Abstract
A novel 22.8 kDa of Opisthorchis viverrini (Ov) calcium-binding EF-hand protein (Ov CaBP) was identified and isolated from an immunoscreening of the adult stage Ov cDNA library by using a human cholangiocarcinoma (CCA) serum. This protein was related to other calcium binding proteins and conserved among the trematodes. Ov CaBP shared 98% amino acid identity to 22.8 kDa of Clonorchis sinensis CaBP and both were classified as a new group of CaBP EF-hand protein by multiple sequence alignment and phylogenetic tree analysis. The open reading frame of Ov CaBP was 585 bp which encoded for 194 amino acids. The N-terminal part is composed of two calcium-binding EF-hand motifs whereas the C-terminal part contains a dynein light chain motif (DLC). In addition, transcription analysis by RT-PCR revealed that it was constitutively transcribed in all stages, including metacercariae, juvenile, and adult. Furthermore, recombinant Ov CaBP protein (rOv CaBP) was expressed as a soluble protein and antibody generated against this rOv CaBP protein was capable of detecting Ov CaBP in the Ov somatic extracts but not in Ov ES products. This anti-rOv CaBP serum was also used to localize Ov CaBP in Ov infected hamster’s liver sections which the distribution of Ov CaBP was located in gut epithelium, miracidia in eggs and slightly in parenchyma. Moreover, rOv CaBP protein showed a calcium binding property in non-denaturing gel mobility shift assay.
Keywords: Opisthorchis viverrini, calcium-binding EF-hand, protein expression, gel mobility shift assay, immunolocalization
1. Introduction
Opisthorchis viverrini (Ov), a human carcinogenic liver fluke, is a major health problem especially in the Northeast of Thailand. A Thailand wide survey showed infection in this area declined to 15.7% in 2001 [1], but in the local endemic areas of Khon Kaen province, in Northeast Thailand, the prevalence can still be as high as 70% [2]. Ov infection is associated with hepatobiliary diseases including hepatomegaly, cholangitis, fibrosis of the periportal system, cholecystitis, gallstones and are major aetiological agents of bile duct cancer (CCA). Moreover, Ov and C. sinensis are classified as Group 1 carcinogens—metazoan parasites that are carcinogenic to humans—by the International Agency for Research on Cancer, World Health Organization (WHO) [3]. The highest incidence of the liver cancer in the world has been reported in the liver fluke endemic area of Khon Kaen province, Northeast Thailand [4]. In our laboratory, the adult stage Ov cDNA was constructed for molecular study of interesting Ov genes [5].
Calcium binding EF-hand protein (CaBP) was first discovered and termed by R.H. Kretsinger in his research on the carp muscle, parvalbumin, a small Ca2+-binding protein which had a helix-loop-helix Ca2+-binding structure [6]. At present, there are more than 3,000 calcium-binding EF-hand related sequences in the NCBI Reference Sequences Data Bank [7]. The CaBP EF-hand proteins can be classified into at least 66 subfamilies [6, 8, 9]. While the functions of EF-hand CaBP protein can be divided into three categories: sensor proteins (calmodulin [10]), buffer proteins (parvalbumin [6]), and Ca2+-stabilized proteins (thermolysin [11]). In trematodes, the CaBP EF-hand containing dynein light chain (DLC) protein motif was parasite specific and was grouped into nematode calcium-binding protein subfamily. Although the function of CaBP EF-hand protein in trematode is still unknown several have shown calcium binding properties in gel mobility shift assays [12, 13, 14]. In this study, Ov CaBP was cloned, expressed, and characterized, for transcriptional patterns and protein properties as well as immunolocalization.
2. Materials and Methods
2.1. Parasites and parasite proteins preparation
Ov metacercariae were obtained naturally from infected cyprinoid fish in an endemic area of Khon Kaen province, Thailand while juvenile and adult worms were obtained after infecting hamsters with metacercariae as described [15]. Ov excretory/secretory (ES) products were collected from Ov culture as previously described [16]. Ov somatic extracts of adult stage were prepared as described [17]. All parasite proteins, ES and somatic, were concentrated by Amicon ultra centrifugal filter devices with a cut-off size of 10 kDa (Millipore, MA, USA) and concentration measured by ND-1000 Spectrophotometer (NanoDrop®, Thermo Fisher Scientific Inc., MA, USA).
2.2. Immunoscreening and sequence analysis
A serum from CCA patient R-091 was used for screening the adult stage of Ov cDNA library. Positive plaques were selected and cDNA inserts were determined by restriction analysis and sequencing in both directions. Similarity searches were done by NCBI-BLAST. Several proteins were identified including 22.8 kDa Ov CaBP. Multiple sequence and amino acid sequence alignments of the homologs of CaBP from other parasites were analyzed by using ClustalX program (Version 1.81). Phylogenetic tree were generated using Phylogeny.fr program for non-specialist [18]. The characteristic of Ov CaBP protein was analyzed by using BioEdit 7.0.1 and Expasy (http://au.expasy.org/tools/). Pfam database search was performed to determine the domain families [19].
2.3. Cloning, expression and purification
The Ov CaBP was amplified using Ov cDNA library as a template, Ov CaBP specific primers (sense primer, 5′-CAGCACCATATGAGCTACACC-3′; antisense primer 5′-GAGGGATCCTCAGTTAAATGAATC-3′) that introduced NdeI and BamHI restriction sites into sense primer and antisense primer (underlined letters), respectively, and Phusion DNA polymerase (Finnzymes, Thermo Scientific, MA, USA). The PCR profile was initial denaturation at 98°C for 30 sec followed by 35 cycles of 98°C for 10 sec, 55°C for 30 s, 72°C for 20 sec and final termination at 72°C for 5 min. The PCR product was then digested with NdeI-BamHI and purified before subcloning into NdeI-BamHI site of pET-15b expression vector. The recombinant plasmid was confirmed by restriction analysis and sequencing. After transforming the recombinant plasmid into Escherichia coli BL21 (DE3), the Ov CaBP protein was expressed as the N-terminus 6×His-tagged when induced with 1 mM isopropyl β-D-thiogalactopyranoside (IPTG). A Ni-NTA column (Ni sepharose™ 6 Fast Flow, GE Healthcare, UK) was used to purify the fusion Ov CaBP protein under native conditions. Eluted fractions of purified recombinant Ov CaBP protein (rOv CaBP) was analyzed by 12.5% SDS-PAGE.
2.4. Transcription analysis of each stage of Ov CaBP
Total Ov RNA from each stage was extracted using TRIzol reagent (Invitrogen Corporation, CA, USA) and reverse transcribed to cDNA using Revert Aid™ First Strand cDNA Synthesis Kit (Fermentas, Thermo Scientific, MA, USA). The cDNA of each stage served as template for PCR amplification of Ov CaBP gene with specific primers as described in section 2.3 and β-actin gene with β-actin primers (sense primer, 5′-AGCCAACCGAGAGAAGATGA-3′; antisense primer, 5′-ACCTGACCATCAGGCAGTTC-3′) as an internal control using the same profile of initial denaturation at 95°C for 2 min followed by 40 cycles of 95°C for 1 min, 55°C for 30 s, 72°C for 1 min and final termination at 72°C for 4 min. PCR were performed using GoTaq®colorless DNA polymerase (Promega Corporation, WI, USA). PCR products were determined by 1% agarose gel electrophoresis.
2.5. Anti-sera preparation
ICR mice were immunized subcutaneously with 100 µg of purified rOv CaBP emulsified with Freud’s complete adjuvant (GIBCO BRL, Gel Company, CA, USA) in the first shot. After two weeks, mice were boosted three times at one week intervals with 50 µg of purified rOv CaBP proteins emulsified with Freud’s incomplete adjuvant (GIBCO BRL, Gel Company, CA, USA). Antisera were obtained one week after the last shot.
2.6. Western blotting
Western blot analysis was performed to determine rOv CaBP protein from the purified fraction and to analyze the presence of Ov CaBP protein in crude Ov somatic extracts as well as in Ov ES products. Proteins were separated on 12.5% SDS-PAGE as described previously [20] and transferred onto nitrocellulose membrane (Amersham™ Hybond™-ECL, GE Healthcare, UK) by semi-dry blotting (Trans-Blot®, Bio-Rad Laboratories, CA, USA). Membrane was blocked with 5% skim milk in Phosphate buffered saline with 0.1% Tween 20 (PBS-T) at room temperature (RT) for 1 hr. and washed three times with PBS-T before incubation at RT for 1 hr with primary antibody diluted in 2% skim milk in PBS-T (1:1,000 for anti-6×His-tag and 1:5,000 for anti-rOv CaBP). Then membrane was washed three times with PBS-T and incubated at RT for 1 hr. with secondary antibody, anti-mouse IgG conjugated with horseradish peroxidase (HRP) (Zymed Laboratories Inc., CA, USA), diluted in PBS (1:1,000). After washing with PBS-T two times, membrane was rinsed with PBS and detected by using diaminobenzidine (DAB) reagent (Sigma-Aldrich Co., MO, USA).
2.7. Gel mobility shift assay
Eluted fraction of purified rOv CaBP protein had the buffer exchanged to PBS while concentrating by using amicon ultra centrifugal filter devices. Dithiothreitol (DTT) was added to a final concentration of 10 mM. Calcium binding activity was analyzed by loading of rOv CaBP protein on 12.5% PAGE (without SDS) in the presence of 20 mM ethylenediaminetetraacetic acid (EDTA) and (a) 1 mM MgCl2 (b) 1 mM CaCl2 or without MgCl2 and CaCl2. Bovine serum albumin (BSA) was used as control.
2.8. Immunolocalization of Ov CaBP
The paraffin-embedded sections (4 µm) of an Ov infected hamster’s liver was obtained as previously described [21]. The native Ov CaBP was localized by immunohistochemistry as described previously [16]. In brief, tissue sections were deparaffined, rehydrated, and retrieved antigen before destroying the endogenous peroxidase. After blocking non-specific binding with normal serum, tissue sections were incubated overnight at 4°C with mouse preimmune serum (negative control) and anti-rOv CaBP serum (1:2,000). The sections were then washed with PBS and subsequently incubated at RT for 1 hr with HRP-conjugated anti-mouse IgG (1:300). After washing with PBS, the sections were detected using DAB reagent, counterstained with Mayer’s hematoxylin, dehydrated, cleared in xylene, and mounted in Permount®. Mounted sections were examined under light microscope and photographed.
3. Results
3.1. Immunoscreening and characterization of the Ov CaBP sequence
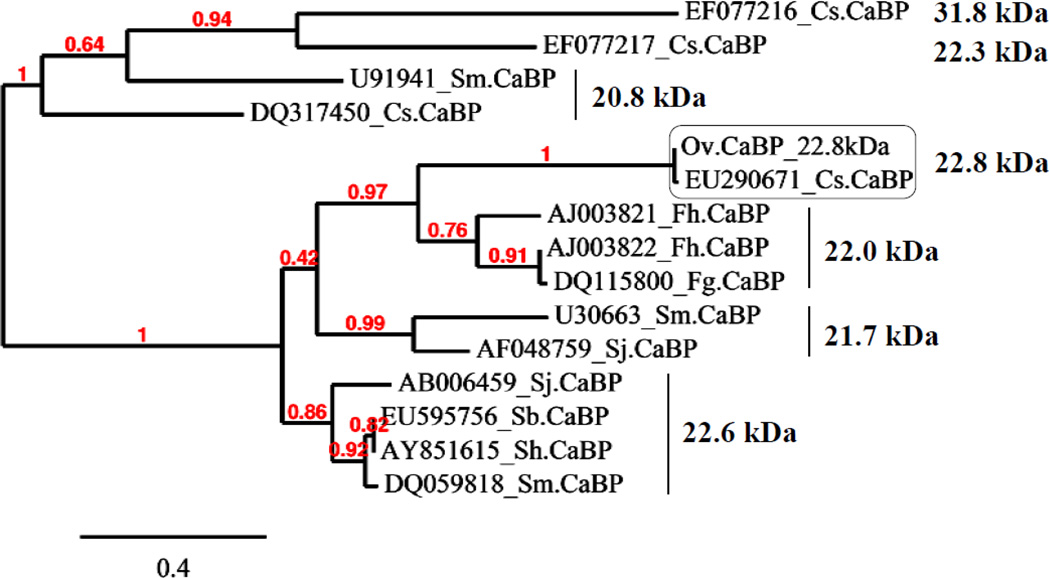
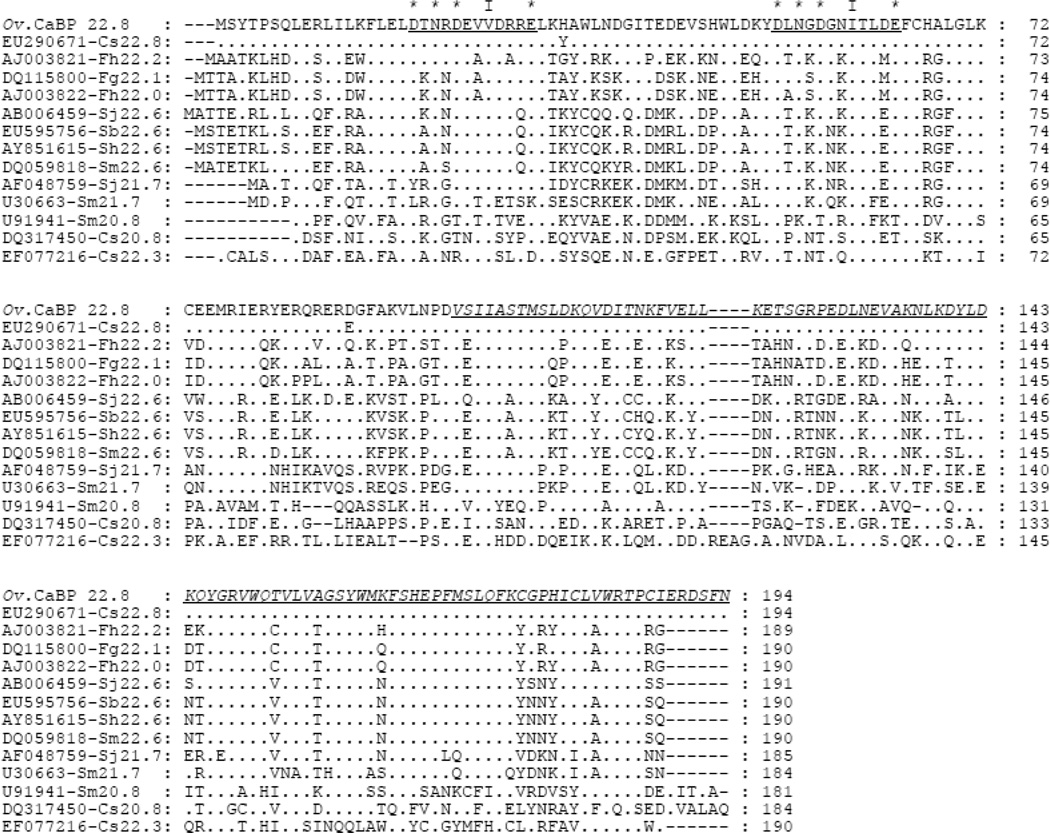
Ov CaBP cDNA was isolated in an immunoscreen of adult stage Ov cDNA library with human CCA serum. The result from NCBI-BLAST (tblastn) showed that Ov CaBP had 98%/99% amino acid identity/similarity to CaBP of C. sinensis 22.8 kDa (GenBank accession no. EU290671). Furthermore, BLAST searches within expressed sequence tags (EST) database of Ov adult stage showed that both Ov and Cs CaBPs were 100% and 99% compared to four sequences (OvAE3718, OvAE3719, OvAE3720, and OvAE1788) at the amino acid level, respectively. On the other hand, the CaBPs of the Fasciola group; F. hepatica 22.2 kDa (AJ003821), F. gigantica 22.1 kDa (DQ115800), and F. hepatica 22 kDa (AJ003822), shared more than 45%/69% amino acid identity/similarity to Ov CaBP. However, when these Fasciola CaBPs sequences were blasted within the Ov EST databases, different sequences with 97% amino acid similarity were hit from those described above. While the Schistosoma CaBPs, S. mansoni 22.6 kDa (DQ059818), S. japonicum 22.6 kDa (AB006459), S. bovis 22.6 kDa (EU595756), S. haematobium 22.6 kDa (AY851615), S. japonicum 21.7 kDa (AF048759), and S. mansoni 21.7 kDa (U30663) had more than 41%/57% amino acid identity/similarity to Ov CaBP, no sequence was hit within the Ov EST database. Whereas the other size of CaBPs from Clonorchis sinensis (Cs): 20.8 kDa (DQ317450) and 22.3 kDa (EF077216) had less than 32%/50% amino acid identity/similarity to the Ov CaBP protein. The phylogenetic classification showed that the Ov and Cs CaBP 22.8 kDa proteins were considered as a new group of CaBP protein (Fig. 1). The Ov CaBP cDNA was 585 bp and encoded for a putative protein of 194 amino acids. Its molecular weight and theoretical isoelectric point predicted from ProtParam program were 22.82 kDa and 5.11, respectively. The Ov CaBP protein was predicted not to contain a signal peptide and transmembrane helix via SignalP 3.0 and DAS program, respectively. Besides, the amino acid sequence of 22.8 kDa Ov CaBP was not found when searching within the secreted (ES products) and surface (tegument) proteomic database of Ov adult stage (personal communication with Dr. Jason Mulvenna) [22]. In addition, the TargetP program predicted that this protein is present in cytoplasm (http://www.cbs.dtu.dk/services/Target). Pfam database search showed that the N-terminus domain of Ov CaBP protein contained two putative EF-hand calcium-binding motifs (Pfam accession no. PF00036, residues 14–72) whereas the C-terminus domain was similar to dynein light chain type 1 (Pfam accession no. PF01221, residues 97–186) (Fig. 2).
Fig. 1.
The phylogenetic tree of Ov CaBP with homologs from other parasites. GenBank accession number is given followed by abbreviation of species name and the right hand side indicates the molecular weight of CaBPs.
Fig. 2.
Multiple sequence alignment between Ov CaBP and the homologs from Clonorchis, Fasciola, and Schistosoma. GenBank accession numbers are given followed by abbreviation of species name and molecular weight of CaBPs. The underlined sequences indicate the loop region between helix-loop-helix motif of EF-hand 1 and 2. The (*) represents the amino acid residues interacting with calcium while (I) represents the conserved hydrophobic residue within loop whereas the underlined italic letters indicated the DLC part of CaBP.
3.2. Transcription analysis of each stage of Ov CaBP
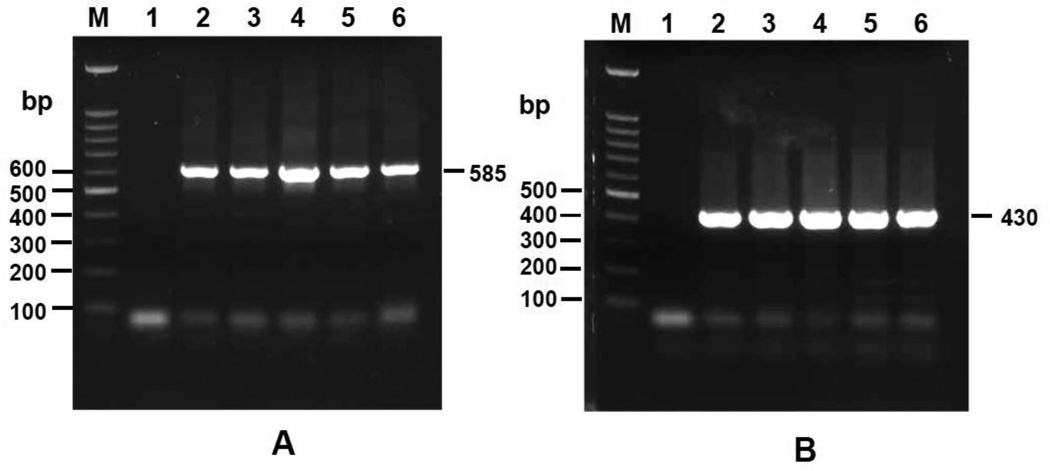
RNA of each stage (metacercariae, 2-week, 3-week, 1-month-old juvenile, 2-month-old adult worm) was extracted and reversed transcribed to cDNA for transcriptional analysis. The RT-PCR product of Ov CaBP gene in all stages showed a single band of 585 bp (Fig.3a). The PCR product of β-actin gene (internal control) showed a single band of 430 bp in all stages and in equal quantity (Fig.3b). Consequently, the transcripts of Ov CaBP were present in all intra-mammalian developmental stages.
Fig. 3.
Transcriptional analysis of stage specific expression of Ov CaBP gene by RT-PCR. Lane M, 1, 2, 3, 4, 5, and 6 show a molecular weight marker, negative control (without template), metacercariae, 2-week-old juvenile, 3-week-old juvenile, 1-month-old juvenile, and 2-month-old adult worm, respectively. Panel A shows the RT-PCR amplification of Ov CaBP gene in each stage whereas panel B shows the amplification of actin gene in each stage as an internal control. Electrophoresis was performed on 1% agarose gel.
3.3. Expression, purification, and western blotting analysis of rOv CaBP
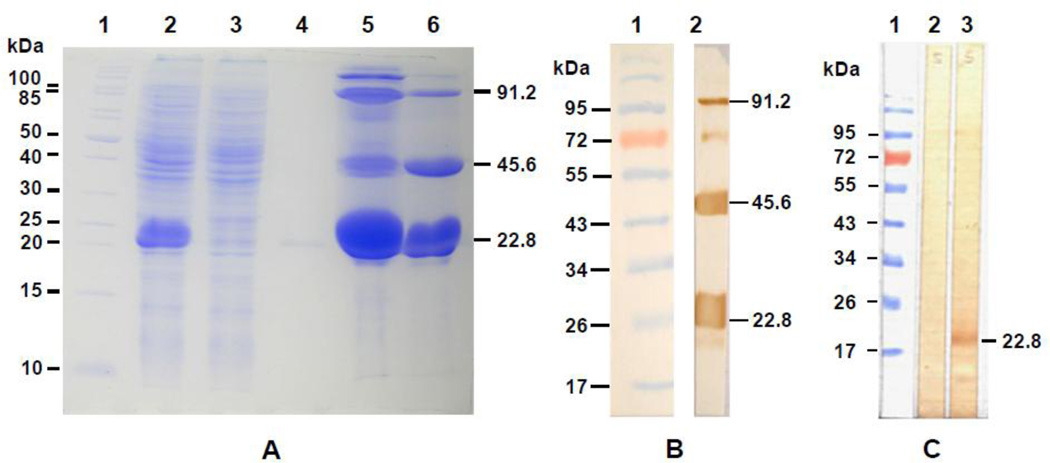
The recombinant plasmid pET15b-Ov CaBP was expressed in E. Coli BL21 (DE3) as a soluble protein with 6×His-tag at the N-terminus under IPTG induction at 25°C. When expressed at 37°C the majority of expressed rOv CaBP protein was found in the inclusion body. A Ni-NTA column was used to purify the fusion rOv CaBP protein under non-denaturing conditions (Fig. 4a). The multimeric forms of rOv CaBP in the eluted fraction of purified protein were observed and confirmed by western blotting with anti-His-tag antibody (Fig. 4b). The purified 6×His-tagged-rOv CaBP protein was used to immunize mice and antibody raised against this fusion rOv CaBP protein was able to detect 22.8 kDa of Ov CaBP in Ov somatic extracts (Fig. 4c) but not in Ov ES products (data not shown).
Fig. 4.
SDS-PAGE of rOv CaBP and western blot analysis of the purified rOv CaBP protein and native Ov CaBP in Ov somatic extracts. In panel A, rOv CaBP protein was purified under the native condition and loaded on 12.5% SDS-PAGE. Lane 1: molecular weight marker, Lane 2: E. coli lysate, Lane 3: flow through, Lane 4–6: eluted fraction 1, 2, and 3 of rOv CaBP protein, respectively. The multimeric forms of rOv CaBP protein were observed and the sizes are shown by arrows. In panel B, western blot was performed to verify the multimer of rOv CaBP protein in the eluted fraction 3 using anti-His tag antibody. Lane 1: molecular weight marker, Lane 2: eluted fraction 3 of purified rOv CaBP protein. In panel C, western blot analysis of native Ov CaBP protein in Ov somatic extracts probed with sera from mice. Lane 1: molecular weight marker, Lane 2: Ov somatic extracts reacted with mouse preimmune serum, Lane 3: Ov somatic extracts reacted with serum from rOv CaBP protein immunized mouse.
3.4. Analysis of rOv CaBP calcium-binding activity
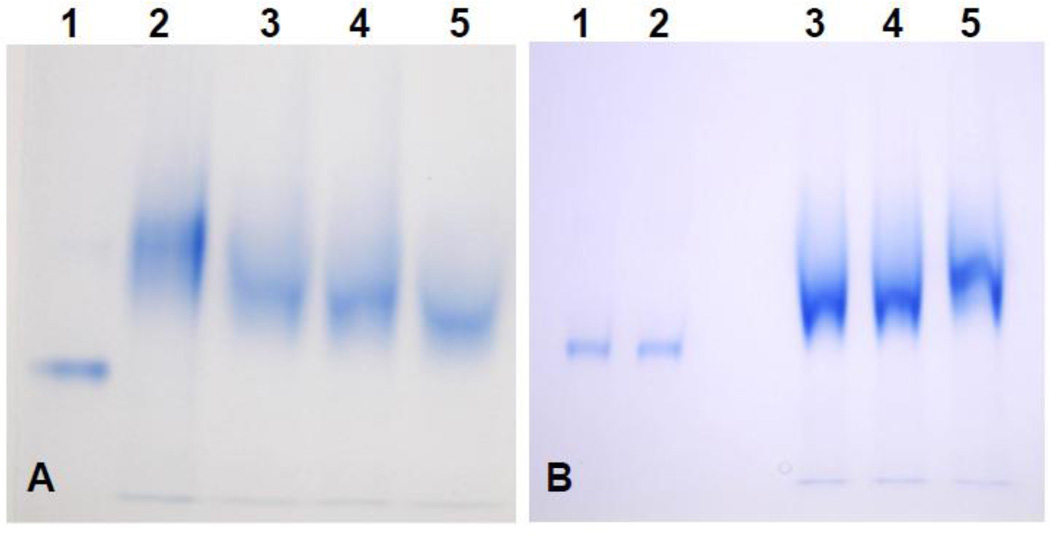
Initially the mobility shift of rOv CaBP protein was not observable. But when EDTA was added to the protein sample at various concentrations (1 mM – 20 mM) before performing 12.5% PAGE, a retardation effect occurred without adding CaCl2 (Fig. 5a). That suggested that Ca2+ ion was already present and saturating the system, probably via the E. coli culture medium. So, without a divalent cation chelating agent such as EDTA we could not observe the mobility shift of rOv CaBP. However, the retardation effect was clearly seen when adding 20 mM EDTA (final concentration) to the protein sample and in the presence of 1 mM CaCl2 while BSA protein used as a control did not undergo a mobility shift (Fig. 5b). Moreover the retardation effect of rOv CaBP protein was not observed in the presence of 1 mM MgCl2 (Fig. 5b).
Fig. 5.
Analysis of rOv CaBP calcium-binding property by gel mobility shift assay (12.5% PAGE). In panel A, a retardation effect was observed before adding CaCl2 to the protein sample due to the contamination of Ca2+ ion in system, consequently, various concentration of EDTA was added to protein sample for chelating out Ca2+. Lane 1: BSA served as control, Lane 2: rOv CaBP without adding EDTA, Lane 3: rOv CaBP with 1 mM EDTA, Lane 4: rOv CaBP with 5 mM EDTA, Lane 5: rOv CaBP with 20 mM EDTA. In panel B, the mobility shift assay was performed by adding 20 mM EDTA to all protein samples loaded on 12.5% PAGE in the presence/absence of 1 mM MgCl2 or 1 mM CaCl2. Lane 1: BSA with 1 mM MgCl2, Lane 2: BSA with 1 mM CaCl2, Lane 3: rOv CaBP as negative control, Lane 4: rOv CaBP with 1 mM MgCl2, Lane 5: the retardation effect of rOv CaBP with 1 mM CaCl2.
3.5. Immunolocalization of Ov CaBP
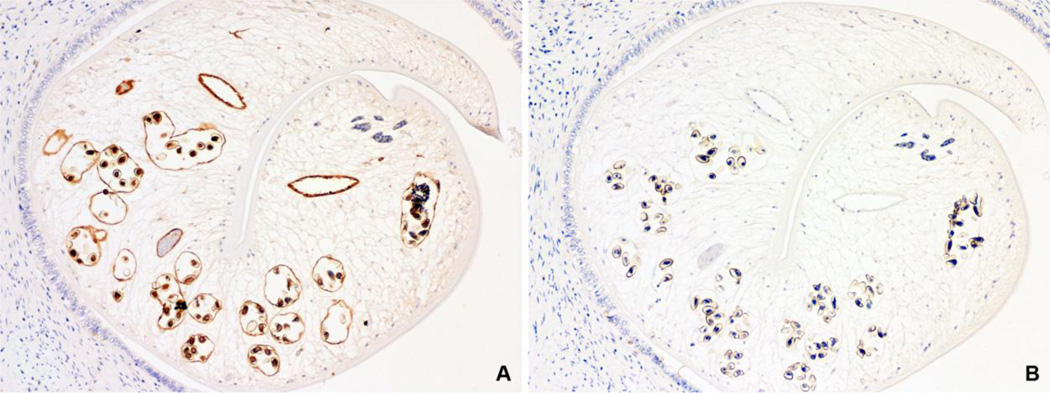
Mouse polyclonal anti-rOv CaBP serum was used to localize the native Ov CaBP in paraffin-embedded Ov infected hamster’s liver sections which revealed that Ov CaBP was located in gut epithelium, miracidia in eggs, and slightly in parenchyma but not in epithelium grand, mature sperm, and tegument while the preimmune serum showed negative staining to all tissues (Fig. 6).
Fig. 6.
Immunolocalization of native Ov CaBP in tissue section of Ov infected hamster’s liver by mouse anti-rOv CaBP serum. Positive immunoperoxidase staining was observed in gut epithelium, miracidia in eggs, and slightly in parenchyma (A), while negative control with mouse preimmune serum showed no staining (B). (Immunoperoxidase, original magnification, ×100)
4. Discussion
In this study, a novel 22.8 kDa of Ov CaBP EF-hand protein was identified from Ov cDNA library with a CCA serum. Moreover, the Ov CaBP protein had 98% amino acid identity to 22.8 kDa of Cs CaBP protein which is found up-regulated when exposing Cs metacercariae to gamma irradiation [23]. However, the 22.8 kDa of Cs CaBP sequence reported in GenBank is a partial mRNA sequence. In contrast, the 22.8 kDa Ov CaBP sequence obtained from the Ov cDNA library was a complete mRNA sequence. From the identity alignment, we concluded that 22.8 kDa of Ov CaBP and Cs CaBP were orthologs while the different MW size of CaBPs from Clonorchis, Fascisola, and Schistosoma, arose from distinct genes which had an amino acid identity less than 46%. In addition, the result from phylogenetic tree analysis classified these two CaBPs 22.8 kDa of Ov and Cs as a new group of CaBP proteins. However, more than one size of CaBP EF-hand proteins is found in any given species [12, 24–28]. In the case of Ov CaBP protein, the homology search within the Ov EST database by NCBI-BLAST using the other size of CaBP sequences from the other parasites (Cs 20.8; DQ317450, Fh 22.2; AJ003821, and Cs 22.3; EF077216) showed that there were at least four sequences of Ov CaBP with more than 88% amino acid identity (20.8, 22.2, 22.3, and 22.8 kDa). On the other hand, the Ov CaBP of 21.7, 22.6 and 31.8 kDa were not found in the Ov EST database when using the sequences from Sm 21.7 (U30663), Sj 22.6 (AB006459), and Cs 31.8 (EF077217) searching.
The 6×His-tagged-rOv CaBP protein was expressed as a soluble protein at 25°C and able to be purified by Ni-NTA column. However, we found the rOv CaBP protein preferred to homodimerize as multimeric forms as observed in SDS-PAGE. This property was also observed in the CaBP proteins from other parasites [12, 13, 14]. The homodimerization of CaBP proteins occurred due to the presence of Cys residues in the CaBPs which form disulfide bonds between inter/intramolecule of proteins. In the case of Ov CaBP protein, there were two Cys residues located on the N-terminus and three residues located on the C-terminus. Nevertheless, only a monomeric form of rOv CaBP protein was observed when 10 mM DTT was added to the protein sample before performing SDS-PAGE (data not shown) as previously described for Fg CaBP protein [13].
Some of the parasite CaBP EF-hand proteins showed a Ca2+ binding property [12, 13, 14, 29]. Binding of calcium to CaBP protein occurs in the loop region between helix-loop-helix motifs of EF-hand structure via an ionic interaction. Consequently, amino acid residues in the loop region at positions 1, 3, 5, and 12 were found to be a conserved negative charge that will bind to the positive charge of Ca2+ [30, 31]. Our Ov CaBP protein is composed of two EF-hand motifs (EF-hand 1 and EF-hand 2) at the N-terminus and both EF-hand motifs showed the conserved negative charged amino acids at positions 1, 3, 5, and 12 which were Asp, Asn, Asp, and Asp, respectively. A loop residue at position 6 provided loop bending and had a highly conserved Gly residue that was found in EF-hand 2 but not EF-hand 1 of Ov CaBP. Whereas a loop residue at position 8 showed a conserved hydrophobic amino acid that provided the β–sheet structure between the pair of EF-hands with Val and Ile residues found in EF-hand 1 and 2 of Ov CaBP, respectively [30]. Moreover, rOv CaBP showed the Ca2+ binding property in non-denaturing gel mobility shift assay in the presence of CaCl2 as well as 8 kDa Sj CaBP, 8 kDa Fh CaBP, 22 kDa Fh CaBP, and 22.1 kDa Fg CaBP [12, 13, 14, 29] whereas S. mansoni 20.8 and 21.7 kDa calcium-binding proteins failed in Ca2+-binding assays [25, 27]. Unlike Fg CaBP, the rOv CaBP did not bind to Mg2+ ion, such that a retardation effect was not observed on PAGE in the presence of MgCl2 [13].
Based on recent literature, some of CaBP EF-hand containing DLC motifs in trematodes were found to be located in the syncytial tegument membrane and/or tegument cell bodies [13, 24, 26–28, 32]. The other two small sizes of 8 kDa Sj and Fh CaBPs, however, were located on the surface of the parasites during cercarial stage [14, 33]. On the contrary, native Ov CaBP could be localized by the anti-rOv CaBP serum in gut epithelium, miracidia in eggs, and slightly in parenchyma (Fig. 6). According to this finding, the 22.8 kDa Ov CaBP might have a different function from the other sizes of trematode CaBPs. Although some of trematode CaBPs including Ov CaBP had more than 30% amino acid similarities in calcium-binding EF-hand motifs to calmodulins of other species [12, 14], the function of CaBP EF-hand proteins in trematodes is still unknown. Furthermore, the 8 kDa Fh CaBP which shared 27%/48% amino acid identity/similarity to Ov CaBP calcium-binding motif has been categorized into a sensor protein like calmodulin [14]. Nevertheless, trematode CaBPs had one pair EF-hand less than calmodulin and they were composed of two motifs, Ca2+ binding at N-terminus and DLC at C-terminus. These motifs were parasite specific and highly conserved among trematode CaBPs. However, the small size 8 kDa trematode CaBP contained only one Ca2+ binding motif. Both calmodulin and trematode CaBPs were multifunctional proteins [34, 35]. The speculated function of trematode CaBPs was based on their sequences and localization. In this study, Ov CaBP could be predicted to be a sensor protein, in which its binding to Ca2+ induced the conformational change in order to bind to its target protein. Furthermore, Ov CaBP was an abundant somatic antigen, highly conserved and diverged in parasite specific CaBP subfamily that could be found in every development stage as a soluble protein and was located mainly in gut epithelium as well as miracidia in eggs. Therefore, Ov CaBP might play roles in nutrient uptake and/or miracidia transformation. Basic knowledge on the Ov CaBP will increase understanding the structure-function relationship as well as to develop novel anti-parasitic drugs, vaccines, and/or to be a potential protein for immunodiagnostic detection of the parasitic disease in the future.
Research highlights.
A novel 22.8 kDa Ov CaBP that reacted with cholangiocarcinoma serum was identified > It was transcribed in all stages and had functional calcium-binding property > Ov CaBP was categorized into a sensor protein like calmodulin based on sequences > This somatic protein was localized mainly in gut epithelium and miracidia in eggs > It might play role in nutrient uptake and/or miracidia transformation
Acknowledgements
Sincere appreciations are expressed to Dr. Jason Mulvenna for his assistance on searching Ov CaBP protein sequence in his proteomic database, Dr. Albert John Ketterman for English proof-reading, and Mr. Suwit Balthaisong for technical support on parasite preparation and mice immunization. This work was supported by NIAID, NIH grant (award no. UO1AI065871) and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS), Khon Kaen University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jongsuksuntigul P, Imsomboon T. Opisthorchiasis control in Thailand. Acta Trop. 2003;88:229–232. doi: 10.1016/j.actatropica.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Sripa B, Kaewkes S, Intapan PM, et al. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72:305–350. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- 3.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens-Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 4.Vatanasapt V, Uttaravichien T, Mairiang EO, et al. Cholangiocarcinoma in north-east Thailand. Lancet. 1990;335:116–117. doi: 10.1016/0140-6736(90)90591-r. [DOI] [PubMed] [Google Scholar]
- 5.Laha T, Pinlaor P, Mulvenna J, et al. Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics. 2007;8:189. doi: 10.1186/1471-2164-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kretsinger RH, Nockolds CE. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973;248:3313–3326. [PubMed] [Google Scholar]
- 7.Grabarek Z. Structural basis for diversity of the EF-hand calcium-binding proteins. J Mol Biol. 2006;359:509–525. doi: 10.1016/j.jmb.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki H, Nakayama S, Kretsinger RH. Classification and evolution of EF-hand proteins. Biometals. 1998;11:277–295. doi: 10.1023/a:1009282307967. [DOI] [PubMed] [Google Scholar]
- 9.Ravasi T, Hsu K, Goyette J, et al. Probing the S100 protein family through genomic and functional analysis. Genomics. 2004;84:10–22. doi: 10.1016/j.ygeno.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Levine BA, Dalgarno DC, Esnouf MP, et al. The mobility of calcium-trigger proteins and its function. Ciba Found Symp. 1983;93:72–97. doi: 10.1002/9780470720752.ch5. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan JD, Corbett RJ, Roche RS. The thermodynamics of calcium binding to thermolysin. Biophys Chem. 1986;23:183–199. doi: 10.1016/0301-4622(86)85003-7. [DOI] [PubMed] [Google Scholar]
- 12.Hu S, Law P, Lv Z, et al. Molecular characterization of a calcium-binding protein SjCa8 from Schistosoma japonicum. Parasitol Res. 2008;103:1047–1053. doi: 10.1007/s00436-008-1090-5. [DOI] [PubMed] [Google Scholar]
- 13.Vichasri-Grams S, Subpipattana P, Sobhon P, et al. An analysis of the calcium-binding protein 1 of Fasciola gigantica with a comparison to its homologs in the phylum Platyhelminthes. Mol Biochem Parasitol. 2006;146:10–23. doi: 10.1016/j.molbiopara.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Fraga H, Faria TQ, Pinto F, et al. FH8-a small EF-hand protein from Fasciola hepatica. FEBS Journal. 2010;277:5072–5085. doi: 10.1111/j.1742-4658.2010.07912.x. [DOI] [PubMed] [Google Scholar]
- 15.Suttiprapa S, Mulvenna J, Huong NT, et al. Ov-APR-1, an aspartic protease from the carcinogenic liver fluke, Opisthorchis viverrini: functional expression, immunolocalization and subsite specificity. Int J Biochem Cell Biol. 2009;41:1148–1156. doi: 10.1016/j.biocel.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suttiprapa S, Loukas A, Laha T, et al. Characterization of the antioxidant enzyme, thioredoxin peroxidase, from the carcinogenic human liver fluke, Opisthorchis viverrini. Mol Biochem Parasitol. 2008;160:116–122. doi: 10.1016/j.molbiopara.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sripa B, Kaewkes S. Relationship between parasite-specific antibody responses and intensity of Opisthorchis viverrini infection in hamsters. Parasite Immunol. 2000;22:139–145. doi: 10.1046/j.1365-3024.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 18.Dereeper A, Guignon V, Blanc G, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finn RD, Mistry J, Tate J, et al. The Pfam protein families database. Nucleic Acids Res. 2010;38:D211–D222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int J Parasitol. 2000;30:735–740. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- 22.Mulvenna J, Sripa B, Brindley PJ, et al. The secreted and surface proteomes of the adult stage of the carcinogenic human liver fluke Opisthorchis viverrini. Proteomics. 2010;10:1063–1078. doi: 10.1002/pmic.200900393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim TI, Cho PY, Song KJ, et al. Gene expression of Clonorchis sinensis metacercaria induced by gamma irradiation. Parasitol Res. 2008;102:1143–1150. doi: 10.1007/s00436-008-0882-y. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Xu H, Zhang Z, et al. Cloning and expression of 21.1-kDa tegumental protein of Clonorchis sinensis and human antibody response to it as a trematode-nematode pan-specific serodiagnosis antigen. Parasitol Res. 2011;108:161–168. doi: 10.1007/s00436-010-2050-4. [DOI] [PubMed] [Google Scholar]
- 25.Francis P, Bickle Q. Cloning of a 21.7-kDa vaccine-dominant antigen gene of Schistosoma mansoni reveals an EF hand-like motif. Mol Biochem Parasitol. 1992;50:215–224. doi: 10.1016/0166-6851(92)90218-9. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Zhou Z, Hu X, et al. A novel tegumental protein 31.8 kDa of Clonorchis sinensis: sequence analysis, expression, and immunolocalization. Parasitol Res. 2007;102:77–81. doi: 10.1007/s00436-007-0728-z. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed MM, Shalaby KA, LoVerde PT, Karim AM. Characterization of Sm20.8, a member of a family of schistosome tegumental antigens. Mol Biochem Parasitol. 1998;96:15–25. doi: 10.1016/s0166-6851(98)00088-7. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z, Xia H, Hu X, et al. Oral administration of a Bacillus subtilis spore-based vaccine expressing Clonorchis sinensis tegumental protein 22.3 kDa confers protection against Clonorchis sinensis. Vaccine. 2008;26:1817–1825. doi: 10.1016/j.vaccine.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz de Eguino AD, Machin A, Casais R, et al. Cloning and expression in Escherichia coli of a Fasciola hepatica gene encoding a calcium-binding protein. Mol Biochem Parasitol. 1999;101:13–21. doi: 10.1016/s0166-6851(99)00012-2. [DOI] [PubMed] [Google Scholar]
- 30.Gifford JL, Walsh MP, Vogel HJ. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem J. 2007;405:199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Yang W, Kirberger M, et al. Prediction of EF-hand calcium-binding proteins and analysis of bacterial EF-hand proteins. Proteins. 2006;65:643–655. doi: 10.1002/prot.21139. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Auliff A, Jones MK, et al. Immunogenicity and immunolocalization of the 22.6 kDa antigen of Schistosoma japonicum. Parasite Immunol. 2000;22:415–424. doi: 10.1046/j.1365-3024.2000.00319.x. [DOI] [PubMed] [Google Scholar]
- 33.Lv ZY, Yang LL, Hu SM, et al. Expression profile, localization of an 8-kDa calcium-binding protein from Schistosoma japonicum (SjCa8), and vaccine potential of recombinant SjCa8 (rSjCa8) against infections in mice. Parasitol Res. 2009;104:733–743. doi: 10.1007/s00436-008-1249-0. [DOI] [PubMed] [Google Scholar]
- 34.Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends in Cell Biology. 2000;10:322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 35.FuDong Y, Bin K, YuanYuan L, et al. Functional analysis of schistosomes EF-hand domain-containing tegument proteins. Chinese Science Bulletin. 2007;52:2100–2107. [Google Scholar]