calmodulin-like activity. Biochim Biophys Acta. 1990 Oct 15;1055(1):75–81. doi: 10.1016/0167-4889(90)90093-s. [DOI] [PubMed] [Google Scholar]
- Biville F., Guiso N. Evidence for the presence of cAMP, cAMP receptor and transcription termination factor rho in different gram-negative bacteria. J Gen Microbiol. 1985 Nov;131(11):2953–2960. doi: 10.1099/00221287-131-11-2953. [DOI] [PubMed] [Google Scholar]
- Black R. A., Hobson A. C., Adler J. Adenylate cyclase is required for chemotaxis to phosphotransferase system sugars by Escherichia coli. J Bacteriol. 1983 Mar;153(3):1187–1195. doi: 10.1128/jb.153.3.1187-1195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazy B., Takahashi M., Baudras A. Binding of CRP to DNA-dependent RNA polymerase from E. coli: modulation by cAMP of the interactions with free and DNA-bound holo and core enzyme. Mol Biol Rep. 1980 Mar 31;6(1):39–43. doi: 10.1007/BF00775753. [DOI] [PubMed] [Google Scholar]
- Blazy B., Ullmann A. Properties of cyclic AMP-independent catabolite gene activator proteins of Escherichia coli. J Biol Chem. 1986 Sep 5;261(25):11645–11649. [PubMed] [Google Scholar]
- Blum P. H., Jovanovich S. B., McCann M. P., Schultz J. E., Lesley S. A., Burgess R. R., Matin A. Cloning and in vivo and in vitro regulation of cyclic AMP-dependent carbon starvation genes from Escherichia coli. J Bacteriol. 1990 Jul;172(7):3813–3820. doi: 10.1128/jb.172.7.3813-3820.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Lee P. C., Wilson S. W., Cutler C. W., Ames B. N. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell. 1984 May;37(1):225–232. doi: 10.1016/0092-8674(84)90318-0. [DOI] [PubMed] [Google Scholar]
- Borowiec J. A., Zhang L., Sasse-Dwight S., Gralla J. D. DNA supercoiling promotes formation of a bent repression loop in lac DNA. J Mol Biol. 1987 Jul 5;196(1):101–111. doi: 10.1016/0022-2836(87)90513-4. [DOI] [PubMed] [Google Scholar]
- Botsford J. L. Cyclic AMP phosphodiesterase in Salmonella typhimurium: characteristics and physiological function. J Bacteriol. 1984 Nov;160(2):826–830. doi: 10.1128/jb.160.2.826-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L., Drexler M. The cyclic 3',5'-adenosine monophosphate receptor protein and regulation of cyclic 3',5'-adenosine monophosphate synthesis in Escherichia coli. Mol Gen Genet. 1978 Sep 20;165(1):47–56. doi: 10.1007/BF00270375. [DOI] [PubMed] [Google Scholar]
- Bracco L., Kotlarz D., Kolb A., Diekmann S., Buc H. Synthetic curved DNA sequences can act as transcriptional activators in Escherichia coli. EMBO J. 1989 Dec 20;8(13):4289–4296. doi: 10.1002/j.1460-2075.1989.tb08615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsch R., Decker K. The effect of gyrase inhibitors and cyclic AMP on induction and glucose repression of the 6-hydroxy-nicotine oxidases in Arthrobacter oxidans. Arch Microbiol. 1982 Dec 3;133(4):274–277. doi: 10.1007/BF00521289. [DOI] [PubMed] [Google Scholar]
- Bremer E., Gerlach P., Middendorf A. Double negative and positive control of tsx expression in Escherichia coli. J Bacteriol. 1988 Jan;170(1):108–116. doi: 10.1128/jb.170.1.108-116.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Crothers D. M. Modulation of the stability of a gene-regulatory protein dimer by DNA and cAMP. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7387–7391. doi: 10.1073/pnas.86.19.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie R. M., Coote J. G., Parton R. Adenylate cyclase activity during phenotypic variation of Bordetella pertussis. J Gen Microbiol. 1985 Jan;131(1):27–38. doi: 10.1099/00221287-131-1-27. [DOI] [PubMed] [Google Scholar]
- Brownlie R. M., Parton R., Coote J. G. The effect of growth conditions on adenylate cyclase activity and virulence-related properties of Bordetella pertussis. J Gen Microbiol. 1985 Jan;131(1):17–25. doi: 10.1099/00221287-131-1-17. [DOI] [PubMed] [Google Scholar]
- Broyles S. S., Pettijohn D. E. Interaction of the Escherichia coli HU protein with DNA. Evidence for formation of nucleosome-like structures with altered DNA helical pitch. J Mol Biol. 1986 Jan 5;187(1):47–60. doi: 10.1016/0022-2836(86)90405-5. [DOI] [PubMed] [Google Scholar]
- Busby S., Buc H. Positive regulation of gene expression by cyclic AMP and its receptor protein in Escherichia coli. Microbiol Sci. 1987 Dec;4(12):371–375. [PubMed] [Google Scholar]
- Calcott P. H. Cyclic AMP and cyclic GMP control of synthesis of constitutive enzymes in Escherichia coli. J Gen Microbiol. 1982 Apr;128(4):705–712. doi: 10.1099/00221287-128-4-705. [DOI] [PubMed] [Google Scholar]
- Catanese C. A., Emerich D. W., Zahler W. L. Adenylate cyclase and cyclic AMP phosphodiesterase in Bradyrhizobium japonicum bacteroids. J Bacteriol. 1989 Sep;171(9):4531–4536. doi: 10.1128/jb.171.9.4531-4536.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M. K., Ganguly U. Comparison of extracellular & intracellular level of cyclic AMP between the toxinogenic & non-toxinogenic strains of Vibrio cholerae. Indian J Exp Biol. 1981 Feb;19(2):179–180. [PubMed] [Google Scholar]
- Chakravarti D., Ghosh A. Reversal by cyclic AMP of the urea-induced inhibition of synthesis of a catabolite-repressible enzyme in Vibrio cholerae. J Gen Microbiol. 1987 Nov;133(11):3265–3270. doi: 10.1099/00221287-133-11-3265. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Vining L. C. Catabolite repression in Streptomyces venezuelae. Induction of beta-galactosidase, chloramphenicol production, and intracellular cyclic adenosine 3',5'-monophosphate concentrations. Can J Microbiol. 1982 Mar;28(3):311–317. doi: 10.1139/m82-046. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Vining L. C. Intracellular adenosine triphosphate and cyclic adenosine 3',5'-monophosphate concentrations during derepression of actinomycin biosynthesis. Can J Microbiol. 1982 Dec;28(12):1396–1399. doi: 10.1139/m82-207. [DOI] [PubMed] [Google Scholar]
- Clark D. Regulation of fatty acid degradation in Escherichia coli: analysis by operon fusion. J Bacteriol. 1981 Nov;148(2):521–526. doi: 10.1128/jb.148.2.521-526.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condemine G., Hugouvieux-Cotte-Pattat N., Robert-Baudouy J. Isolation of Erwinia chrysanthemi kduD mutants altered in pectin degradation. J Bacteriol. 1986 Mar;165(3):937–941. doi: 10.1128/jb.165.3.937-941.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P., Gicquel-Sanzey B. Cloning and sequence of the crp gene of Escherichia coli K 12. Nucleic Acids Res. 1982 Feb 25;10(4):1363–1378. doi: 10.1093/nar/10.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P., Gicquel-Sanzey B. Regulation of expression of the crp gene of Escherichia coli K-12: in vivo study. J Bacteriol. 1985 Jan;161(1):454–457. doi: 10.1128/jb.161.1.454-457.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P., Groisman E. A., Serre M. C., Casadaban M. J., Gicquel-Sanzey B. crp genes of Shigella flexneri, Salmonella typhimurium, and Escherichia coli. J Bacteriol. 1986 Aug;167(2):639–646. doi: 10.1128/jb.167.2.639-646.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P. A., Gunsalus R. P. Oxygen, nitrate, and molybdenum regulation of dmsABC gene expression in Escherichia coli. J Bacteriol. 1989 Jul;171(7):3817–3823. doi: 10.1128/jb.171.7.3817-3823.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzone A. J. Protein phosphorylation in prokaryotes. Annu Rev Microbiol. 1988;42:97–125. doi: 10.1146/annurev.mi.42.100188.000525. [DOI] [PubMed] [Google Scholar]
- Crasnier M., Danchin A. Characterization of Escherichia coli adenylate cyclase mutants with modified regulation. J Gen Microbiol. 1990 Sep;136(9):1825–1831. doi: 10.1099/00221287-136-9-1825. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Goldschmidt R. M., Fletchall N. B., Kelly S. M. Avirulent Salmonella typhimurium delta cya delta crp oral vaccine strains expressing a streptococcal colonization and virulence antigen. Vaccine. 1988 Apr;6(2):155–160. doi: 10.1016/s0264-410x(88)80020-3. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M. S., Thorner J. Putting it on and taking it off: phosphoprotein phosphatase involvement in cell cycle regulation. Cell. 1989 Jun 16;57(6):891–893. doi: 10.1016/0092-8674(89)90325-5. [DOI] [PubMed] [Google Scholar]
- D'Ari R., Jaffé A., Bouloc P., Robin A. Cyclic AMP and cell division in Escherichia coli. J Bacteriol. 1988 Jan;170(1):65–70. doi: 10.1128/jb.170.1.65-70.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalma-Weiszhausz D. D., Gartenberg M. R., Crothers D. M. Sequence-dependent contribution of distal binding domains to CAP protein-DNA binding affinity. Nucleic Acids Res. 1991 Feb 11;19(3):611–616. doi: 10.1093/nar/19.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin A., Dondon L., Daniel J. Metabolic alterations mediated by 2-ketobutyrate in Escherichia coli K12. Mol Gen Genet. 1984;193(3):473–478. doi: 10.1007/BF00382086. [DOI] [PubMed] [Google Scholar]
- Danchin A., Sezer O., Glaser P., Chalon P., Caput D. Cloning and expression of mouse-brain calmodulin as an activator of Bordetella pertussis adenylate cyclase in Escherichia coli. Gene. 1989 Aug 1;80(1):145–149. doi: 10.1016/0378-1119(89)90259-x. [DOI] [PubMed] [Google Scholar]
- Daniel J., Joseph E., Danchin A. Role of 2-ketobutyrate as an alarmone in E. coli K12: inhibition of adenylate cyclase activity mediated by the phosphoenolpyruvate: glycose phosphotransferase transport system. Mol Gen Genet. 1984;193(3):467–472. doi: 10.1007/BF00382085. [DOI] [PubMed] [Google Scholar]
- Dassa E., Cahu M., Desjoyaux-Cherel B., Boquet P. L. The acid phosphatase with optimum pH of 2.5 of Escherichia coli. Physiological and Biochemical study. J Biol Chem. 1982 Jun 25;257(12):6669–6676. [PubMed] [Google Scholar]
- De Lorenzo V., Herrero M., Giovannini F., Neilands J. B. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur J Biochem. 1988 May 2;173(3):537–546. doi: 10.1111/j.1432-1033.1988.tb14032.x. [DOI] [PubMed] [Google Scholar]
- De Reuse H., Danchin A. Positive regulation of the pts operon of Escherichia coli: genetic evidence for a signal transduction mechanism. J Bacteriol. 1991 Jan;173(2):727–733. doi: 10.1128/jb.173.2.727-733.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Reuse H., Danchin A. The ptsH, ptsI, and crr genes of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: a complex operon with several modes of transcription. J Bacteriol. 1988 Sep;170(9):3827–3837. doi: 10.1128/jb.170.9.3827-3837.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrazia H., Harman J. G., Tan G. S., Wartell R. M. Investigation of the cAMP receptor protein secondary structure by Raman spectroscopy. Biochemistry. 1990 Apr 10;29(14):3557–3562. doi: 10.1021/bi00466a019. [DOI] [PubMed] [Google Scholar]
- Delaney J. M. A cya deletion mutant of Escherichia coli develops thermotolerance but does not exhibit a heat-shock response. Genet Res. 1990 Feb;55(1):1–6. doi: 10.1017/s001667230002512x. [DOI] [PubMed] [Google Scholar]
- DiRusso C. C. Primary sequence of the Escherichia coli fadBA operon, encoding the fatty acid-oxidizing multienzyme complex, indicates a high degree of homology to eucaryotic enzymes. J Bacteriol. 1990 Nov;172(11):6459–6468. doi: 10.1128/jb.172.11.6459-6468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan M. G., Masure H. R., Storm D. R. Isolation of a protein fraction from Bordetella pertussis that facilitates entry of the calmodulin-sensitive adenylate cyclase into animal cells. Biochemistry. 1989 Oct 3;28(20):8124–8129. doi: 10.1021/bi00446a024. [DOI] [PubMed] [Google Scholar]
- Dorman C. J., Barr G. C., Ni Bhriain N., Higgins C. F. DNA supercoiling and the anaerobic and growth phase regulation of tonB gene expression. J Bacteriol. 1988 Jun;170(6):2816–2826. doi: 10.1128/jb.170.6.2816-2826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C. M., Arp D. J. Regulation of H2 oxidation activity and hydrogenase protein levels by H2, O2, and carbon substrates in Alcaligenes latus. J Bacteriol. 1987 Oct;169(10):4463–4468. doi: 10.1128/jb.169.10.4463-4468.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dripps D., Wartell R. M. DNA bending induced by the catabolite activator protein allows ring formation of a 144 bp DNA. J Biomol Struct Dyn. 1987 Aug;5(1):1–13. doi: 10.1080/07391102.1987.10506370. [DOI] [PubMed] [Google Scholar]
- Drummond M., Clements J., Merrick M., Dixon R. Positive control and autogenous regulation of the nifLA promoter in Klebsiella pneumoniae. Nature. 1983 Jan 27;301(5898):302–307. doi: 10.1038/301302a0. [DOI] [PubMed] [Google Scholar]
- Dunlap P. V., Greenberg E. P. Control of Vibrio fischeri luminescence gene expression in Escherichia coli by cyclic AMP and cyclic AMP receptor protein. J Bacteriol. 1985 Oct;164(1):45–50. doi: 10.1128/jb.164.1.45-50.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V., Greenberg E. P. Control of Vibrio fischeri lux gene transcription by a cyclic AMP receptor protein-luxR protein regulatory circuit. J Bacteriol. 1988 Sep;170(9):4040–4046. doi: 10.1128/jb.170.9.4040-4046.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V. Regulation of luminescence by cyclic AMP in cya-like and crp-like mutants of Vibrio fischeri. J Bacteriol. 1989 Feb;171(2):1199–1202. doi: 10.1128/jb.171.2.1199-1202.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Nakazawa A. Cyclic AMP-dependent initiation and rho-dependent termination of colicin E1 gene transcription. J Biol Chem. 1983 Jun 10;258(11):7072–7078. [PubMed] [Google Scholar]
- Ebright R. H., Ebright Y. W., Gunasekera A. Consensus DNA site for the Escherichia coli catabolite gene activator protein (CAP): CAP exhibits a 450-fold higher affinity for the consensus DNA site than for the E. coli lac DNA site. Nucleic Acids Res. 1989 Dec 25;17(24):10295–10305. doi: 10.1093/nar/17.24.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Beachey E. H., Solomon S. S. Divergent effects of cyclic adenosine 3',5'-monophosphate on formation of type 1 fimbriae in different K-12 strains of Escherichia coli. J Bacteriol. 1981 Jan;145(1):620–623. doi: 10.1128/jb.145.1.620-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Dodd D. C. Pseudocatabolite repression of type 1 fimbriae of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1560–1567. doi: 10.1128/jb.151.3.1560-1567.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escuyer V., Duflot E., Sezer O., Danchin A., Mock M. Structural homology between virulence-associated bacterial adenylate cyclases. Gene. 1988 Nov 30;71(2):293–298. doi: 10.1016/0378-1119(88)90045-5. [DOI] [PubMed] [Google Scholar]
- Fandl J. P., Thorner L. K., Artz S. W. Mutations that affect transcription and cyclic AMP-CRP regulation of the adenylate cyclase gene (cya) of Salmonella typhimurium. Genetics. 1990 Aug;125(4):719–727. doi: 10.1093/genetics/125.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfel Z., Friedman E., Hanski E. The invasive adenylate cyclase of Bordetella pertussis. Intracellular localization and kinetics of penetration into various cells. Biochem J. 1987 Apr 1;243(1):153–158. doi: 10.1042/bj2430153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., Arnosti D. N., Chamberlin M. J., Ames B. N. An apaH mutation causes AppppA to accumulate and affects motility and catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5010–5014. doi: 10.1073/pnas.86.13.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim D. A., Chin A. M., Nierva C. T., Feucht B. U., Cao Y. W., Xu Y. F., Sutrina S. L., Saier M. H., Jr Physiological consequences of the complete loss of phosphoryl-transfer proteins HPr and FPr of the phosphoenolpyruvate:sugar phosphotransferase system and analysis of fructose (fru) operon expression in Salmonella typhimurium. J Bacteriol. 1990 Sep;172(9):5459–5469. doi: 10.1128/jb.172.9.5459-5469.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennington G., Neubauer D., Stutzenberger F. Cellulase biosynthesis in a catabolite repression-resistant mutant of Thermomonospora curvata. Appl Environ Microbiol. 1984 Jan;47(1):201–204. doi: 10.1128/aem.47.1.201-204.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flashner Y., Gralla J. D. DNA dynamic flexibility and protein recognition: differential stimulation by bacterial histone-like protein HU. Cell. 1988 Aug 26;54(5):713–721. doi: 10.1016/s0092-8674(88)80016-3. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Aliabadi Z. pH-regulated gene expression in Salmonella: genetic analysis of aniG and cloning of the earA regulator. Mol Microbiol. 1989 Nov;3(11):1605–1615. doi: 10.1111/j.1365-2958.1989.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Friden P., Newman T., Freundlich M. Nucleotide sequence of the ilvB promoter-regulatory region: a biosynthetic operon controlled by attenuation and cyclic AMP. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6156–6160. doi: 10.1073/pnas.79.20.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E., Farfel Z., Hanski E. The invasive adenylate cyclase of Bordetella pertussis. Properties and penetration kinetics. Biochem J. 1987 Apr 1;243(1):145–151. doi: 10.1042/bj2430145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L. Bordetella pertussis adenylate cyclase: isolation and purification by calmodulin-sepharose 4B chromatography. Infect Immun. 1987 Jan;55(1):129–134. doi: 10.1128/iai.55.1.129-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry I. J., Villa L., Kuehn G. D., Hageman J. H. Calmodulin-like protein from Bacillus subtilis. Biochem Biophys Res Commun. 1986 Jan 14;134(1):212–217. doi: 10.1016/0006-291x(86)90549-8. [DOI] [PubMed] [Google Scholar]
- Garges S., Adhya S. Cyclic AMP-induced conformational change of cyclic AMP receptor protein (CRP): intragenic suppressors of cyclic AMP-independent CRP mutations. J Bacteriol. 1988 Apr;170(4):1417–1422. doi: 10.1128/jb.170.4.1417-1422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garges S., Adhya S. Sites of allosteric shift in the structure of the cyclic AMP receptor protein. Cell. 1985 Jul;41(3):745–751. doi: 10.1016/s0092-8674(85)80055-6. [DOI] [PubMed] [Google Scholar]
- Gemmill R. M., Tripp M., Friedman S. B., Calvo J. M. Promoter mutation causing catabolite repression of the Salmonella typhimurium leucine operon. J Bacteriol. 1984 Jun;158(3):948–953. doi: 10.1128/jb.158.3.948-953.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile F., Raptis A., Knipling L. G., Wolff J. Bordetella pertussis adenylate cyclase. Penetration into host cells. Eur J Biochem. 1988 Aug 15;175(3):447–453. doi: 10.1111/j.1432-1033.1988.tb14215.x. [DOI] [PubMed] [Google Scholar]
- Gentile F., Raptis A., Knipling L. G., Wolff J. Extracellular cAMP formation from host cell ATP by Bordetella pertussis adenylate cyclase. Biochim Biophys Acta. 1988 Aug 19;971(1):63–71. doi: 10.1016/0167-4889(88)90162-0. [DOI] [PubMed] [Google Scholar]
- George S. E., Melton T. Cloning and molecular characterization of csm mutations allowing expression of catabolite-repressible operons in the absence of exogenous cyclic AMP. J Bacteriol. 1986 May;166(2):533–540. doi: 10.1128/jb.166.2.533-540.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber L., Neubauer D. G., Stutzenberger F. J. Cyclic AMP phosphodiesterase in Thermomonospora curvata. J Bacteriol. 1987 May;169(5):2267–2271. doi: 10.1128/jb.169.5.2267-2271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert I., Llagostera M., Barbé J. Regulation of ubiG gene expression in Escherichia coli. J Bacteriol. 1988 Mar;170(3):1346–1349. doi: 10.1128/jb.170.3.1346-1349.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa-Ron A., Rogel A., Hanski E. Bordetella pertussis adenylate cyclase inactivation by the host cell. Biochem J. 1989 Aug 15;262(1):25–31. doi: 10.1042/bj2620025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Elmaoglou-Lazaridou A., Krin E., Ladant D., Bârzu O., Danchin A. Identification of residues essential for catalysis and binding of calmodulin in Bordetella pertussis adenylate cyclase by site-directed mutagenesis. EMBO J. 1989 Mar;8(3):967–972. doi: 10.1002/j.1460-2075.1989.tb03459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldie H. Regulation of transcription of the Escherichia coli phosphoenolpyruvate carboxykinase locus: studies with pck-lacZ operon fusions. J Bacteriol. 1984 Sep;159(3):832–836. doi: 10.1128/jb.159.3.832-836.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick P., Yanofsky C. tRNA(Trp) translation of leader peptide codon 12 and other factors that regulate expression of the tryptophanase operon. J Bacteriol. 1990 Jun;172(6):3100–3107. doi: 10.1128/jb.172.6.3100-3107.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon V. M., Leppla S. H., Hewlett E. L. Inhibitors of receptor-mediated endocytosis block the entry of Bacillus anthracis adenylate cyclase toxin but not that of Bordetella pertussis adenylate cyclase toxin. Infect Immun. 1988 May;56(5):1066–1069. doi: 10.1128/iai.56.5.1066-1069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon V. M., Young W. W., Jr, Lechler S. M., Gray M. C., Leppla S. H., Hewlett E. L. Adenylate cyclase toxins from Bacillus anthracis and Bordetella pertussis. Different processes for interaction with and entry into target cells. J Biol Chem. 1989 Sep 5;264(25):14792–14796. [PubMed] [Google Scholar]
- Goss T. J., Datta P. Escherichia coli K-12 mutation that inactivates biodegradative threonine dehydratase by transposon Tn5 insertion. J Bacteriol. 1984 Jun;158(3):826–831. doi: 10.1128/jb.158.3.826-831.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S. Bacterial regulation: global regulatory networks. Annu Rev Genet. 1984;18:415–441. doi: 10.1146/annurev.ge.18.120184.002215. [DOI] [PubMed] [Google Scholar]
- Griggs D. W., Kafka K., Nau C. D., Konisky J. Activation of expression of the Escherichia coli cir gene by an iron-independent regulatory mechanism involving cyclic AMP-cyclic AMP receptor protein complex. J Bacteriol. 1990 Jun;172(6):3529–3533. doi: 10.1128/jb.172.6.3529-3533.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R., Aricò B., Rappuoli R. Families of bacterial signal-transducing proteins. Mol Microbiol. 1989 Nov;3(11):1661–1667. doi: 10.1111/j.1365-2958.1989.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Gross R., Rappuoli R. Positive regulation of pertussis toxin expression. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3913–3917. doi: 10.1073/pnas.85.11.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grübl G., Vogler A. P., Lengeler J. W. Involvement of the histidine protein (HPr) of the phosphotransferase system in chemotactic signaling of Escherichia coli K-12. J Bacteriol. 1990 Oct;172(10):5871–5876. doi: 10.1128/jb.172.10.5871-5876.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E. Invasive adenylate cyclase toxin of Bordetella pertussis. Trends Biochem Sci. 1989 Nov;14(11):459–463. doi: 10.1016/0968-0004(89)90106-0. [DOI] [PubMed] [Google Scholar]
- Harman J. G., Dobrogosz W. J. Mechanism of CRP-mediated cya suppression in Escherichia coli. J Bacteriol. 1983 Jan;153(1):191–199. doi: 10.1128/jb.153.1.191-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman J. G., McKenney K., Peterkofsky A. Structure-function analysis of three cAMP-independent forms of the cAMP receptor protein. J Biol Chem. 1986 Dec 15;261(35):16332–16339. [PubMed] [Google Scholar]
- Harman J. G., Peterkofsky A., McKenney K. Arginine substituted for leucine at position 195 produces a cyclic AMP-independent form of the Escherichia coli cyclic AMP receptor protein. J Biol Chem. 1988 Jun 15;263(17):8072–8077. [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Haughn G. W., Calvo J. M., Wallace J. C. A large family of bacterial activator proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Gordon V. M., McCaffery J. D., Sutherland W. M., Gray M. C. Adenylate cyclase toxin from Bordetella pertussis. Identification and purification of the holotoxin molecule. J Biol Chem. 1989 Nov 15;264(32):19379–19384. [PubMed] [Google Scholar]
- Heyduk T., Lee J. C. Application of fluorescence energy transfer and polarization to monitor Escherichia coli cAMP receptor protein and lac promoter interaction. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1744–1748. doi: 10.1073/pnas.87.5.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyduk T., Lee J. C. Escherichia coli cAMP receptor protein: evidence for three protein conformational states with different promoter binding affinities. Biochemistry. 1989 Aug 22;28(17):6914–6924. doi: 10.1021/bi00443a021. [DOI] [PubMed] [Google Scholar]
- Hosono K., Suzuki H. Morphological transformation of Chinese hamster cells by acylpeptides, inhibitors of cAMP phosphodiesterase, produced by Bacillus subtilis. J Biol Chem. 1985 Sep 15;260(20):11252–11255. [PubMed] [Google Scholar]
- Hudson J. M., Crowe L. G., Fried M. G. A new DNA binding mode for CAP. J Biol Chem. 1990 Feb 25;265(6):3219–3225. [PubMed] [Google Scholar]
- Hudson J. M., Fried M. G. Co-operative interactions between the catabolite gene activator protein and the lac repressor at the lactose promoter. J Mol Biol. 1990 Jul 20;214(2):381–396. doi: 10.1016/0022-2836(90)90188-R. [DOI] [PubMed] [Google Scholar]
- Hughes P., Landoulsi A., Kohiyama M. A novel role for cAMP in the control of the activity of the E. coli chromosome replication initiator protein, DnaA. Cell. 1988 Oct 21;55(2):343–350. doi: 10.1016/0092-8674(88)90057-8. [DOI] [PubMed] [Google Scholar]
- Inouye S., Franceschini T., Inouye M. Structural similarities between the development-specific protein S from a gram-negative bacterium, Myxococcus xanthus, and calmodulin. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6829–6833. doi: 10.1073/pnas.80.22.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani M., Musso R., Adhya S. Cyclic-AMP-dependent switch in initiation of transcription from the two promoters of the Escherichia coli gal operon: identification and assay of 5'-triphosphate ends of mRNA by GTP:RNA guanyltransferase. J Bacteriol. 1989 Mar;171(3):1623–1630. doi: 10.1128/jb.171.3.1623-1630.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin N., Ptashne M. Mutants of the catabolite activator protein of Escherichia coli that are specifically deficient in the gene-activation function. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8315–8319. doi: 10.1073/pnas.84.23.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa Y., Yonemitsu K., Matsui K., Fukunaga K., Miyamoto E. Calmodulin-like activity in the soluble fraction of Escherichia coli. Biochem Biophys Res Commun. 1981 Feb 12;98(3):656–660. doi: 10.1016/0006-291x(81)91164-5. [DOI] [PubMed] [Google Scholar]
- James D. W., Jr, Gutterson N. I. Multiple antibiotics produced by Pseudomonas fluorescens HV37a and their differential regulation by glucose. Appl Environ Microbiol. 1986 Nov;52(5):1183–1189. doi: 10.1128/aem.52.5.1183-1189.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Sawers R. G., Rugman P. A., Boxer D. H., Higgins C. F. Effects of anaerobic regulatory mutations and catabolite repression on regulation of hydrogen metabolism and hydrogenase isoenzyme composition in Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):405–411. doi: 10.1128/jb.168.1.405-411.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. E., Chaisson S. A., Matin A. Starvation-induced cross protection against osmotic challenge in Escherichia coli. J Bacteriol. 1990 May;172(5):2779–2781. doi: 10.1128/jb.172.5.2779-2781.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M. P., Beacham I. R. Analysis of the Escherichia coli gene encoding L-asparaginase II, ansB, and its regulation by cyclic AMP receptor and FNR proteins. J Bacteriol. 1990 Mar;172(3):1491–1498. doi: 10.1128/jb.172.3.1491-1498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. L., Agarwal A. K., Keister D. L. Inhibition of growth of Rhizobium japonicum by cyclic GMP. J Bacteriol. 1985 Nov;164(2):757–761. doi: 10.1128/jb.164.2.757-761.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. M., Gunsalus R. P. Transcription of the Escherichia coli fumarate reductase genes (frdABCD) and their coordinate regulation by oxygen, nitrate, and fumarate. J Bacteriol. 1985 Dec;164(3):1100–1109. doi: 10.1128/jb.164.3.1100-1109.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovich S. B. Regulation of a cya-lac fusion by cyclic AMP in Salmonella typhimurium. J Bacteriol. 1985 Feb;161(2):641–649. doi: 10.1128/jb.161.2.641-649.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez A., Parés R., Vives-Rego J. Effect of the carbon source and cyclic AMP on isocitrate dehydrogenase, succinate dehydrogenase, and malate dehydrogenase in Klebsiella pneumoniae C3. Can J Microbiol. 1982 Oct;28(10):1101–1106. doi: 10.1139/m82-164. [DOI] [PubMed] [Google Scholar]
- Kalman L. V., Gunsalus R. P. Identification of a second gene involved in global regulation of fumarate reductase and other nitrate-controlled genes for anaerobic respiration in Escherichia coli. J Bacteriol. 1989 Jul;171(7):3810–3816. doi: 10.1128/jb.171.7.3810-3816.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul R., Wenman W. M. Cyclic AMP inhibits developmental regulation of Chlamydia trachomatis. J Bacteriol. 1986 Nov;168(2):722–727. doi: 10.1128/jb.168.2.722-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamukai M., Kishimoto J., Utsumi R., Himeno M., Komano T., Aiba H. Negative regulation of adenylate cyclase gene (cya) expression by cyclic AMP-cyclic AMP receptor protein in Escherichia coli: studies with cya-lac protein and operon fusion plasmids. J Bacteriol. 1985 Nov;164(2):872–877. doi: 10.1128/jb.164.2.872-877.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamukai M., Matsuda H., Fujii W., Utsumi R., Komano T. Nucleotide sequences of fic and fic-1 genes involved in cell filamentation induced by cyclic AMP in Escherichia coli. J Bacteriol. 1989 Aug;171(8):4525–4529. doi: 10.1128/jb.171.8.4525-4529.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T., Dahlbeck D., Staskawicz B., Belser W. Molecular cloning of pectate lyase genes from Erwinia chrysanthemi and their expression in Escherichia coli. J Bacteriol. 1984 Sep;159(3):825–831. doi: 10.1128/jb.159.3.825-831.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Zwieb C., Wu C., Adhya S. Bending of DNA by gene-regulatory proteins: construction and use of a DNA bending vector. Gene. 1989 Dec 21;85(1):15–23. doi: 10.1016/0378-1119(89)90459-9. [DOI] [PubMed] [Google Scholar]
- Kotlarz D., Fritsch A., Buc H. Variations of intramolecular ligation rates allow the detection of protein-induced bends in DNA. EMBO J. 1986 Apr;5(4):799–803. doi: 10.1002/j.1460-2075.1986.tb04284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Agarwal K. N., Hazela S. Regulation of envelope-growth in Escherichia coli: horizontal envelope-growth by a process under cyclic AMP control. Indian J Exp Biol. 1981 Jul;19(7):640–642. [PubMed] [Google Scholar]
- Kumar S., Prakash N., Sharma V. K. Control of minicell producing cell division by cAMP-receptor protein complex in Escherichia coli. Mol Gen Genet. 1979 Nov;176(3):449–450. doi: 10.1007/BF00333110. [DOI] [PubMed] [Google Scholar]
- Kumar S. Properties of adenyl cyclase and cyclic adenosine 3',5'-monophosphate receptor protein-deficient mutants of Escherichia coli. J Bacteriol. 1976 Feb;125(2):545–555. doi: 10.1128/jb.125.2.545-555.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Srivastava S. Cyclic AMP and its receptor protein are required for expression of transfer genes of conjugative plasmid F in Escherichia coli. Mol Gen Genet. 1983;190(1):27–34. doi: 10.1007/BF00330320. [DOI] [PubMed] [Google Scholar]
- Kustu S., Santero E., Keener J., Popham D., Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989 Sep;53(3):367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsukake K., Ohya Y., Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypr J., Mrázek J. Possible mechanism of the allosteric activation of cAMP receptor protein. Biochem Biophys Res Commun. 1985 Sep 16;131(2):780–785. doi: 10.1016/0006-291x(85)91307-5. [DOI] [PubMed] [Google Scholar]
- Ladant D., Michelson S., Sarfati R., Gilles A. M., Predeleanu R., Bârzu O. Characterization of the calmodulin-binding and of the catalytic domains of Bordetella pertussis adenylate cyclase. J Biol Chem. 1989 Mar 5;264(7):4015–4020. [PubMed] [Google Scholar]
- Lagos R., Goldstein R. Phasmid P4: manipulation of plasmid copy number and induction from the integrated state. J Bacteriol. 1984 Apr;158(1):208–215. doi: 10.1128/jb.158.1.208-215.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathigra R., O'Regan M., Kiely B., Boesten B., O'Gara F. Organization of the adenyl cyclase (cya) locus of Rhizobium meliloti. Gene. 1986;44(1):89–96. doi: 10.1016/0378-1119(86)90046-6. [DOI] [PubMed] [Google Scholar]
- Lau T. M., Chan K. Y. Physiological responses of Bacillus species to concanavalin A. 2. Effect on growth, oxygen uptake, enzyme activities and intracellular cyclic guanosine 3',5'-monophosphate level of B. cereus ATCC 14579. Microbios. 1984;39(157-158):137–150. [PubMed] [Google Scholar]
- Le Grice S. F., Matzura H., Marcoli R., Iida S., Bickle T. A. The catabolite-sensitive promoter for the chloramphenicol acetyl transferase gene is preceded by two binding sites for the catabolite gene activator protein. J Bacteriol. 1982 Apr;150(1):312–318. doi: 10.1128/jb.150.1.312-318.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckie M. P., Tieber V. L., Porter S. E., Roth W. G., Dietzler D. N. Independence of cyclic AMP and relA gene stimulation of glycogen synthesis in intact Escherichia coli cells. J Bacteriol. 1985 Jan;161(1):133–140. doi: 10.1128/jb.161.1.133-140.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Dobrogosz W. J. Effects of aerobic and anaerobic shock on catabolite repression in cyclic AMP suppressor mutants of Escherichia coli. J Bacteriol. 1983 May;154(2):992–994. doi: 10.1128/jb.154.2.992-994.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7496–7500. doi: 10.1073/pnas.80.24.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichtling B. H., Rickenberg H. V., Seely R. J., Fahrney D. E., Pace N. R. The occurrence of cyclic AMP in archaebacteria. Biochem Biophys Res Commun. 1986 May 14;136(3):1078–1082. doi: 10.1016/0006-291x(86)90443-2. [DOI] [PubMed] [Google Scholar]
- Lengeler J. W., Vogler A. P. Molecular mechanisms of bacterial chemotaxis towards PTS-carbohydrates. FEMS Microbiol Rev. 1989 Jun;5(1-2):81–92. doi: 10.1016/0168-6445(89)90011-9. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Bacillus anthracis calmodulin-dependent adenylate cyclase: chemical and enzymatic properties and interactions with eucaryotic cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:189–198. [PubMed] [Google Scholar]
- Lichenstein H. S., Hamilton E. P., Lee N. Repression and catabolite gene activation in the araBAD operon. J Bacteriol. 1987 Feb;169(2):811–822. doi: 10.1128/jb.169.2.811-822.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. T., Hennecke H., Scott D. B. Effect of cyclic guanosine 3',5'-monophosphate on nitrogen fixation in Rhizobium japonicum. J Bacteriol. 1979 Jul;139(1):256–263. doi: 10.1128/jb.139.1.256-263.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. T., Shanmugam K. T. Regulation of hydrogen utilisation in Rhizobium japonicum by cyclic AMP. Biochim Biophys Acta. 1979 May 16;584(3):479–492. doi: 10.1016/0304-4165(79)90121-1. [DOI] [PubMed] [Google Scholar]
- Liu J., Beacham I. R. Transcription and regulation of the cpdB gene in Escherichia coli K12 and Salmonella typhimurium LT2: evidence for modulation of constitutive promoters by cyclic AMP-CRP complex. Mol Gen Genet. 1990 Jun;222(1):161–165. doi: 10.1007/BF00283039. [DOI] [PubMed] [Google Scholar]
- Liu J., Burns D. M., Beacham I. R. Isolation and sequence analysis of the gene (cpdB) encoding periplasmic 2',3'-cyclic phosphodiesterase. J Bacteriol. 1986 Mar;165(3):1002–1010. doi: 10.1128/jb.165.3.1002-1010.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell R. B., Schleif R. F. DNA looping and unlooping by AraC protein. Science. 1990 Oct 26;250(4980):528–532. doi: 10.1126/science.2237403. [DOI] [PubMed] [Google Scholar]
- Lobell R. B., Schleif R. F. DNA looping and unlooping by AraC protein. Science. 1990 Oct 26;250(4980):528–532. doi: 10.1126/science.2237403. [DOI] [PubMed] [Google Scholar]
- Lopes J. M., Lawther R. P. Physical identification of an internal promoter, ilvAp, in the distal portion of the ilvGMEDA operon. Gene. 1989;76(2):255–269. doi: 10.1016/0378-1119(89)90166-2. [DOI] [PubMed] [Google Scholar]
- Lévy S., Zeng G. Q., Danchin A. Cyclic AMP synthesis in Escherichia coli strains bearing known deletions in the pts phosphotransferase operon. Gene. 1990 Jan 31;86(1):27–33. doi: 10.1016/0378-1119(90)90110-d. [DOI] [PubMed] [Google Scholar]
- Macaluso A., Best E. A., Bender R. A. Role of the nac gene product in the nitrogen regulation of some NTR-regulated operons of Klebsiella aerogenes. J Bacteriol. 1990 Dec;172(12):7249–7255. doi: 10.1128/jb.172.12.7249-7255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S. K., Vartak N. B., Datta A. R. A new pleiotropic mutation causing defective carbohydrate uptake in Escherichia coli K-12: isolation, mapping, and preliminary characterization. J Bacteriol. 1988 Jun;170(6):2568–2574. doi: 10.1128/jb.170.6.2568-2574.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S., Bose S. K. Derepression of sporulation and synthesis of mycobacillin and dipicolinic acid by guanosine 3':5'-cyclic monophosphate under conditions of glucose repression in Bacillus subtilis. J Gen Microbiol. 1985 Oct;131(10):2783–2788. doi: 10.1099/00221287-131-10-2783. [DOI] [PubMed] [Google Scholar]
- Mallick U., Herrlich P. Regulation of synthesis of a major outer membrane protein: cyclic AMP represses Escherichia coli protein III synthesis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5520–5523. doi: 10.1073/pnas.76.11.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez De Drets G., Arias A. Metabolism of some polyols by Rhizobium meliloti. J Bacteriol. 1970 Jul;103(1):97–103. doi: 10.1128/jb.103.1.97-103.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cadena M. G., Guzman-Verduzco L. M., Stieglitz H., Kupersztoch-Portnoy Y. M. Catabolite repression of Escherichia coli heat-stable enterotoxin activity. J Bacteriol. 1981 Feb;145(2):722–728. doi: 10.1128/jb.145.2.722-728.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masure H. R., Shattuck R. L., Storm D. R. Mechanisms of bacterial pathogenicity that involve production of calmodulin-sensitive adenylate cyclases. Microbiol Rev. 1987 Mar;51(1):60–65. doi: 10.1128/mr.51.1.60-65.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masure H. R., Storm D. R. Characterization of the bacterial cell associated calmodulin-sensitive adenylate cyclase from Bordetella pertussis. Biochemistry. 1989 Jan 24;28(2):438–442. doi: 10.1021/bi00428a005. [DOI] [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- Matin A., Matin M. K. Cellular levels, excretion, and synthesis rates of cyclic AMP in Escherichia coli grown in continuous culture. J Bacteriol. 1982 Mar;149(3):801–807. doi: 10.1128/jb.149.3.801-807.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura A., Treinin M., Mitsuzawa H., Kassir Y., Uno I., Simchen G. The adenylate cyclase/protein kinase cascade regulates entry into meiosis in Saccharomyces cerevisiae through the gene IME1. EMBO J. 1990 Oct;9(10):3225–3232. doi: 10.1002/j.1460-2075.1990.tb07521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGetrick A. M., Goulding C. F., Manian S. S., O'Gara F. Catabolite repression and role of cyclic AMP in CO2 fixation and H2 metabolism in Rhizobium spp. J Bacteriol. 1985 Sep;163(3):1282–1284. doi: 10.1128/jb.163.3.1282-1284.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiklejohn A. L., Gralla J. D. Activation of the lac promoter and its variants. Synergistic effects of catabolite activator protein and supercoiling in vitro. J Mol Biol. 1989 Jun 20;207(4):661–673. doi: 10.1016/0022-2836(89)90236-2. [DOI] [PubMed] [Google Scholar]
- Mock M., Labruyère E., Glaser P., Danchin A., Ullmann A. Cloning and expression of the calmodulin-sensitive Bacillus anthracis adenylate cyclase in Escherichia coli. Gene. 1988 Apr 29;64(2):277–284. doi: 10.1016/0378-1119(88)90342-3. [DOI] [PubMed] [Google Scholar]
- Mori K., Aiba H. Evidence for negative control of cya transcription by cAMP and cAMP receptor protein in intact Escherichia coli cells. J Biol Chem. 1985 Nov 25;260(27):14838–14843. [PubMed] [Google Scholar]
- Movva R. N., Green P., Nakamura K., Inouye M. Interaction of cAMP receptor protein with the ompA gene, a gene for a major outer membrane protein of Escherichia coli. FEBS Lett. 1981 Jun 15;128(2):186–190. doi: 10.1016/0014-5793(81)80077-4. [DOI] [PubMed] [Google Scholar]
- Mulvey M. R., Switala J., Borys A., Loewen P. C. Regulation of transcription of katE and katF in Escherichia coli. J Bacteriol. 1990 Dec;172(12):6713–6720. doi: 10.1128/jb.172.12.6713-6720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namdari H., Cabelli V. J. Glucose-mediated catabolite repression of the tricarboxylic acid cycle as an explanation for increased acetic acid production in suicidal Aeromonas strains. J Bacteriol. 1990 Aug;172(8):4721–4724. doi: 10.1128/jb.172.8.4721-4724.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop A. J., Boylan S. A., Bender R. A. Regulation of hutUH operon expression by the catabolite gene activator protein-cyclic AMP complex in Klebsiella aerogenes. J Bacteriol. 1984 Sep;159(3):934–939. doi: 10.1128/jb.159.3.934-939.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon B. T., Ronson C. W., Ausubel F. M. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7850–7854. doi: 10.1073/pnas.83.20.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan M., Kiely B., O'Gara F. Expression of the adenyl cyclase-encoding gene (cya) of Rhizobium meliloti F34: existence of two cya genes? Gene. 1989 Nov 30;83(2):243–249. doi: 10.1016/0378-1119(89)90110-8. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Freundlich M. Mechanism for the autogenous control of the crp operon: transcriptional inhibition by a divergent RNA transcript. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5000–5004. doi: 10.1073/pnas.83.14.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Hara S., Bhasin R., Freundlich M. Evidence in vivo for autogenous control of the cyclic AMP receptor protein gene (crp) in Escherichia coli by divergent RNA. J Bacteriol. 1988 Nov;170(11):5076–5079. doi: 10.1128/jb.170.11.5076-5079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega B., Stegehuis F., van Tiel-Menkveld G. J., de Graaf F. K. Protein H encoded by plasmid CloDF13 is involved in excretion of cloacin DF13. J Bacteriol. 1982 Jun;150(3):1115–1121. doi: 10.1128/jb.150.3.1115-1121.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall M. L. Adenosine 3',5'-phosphate in fungi. Microbiol Rev. 1981 Sep;45(3):462–480. doi: 10.1128/mr.45.3.462-480.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J. M., Dobrogosz W. J. The effect of cyclic AMP on anaerobic growth of Escherichia coli. Biochem Biophys Res Commun. 1973 Sep 18;54(2):555–561. doi: 10.1016/0006-291x(73)91458-7. [DOI] [PubMed] [Google Scholar]
- Pinkney M., Hoggett J. G. Binding of the cyclic AMP receptor protein of Escherichia coli to RNA polymerase. Biochem J. 1988 Mar 15;250(3):897–902. doi: 10.1042/bj2500897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla J., Dopazo A., Vicente M. The native form of FtsA, a septal protein of Escherichia coli, is located in the cytoplasmic membrane. J Bacteriol. 1990 Sep;172(9):5097–5102. doi: 10.1128/jb.172.9.5097-5102.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polayes D. A., Rice P. W., Garner M. M., Dahlberg J. E. Cyclic AMP-cyclic AMP receptor protein as a repressor of transcription of the spf gene of Escherichia coli. J Bacteriol. 1988 Jul;170(7):3110–3114. doi: 10.1128/jb.170.7.3110-3114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S., Miller R. E., Valentine R. C. Adenosine 3':5'-cyclic monophosphate control of the enzymes of glutamine metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2922–2926. doi: 10.1073/pnas.69.10.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Rosenberg M. A promoter of pBR322 activated by cAMP receptor protein. Nucleic Acids Res. 1981 Jul 24;9(14):3365–3377. doi: 10.1093/nar/9.14.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Vidal-Ingigliardi D., Richet E. A complex nucleoprotein structure involved in activation of transcription of two divergent Escherichia coli promoters. J Mol Biol. 1989 Feb 5;205(3):471–485. doi: 10.1016/0022-2836(89)90218-0. [DOI] [PubMed] [Google Scholar]
- Reddy P., Meadow N., Roseman S., Peterkofsky A. Reconstitution of regulatory properties of adenylate cyclase in Escherichia coli extracts. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8300–8304. doi: 10.1073/pnas.82.24.8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P., Peterkofsky A., McKenney K. Translational efficiency of the Escherichia coli adenylate cyclase gene: mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5656–5660. doi: 10.1073/pnas.82.17.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Expression of glnA in Escherichia coli is regulated at tandem promoters. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1979–1983. doi: 10.1073/pnas.82.7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y. L., Garges S., Adhya S., Krakow J. S. Characterization of the binding of cAMP and cGMP to the CRP*598 mutant of the E. coli cAMP receptor protein. Nucleic Acids Res. 1990 Sep 11;18(17):5127–5132. doi: 10.1093/nar/18.17.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y. L., Garges S., Adhya S., Krakow J. S. Cooperative DNA binding of heterologous proteins: evidence for contact between the cyclic AMP receptor protein and RNA polymerase. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4138–4142. doi: 10.1073/pnas.85.12.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Regulation of genes coding for enzyme constituents of the bacterial phosphotransferase system. J Bacteriol. 1980 Feb;141(2):658–663. doi: 10.1128/jb.141.2.658-663.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. L. Relationships between the calmodulin-dependent adenylate cyclases produced by Bacillus anthracis and Bordetella pertussis. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1027–1032. doi: 10.1016/s0006-291x(88)80977-x. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Tippetts M. T., Leppla S. H. Nucleotide sequence of the Bacillus anthracis edema factor gene (cya): a calmodulin-dependent adenylate cyclase. Gene. 1988 Dec 20;73(2):363–371. doi: 10.1016/0378-1119(88)90501-x. [DOI] [PubMed] [Google Scholar]
- Rogel A., Farfel Z., Goldschmidt S., Shiloach J., Hanski E. Bordetella pertussis adenylate cyclase. Identification of multiple forms of the enzyme by antibodies. J Biol Chem. 1988 Sep 15;263(26):13310–13316. [PubMed] [Google Scholar]
- Romeo T., Preiss J. Genetic regulation of glycogen biosynthesis in Escherichia coli: in vitro effects of cyclic AMP and guanosine 5'-diphosphate 3'-diphosphate and analysis of in vivo transcripts. J Bacteriol. 1989 May;171(5):2773–2782. doi: 10.1128/jb.171.5.2773-2782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkrantz M. S., Dingman D. W., Sonenshein A. L. Bacillus subtilis citB gene is regulated synergistically by glucose and glutamine. J Bacteriol. 1985 Oct;164(1):155–164. doi: 10.1128/jb.164.1.155-164.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P., Weinhouse H., Aloni Y., Michaeli D., Weinberger-Ohana P., Mayer R., Braun S., de Vroom E., van der Marel G. A., van Boom J. H. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature. 1987 Jan 15;325(6101):279–281. doi: 10.1038/325279a0. [DOI] [PubMed] [Google Scholar]
- Roy A., Glaser P., Danchin A. Aspects of the regulation of adenylate cyclase synthesis in Escherichia coli K12. J Gen Microbiol. 1988 Feb;134(2):359–367. doi: 10.1099/00221287-134-2-359. [DOI] [PubMed] [Google Scholar]
- Roy A., Haziza C., Danchin A. Regulation of adenylate cyclase synthesis in Escherichia coli: nucleotide sequence of the control region. EMBO J. 1983;2(5):791–797. doi: 10.1002/j.1460-2075.1983.tb01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L., Yamazaki H. The dependence of Escherichia coli asparaginase II formation on cyclic AMP and cyclic AMP receptor protein. Can J Microbiol. 1978 May;24(5):629–631. doi: 10.1139/m78-104. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol Rev. 1989 Mar;53(1):109–120. doi: 10.1128/mr.53.1.109-120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles B., Weisemann J. M., Weinstock G. M. Temporal control of colicin E1 induction. J Bacteriol. 1987 Nov;169(11):5028–5034. doi: 10.1128/jb.169.11.5028-5034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll T., Ott M., Oudega B., Hacker J. Use of a wild-type gene fusion to determine the influence of environmental conditions on expression of the S fimbrial adhesin in an Escherichia coli pathogen. J Bacteriol. 1990 Sep;172(9):5103–5111. doi: 10.1128/jb.172.9.5103-5111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. E., Latter G. I., Matin A. Differential regulation by cyclic AMP of starvation protein synthesis in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3903–3909. doi: 10.1128/jb.170.9.3903-3909.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H., Larson T. J. Cloning and characterization of the aerobic sn-glycerol-3-phosphate dehydrogenase structural gene glpD of Escherichia coli K-12. J Bacteriol. 1987 Feb;169(2):507–513. doi: 10.1128/jb.169.2.507-513.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Sacks L. E. Cyclic AMP is not detectable in Clostridium perfringens. Can J Microbiol. 1983 Sep;29(9):1228–1230. doi: 10.1139/m83-189. [DOI] [PubMed] [Google Scholar]
- Shanblatt S. H., Revzin A. Two catabolite activator protein molecules bind to the galactose promoter region of Escherichia coli in the presence of RNA polymerase. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1594–1598. doi: 10.1073/pnas.80.6.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatters R. G., Kahn M. L. Glutamine synthetase II in Rhizobium: reexamination of the proposed horizontal transfer of DNA from eukaryotes to prokaryotes. J Mol Evol. 1989 Nov;29(5):422–428. doi: 10.1007/BF02602912. [DOI] [PubMed] [Google Scholar]
- Shaw D. J., Rice D. W., Guest J. R. Homology between CAP and Fnr, a regulator of anaerobic respiration in Escherichia coli. J Mol Biol. 1983 May 15;166(2):241–247. doi: 10.1016/s0022-2836(83)80011-4. [DOI] [PubMed] [Google Scholar]
- Shioi J., Tribhuwan R. C., Berg S. T., Taylor B. L. Signal transduction in chemotaxis to oxygen in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1988 Dec;170(12):5507–5511. doi: 10.1128/jb.170.12.5507-5511.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirabe K., Ebina Y., Miki T., Nakazawa T., Nakazawa A. Positive regulation of the colicin E1 gene by cyclic AMP and cyclic AMP receptor protein. Nucleic Acids Res. 1985 Jul 11;13(13):4687–4698. doi: 10.1093/nar/13.13.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., Singer J. T. Studies on deo operon regulation in Escherichia coli: cloning and expression of the deoR structural gene. Gene. 1984 Nov;31(1-3):205–211. doi: 10.1016/0378-1119(84)90211-7. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by anaerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):336–343. doi: 10.1128/jb.154.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaiman D., Uffen R. L. Influence of cyclic AMP on photosynthetic development in Rhodospirillum rubrum. J Bacteriol. 1984 Aug;159(2):790–792. doi: 10.1128/jb.159.2.790-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector M. P., Aliabadi Z., Gonzalez T., Foster J. W. Global control in Salmonella typhimurium: two-dimensional electrophoretic analysis of starvation-, anaerobiosis-, and heat shock-inducible proteins. J Bacteriol. 1986 Oct;168(1):420–424. doi: 10.1128/jb.168.1.420-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector M. P., Park Y. K., Tirgari S., Gonzalez T., Foster J. W. Identification and characterization of starvation-regulated genetic loci in Salmonella typhimurium by using Mu d-directed lacZ operon fusions. J Bacteriol. 1988 Jan;170(1):345–351. doi: 10.1128/jb.170.1.345-351.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Activation of the lac operon of Escherichia coli by a mutant FNR protein. Mol Microbiol. 1987 Jul;1(1):53–58. doi: 10.1111/j.1365-2958.1987.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Regulation and over-expression of the fnr gene of Escherichia coli. J Gen Microbiol. 1987 Dec;133(12):3279–3288. doi: 10.1099/00221287-133-12-3279. [DOI] [PubMed] [Google Scholar]
- Spiteri A., Viratelle O. M., Raymond P., Rancillac M., Labouesse J., Pradet A. Artefactual Origins of Cyclic AMP in Higher Plant Tissues. Plant Physiol. 1989 Oct;91(2):624–628. doi: 10.1104/pp.91.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. J., Higgins C. F., Ames G. F. Isolation and characterization of lac fusions to two nitrogen-regulated promoters. Mol Gen Genet. 1984;195(1-2):219–227. doi: 10.1007/BF00332750. [DOI] [PubMed] [Google Scholar]
- Stewart V. Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol Rev. 1988 Jun;52(2):190–232. doi: 10.1128/mr.52.2.190-232.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus L., Wilcox G. Effect of mutations in the cyclic AMP receptor protein-binding site on araBAD and araC expression. J Bacteriol. 1989 Feb;171(2):1178–1184. doi: 10.1128/jb.171.2.1178-1184.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straney D. C., Straney S. B., Crothers D. M. Synergy between Escherichia coli CAP protein and RNA polymerase in the lac promoter open complex. J Mol Biol. 1989 Mar 5;206(1):41–57. doi: 10.1016/0022-2836(89)90522-6. [DOI] [PubMed] [Google Scholar]
- Straney S. B., Crothers D. M. Lac repressor is a transient gene-activating protein. Cell. 1987 Dec 4;51(5):699–707. doi: 10.1016/0092-8674(87)90093-6. [DOI] [PubMed] [Google Scholar]
- Swan D. G., Hale R. S., Dhillon N., Leadlay P. F. A bacterial calcium-binding protein homologous to calmodulin. Nature. 1987 Sep 3;329(6134):84–85. doi: 10.1038/329084a0. [DOI] [PubMed] [Google Scholar]
- Søgaard-Andersen L., Martinussen J., Møllegaard N. E., Douthwaite S. R., Valentin-Hansen P. The CytR repressor antagonizes cyclic AMP-cyclic AMP receptor protein activation of the deoCp2 promoter of Escherichia coli K-12. J Bacteriol. 1990 Oct;172(10):5706–5713. doi: 10.1128/jb.172.10.5706-5713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard-Andersen L., Møllegaard N. E., Douthwaite S. R., Valentin-Hansen P. Tandem DNA-bound cAMP-CRP complexes are required for transcriptional repression of the deoP2 promoter by the CytR repressor in Escherichia coli. Mol Microbiol. 1990 Sep;4(9):1595–1601. [PubMed] [Google Scholar]
- Takahashi M., Blazy B., Baudras A., Hillen W. Ligand-modulated binding of a gene regulatory protein to DNA. Quantitative analysis of cyclic-AMP induced binding of CRP from Escherichia coli to non-specific and specific DNA targets. J Mol Biol. 1989 Jun 20;207(4):783–796. doi: 10.1016/0022-2836(89)90244-1. [DOI] [PubMed] [Google Scholar]
- Thorner L. K., Fandl J. P., Artz S. W. Analysis of sequence elements important for expression and regulation of the adenylate cyclase gene (cya) of Salmonella typhimurium. Genetics. 1990 Aug;125(4):709–717. doi: 10.1093/genetics/125.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippetts M. T., Robertson D. L. Molecular cloning and expression of the Bacillus anthracis edema factor toxin gene: a calmodulin-dependent adenylate cyclase. J Bacteriol. 1988 May;170(5):2263–2266. doi: 10.1128/jb.170.5.2263-2266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribhuwan R. C., Johnson M. S., Taylor B. L. Evidence against direct involvement of cyclic GMP or cyclic AMP in bacterial chemotactic signaling. J Bacteriol. 1986 Nov;168(2):624–630. doi: 10.1128/jb.168.2.624-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G., Duchene A. On the role of cyclic AMP and the Fnr protein in Escherichia coli growing anaerobically. Arch Microbiol. 1987 Mar;147(2):195–200. doi: 10.1007/BF00415284. [DOI] [PubMed] [Google Scholar]
- Unden G., Guest J. R. Cyclic AMP and anaerobic gene expression in E. coli. FEBS Lett. 1984 May 21;170(2):321–325. doi: 10.1016/0014-5793(84)81336-8. [DOI] [PubMed] [Google Scholar]
- Ushida C., Aiba H. Helical phase dependent action of CRP: effect of the distance between the CRP site and the -35 region on promoter activity. Nucleic Acids Res. 1990 Nov 11;18(21):6325–6330. doi: 10.1093/nar/18.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R., Kawamukai M., Aiba H., Himeno M., Komano T. Expression of the adenylate cyclase gene during cell elongation in Escherichia coli K-12. J Bacteriol. 1986 Dec;168(3):1408–1414. doi: 10.1128/jb.168.3.1408-1414.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R., Nakamoto Y., Kawamukai M., Himeno M., Komano T. Involvement of cyclic AMP and its receptor protein in filamentation of an Escherichia coli fic mutant. J Bacteriol. 1982 Aug;151(2):807–812. doi: 10.1128/jb.151.2.807-812.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R., Noda M., Kawamukai M., Komano T. Control mechanism of the Escherichia coli K-12 cell cycle is triggered by the cyclic AMP-cyclic AMP receptor protein complex. J Bacteriol. 1989 May;171(5):2909–2912. doi: 10.1128/jb.171.5.2909-2912.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Holst B., Josephsen J., Hammer K., Albrechtsen B. CRP/cAMP- and CytR-regulated promoters in Escherichia coli K12: the cdd promoter. Mol Microbiol. 1989 Oct;3(10):1385–1390. doi: 10.1111/j.1365-2958.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P., Holst B., Søgaard-Andersen L., Martinussen J., Nesvera J., Douthwaite S. R. Design of cAMP-CRP-activated promoters in Escherichia coli. Mol Microbiol. 1991 Feb;5(2):433–437. doi: 10.1111/j.1365-2958.1991.tb02126.x. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P. Tandem CRP binding sites in the deo operon of Escherichia coli K-12. EMBO J. 1982;1(9):1049–1054. doi: 10.1002/j.1460-2075.1982.tb01295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler A. P., Lengeler J. W. Indirect role of adenylate cyclase and cyclic AMP in chemotaxis to phosphotransferase system carbohydrates in Escherichia coli K-12. J Bacteriol. 1987 Feb;169(2):593–599. doi: 10.1128/jb.169.2.593-599.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wanner B. L. Overlapping and separate controls on the phosphate regulon in Escherichia coli K12. J Mol Biol. 1983 May 25;166(3):283–308. doi: 10.1016/s0022-2836(83)80086-2. [DOI] [PubMed] [Google Scholar]
- Weber I. T., Steitz T. A. Structure of a complex of catabolite gene activator protein and cyclic AMP refined at 2.5 A resolution. J Mol Biol. 1987 Nov 20;198(2):311–326. doi: 10.1016/0022-2836(87)90315-9. [DOI] [PubMed] [Google Scholar]
- Webster C., Gaston K., Busby S. Transcription from the Escherichia coli melR promoter is dependent on the cyclic AMP receptor protein. Gene. 1988 Sep 7;68(2):297–305. doi: 10.1016/0378-1119(88)90032-7. [DOI] [PubMed] [Google Scholar]
- Whitson P. A., Hsieh W. T., Wells R. D., Matthews K. S. Supercoiling facilitates lac operator-repressor-pseudooperator interactions. J Biol Chem. 1987 Apr 15;262(11):4943–4946. [PubMed] [Google Scholar]
- Williams A. L. Regulation of acetohydroxy acid synthase activities in adenyl cyclase-deficient strains of Escherichia coli K-12. Mol Gen Genet. 1983;191(3):353–357. doi: 10.1007/BF00425745. [DOI] [PubMed] [Google Scholar]
- Wong K. K., Suen K. L., Kwan H. S. Transcription of pfl is regulated by anaerobiosis, catabolite repression, pyruvate, and oxrA: pfl::Mu dA operon fusions of Salmonella typhimurium. J Bacteriol. 1989 Sep;171(9):4900–4905. doi: 10.1128/jb.171.9.4900-4905.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D., Darlison M. G., Wilde R. J., Guest J. R. Nucleotide sequence encoding the flavoprotein and hydrophobic subunits of the succinate dehydrogenase of Escherichia coli. Biochem J. 1984 Sep 1;222(2):519–534. doi: 10.1042/bj2220519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. E., Neubauer D. G., Stutzenberger F. J. Cyclic AMP levels during induction and repression of cellulase biosynthesis in Thermomonospora curvata. J Bacteriol. 1984 Dec;160(3):1047–1054. doi: 10.1128/jb.160.3.1047-1054.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. M., Boyle S. M. Negative control of ornithine decarboxylase and arginine decarboxylase by adenosine-3':5'-cyclic monophosphate in Escherichia coli. Mol Gen Genet. 1982;186(4):482–487. doi: 10.1007/BF00337952. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Zinkel S. S., Crothers D. M. Comparative gel electrophoresis measurement of the DNA bend angle induced by the catabolite activator protein. Biopolymers. 1990 Jan;29(1):29–38. doi: 10.1002/bip.360290106. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crecy-Lagard V., Glaser P., Lejeune P., Sismeiro O., Barber C. E., Daniels M. J., Danchin A. A Xanthomonas campestris pv. campestris protein similar to catabolite activation factor is involved in regulation of phytopathogenicity. J Bacteriol. 1990 Oct;172(10):5877–5883. doi: 10.1128/jb.172.10.5877-5883.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]
- van den Elzen P. J., Maat J., Walters H. H., Veltkamp E., Nijkamp H. J. The nucleotide sequence of the bacteriocin promoters of plasmids Clo DF13 and Co1 E1: role of lexA repressor and cAMP in the regulation of promoter activity. Nucleic Acids Res. 1982 Mar 25;10(6):1913–1928. doi: 10.1093/nar/10.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]



