Abstract
Fungi are among the most degradative organisms inducing biodeterioration of paper-based items of cultural heritage. Appropriate conservation measures and restoration treatments to deal with fungal infections include mechanical, chemical, and biological methods, which entail effects on the paper itself and health hazards for humans. Three different conservation treatments, namely freeze-drying, gamma rays, and ethylene oxide fumigation, were compared and monitored to assess their short- (one month, T1) and long-term (one year, T2) effectiveness to inhibit fungal growth. After the inoculation with fungi possessing cellulose hydrolysis ability — Chaetomium globosum, Trichoderma viride, and Cladosporium cladosporioides — as single strains or as a mixture, different quality paper samples were treated and screened for fungal viability by culture-dependent and -independent techniques.
Results derived from both strategies were contradictory. Both gamma irradiation and EtO fumigation showed full efficacy as disinfecting agents when evaluated with cultivation techniques. However, when using molecular analyses, the application of gamma rays showed a short-term reduction in DNA recovery and DNA fragmentation; the latter phenomenon was also observed in a minor degree in samples treated with freeze-drying. When RNA was used as an indicator of long-term fungal viability, differences in the RNA recovery from samples treated with freeze-drying or gamma rays could be observed in samples inoculated with the mixed culture. Only the treatment with ethylene oxide proved negative for both DNA and RNA recovery. Therefore, DNA fragmentation after an ethylene oxide treatment can hamper future paleogenetic and archaeological molecular studies on the objects.
Keywords: Paper, Biodeterioration, Treatments, Freeze-drying, Gamma rays, Ethylene oxide fumigation, Monitoring
Highlights
► Freeze-drying, gamma rays and ethylene oxide fumigation: evaluation of effectiveness to inhibit fungal growth. ► Screening with DGGE for the short- and long-term recovery from infected paper of fungal DNA and RNA. ► Freeze-drying: no efficacy as disinfecting agent, no effect on DNA recovery either after short- or long-term monitoring. ► Gamma rays: full efficacy as disinfecting agent, short-term reduction of DNA recovery and DNA fragmentation. ► Ethylene oxide: full efficacy as disinfecting agent. DNA fragmentation: negative for both DNA and RNA recovery.
1. Introduction
A major part of our cultural heritage has been recorded in various forms on paper for centuries and is therefore vulnerable to biodeterioration of its organic components through fungi (Nol et al., 2001; Corte et al., 2003; Cappitelli and Sorlini, 2005; Michaelsen et al., 2006, 2009; Rakotonirainy et al., 2007; Zotti and Ferroni, 2008; Mesquita et al., 2009) and, in a minor way, bacteria (De Paolis and Lippi, 2008; Jurado et al., 2010; Michaelsen et al., 2010), resulting in both structural and aesthetic damage.
Fungal contamination is considered a major concern for libraries or archives full of paper-based books and documents. For the storage and maintenance of this often valuable material, it is crucial not only to control active fungal growth, i.e., hyphae, mycelium, or mould, but also to remove or reduce the amount of fungal ascospores and conidia. The structural nature of ascospores as progenitors of future growth allows the fungi to survive severe conditions, and ascospores are consequently harder to inactivate than the vegetative hyphae. In unfavorable conditions resting spores have low water content and their metabolism is inactivated but reversible (Florian, 1993; Deacon, 2005). Hence, any treatment to conserve objects of cultural value should be directed toward the spores, as the vegetative hyphae are relatively easy to control by physical removal and through monitoring of the storage climate, in terms of temperature, relative humidity, and activity of water (Nitterus, 2000). Fungi are an increasing and dominant problem for archives and museums and thus the prevention of their growth and the development of appropriate treatment measures for contaminated objects are a challenge for restorers, curators, and scientists (Sterflinger, 2010).
A broad spectrum of chemical and non-chemical components has been used to sanitize microfungi attacking paper-made objects in an attempt to inhibit degradation (Magaudda, 2004). In this study we chose three different conservation treatments for paper to compare their effectiveness over the short- and long-term, namely freeze-drying, gamma rays, and ethylene oxide fumigation.
A commonly used strategy in paper conservation is freeze-drying, a method in which the water is frozen and then removed by sublimation, i.e., it goes from the solid phase to water vapor, bypassing the liquid phase. Sublimation allows the water to be removed without the effects of water evaporative forces, which can cause dimensional changes. In addition, freeze-drying with dehydration can kill hydrated conidia and germinating conidia and it stops the growth of fungal mycelia and bacterial cells (Sussman, 1966; Mazur, 1968; Florian, 2002). Yet if the moisture content in the thawed materials remains high, resting dry conidia may be activated. Freezing can also increase the porosity and thickness of organic materials and make them more hygroscopic. Although it cannot be considered a disinfecting treatment, freeze-drying is still the most effective method known for the physical, chemical, and biological stabilization of water-damaged archival and library materials, especially when large quantities are involved and time is of the essence (Schmidt, 1985; McCleary, 1987; Walsh, 1988; Parker, 1989; Florian, 1990).
The application of gamma rays in conservation science dates back to the 1960s, when the radio-resistance of significant mould fungi from goods of cultural value was tested (Belyakova, 1960). High-energy electromagnetic radiation is deeply penetrating and biocidal through the denaturation and cleavage of nucleic acids, which leads to a simultaneous and indiscriminate devitalisation of all living organisms (Magaudda, 2004). Gamma irradiation is successfully used to sterilize laboratory and hospital utensils and food, but it can have unwanted side effects when applied in paper conservation where high irradiation doses, which are often required in repeated doses, can result in cumulative depolymerization of the underlying cellulose in the paper. Severe aging characteristics, such as lowered folding endurance and tear resistance, increased yellowing, and general embrittlement, have been reported in paper treated with gamma rays (Butterfield, 1987; Adamo et al., 1998), whereas more recent studies have suggested that the damage in terms of mechanical–physical properties is not significant (Adamo et al., 2001; Gonzalez et al., 2002). The observed effects of gamma rays on fungi from paper have confirmed that radiation treatment of books and documents is effective, as there was no fungal growth detectable in cultivation studies (Jörg et al., 1992; Da Silva et al., 2006).
Finally, ethylene oxide (EtO) has been widely used for sterilization of objects of cultural heritage since 1933 (Ballard and Baer, 1986). Ethylene oxide does not require activation energy, is used at room temperature, and expresses the high reactivity and diffusivity required for the inactivation of microorganisms (Mendes et al., 2007). By adding alkyl groups to DNA, RNA, or proteins, EtO prevents normal cellular metabolism and the ability to reproduce (Rutala and Weber, 1999). This ability to act as an alkylating agent was widely taken to indicate a carcinogenic potential, an assumption that has subsequently been proven for EtO and some of its residuals (Bolt, 1996; Angerer et al., 1998), leading to a ban on EtO for the practice of paper conservation in many countries. Additionally, a study found paper material fumigated with EtO to be more susceptible to microbial attack after fumigation (Valentin, 1986). This phenomenon is not fully understood but it is claimed that ethylene glycol, formed as a by-product during fumigation, activates spores that contaminate the object in further storage (Florian, 1993). In this study, the effects of these three conservation treatments on fungal spores and mycelium viability and activity were investigated by culture-dependent and -independent strategies. The main goals were: (a) to compare the efficacy of molecular versus cultivation techniques as models for the monitoring of conservation treatments, (b) to evaluate massive and aggressive disinfecting treatments on DNA integrity, and (c) to use rRNA analyses as a method of determining long-term viability of fungi.
With this end, two paper grades were used for the in-vitro inoculation of spores from different fungal species to obtain infected paper samples. The physiological state of the fungal strains was monitored over the short- and long-term after the treatments to determine time-dependent effects of the treatments on fungal viability. These effects were examined by culturing and on a molecular level by generating DNA-denaturing gradient gel electrophoresis (DNA-DGGE) profiles. As only metabolically active cells produce RNA, we used the ability to retrieve fungal-specific rRNA directly from the paper samples as an indicator of the viability of the respective fungi after the samples had been left for a year for eventual regrowth. This strategy is commonly used in bacterial ecology and is based on the fact that metabolically active species transcribe more rRNA for ribosome synthesis than do inactive species (Prosser, 2002). As RNA is also highly unstable in the environment, the detection of RNA in an environmental sample has been used as a strategy for detecting the most active microorganisms within a natural community (Gonzalez et al., 2006). To our knowledge this is the first study combining molecular methods including RNA detection of fungi with monitoring of conservation treatments for paper-made objects.
2. Materials and methods
2.1. Sample preparation and cultivation
Fungal growth was induced in vitro by inoculating two types of paper with three fungal strains previously isolated: Chaetomium globosum Kunze, Cladosporium cladosporioides (Fres.) de Vries, and Trichoderma viride Pers. (Michaelsen et al., 2006). Paper A was a Whatman paper type (Whatman 1 CHR category No. 3001917, not glued and not sized, 100% cotton linter, pH 6.5–7.0) consisting of pure cellulose with low ash content, and paper D was a “Mezzofino” type paper with a high lignin content, namely a naturally aged rag paper produced by the Istituto Poligrafico dello Stato in 1976 (Gallo et al., 1999), No. 200953, consisting of bleached cellulose (45%), wood pulp treated with sulphites (25%), wood pulp from softwood (20%), glue (3%), aluminum sulfate (5%), and kaolin (2%), pH 4.5. Each paper sample existed as a set of small discs (about 1 cm diameter) cut with a puncher (12.5 mm disc punch n. T5443, Agar Scientific Ltd., Stansted, Essex, England).
Fungal strains were grown on malt extract agar (2%) to obtain colonies with mature fruiting bodies or reproductive structures. Conidia and ascospores were harvested with a sterile cotton swab and diluted in water with Tween 80 (0.01%, Sigma, Italia) to obtain solutions with a standard concentration of about 5000 spores/ml. A further dilution was performed with a Czapek broth, to obtain 50 spores μl−1. The water vapor-sterilized paper samples were inoculated with 50 μl of broth each to provide a physiological starter for germination. Each inoculum was applied to the sample disc in a single spot. About 100% relative humidity (RH) was maintained with distilled water during fungal growth in double-bottom glass containers. Samples were kept in a thermostatic cell at 27 °C for 7 days (C. cladosporioides and T. viride), and 14 days (Ch. globosum and mixed inocula).
Chaetomium is a genus of filamentous fungi (phylum Ascomycota, class Sordariomycetes) encompassing species that typically possess densely setose, ovoid to pyriform ostiolate ascomata, clavate asci, and pigmented, one-celled ascospores (Samson et al., 2000). Species of Chaetomium are important in the decomposition of cellulose-rich materials. T. viride is the conidial stage of Hypocrea rufa. Several strains of Trichoderma have been developed as biocontrol agents against fungal diseases of plants. The various mechanisms include antibiosis, parasitism, inducing host-plant resistance, and competition. The T. viride strain used in the production of paper samples was characterized by producing only conidia, and not the ascospores typical of its prefect state (Hypocrea). Cladosporium is a dematiaceous (pigmented) mould widely distributed in air and rotten organic material. The genus Cladosporium is one of the largest genera of dematiaceous hyphomycetes, and is characterized by conidia in acropetal chains and Davidiella teleomorphs. C. cladosporioides has conidiophora distinct from vegetative hypahe and conidia that are one-celled, thick-walled, and finely roughed, produced in branched chains (Samson et al., 2000).
One replica of each set was used as a control sample and not subjected to any treatment. The other replicas were treated with freeze-drying, gamma rays, or EtO. Viability of fungal spores was tested one month after treatment for all samples (T1) and one year later (T2) for the untreated control samples with the 25 points inoculum method.
2.2. Treatments of paper samples
To avoid contamination from airborne fungi after the treatment, all samples were placed into sterile paper air-permeable envelopes before being treated and left therein until further testing. Sample envelopes were stored in petri dishes in dark conditions at room temperature. Gamma irradiation and EtO fumigation are hazardous and consequently conducted by specialists and approved companies.
2.2.1. Freeze-drying
The envelopes containing samples (a single envelope for each paper grade and each fungal inoculum) were positioned inside blocks of copying paper in order to simulate the treatment of a thicker volume and to avoid a direct heating of the samples on the warming grid of the freeze-dryer. A set of five paper leaves was interposed between each envelope and a 2-cm layer of paper leaves was used to isolate the envelopes from the heating plate. The treatment was conducted at the Centro di Legatoria e Restauro Frati e Livi, s.r.l. (Castelmaggiore, Bologna, Italy) in a 5-h cycle with warming temperatures of 50 °C maximum.
2.2.2. Gamma rays
Irradiation treatment was performed at the Research Centre of ENEA Casaccia, Rome, Italy, by Dr. Marianna Adamo. Inoculated paper samples underwent a gamma ray dose of 4756 Gy/h with an exposure time of 1 h and 3 min (Adamo et al., 2001).
2.2.3. Ethylene oxide
Fumigation treatment was performed by Spix Italia s.r.l. in a vacuum cell device with a mixture of EtO (10%) and CO2 (90%) for 48 h at a temperature between 20 and 22 °C. After gas abatement, 20 washing cycles with air were performed. The treatment was verified with chemical (EO stripes n. 6615 EtO chemical indicator, VWR International PBI Srl) and biological (EO vials n. 3166 EtO biological indicator, VWR International PBI Srl) standard indicators (Ballard and Baer, 1986).
2.2.4. Control samples
Sets of inoculated paper samples that developed fungal mycelia were air-dried in sterile polystyrene ventilated petri dishes at about 21 °C and 50% RH for one week to stop active growth of fungal mycelia, but to keep conidia and ascospores alive. Following natural dehydration the samples were sterilely introduced into paper envelopes and kept in petri dishes in the same manner as the treated samples.
2.2.5. Culturing of treated and untreated samples
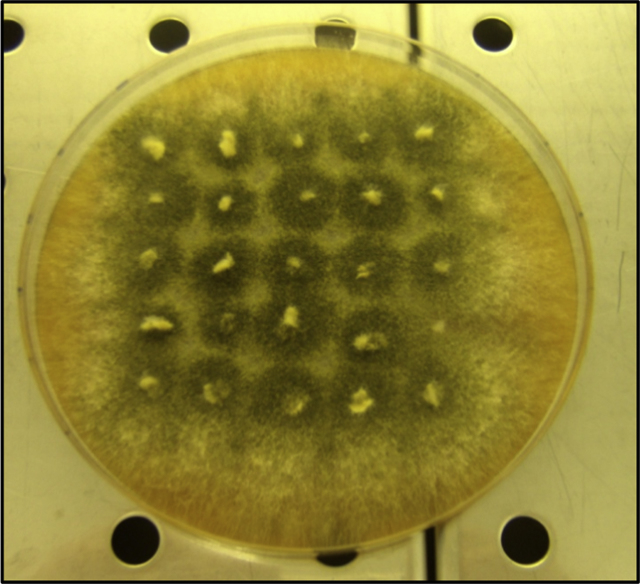
The treated and the untreated control samples were tested for viability of the fungal spores by means of the 25 points inoculum method: Sets of inoculated and treated paper, three each, were washed three times in sterile water and divided into 25 sub-samples that were inoculated directly on solid nutritive agar (malt extract agar, 20 g l−1) distributed according to a geometric scheme (Fig. 1). The frame consists of a grid with 25 nodes spaced out equally. The development of the fungal mycelium in all or a fraction of the 25 inoculum points allows for a statistical comparison between different treatments and untreated samples (Dix and Webster, 1995, after adaptation on cellulosic materials). One-way ANOVA was computed and followed by the Fisher test according to Massart et al. (1998) using XLSTAT statistical software (Addinsoft, Paris) to determine the significant differences between the treatments. The inocula on agar were performed directly after the treatments and 1 year later (long-term monitoring, T2).
Fig. 1.
Example of a 25-point inoculum of Chaetomium globosum on paper A taken from control paper samples. The development of mycelium in the points allows for a statistical comparison between different treatments and untreated control samples. In the control sample all the 25 fragments of paper produced a colony after the inoculation on nutritive agar.
2.3. DNA extraction from paper material
DNA was extracted directly from the prepared small paper discs (about 1 cm in diameter) by using the FastDNA SPIN Kit for Soil (MP Biomedicals, Solon, OH, USA). The protocol of the manufacturer was slightly modified, as described by Michaelsen et al. (2006). To ensure the lysis of all cells and spores on the paper surface, the samples were pre-treated with lysozyme and proteinase K as described by Schabereiter-Gurtner et al. (2001) before the application of the kit. DNA crude extracts were used directly for PCR amplification analysis. DNA extraction was performed one month after treatment (short-term monitoring, T1) and again one year later (long-term monitoring, T2) to test the efficiency and long-term effects of the different treatments on the DNA of the inoculated fungi.
2.4. DNA amplification and DGGE analyses
All PCR reactions were executed using PCR Master Mix (Promega, Mannheim, Germany). Fragments of about 500–700 bp size corresponding to the ITS1–ITS2 region of the ribosomal-DNA internal transcribed spacer region were amplified with the primer pair ITS1 and ITS4 (White et al., 1990). For DGGE analysis of the DNA fragments, a nested PCR was performed with the PCR product of the first round as template DNA using the primers ITS1GC with a 37-base GC clamp attached to the 5′-end and ITS2 (White et al., 1990; Muyzer et al., 1993). All reactions were carried out as described in Michaelsen et al. (2006) and PCR products were visualized by electrophoresis in a 2% (w/v) agarose gel. Denaturing gradient gel electrophoresis (DGGE) was performed as previously described by Muyzer et al. (1993) in 0.5 × TAE (20 mM Tris, 10 mM acetate, 0.5 mM Na2EDTA; pH 7.8) with 8% (w/v) acrylamide gels containing a gradient of 30–50% denaturants in a DGGE 2401 system (C.B.S. Scientific Co., USA). Gels were run at 60 °C and 75 V for 14 h, stained with ethidium bromide, and visualized using a Bio-Rad Gel Doc imaging system (Bio-Rad Laboratories Pty., Ltd, Australia).
2.5. RNA extraction
RNA was extracted from the paper samples using a combination of the method developed by Griffiths et al. (2000) and the RNeasy Plant Mini Kit (Qiagen, Australia) with some changes to the manufacturer's protocol. One disc per treatment was placed in a Lysing Matrix E tube (MP Biomedicals, Solon, OH, USA) and 600 μl of buffer RLC from the RNeasy Plant Mini Kit was added. The tubes were processed on the FastPrep beadbeater for 30 s at 5.5, followed by a centrifugation for 2 min at 13,000 rpm. The supernatant was then used with the RNeasy kit following the protocol for fungi and plants from step 4 on, according to the manufacturer's instruction. An on-column DNAase treatment was performed using the Qiagen RNase-free DNase set (Qiagen, Australia). The final elution step was carried out twice with the provided water and stored at −80 °C. RNA extraction was performed one year after the treatment of samples (long-term monitoring, T2) to allow conclusions on the viability and actual growth of the inoculated and treated strains to be compared with the DNA results.
In all cases, PCR controls with the crude RNA extracts were performed to exclude DNA contamination.
2.6. RNA amplification
RT-PCR was carried out using 2 μl RNA template for the initial denaturing step with 1 μl of reverse primer 18Sr (5′-GATCCTTCTGCAGGTTCACCTAC-3′) for 5 min at 65 °C. For DNA synthesis, 1 μl of AMV Reverse Transcriptase (Promega, Australia), 0.5 μl dNTPs (100 mM) and 2.5 μl buffer Omniscript were used in a 10-μl reaction volume with molecular grade water (all Qiagen, Australia) for 60 min incubation at 37 °C.
The cDNA was used to amplify approximately 250 bp products of the 18S rRNA with primers EK555sr (5′-GCTGCTGGCACCAGACT-3′) and 18S-300f GC, which includes a 39-bp GC clamp on the 5′ end to be used in DGGE analysis (Moreno et al., 2010). The PCR reaction contained 2 μl of cDNA with 2.5 μl AmpliTaqTM10× buffer, 1.5 μl MgCl2 solution, 0.1 μl AmpliTaqTM DNA polymerase (all Applied Biosystems), 10 pmol of each primer, 0.5 μl dNTPs (10 mM), and 1.25 μl DMSO in a 25-μl volume. The PCR conditions used included an initial denaturation step at 95 °C for 2 min, followed by 30 cycles of 95 °C for 30 s, 61 °C for 30 s, and 72 °C for 30 s, and a final extension cycle of 10 min at 72 °C. The products were purified using the Qiagen PCR purification kit. The GC-clamped PCR products were separated using DGGE on 10% (wt/vol) polyacrylamide gels with a 30–50% urea/formamide denaturing gradient using the DGGE 2401 system (C.B.S. Scientific Co., USA). Gels were run at 60 °C and 55 V for 17 h, stained with ethidium bromide, and visualized using a Bio-Rad Gel Doc imaging system (Bio-Rad Laboratories Pty., Ltd, Australia).
3. Results and discussion
3.1. Cultivation of fungal spores from treated and non-treated control samples
The development of fungal mycelium in the 25-spot grid enabled statistical comparisons between the treatments and the control samples and among the control samples as presented in Fig. 1 and Table 1, according to the analysis of variance (ANOVA) method and Fisher test. Non-treated control samples showed good growth for all fungi with no statistical difference, but different inoculation results were obtained from paper samples subjected to freeze-drying, dependent on the species. C. cladosporioides appeared to be no longer cultivable and the recorded growth for T. viride was low and statistically different from both the control sample and Ch. globosum. No statistical difference was found for Ch. globosum and mixed inocula samples with respect to the control samples. Both gamma irradiation and EtO fumigation did suppress fungal growth of all single species and the mixed inocula over the short-term and showed full efficacy as disinfecting agents when evaluated with cultivation techniques (Table 1).
Table 1.
Statistical analysis of the 25-inoculum growth test. Three replicates of 25 inoculum grids were considered (25 × 3 inoculum points for each treatment/paper/fungus). Average value ± standard deviation. Different letters indicate significant differences (Fisher, p < 0.05) between the means of independent replicates. Paper grades: A (Whatman) and D (Mezzofino). Treatments: nt T1, control after 1 month from fungal inoculums; nt T2 control after 1 year of natural drying; O ethylene oxide treatment; γ, gamma rays treatment; L, freeze-drying treatment. Fungi: Ch = Chaetomium globosum; Cl = Cladosporium cladosporioides; Tr = Trichoderma viride; Mix = the three strains together.
| Treatment | Average paper A ± St. Dev. | Average paper D ± St.Dev. |
|---|---|---|
| nt T1_Ch | 25.0 ± 0.0 A | 23.3 ± 1.5 A |
| nt T1_Cl | 25.0 ± 0.0 A | 25.0 ± 0.0 A |
| nt T1_Mix | 24.0 ± 1.7 A | 24.3 ± 1.2 A |
| nt T1_Tr | 19.0 ± 1.0 B | 17.3 ± 0.6 B |
| nt T2_Ch | 25.0 ± 0.0 A | 7.7 ± 4.9 D |
| nt T2_Cl | 0.0 ± 0.0 E | 0.0 ± 0.0 E |
| nt T2_Mix | 24.0 ± 1.7 A | 2.7 ± 0.6 E |
| nt T2_Tr | 0.0 ± 0.0 E | 0.0 ± 0.0 E |
| O_Ch | 0.0 ± 0.0 E | 0.0 ± 0.0 E |
| O_Cl | 0.0 ± 0.0 E | 0.0 ± 0.0 E |
| O_Mix | 0.0 ± 0.0 E | 0.0 ± 0.0 E |
| O_Tr | 0.0 ± 0.0 E | 0.0 ± 0.0 E |
| γ_Ch | 0.0 ± 0.0 E | 0.0 ± 0.0 E |
| γ_Cl | 0.0 ± 0.0 E | 0.0 ± 0.0 E |
| γ_Mix | 0.0 ± 0.0 E | 0.0 ± 0.0 E |
| γ_Tr | 0.0 ± 0.0 E | 0.0 ± 0.0 E |
| L_Ch | 25.0 ± 0.0 A | 12.3 ± 1.5 C |
| L_Cl | 0.0 ± 0.0 E | 0.0 ± 0.0 E |
| L_Mix | 16.7 ± 3.8 B | 0.3 ± 0.6 E |
| L_Tr | 0.0 ± 0.0 E | 2.3 ± 1.5 E |
Long-term monitoring showed differences in the growth of the singly inoculated fungal strains stored without treatment (T2). T. viride and C. cladosporioides were not able to grow after one year at room temperature. Ch. globosum did not behave in a statistically different manner compared to the sample one year before. These results can be explained by the higher resistance of Chaetomium ascospores with respect to the conidia of Trichoderma and Cladosporium strains. Ascospores are typically thick-walled and protected in a fruiting body, as in Chaetomium spp. (Deacon, 2005).
Comparing the two types of paper tested, statistically significant differences (Table 1) were also noted in a general favor of paper A, the Whatman paper, for fungal growth after treatment. However, as the same effect was observed for the control samples of paper A, it indicates that cotton linter generally supported fungal growth better than rag paper D, the Mezzofino paper made from cellulose and wood pulp, and the differences evaluated can be independent of the treatments.
3.2. DGGE fingerprints to monitor and compare the effect of the treatments
Denaturing gradient gel electrophoresis is the technique most often used to study the structure of the microbial communities colonizing artworks (Gonzalez and Saiz-Jimenez, 2004). Furthermore, it has recently been implemented to monitor structural changes in microbial communities colonizing stone-works after conservation treatments (Piñar et al., 2010; Ettenauer et al., 2011). Hence, in this study we applied DGGE for the first time to monitor paper samples that were inoculated with fungal spores and further subjected to different conservation treatments. This allowed monitoring of changes on the DNA level of different fungal species in relation to the treatments, and determination of the treatments' efficiency.
To this end, the internal transcribed spacer region 1 (ITS1) was amplified from template DNA extracted directly from the treated paper samples as well as from non-treated control samples and subsequently separated in DGGE (Michaelsen et al., 2006).
Interestingly, our results show only minor effects on the recovery of fungal DNA from samples treated with freeze-drying or gamma rays, as minor differences between the DGGE profiles of treated and non-treated control samples are mostly restricted to the mixed inocula samples (Fig. 2). This fact may indicate a result of the competition between fungal strains during incubation.
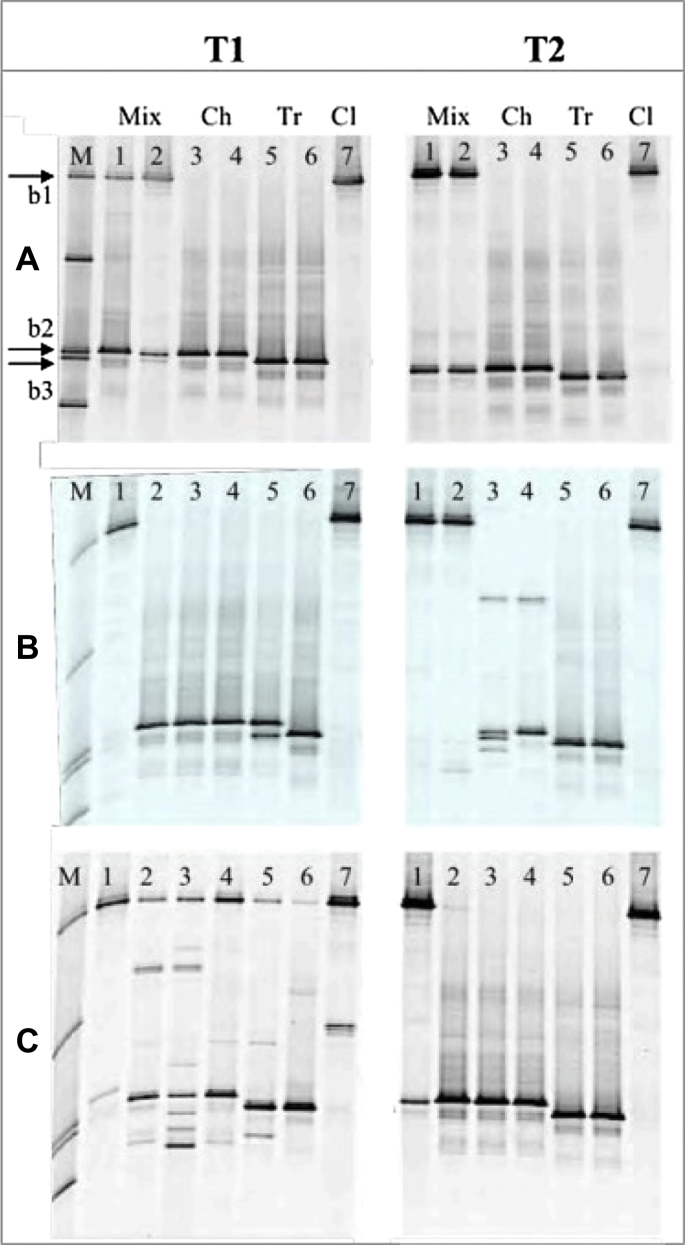
Fig. 2.
DGGE profiles generated from ITS1 derived DNA of untreated control (a), freeze-dried (b), and gamma ray- (c) treated paper samples extracted after one month of the application of treatments (T1) and one year later (T2). Lanes contain marker (M), mixed inocula (Mix) on paper types A and D (lanes 1 and 2), Chaetomium globosum (Ch) on paper types A and D (lanes 3 and 4), Trichoderma viride (Tr) on paper types A and D (lanes 5 and 6), and Cladosporium cladosporioides (Cl) on paper type A (lanes 7). No DNA was obtained for any sample treated with EtO. The marker contains, among others, fragments from C. cladosporioides (b1), Ch. globosum (b2), and T. viride (b3).
3.2.1. Control samples
Denaturing gradient gel electrophoresis fingerprints derived from untreated control samples one month after the treatment (T1) and one year later (T2) are nearly identical, as shown in Fig. 2a. DNA from T. viride (lanes 5 and 6) was not detected in any sample when inoculated in a mixture with Ch. globosum and C. cladosporioides independently of the nature of treatment. However, it should be considered that T. viride was initially not competitive enough and was outgrown by the mycelium of the other two fungi, probably due to the antifungal effect of Chaetomium (Kanokmedhakul et al., 2002). The amount of fungal spores and mycelium from T. viride on the samples was below the detection limit of our PCR and DGGE conditions.
DGGE bands representing C. globosum (lanes 3 and 4) were observed in both single and mixed inoculated control samples of both paper types, at both T1 and T2, whereas T. viride only showed clear bands when inoculated as a single strain at both time points monitored. Unexpectedly, no DGGE band could be obtained for C. cladosporioides inoculated as a single strain on paper D throughout the experiment, probably due to the difficulty of this species to grow as a single strain on the lignin containing/acidic paper. The conidia of this fungus deposited on paper D with the inoculum did not germinate but as conidia were possibly around they were probably below the detection limit of our PCR and DGGE conditions. Therefore, this sample is not shown in Figs. 2 and 3.
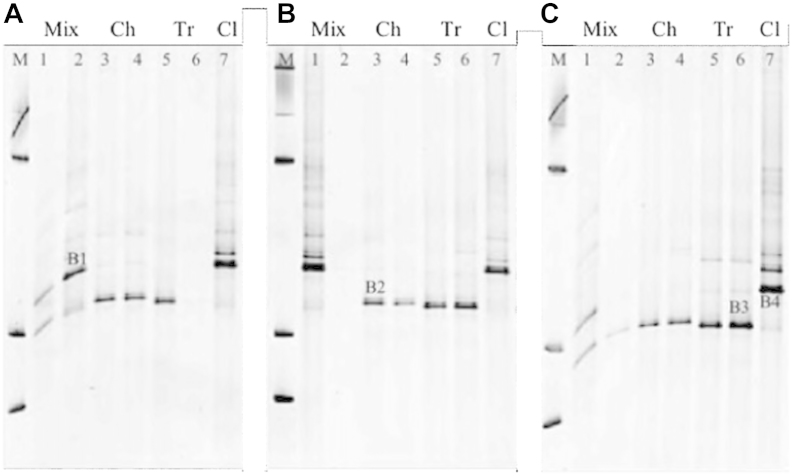
Fig. 3.
DGGE profiles generated from 18S cDNA of untreated control (a), freeze-dried (b), and gamma ray- (c) treated paper samples one year after the application of treatments (T2). Lanes contain marker (M), mixed inocula (Mix) on paper types A and D (lanes 1 and 2), Chaetomium globosum (Ch) on paper types A and D (lanes 3 and 4), Trichoderma viride (Tr) on paper types A and D (lanes 5 and 6), and Cladosporium cladosporioides (Cl) on paper types A (lane 7). Marked bands indicate the position of each fungus on DGGE: C. Cladosporioides (B1, in A and B4 in 3C), Ch. globosum (B2), and T. viride (B3).
3.2.2. Samples treated with freeze-drying
Temperature has an effect on the growth and survival of fungi, as it dictates the rate of the reactions catalyzed by enzymes, but it is important to note that a temperature below which no growth occurs should not be understood as a lethal temperature for fungi (Nitterus, 2000). Reducing the temperature to subzero values, as happens in freeze-drying cycles, reduces the viability of many fungi and can finally exhaust even dormant spores if these temperatures are maintained over long periods after initially activating them (Florian, 1993). As spores and hyphae in mature and active fungal colonies contain considerable amounts of water, the majority of them should be killed at temperatures below 0 °C (Nitterus, 2000). But our results show that, on the contrary, cultivation and molecular techniques both produced positive results for fungal growth or activity on samples subjected to freeze-drying, which are comparable with those of untreated samples. This explains also why freeze-drying is not considered a conservation treatment but an emergency mass-rescue procedure for flooding and other water-related incidents (Florian, 1990, 2002).
DGGE fingerprints derived from samples treated with freeze-drying showed some differences compared to the control samples (Fig. 2b). One month after the treatment (T1) the fingerprints derived from samples inoculated with the mixed culture differed from the control and between paper A, Whatman, and paper D, Mezzofino. The C. cladosporioides was represented by a strong band on paper A (T1, lane 1) but it was missing on paper D (T1, lane 2). By contrast, Ch. globosum appeared as a strong band on paper D but was very faint on paper A, whereas T. viride was not detected in any mixed inoculated sample, as observed in the control samples. Mixed inocula showed a dominance of Ch. globosum that exhibited a strong antifungal tendency against T. viride. The bands representing each single fungus inoculated as pure culture did not show differences due to treatment.
Long-term effects due to the freeze-drying treatment were only observed for Ch. globosum when inoculated as a single strain, showing an unspecific band-pattern (Fig. 2b, T2, lanes 3 and 4). Furthermore, no DGGE band corresponding to this fungus was detected when it was inoculated in a mixture with the other two fungi (Fig. 2b, T2, lanes 1 and 2). The other fungal bands showed patterns in accordance with the control samples.
3.2.3. Samples treated with gamma rays
Different functions of the paper or its components, such as cellulose, are affected by gamma irradiation. Furthermore, its germicidal power on fungal microflora has been reported to be dose-dependent (Magaudda et al., 2000; Adamo et al., 2001). A dose of about 5 kGy, as applied in our experiment, was found to cause statistically significant variations on almost all paper properties, such as tensile strength, tearing resistance, and folding endurance, but also reduced the fungal population down to the “blank” samples level (Adamo et al., 2001; Magaudda, 2004). Relatively low irradiation doses of 2–3 kGy delivered comparable decontaminating power without the increasingly depolymerizing effects of gamma rays on cellulose.
Samples treated with gamma rays showed DGGE patterns with numerous unspecific bands one month after treatment (Fig. 2c, T1). Bands corresponding to the individual fungi Ch. globosum, T. viride, and C. cladosporioides were still observed, but their identities were indecisive in some samples. In addition, new faint bands were observed. These patterns indicate a mixed population of intact DNA from surviving fungi and DNA artifacts, as a consequence of DNA fragmentation due to the irradiation, from affected fungi.
One year later (Fig. 2c, T2), no more visible effect of gamma radiation on the DNA of pure strains, compared to the control samples, was observed. This indicates that the surviving fungal population recovered with time and only the intact DNA of this population was detected. For paper samples inoculated with mixed inocula, only the band for C. cladosporioides was shown to be weak on paper D (Fig. 2c, T2, lane 2).
3.2.4. Samples treated with EtO fumigation
The observation that EtO can significantly reduce or erase the amount of amplifiable DNA on small items has been reported before. Ethylene oxide is successfully applied in forensics to remove DNA contamination on items used both at crime scenes and in the forensics laboratory, without significantly affecting any needed downstream DNA analysis when used to sterilize equipment (Shaw et al., 2008). The gaseous EtO is considered a radiomimetic agent, which means it is similar to ionizing radiation by virtue of its ability to induce the same biological end-points, such as gene mutations, mainly by alkylating and reacting with nucleophile centers, such as nitrogen and oxygen atoms in the DNA bases (Bolt, 1996; Rutala and Weber, 1999; Chovanec et al., 2001). Double-strand-breakages are induced, leading to fragmentation of the DNA helix. Our results could be explained by such a fragmentation of fungal DNA through EtO fumigation, resulting in PCR not delivering any evidence of fungal DNA or RNA on all samples submitted to fumigation. In accordance with this, a study on viability of fungal spores on paper after EtO treatment indicated that fungal spores became non-cultivable and non-viable with EtO inhibiting spore activity (Rakotonirainy et al., 2003).
As Shaw et al. (2008) did, we observed that EtO fumigation was more effective than the application of gamma rays in reducing DNA from the surface of samples. The superiority of EtO as a sterilizing agent compared to gamma radiation was explained by Chovanec et al. (2001) as a combination of differences in the nature of damage caused and the distribution of the target. Ionizing radiation can react more locally and focused with free radicals following tracks along covalent bonds, whereas small molecules such as EtO can diffuse quite freely in the cell nucleus, causing damage on a broader scale. Although EtO application is banned for use in conservation, it should still be recommended in certain cases where the careful restoration and conservation of valued material is assessed.
The fumigation of paper with EtO caused the loss of amplifiable fungal DNA from all spore-inoculated samples tested in both single and mixed cultures. General EtO inhibition properties on PCR performance were ruled out with PCR controls with DNA extracts spiked with pure fungal DNA as template (data not shown).
3.3. RNA as an indicator for metabolically active fungi
As we confirmed the presence of DNA on most samples, with the exception of those treated with EtO, the next consequent step was to investigate if the fungi inoculated on the samples were still metabolically active. Several methods are available to test fungal and microbial viability, such as the quantitation of cell's ATP content (Rakotonirainy et al., 2003) or fluorescein diacetate (FDA) and acridine-orange stainings that provide a qualitative observation of a microorganism's active structures by means of an epifluorescent microscope, equipped with specific filters (Pinzari et al., 2011).
In this study we decided to use nucleic acids as markers of fungal viability. The existence of ribosomal-DNA alone does not lead to assumptions about viability because rRNA genes can persist in environmental DNA pools for species that are metabolically inactive and functionally less important (Ostle et al., 2003). Thus, if responses of microbial communities to environmental perturbations are investigated, the rRNA gene approach seems problematic, as rRNA genes may be detected in DNA pools for species whose growth or cellular activity has declined (Anderson and Parkin, 2007). To test the viability of the inoculated fungi, we targeted the fungal rRNA directly, as it allows the detection of metabolically active and functionally important species, as they transcribe more rRNA, a principle that is commonly exploited in bacterial ecology (Prosser, 2002). Determination of the viability of the causative agent is important in determining the active infection and in the evaluation of the efficacy of a particular treatment.
In this study, RNA was extracted from paper samples one year after the application of the respective treatments (T2), and subsequently transcribed into cDNA for further DGGE analyses. The DGGE profiles generated with 18S primers from the fungal cDNA are shown in Fig. 3. Untreated control samples and those subjected to freeze-drying and gamma irradiation were all positive for amplification of fungal 18S cDNA, whereas no positive RNA extraction or PCR could be established for EtO-treated samples. Strong signals could be observed for all pure strains, with the exception of T. viride on paper D, in the control samples (Fig. 3a). The pattern of single bands obtained for the pure inoculated strains indicated the presence of only one fungus on each paper sample. On samples inoculated with the mixed cultures, the DGGE band corresponding to T. viride was not observable, a pattern that is congruent with the DNA-generated gels.
RNA analyses revealed a minimal long-term impact of either gamma rays or freeze-drying treatments on the viability of the fungal spores, when inoculated as single strains (Fig. 3b and c, lanes 3–7). All samples deliver clear bands with a pattern that matches the expectations and consistent for the different fungi. However, when fungi were inoculated as mixed cultures, no RNA band at all or very faint signals were observed for paper D after freeze-drying and gamma ray treatments, respectively (Fig. 3b and c, lane 2).
Unfortunately, an amplification of the cDNA with ITS primers, as used for the DGGE analysis of DNA, failed. Therefore we used primers targeting the 18S rRNA to obtain DGGE profiles of cDNA. ITS sequences are the most popular choice for species identification of fungi in environmental DNA pools (White et al., 1990), but due to post-transcriptional processing of the main precursor rRNA molecules containing 18S, ITS1, 5.8S, ITS2, and 28S rRNA, the ITS spacer regions are spliced out to leave the rRNA genes for ribosome synthesis (Anderson and Parkin, 2007). Even if there is evidence that the ITS regions can be used for the detection of active fungi, as was done by Anderson and Parkin (2007), it is generally considered impossible to detect ITS sequences in RNA pools (Hibbett, 1992). A successful amplification of ITS rRNA was performed by other groups from fungal isolate extracts and soil samples. We assumed, during the experimental work, that there was more rRNA available in the soil samples themselves, as soils are active environments compared to the paper environment, which resembles starving conditions. While the present paper was under review, a paper by Rajala et al. (2011) was published, which demonstrated that internal transcribed spacers (ITS) precursor rRNA are a better marker of active fungi than small subunit rRNA. This is because the ITS sequences, after being excised from the subunits turnover rapidly, and small and large subunit rRNA particles are relatively stable in the environment. The work by Rajala et al. (2011) may be important for follow-up elaboration of the present work.
3.4. Comparison of methods used for the monitoring of treatments — cultivation versus molecular methods
Our molecular results generally contradicted the cultivation results obtained in this study. By using culture-dependent techniques, samples subjected to freeze-drying showed growth in the case of T. viride, but is was recorded as low and statistically different from the control sample, whereas no statistical difference was found for C. globosum and the mixed inocula sample with respect to the control samples (see Table 1). However, by using culture-independent techniques we could observe some differences on samples inoculated with the mixed culture samples one month after the freeze-drying treatment (T1) compared to the control samples (see Fig. 2b). DGGE bands representing each single fungus inoculated as pure culture did not show differences due to treatment, but a long-term effect was only observed for Ch. globosum when it was inoculated as a single strain, showing an unspecific band-pattern.
Samples subjected to gamma irradiation and EtO fumigation showed no fungal growth, either of all single species or the mixed inocula. Therefore, the last two treatments indicated a full efficacy as disinfecting agents when evaluated with cultivation techniques (see Table 1). However, when using culture-independent techniques, gamma rays were not able to eliminate fungal colonization of paper even if relatively high doses (5 kGy) were applied, but they could potentially reduce or slow down the growing process. DGGE profiles obtained from DNA extracted shortly after the irradiation process display a certain amount of fragmentation and unspecific bands that could be related to the effect of gamma radiation on the tertiary or secondary structure of the DNA itself, but no long-term effect of gamma rays on DNA or RNA was observed, indicating the recovering of the surviving fungal fraction. Gamma rays have been reported to inactivate fungi from and related to books (Jörg et al., 1992; Da Silva et al., 2006). However, Da Silva et al. (2006) determined the efficiency of the radiation treatment on fungal spores by cultivation attempts only, and therefore described a higher degree of reduction than our DNA- and RNA-based studies. Our cultivation results are in accordance with these observations, but are proven wrong when molecular data are included.
Only in the case of EtO fumigation did both culture-dependent and -independent techniques indicate the total inactivation of the inoculated fungi.
Comparing DNA and RNA recovery, we could observe no differences in the case of samples inoculated with pure strains, being able to recover both DNA and RNA, which indicates that the fungi were indeed viable. However, differences in the RNA recovery from samples treated with freeze-drying or gamma rays were observed when samples were inoculated with the mixed culture, especially on paper type D, indicating the inactivation of the fungi on this kind of paper and the higher sensitivity of RNA- versus DNA-based analyses for the detection of viability.
4. Conclusions
The differences between culture and molecular results obtained in this study highlight the importance of molecular tools to complement conventional techniques, as they display higher sensitivity and bypass culturing methods. Retrieval of fungal-specific rRNA directly from paper can, in fact, be successfully used as an indicator for the viability of fungi after disinfecting treatments.
Information on the effects of mass treatments on the DNA of paper-spoiling fungi is also provided. Our results show, in fact, that the freeze-drying treatment displays only minimal effect on DNA recovery over both the short- or long-term when compared to control samples, whereas the application of gamma rays shows a short-term slight reduction of DNA recovery and DNA fragmentation but no significant difference in long-term effect.
When RNA was used as an indicator for long-term fungal viability, differences in the RNA recovery from samples treated with freeze-drying or gamma ray-treated samples were observed when samples were inoculated with the mixed culture, specially on paper type D. Samples inoculated with the single strains showed no difference in the RNA recovery compared to control samples. Only treatment with ethylene oxide proved negative for both DNA and RNA recovery. These results show the higher sensitivity of RNA- over DNA-based molecular analyses for the detection of viability of fungi on disinfected paper materials.
Considering our results, we can assume that gamma rays can be used to treat large amounts of paper simultaneously and without subsequent chemical hazard, but this can only be considered a decontamination treatment, a method to remove biodeteriogenous microorganisms to a controllable or blank level. This target of reducing and holding the biodeteriogens at under the danger threshold is crucial in paper conservation, and it can be achieved with gamma radiation, whereas freeze-drying can be applied only to stop heavy mould before further treatment.
Furthermore, our results point to ethylene oxide fumigation as the only effective and long-lasting treatment when the sterilization of a fungal infected paper object is needed. Subsequent contamination is, however, possible, as ethylene oxide is not a chemical that remains as a preventive biocide on materials.
It is worth emphasizing that no DNA or RNA could be recovered from paper samples fumigated with EtO, a fact that is of particular importance for studies that address a molecular analysis of old contaminations or biological materials in cultural heritage characterization and diagnostics, including paleogenetic and archaeological molecular studies. Ethylene oxide fumigation was used by museums and libraries in the recent past to treat insect or mould infestations, but it is often not traceable or not clearly mentioned in the reports that accompany the treated objects. Consequently, misleading diagnostics could be produced in subsequent molecular studies. What is needed is improved documentation of the conservation history of objects of cultural value, in order to account for treatments that can alter future studies and analysis and to avoid any impediments by past treatment based on EtO.
Generally, any treatment that is applied to prevent the biodeterioration of paper documents or books has to be carefully assessed beforehand. The type of biological attack and the urgency of intervention have to be considered when choosing among treatments. Economic factors such as cost of treatment, quantity of objects to be treated, and their historical or commercial value also have also an impact on the decision of conservators.
Acknowledgements
The molecular analysis included in this study and Astrid Michaelsen were financed by the Austrian Science Fund (FWF) within the framework of project P17328-B12. Guadalupe Piñar is currently financed by the Austrian Science Fund (FWF) project “Elise-Richter V194-B20.” Part of this work was performed at the Centre for Marine Bioinnovation (CMB) at the University of New South Wales (UNSW) in Sydney, Australia. We thank Prof. Staffan Kjelleberg, Dr. Thorsten Thomas, and Dr. Mike Manefield for their kind support. The authors are grateful to Pietro Livi (Centro di Legatoria e Restauro Frati e Livi s.r.l., Italy), Dr. Marianna Adamo (Research Centre of ENEA Casaccia, Rome, Italy), and the people from Spix Italia s.r.l. for the treatment of paper samples.
References
- Adamo M., Giovanotti M., Magaudda G., Plossi Zappala M., Rocchetti F., Rossi G. Effect of gamma rays on pure cellulose paper as a model for the study of treatment of biological recovery of biodeteriorated books. Restaurator. 1998;19:41–59. [Google Scholar]
- Adamo M., Brizzi M., Magaudda G., Martinelli G., Plossi Zappala M., Rocchetti F., Savagnone F. Gamma radiation treatment of paper in different environmental conditions: chemical, physical and microbiological analysis. Restaurator. 2001;22:107–131. [Google Scholar]
- Anderson I.C., Parkin P.I. Detection of active soil fungi by RT-PCR amplification of precursor rRNA molecules. Journal of Microbiological Methods. 2007;68:248–253. doi: 10.1016/j.mimet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Angerer J., Bader M., Kramer A. Ambient and biochemical effect monitoring of workers exposed to ethylene oxide. International Archives of Occupational and Environmental Health. 1998;71:14–18. doi: 10.1007/s004200050244. [DOI] [PubMed] [Google Scholar]
- Ballard M.W., Baer N.S. Ethylene oxide fumigation: results and risk assessment. Restaurator. 1986;7:143–168. [Google Scholar]
- Belyakova L.A. Gamma radiation as a means of disinfection of books against spores of mould fungi. Mikrobiologiya. 1960;29:762–765. [Google Scholar]
- Bolt H.M. Quantification of endogenous carcinogens. The ethylene oxide paradox. Biochemical Pharmacology. 1996;52:1–5. doi: 10.1016/0006-2952(96)00085-8. [DOI] [PubMed] [Google Scholar]
- Butterfield F.J. The potential long-term effects of gamma radiation on paper. Studies in Conservation. 1987;32:181–191. [Google Scholar]
- Cappitelli F., Sorlini C. From papyrus to compact disc: the microbial deterioration of documentary heritage. Critical Reviews in Microbiology. 2005;31:1–10. doi: 10.1080/10408410490884766. [DOI] [PubMed] [Google Scholar]
- Chovanec M., Cedervall B., Kolma A. DNA damage induced by gamma-radiation in combination with ethylene oxide or propylene oxide in human fibroblasts. Chemico-Biological Interactions. 2001;137:259–268. doi: 10.1016/s0009-2797(01)00258-7. [DOI] [PubMed] [Google Scholar]
- Corte M.A., Ferrroni A., Salvo V.S. Isolation of fungal species from test samples and maps damaged by foxing, and correlation between these species and the environment. International Biodeterioration and Biodegradation. 2003;51:167–173. [Google Scholar]
- Da Silva M., Moraes A.M.L., Nishikawa M.M., Gatti M.J.A., Vallim de Alencar M.A., Brandao L.E., Nobrega A. Inactivation of fungi from deteriorated paper materials by radiation. International Biodeterioration and Biodegradation. 2006;57:163–167. [Google Scholar]
- De Paolis M.R., Lippi D. Use of metabolic and molecular methods for the identification of a Bacillus strain isolated from paper affected by foxing. Microbiological Research. 2008;163:121–131. doi: 10.1016/j.micres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Deacon J. Blackwell Publishers; Cambridge, Massachusetts: 2005. Fungal Biology. [Google Scholar]
- Dix N.J., Webster J. Chapman & Hall; Londres: 1995. Fungal Ecology. p. 549. [Google Scholar]
- Ettenauer J., Piñar G., Sterflinger K., Gonzalez-Muñoz M.T., Jroundi F. Molecular monitoring of the microbial dynamics occurring on historical limestone buildings during and after the in situ application of different bio-consolidation treatments. Science of the Total Environment. 2011;409:5337–5352. doi: 10.1016/j.scitotenv.2011.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian M.L. The effects of freezing and freeze-drying on natural history specimens. Collection Forum. 1990;6:45–52. [Google Scholar]
- Florian, M.L., 1993. Conidial fungi (mould) activity on artifact materials – a new look at prevention, control and eradication. ICOM. Committee for Conservation 19th Triennal Meeting. Washington, pp. 868–874.
- Florian M.L. Archetype publications; London, UK: 2002. Fungal Facts. p. 146. [Google Scholar]
- Gallo F., Valenti P., Coalizzi P., Pasquariello G., Scorrano M., Sclocchi M.C., Maggi O., Persiani A.M. Research on the viability of fungal spores in relation to different microclimates and different materials. In: Federici C., Munafo P.F., editors. International Conference on Conservation and Restoration of Archival and Library Materials, Erice, 22nd–29th April 1996. G.P. Palumbo; Palermo: 1999. pp. 213–230. [Google Scholar]
- Gonzalez J.M., Saiz-Jimenez C. Microbial activity in biodeteriorated monuments as studied by denaturing gradient gel electrophoresis. Journal of Separation Sciences. 2004;27:174–180. doi: 10.1002/jssc.200301609. [DOI] [PubMed] [Google Scholar]
- Gonzalez M.E., Calvo A.M., Kairiyama E. Gamma radiation for preservation of biologically damaged paper. Radiation Physics and Chemistry. 2002;63:263–265. [Google Scholar]
- Gonzalez J.M., Portillo M.C., Saiz-Jimenez C. Metabolically active Crenarchaeota in Altamira Cave. Naturwissenschaften. 2006;93:42–45. doi: 10.1007/s00114-005-0060-3. [DOI] [PubMed] [Google Scholar]
- Griffiths R.I., Whiteley A.S., O'Donnell A.G., Bailey M.J. Rapid method for co-extraction of DNA and RNA from natural environments for analysis of ribosomal DNA and rRNA-based microbial community composition. Applied and Environmental Microbiology. 2000;66:5488–5491. doi: 10.1128/aem.66.12.5488-5491.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbett D.S. Ribosomal RNA and fungal systematics. Transactions of the Mycological Society Japan. 1992;33:533–556. [Google Scholar]
- Jörg M., Wildführ W., Langguth H., Teichert E. Gammastrahlen zur Schimmelpilzbekämpfung bei Büchern: Versuche an der Universitätsbibliothek zu Leipzig. Restauro. 1992;98:114–119. [Google Scholar]
- Jurado V., Porca E., Pastrana M.P., Cuezva S., Fernandez-Cortes A., Saiz-Jimenez C. Microbiological study of bulls of indulgence of the 15th–16th centuries. Science of the Total Environment. 2010;408:3711–3715. doi: 10.1016/j.scitotenv.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Kanokmedhakul S., Kanokmedhakul K., Phonkerd N., Soytong K., Kongsaeree P., Suksamrarn A. Antimycobacterial anthraquinone-chromanone compound and diketopiperazine alkaloid from the fungus Chaetomium globosum KMITL-N0802. Planta Medica. 2002;68:834–836. doi: 10.1055/s-2002-34415. [DOI] [PubMed] [Google Scholar]
- Magaudda G., Adamo M., Pasquali A., Rossi G. The effect of ionising gamma ray radiation on the biology of the Periplaneta Americana. Restaurator. 2000;21:41–45. [Google Scholar]
- Magaudda G. The recovery of biodeteriorated books and archive documents through gamma radiation: some considerations on the results achieved. Journal of Cultural Heritage. 2004;5:113–118. [Google Scholar]
- Massart D.L., Vandeginste B.G.M., Buydens L.M.C., de Jong S., Lewi P.J., Smeyers-Verbeke J. Elsevier Science; Amsterdam: 1998. Handbook of Chemometrics and Qualimetrics. Part B. [Google Scholar]
- Mazur P. Survival of fungi after freezing and desiccation. In: Ainsworth G.C., Sussman A.S., editors. vol. III. Academic Press; London and New York: 1968. pp. 325–394. (The Fungi: An Advanced Treatise). [Google Scholar]
- McCleary J.P. UNESCO; Paris: 1987. Vacuum Freeze-drying, a Method Used to Salvage Water-damaged Archival and Library Materials: A RAMP Study with Guidelines. (PGI-87/WS/7) [Google Scholar]
- Mendes G., Brandao T.R.S., Silva C.L.M. Ethylene oxide sterilization of medical devices: a review. American Journal of Infection Control. 2007;35:574–581. doi: 10.1016/j.ajic.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Mesquita N., Portugal A., Videira S., Rodríguez-Echeverría S., Bandeira A.M.L., Santos M.J.A., Freitas H. Fungal diversity in ancient documents: a case study on the Archive of the University of Coimbra. International Biodeterioration and Biodegradation. 2009;63:626–629. [Google Scholar]
- Michaelsen A., Pinzari F., Ripka K., Lubitz W., Piñar G. Application of molecular techniques for identification of fungal communities colonising paper material. International Biodeterioration and Biodegradation. 2006;58:33–141. [Google Scholar]
- Michaelsen A., Piñar G., Montanari M., Pinzari F. Biodeterioration and restoration of a 16th-century book using a combination of conventional and molecular techniques: a case study. International Biodeterioration and Biodegradation. 2009;63:161–168. [Google Scholar]
- Michaelsen A., Piñar G., Pinzari F. Molecular and microscopical investigation of the microflora inhabiting a deteriorated Italian manuscript dated from the thirteenth century. Microbial Ecology. 2010;60:69–80. doi: 10.1007/s00248-010-9667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A.M., Matz C., Kjelleberg S., Manefield M. Identification of ciliate grazers of autotrophic bacteria in ammonia-oxidizing activated sludge by RNA stable isotope probing. Applied and Environmental Microbiology. 2010;76:2203–2211. doi: 10.1128/AEM.02777-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyzer G., de Waal E.C., Uitterlinden A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes encoding for 16S rRNA. Applied and Environmental Microbiology. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitterus M. Fungi in archives and libraries. A literary survey. Restaurator. 2000;21:25–40. [Google Scholar]
- Nol L., Henis Y., Kenneth R.G. Biological factors of foxing in postage stamp paper. International Biodeterioration and Biodegradation. 2001;48:94–97. [Google Scholar]
- Ostle N., Whiteley A.S., Bailey M.J., Sleep D., Ineson P., Manefield M. Active microbial RNA turnover in a grassland soil estimated using a 13CO2 spike. Soil Biology and Biochemistry. 2003;35:877–885. [Google Scholar]
- Parker A.E. The freeze-drying process. Some conclusions. Library Conservation News. 1989;23:4–8. [Google Scholar]
- Piñar G., Jimenez-Lopez C., Sterflinger K., Ettenauer J., Jroundi F., Fernandez-Vivas A., Gonzalez-Muñoz M.T. Bacterial community dynamics during the application of a Myxococcus xanthus-inoculated culture medium used for consolidation of ornamental limestone. Microbial Ecology. 2010;60:15–28. doi: 10.1007/s00248-010-9661-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzari F., Troiano F., Piñar G., Sterflinger K., Montanari M. The contribution of microbiological research in the field of book, paper and parchment conservation. In: Engel P., Schirò J., Larsen R., Moussakova E., Kecskeméti I., editors. New Approaches to Book and Paper Conservation-Restoration. Verlag Berger; Horn/Wien: 2011. pp. 575–594. [Google Scholar]
- Prosser J.I. Molecular and functional diversity in soil microorganisms. Plant Soil. 2002;244:9–17. [Google Scholar]
- Rajala T., Peltoniemi M., Hantula J., Mäkipää R., Pennanen T. RNA reveals a succession of active fungi during the decay of Norway spruce logs. Fungal Ecology. 2011;4:437–448. [Google Scholar]
- Rakotonirainy M.S., Heraud C., Lavedrine B. Detection of viable fungal spores contaminant on documents and rapid control of the effectiveness of an ethylene oxide disinfection using ATP assay. Luminescence. 2003;18:113–121. doi: 10.1002/bio.710. [DOI] [PubMed] [Google Scholar]
- Rakotonirainy M.S., Heude E., Lavedrine B. Isolation and attempts of biomolecular characterisation of fungal strains associated to foxing on a 19th century book. Journal of Cultural Heritage. 2007;8:126–133. [Google Scholar]
- Rutala W.A., Weber D.J. Infection control: the role of disinfection and sterilisation. Journal of Hospital Infection. 1999;43:43–55. doi: 10.1016/s0195-6701(99)90065-8. [DOI] [PubMed] [Google Scholar]
- Samson R.A., Hoekstra E.S., Frisvad J.C., Filtenborg O. sixth ed. Centraalbureau voor Schimmelcultures; Utrecht: 2000. Introduction to a Food and Airborne Fungi. [Google Scholar]
- Schabereiter-Gurtner C., Piñar G., Lubitz W., Rölleke S. An advanced molecular strategy to identify bacterial communities on art objects. Journal of Microbiological Methods. 2001;47:345–354. doi: 10.1016/s0167-7012(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Schmidt J.D. Freeze drying of historical cultural properties. Technology and Conservation (Spring) 1985:20–26. [Google Scholar]
- Shaw K., Sesardic I., Bristol N., Ames C., Dagnall K., Ellis C., Whittaker F., Daniel B. Comparison of the effects of sterilisation techniques on subsequent DNA profiling. International Journal of Legal Medicine. 2008;122:29–33. doi: 10.1007/s00414-007-0159-5. [DOI] [PubMed] [Google Scholar]
- Sterflinger K. Fungi: their role in deterioration of cultural heritage. Fungal Biology Reviews. 2010;24:47–55. [Google Scholar]
- Sussman A.S. Longevity and survivability of fungi. In: Ainsworth G.C., Sussman A.S., editors. vol. II. Academic Press; London and New York: 1966. pp. 477–486. (The Fungi: An Advanced Treatise). [Google Scholar]
- Valentin N. Biodeterioration of library materials. Disinfection methods and new alternatives. The Paper Conservator. 1986;10:40–45. [Google Scholar]
- Walsh B. Salvage of water-damaged archival collections: salvage at a glance. Western Association for Art Conservation Newsletter. 1988;10:2–5. [Google Scholar]
- White T.J., Bruns T.D., Lee S., Taylor J. Analysis of phylogenetic relationships by amplification and direct sequencing of ribosomal RNA genes. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR Protocols: A Guide to Methods and Applications. Academic Press; New York: 1990. pp. 315–322. [Google Scholar]
- Zotti M., Ferroni A., Calvini P. Microfungal biodeterioration of historic paper: preliminary FTIR and microbiological analyses. International Biodeterioration and Biodegradation. 2008;62:186–194. [Google Scholar]