Abstract
The gluten-sensitive enteropathy celiac disease is tightly associated with the production of autoantibodies specific for the enzyme transglutaminase 2 (TG2)5. The mechanisms underlying the activation of autoreactive B cells, however, are not well defined. To gain more insight into this autoimmune response we have characterized the binding of TG2 by a panel of human monoclonal antibodies generated by expression cloning of immunoglobulin genes from single plasma cells of the celiac disease lesion. The antibodies were highly specific to TG2 and bound preferentially to the “open”, Ca2+-activated enzyme conformation. Epitope mapping revealed that they recognize few distinct conformational epitopes that cluster in the N-terminal half of the enzyme. Two of the epitopes were overlapping with the fibronectin binding site in TG2, and none of the epitopes was accessible when TG2 was in a cell surface-bound form. Based on our findings we propose that the autoantibodies are generated against the soluble, catalytically active enzyme, whereas antibodies reactive with cell surface-associated TG2 are absent from the response due to negative selection of B cells recognizing membrane-bound self-antigen. The findings give insight into the mechanisms controlling the formation of anti-TG2 autoantibodies in celiac disease.
INTRODUCTION
Autoantibodies against the enzyme transglutaminase 2 (TG2) are a hallmark of the gluten-sensitive enteropathy celiac disease (1), a disorder that affects genetically susceptible individuals upon exposure to dietary cereal proteins. The pathogenesis is driven by CD4+ T cells that react with gluten-derived peptides when presented on the disease-associated HLA molecules, HLA-DQ2 and -DQ8 (2). It is uncertain if the TG2-specific autoantibodies play a pathogenic role in the disease, but the antibodies serve as very accurate diagnostic markers. Tests measuring anti-TG2 serum antibodies, especially of the IgA isotype, are widely used and have sensitivities and specificities close to 100% (3). For childhood celiac disease, the recently launched official European diagnostic guidelines are principally based on positive anti-TG2 serology and no longer include biopsy-proven altered gut histology as a mandatory criterion (4). In addition to being an autoantigen, TG2 plays a role in the generation of gluten T-cell epitopes through conversion of peptide glutamine residues into glutamic acid in a reaction known as deamidation. The TG2-mediated introduction of negative charges in gluten-derived peptides by deamidation raises their binding affinity to the disease-associated HLA molecules, thereby increasing gluten antigenicity (5, 6). The link between gluten ingestion and production of autoantibodies against TG2 is not well understood, but it has been suggested that TG2-reactive B cells receive help from gluten-reactive CD4+ T cells through receptor-mediated uptake of covalent complexes between TG2 and gluten followed by presentation of gluten-derived peptides on HLA-DQ2 or HLA-DQ8 (7, 8).
In addition to glutamine deamidation, TG2 catalyzes protein crosslinking through the formation of Nε(γ-glutamyl)lysine isopeptide bonds in a reaction termed transamidation. Both deamidation and transamidation are Ca2+-dependent reactions taking place in the extracellular environment (9). The enzyme is synthesized in the cytosol but is also found in the nucleus as well as outside of cells on the plasma membrane and in the extracellular matrix (ECM). Intracellularly, TG2 works as a GTPase and presumably acts as a G protein involved in signal transduction (10). GTP/GDP binds to a pocket on the surface of the protein and inhibits the transamidation/deamidation activity of the enzyme (11, 12). In the reported crystal structure of TG2 in complex with GDP, the enzyme adopts a “closed” conformation where the C-terminal end is folded in on the core domain and covers the active site (13). The structure of TG2 with a synthetic peptide inhibitor covalently bound to the active site cysteine has also been solved, revealing an “open”, extended conformation where the C-terminal end is displaced 120 Å, and the active site is accessible (14). The closed conformation presumably represents intracellular TG2, whereas the open conformation is induced extracellularly by the binding of Ca2+.
TG2 is exported to the extracellular environment by an unconventional mechanism involving binding to phosphoinositides in endosomal membranes (15). As a result, the enzyme ends up in a complex with integrins on the cell surface where it has been suggested to act as a fibronectin coreceptor mediating cell adhesion and migration (16). It is also secreted from cells in a soluble form that binds specifically to fibronectin and possibly other components of the ECM. The finding that extracellular TG2 exists both in soluble and membrane-associated forms could have implications for the activation of autoreactive B cells in celiac disease as it has earlier been demonstrated in mice that B cells reactive with membrane-bound self-antigens are negatively selected during development, whereas B cells recognizing soluble self-antigens are present in the periphery and are capable of initiating an immune response (17, 18).
The epitopes recognized by TG2-specific autoantibodies in celiac disease are known to be conformational, thus making it challenging to determine the specific structural regions that are targeted. Recently, however, a single epitope made up of residues from different structural domains was reported as the main epitope in celiac disease based on the loss of serum antibody reactivity toward mutated versions of TG2 (19). Here we have used a panel of TG2-specific mAbs generated from plasma cells from celiac disease patient small intestinal biopsies to characterize the targeting of TG2 by autoantibodies in detail. We show that almost all of the antibodies bind one of four different epitope regions that seem to be clustered closely together in the N-terminal end of the enzyme. This is hardly coincidental and speaks to a common mechanism regulating the recruitment of TG2-reactive B cells in this autoimmune response.
MATERIALS AND METHODS
Human and animal material
Participating human subjects were diagnosed with celiac disease according to the guidelines of the American Gastroenterology Association. The subjects gave their informed consent before the donation of blood and small intestinal biopsies. Collected serum samples were stored at −20°C. Biopsy specimens were cultured in 5% FCS/RPMI-1640 at 37°C in the presence of 5% CO2 as previously described (20). Collected supernatants were stored at −20°C. The study was approved by the Regional Ethics Committee of South-Eastern Norway (approval S-97201). C57BL/6 wild-type and TG2 knockout mice (21) were handled according to a locally approved protocol.
Antigens
His-tagged recombinant human TG2 was either expressed in E. coli and purified as previously described (22) or obtained from Phadia as purified protein expressed in Sf9 insect cells. Mouse TG2 was obtained by extraction of RNA from mouse liver biopsies and cloning of TG2-encoding cDNA into the pET-28a vector (Novagen) as described for human TG2 (23). Mouse TG2 and human TG2 mutants were produced in E. coli in the same way as wild-type human TG2. Mutations were introduced in the TG2 sequence using the QuickChange Site-directed Mutagenesis Kit (Stratagene) and the mutations were confirmed by sequencing (GATC Biotech). The mutants were produced in parallel with the wild-type enzyme. A truncated version of TG2 consisting of aa 1–465 was obtained by PCR amplification of the TG2 wild-type construct using the forward primer 5’-TGCCCATATGGCCGAGGAGCTGGTCTT-3’ and reverse primer 5’-GTCAAAGCTTTTACAGTTTGTTCAGGTGGTTCG-3’ followed by subcloning into the pET-28a vector between the NdeI and HindIII sites. The human transglutaminases TG3 and TG6 were obtained from Zedira and the Jo-1 autoantigen was from Phadia.
Antibodies
Human mAbs were produced as previously reported (20, 24). In brief, IgG1 and Igκ expression vectors containing the cloned antibody heavy and light chain variable regions, respectively, were co-transfected into HEK293 cells. The antibodies were subsequently purified from cell supernatants on Protein A or Protein G sepharose (GE Healthcare). The mouse mAbs CUB7402 and TG100 were obtained from NeoMarkers and the 4G3 mAb was from Millipore.
ELISA assays
Binding of mAbs to the various antigens was assessed essentially as previously described for TG2 (20). In general, microtiter plates were coated over night at 4°C and blocked by washing the plates with 0.1% Tween20, which was also included in all following incubations with antibodies. Alkaline phosphatase-conjugated rabbit anti-human IgG or rabbit anti-mouse IgG (Abcam) was used to detect binding of mAbs. Goat anti-human IgA (Sigma) was used to detect polyclonal IgA. After addition of phosphatase substrate, absorbance was measured at 405 nm in a microplate reader (Thermo). Saturation binding curves were produced by non-linear regression analysis of measured OD values at the indicated antibody concentrations. In the cases where only a single or two mAb concentrations were used, these were picked so that sub-saturating levels of mAb were achieved. In one set of experiments, TG2 was pre-incubated with 5 mM iodoacetamide for 30 min prior to coating of microtiter plates with 5 µg/ml of protein. To investigate the effects of Ca2+ and GTP, 0.1 mg/ml TG2 was pre-incubated with 1 mM GTP or 5 mM CaCl2 in TBS for 1 hour at room temperature prior to coating. The TG2 inhibitor KCC009 (25) was included at a concentration of 1 mM in samples with CaCl2 to avoid auto-crosslinking. During coating and incubations with antibodies, the GTP concentration was kept at 50 µM and the CaCl2 concentration was kept at 5 mM. Antibody incubations were done in TBST. In experiments where TG2 was captured on the 45 kDa gelatin-binding fragment of fibronectin (Sigma), microtiter plates were coated with 2 µg/ml of the fibronectin fragment, followed by addition of 1 µg/ml TG2 produced in Sf9 insect cells. The signals were compared to the ones obtained when plates were coated directly with 3 µg/ml of TG2. In experiments where whole human fibronectin (Sigma) was used to capture TG2, microtiter plates were coated with 10 µg/ml of fibronectin followed by addition of 10 µg/ml TG2 in the presence or absence of 5 mM CaCl2 in TBST. Crosslinking was then allowed to occur for 1 hour at 37°C before incubation with antibodies in the absence of Ca2+.
Competition ELISA
Small aliquots of purified mAbs were biotinylated with the EZ-link sulfo-NHS-LC-biotin reagent (Pierce) following the standard protocol. Binding of biotinylated mAbs to TG2 was assessed as described above except that alkaline phosphatase-conjugated streptavidin (Southern Biotech) was used to detect bound mAbs. For each biotinylated mAb, a concentration falling in the dynamic range of the ELISA assay was chosen and used in the subsequent competition experiments. These were performed in the following way: 5–10 µg/ml of unlabeled competitor mAb was added to TG2-coated microtiter plates. After 30 min incubation at 37°C, one tenth of the volume was removed and replaced with an equal volume of biotinylated mAb giving the optimal, predetermined concentration of labeled mAb. The resulting competitor mAb / biotinylated mAb ratio was between 10 and 50 in all experiments. After addition of labeled mAb, incubation was continued for 1 hour at 37°C and the amount of TG2-bound biotinylated mAb was determined as described above. Competition between IgG mAbs and polyclonal IgA was performed in a similar way. After pre-incubation with a mixture of mAbs, each in a concentration of 2 µg/ml, serum or biopsy supernatant was added to the TG2-coated microtiter plate. Bound IgA was subsequently detected as described above.
Non-denaturing PAGE
The conformational states of TG2 were analyzed by non-denaturing PAGE as previously reported (22). TG2 was either loaded directly or pre-incubated with different reagents prior to mixing with loading buffer and commencement of electrophoresis. Incubations were carried out at room temperature for 30 min in TBS including either 5 mM iodoacetamide, 1 mM GTP or 5 mM CaCl2 in combination with 1 mM KCC009 inhibitor.
Immunofluorescence
Mouse small intestine was embedded in optimal cutting temperature (OCT) medium (Tissue-Tek) and snap frozen in liquid nitrogen. Dried, unfixed 6 µm cryosections were stained with 6 or 3 µg/ml of each of the indicated mAbs in 1.25% BSA/PBS at room temperature for 1 hour followed by FITC-conjugated goat anti-human IgG (Southern Biotech). Nuclei were stained with Hoechst and the slides were mounted with home-made polyvinyl alcohol. Images were acquired on an inverted fluorescence microscope (Nikon Eclipse Ti-S) with a Nikon 10x/0.3 Plan Fluor lens using the NIS-elements BR3.2 software. TG2 knockout tissue sections were incubated with or without 50 µg/ml recombinant human or mouse TG2 produced in E. coli for 1 hour at room temperature before detection with antibodies as described above. In one set of experiments the TG2 concentration was decreased to 10 µg/ml during incubation with tissue sections. This did not have an effect on the staining results.
Culturing of dendritic cells from monocytes
iDCs were generated from peripheral blood mononuclear cells of healthy blood donors essentially as previously described (26). Briefly, CD14+ cells were plated into 24-well culture dishes with 106 cells per well and cultured in 1 ml 10% FCS/RPMI-1640 supplemented with 1000 U/ml GM-CSF and 500 U/ml IL-4. On day 2 and 5, half of the culture medium was replaced with fresh medium. The cells were used for analysis on day 7 or 8.
Flow cytometry
iDCs were incubated with 10 µg/ml of each of the indicated mAbs or 100 µg/ml of IgA purified from serum on goat anti-human IgA-agarose (Sigma) for 30 min on ice in 2% FCS/PBS followed by FITC-conjugated goat anti-mouse IgG1 or goat anti-human IgG (Southern Biotech). For intracellular staining, the cells were fixed in 1.7% formaldehyde for 5 min at room temperature and permeabilized with methanol at −80°C for 30 min prior to incubation with antibodies. The stained cells were analyzed on a FACS Calibur instrument (Becton Dickinson).
RESULTS
Most anti-TG2 autoantibodies bind one of four distinct epitopes that correlate with antibody VH gene segment usage
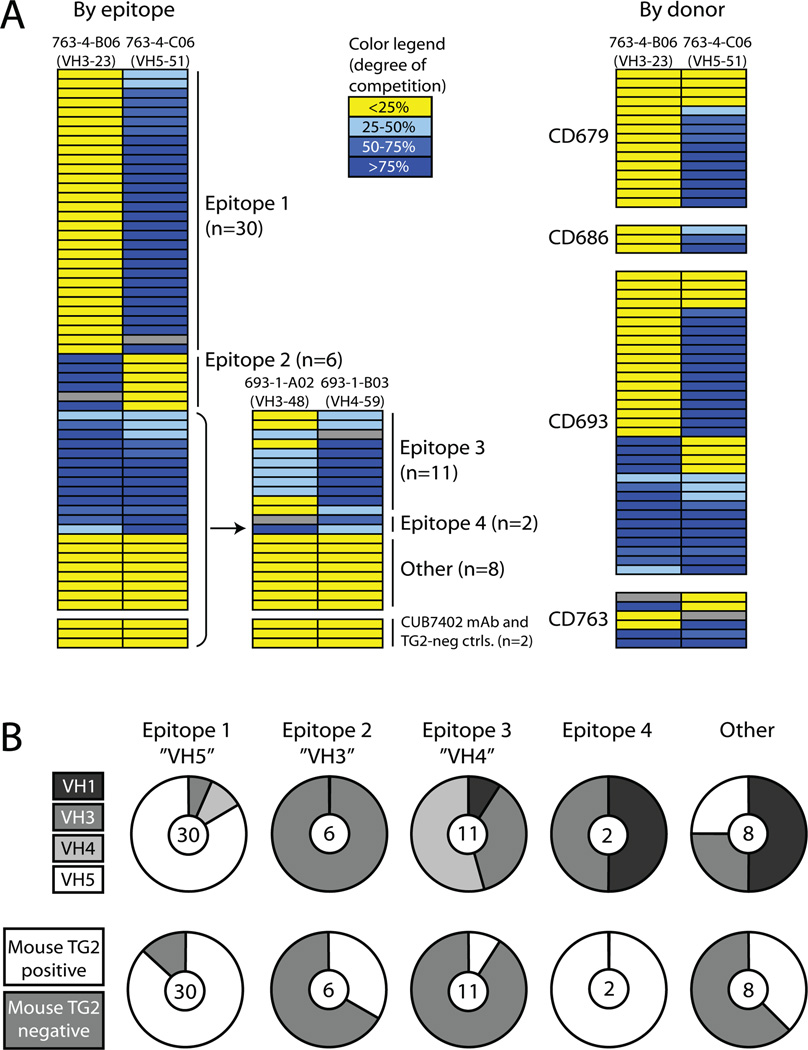
Recently, we reported the cloning of antibody variable regions of single TG2-reactive plasma cells isolated from small intestinal biopsies from four adult individuals with active celiac disease (20). By expressing the antibodies recombinantly in a human IgG1 format we obtained a panel of anti-TG2 mAbs. The antibodies generally had few somatic mutations and showed bias toward usage of the VH5-51 gene segment. Interestingly, the VH5-51 mAbs had fewer mutations and bound TG2 with lower affinity than mAbs using other VH segments. Another surprising feature was that none of 47 tested mAbs had an inhibitory effect on TG2 enzymatic activity. We wished to understand more about this autoimmune response and find out if the TG2-reactive autoantibodies share other characteristics. We therefore carried out competitive binding studies to investigate if the antibodies target common epitopes. Initially, 18 mAbs representing each patient were selected randomly, and their ability to compete with each other was analyzed. Several mAbs were found to compete for TG2 binding, but we were able to pick four that showed different competition patterns and therefore most likely recognize different epitopes. Based on the ability of the rest of the mAbs to compete with these four, we divided the entire panel into five distinct groups (Fig. 1A). The largest group, which was assigned to epitope 1, consists of antibodies competing with mAb 763-4-C06 but not with mAb 763-4-B06. Notably, this group contains almost all of the VH5-51 antibodies in the panel (Fig. 1B). Likewise, the group with the opposite reaction pattern, assigned to epitope 2, is made up entirely of antibodies belonging to the VH3 family. Thus, the targeted epitopes largely reflect the VH gene segment usage of the antibodies. Epitope 1 antibodies were represented in all four donors, from whom mAbs were generated, whereas the epitope 2 antibodies came from just two of the donors. Antibodies assigned to epitope 3 and 4 competed with both 763-4-C06 and 763-4-B06, suggesting that these epitopes are overlapping with epitope 1 and 2. However, these antibodies could be distinguished based on their ability to compete with two other mAbs, 693-1-A02 and 693-1-B03. Only eight out of 57 mAbs did not compete with any of the labeled antibodies and thus react with one or more different epitopes.
FIGURE 1.
Grouping of TG2-reactive mAbs according to epitope binding. (A) Assignment of antibody epitopes according to competition for TG2-binding by mAbs. Each row represents a mAb and the color code indicates the degree to which it inhibits TG2 binding of the mAb indicated at the top of each column. The mAb representing each column was biotinylated and TG2 binding in the presence of an excess of competing unlabeled mAb was determined in an ELISA binding assay. Gray fields indicate competition between labeled and unlabeled versions of the same mAb. The degree of inhibition by all other mAbs was calculated from the reduction in signal relative to the signal reduction that was measured when each reference mAb competed with itself. The TG2-reactive mouse mAb CUB7402 and two TG2-negative mAbs cloned from celiac disease patient small intestinal plasma cells were included as controls. The four biotinylated mAbs reported here were chosen on the basis of extensive preliminary competition experiments suggesting that they represent four different epitopes. In the right panel, the competition pattern of the mAbs is shown in relation to the four patients they were derived from. (B) The upper panel shows the number of mAbs in each epitope group and their distribution among different VH families. Epitope 1, 2 and 3 are mainly represented by VH5, VH3 and VH4 antibodies, respectively. The lower panel shows the reactivity of mAbs in each epitope group with mouse TG2 in ELISA. The mAbs were scored either as mouse TG2 positive or negative.
To further characterize the epitopes, the reactivity of the mAbs with mouse TG2 (sharing 84% sequence identity with human TG2) was assessed (Fig. 1B). Most of the antibodies in the epitope 1 group and both epitope 4 antibodies were reactive with mouse TG2, whereas most of the antibodies binding epitope 3 did not react with mouse TG2. The epitope 2 antibodies showed varying reactivity, suggesting that the antibodies in each group do not necessarily bind TG2 in the same way. The assigned epitopes represent targeted regions rather than exact binding motifs and differences in binding mode can exist within each group.
TG2-reactive autoantibodies are specific and do not cross-react with other transglutaminases
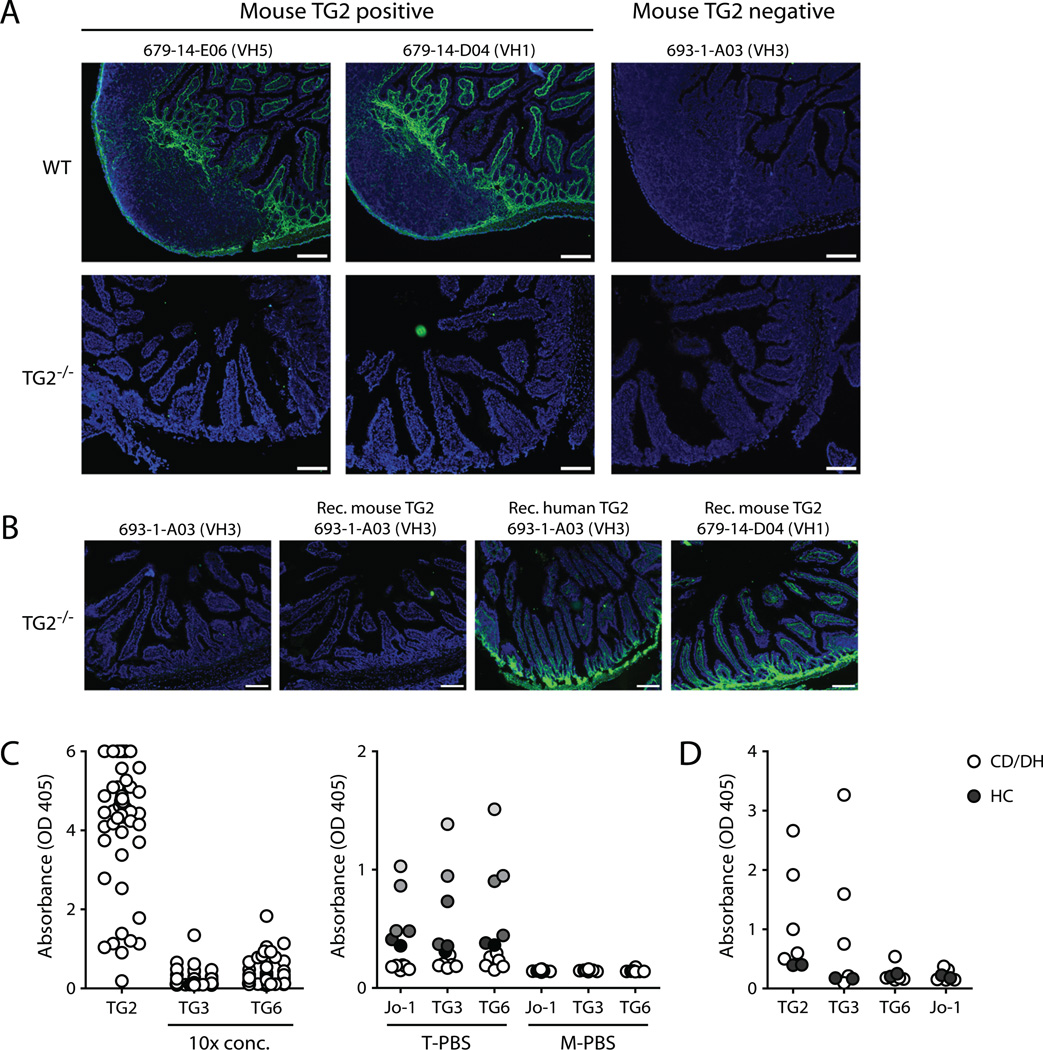
Immunofluorescence staining of small intestinal tissue sections from wild-type or TG2 knockout mice with mAbs reactive or non-reactive with mouse TG2 was found to correspond closely to the expected patterns (Fig. 2A). Notably, complete loss of reactivity to TG2-free tissue suggests that the TG2-reactive autoantibodies in our panel are highly specific. Antibody reactivity with TG2 knockout tissue could, however, be established by the addition of recombinant TG2 to the sections, indicating that antibody-targeted TG2 in the tissue is bound to the ECM via specific interactions (Fig. 2B). To further explore the specificity of the mAbs, we tested their reactivity with other members of the transglutaminase family (Fig. 2C). Whereas TG2 is ubiquitously expressed, TG3 and TG6 are found in the skin and brain, respectively. These transglutaminases have also been implicated in autoimmunity as anti-TG3 autoantibodies are associated with dermatitis herpetiformis (ref. 27, Fig. 2D) and TG6 autoantibodies have been found in gluten ataxia (28). Both conditions are associated with gluten sensitivity and anti-TG3 and anti-TG6 autoantibodies were also described in celiac disease. When testing our antibody panel for binding to TG3 and TG6 we found little, if any, reactivity indicating that the antibodies generated against TG2 are not cross-reactive with TG3 and TG6. Moreover, all reactivity was lost when antibody incubations were done in 2% dry milk, indicating that the low degree of binding we saw for some mAbs is due to slight polyreactivity. The same picture was seen for the nuclear autoantigen Jo-1 which is a target for autoantibodies in myositis (29) and was used here as an irrelevant control antigen.
FIGURE 2.
Specificity of TG2-reactive mAbs. (A and B) Representative micrographs showing immunofluorescence staining of mouse intestinal tissue cryosections with TG2-reactive mAbs. Human IgG is shown in green and cell nuclei are shown in blue (Hoechst staining). Scale bar, 100 µm. (A) Staining of wild-type (WT) or TG2 knockout (TG2−/−) (21) mouse tissue with three different mAbs. The wild-type tissue contains a Peyer’s patch. Two of the mAbs reacted with mouse TG2 in ELISA, whereas the third one did not. (B) Reactivity of TG2-specific mAbs with TG2−/− tissue could be achieved by incubating the cryosections with recombinant mouse or human TG2. After washing, added TG2 remains bound to the ECM. (C) The left panel shows the reactivity of 57 mAbs with TG2, TG3 and TG6 in ELISA. Each circle represents a single mAb tested in one concentration. The mAb concentrations used with TG3 and TG6 were 10 times higher than the concentrations used with TG2 as antigen. The right panel shows the reactivity of 12 selected mAbs with the autoantigens Jo-1, TG3 and TG6 in either PBS with 0.1% tween (T-PBS) or PBS with 2% dry milk (M-PBS). The data points are color-coded so that the reactivity of individual mAbs can be followed with different antigens. (D) Reactivity of IgA antibodies in serum of patients with celiac disease and dermatitis herpetiformis (CD/DH) (n=5) or healthy controls (HC) (n=2) with TG2, TG3, TG6 and Jo-1 determined by ELISA. Both treated and untreated patient sera were used. The sera were used in a dilution of 1:20 and incubated with the antigens in the presence of 2% dry milk.
Autoantibodies target an open rather than a closed TG2 conformation
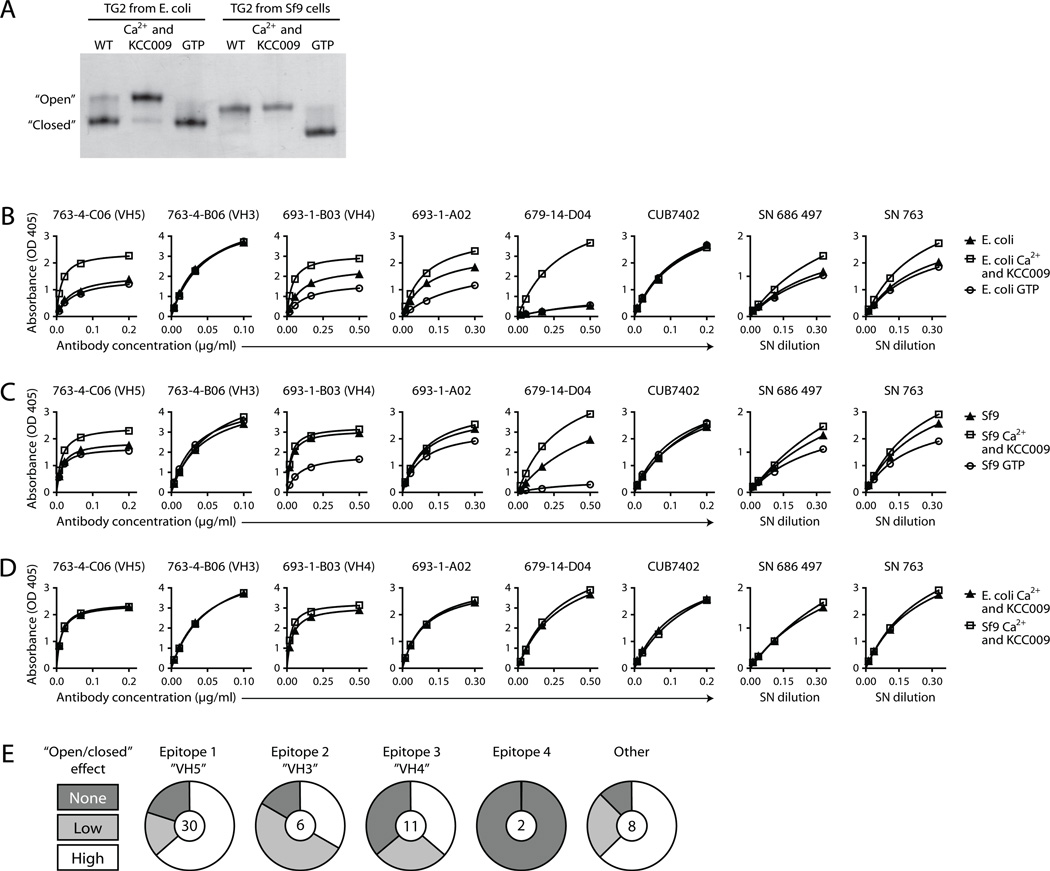
We observed that the reactivity of several antibodies in our panel was dependent on the source of recombinant human TG2 (Supplemental Fig. 1A, 1B). The antibodies generally bound with lower affinity to TG2 produced in E. coli than TG2 produced in Sf9 insect cells although individual antibodies showed different reaction patterns. Interestingly, when assessing the conformational distribution within each TG2 preparation, we saw that the E. coli-produced enzyme existed mostly in a closed conformation, whereas Sf9-produced TG2 took an open conformation (Fig. 3A, Supplemental Fig. 1C). To further address the importance of TG2 conformation for antibody binding, we investigated the effect of the natural allosteric regulators, Ca2+ and GTP. In agreement with the published crystal structures and several other reports (12–14, 30), we saw that TG2 takes a closed conformation in the presence of GTP, whereas Ca2+ in combination with a specific active site irreversible inhibitor induces an open conformation (Fig. 3A). In line with the observed effects of the TG2 expression system, GTP and Ca2+ were found to have opposite effects on antibody binding. For most antibodies, binding affinity was decreased by GTP and increased by Ca2+. The extent to which the addition of ligands changed binding strength varied among the mAbs but in those cases where a change was observed, the Ca2+-bound state was always favored over the GTP-bound state. The same effects were seen for TG2 produced in E. coli and Sf9 cells (Fig. 3B, 3C). Notably, the two TG2 preparations became equally potent antigens in the presence of Ca2+ (Fig. 3D). To rule out the possibility that the reaction pattern we observed in ELISA was due to differences in coating between the open and closed TG2 conformations, in one set of experiments we used fibronectin-coated ELISA plates to capture the different TG2 conformations instead of coating them directly. Again, we observed increased binding to the Ca2+-bound state for selected antibodies (not shown), thus confirming that the open conformation is the preferred antigen.
FIGURE 3.
The effects of Ca2+ and GTP on TG2 conformation and antibody reactivity. (A) The open and closed TG2 conformations can be separated by non-denaturing PAGE. This shows that incubation of TG2 with Ca2+ and the dihydroisoxazole derivative KCC009 (25), which binds irreversibly to the active site cysteine, induces an open conformation, whereas GTP makes the enzyme adopt a closed conformation. KCC009 was added to all samples with Ca2+ to prevent extensive auto-crosslinking of catalytically active TG2. In the absence of regulators TG2 produced in E. coli primarily takes the closed conformation, whereas TG2 produced in Sf9 insect cells is in the open conformation. The two TG2 preparations migrate a bit differently in the gel due to differences in the composition of their purification tags. The E. coli-produced enzyme has an N-terminal His-tag followed by a thrombin cleavage site, whereas the Sf9-produced enzyme has a C-terminal His-tag and no cleavage site. (B–D) Saturation binding curves for selected mAbs and polyclonal IgA from cultured biopsy supernatants (SN) with TG2 produced in E. coli (B) or Sf9 insect cells (C) determined by ELISA. TG2 was either left untreated or incubated with Ca2+ or GTP as indicated. The mouse mAb CUB7402, which recognizes a linear TG2 epitope, was included as a control that should give the same signal irrespective of TG2 conformation. (E) The increase in binding to insect cell-produced TG2 that was observed in the presence of Ca2+ compared to GTP was evaluated for all 57 TG2-specific mAbs based on ELISA signals obtained with two different concentrations of each mAb. The influence of the conformational state of TG2 on the binding of mAb was ranked as no effect (none), low or high.
Preferential binding of the open, Ca2+-bound conformation was observed for the majority of mAbs in our panel, regardless of the targeted epitope (Fig. 3E). However, for a number of mAbs in each group the affinity was only weakly affected or not affected at all by the allosteric regulators. This suggests that the regions that make up the assigned epitopes are susceptible to small structural variations induced by ligand binding but they are most likely not directly involved in the major conformational changes taking place upon switching between an open and a closed TG2 state.
Epitopes can be located according to introduced mutations and competition with fibronectin for TG2 binding
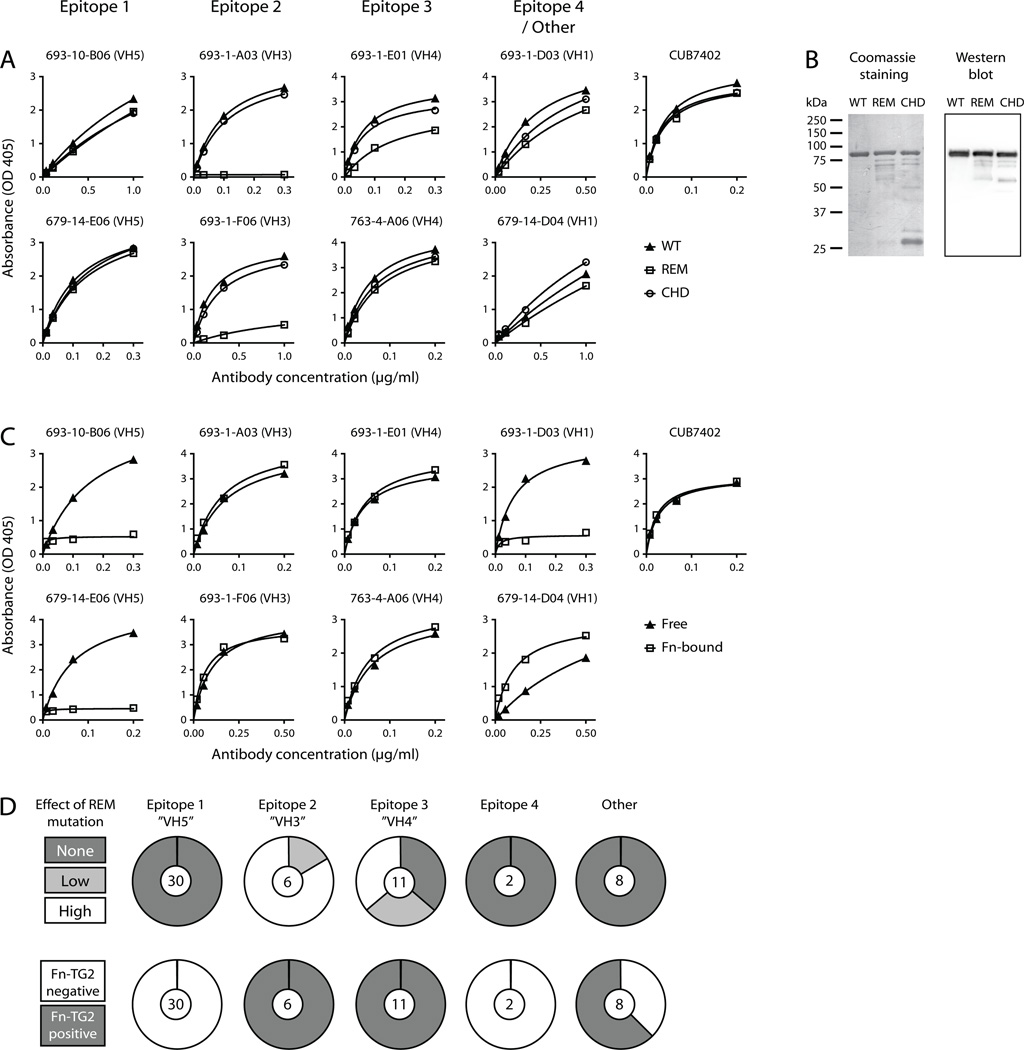
To see if any of the epitopes we have assigned to our panel of mAbs coincide with the recently discovered epitope targeted by celiac disease serum autoantibodies (19), we constructed the reported R19S E153S M659S TG2 triple mutant and tested it for binding to mAbs from each epitope group. Introduction of the mutations caused loss of all or most reactivity for the antibodies assigned to epitope 2 and also affected some of the antibodies binding to the overlapping epitope 3 region (Fig. 4A, 4D). The rest of the antibodies, on the other hand, had comparable reactivity to the mutant and wild-type enzymes, suggesting that the mutations do not interfere with the overall conformation of TG2 but selectively target the epitope 2/3 region.
FIGURE 4.
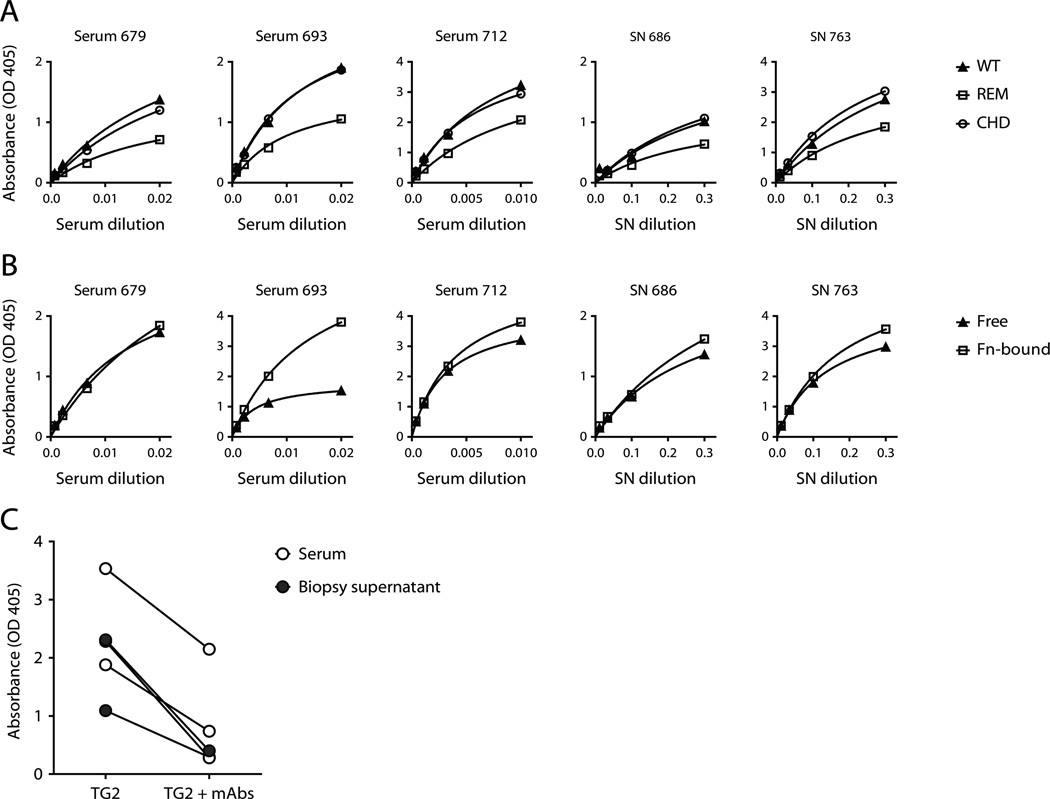
Localization of targeted epitopes in TG2. (A and C) Saturation binding curves comparing reactivity of mAbs to different TG2 variants in ELISA. mAbs representing each epitope group were selected and the mouse mAb CUB7402 was included as control for equal coating of the proteins. (A) Comparison of antibody reactivity to wild-type (WT) and mutant TG2. The tested mutants were the recently described R19S E153S M659S (REM) triple mutant and the C277A H335A D358A (CHD) catalytic triad mutant. (B) SDS-PAGE analysis of wild-type and mutant TG2. The left panel shows a coomassie stained gel, and the right panel shows a western blot done with CUB7402 as primary antibody followed by horseradish peroxidase-conjugated goat anti-mouse IgG. The mutant protein preparations contain both TG2 degradation products and E. coli proteins derived from the expression system. The lower effective TG2 concentration in the mutant preparations was adjusted for in the binding assays. (C) Comparison of antibody reactivity to directly coated TG2 (Free) and TG2 captured on the 45 kDa gelatin-binding fragment of human fibronectin (Fn-bound). (D) The effect of the REM triple mutation and the reactivity to Fn-bound TG2 was evaluated for all 57 TG2-specific mAbs based on ELISA signals obtained with two different concentrations of each mAb. The signals were compared to the corresponding results with directly coated, wild-type TG2.
It has also been suggested that TG2-specific antibodies in celiac disease target the enzyme’s active site based on observed loss of reactivity of serum IgA upon replacement of the catalytic triad residues Cys277, His335 and Asp358 with alanine (31). In contrast to the reported effect on serum antibodies, introduction of these mutations in TG2 did not affect the reactivity of mAbs assigned to the different epitopes to a large degree (Fig. 4A). Importantly, the two generated triple mutants were both produced with lower yields than the wild-type enzyme and also appeared less pure by SDS-PAGE analysis (Fig. 4B). Thus, lower quality of the mutant proteins could explain why they in some cases can appear less reactive than the wild-type in ELISA. Unless protein quality is taken into consideration, it can lead to a “false” effect of the mutations being recorded. Accordingly, it can be difficult to distinguish between effects of mutations on the overall protein quality and specific effects on targeted epitopes, especially when one is analyzing polyclonal antibody responses.
The specific binding between TG2 and fibronectin in the ECM involves the gelatin-binding domain of fibronectin (32) and the N-terminal β-sandwich domain of TG2 (33). More specifically, in TG2, aa 88–106, which make up a β-hairpin motif, have been implicated in fibronectin binding (34). To see if the association with fibronectin influences the binding between TG2 and antibody, we tested the ability of mAbs from each assigned epitope group to react with fibronectin-bound TG2. mAbs targeting epitope 1 and 4 were found to lose reactivity when TG2 was associated with fibronectin, suggesting that these epitopes overlap with the fibronectin binding site in TG2 (Fig. 4C, 4D). This finding was somewhat surprising, given that all the tested mAbs have been scored as positive in an EMA (EndoMysium Antibody) staining assay where the reactivity of antibodies to natural TG2 in situ is tested (20). TG2 co-localizes with fibronectin in the ECM and is therefore expected to exist predominantly in a fibronectin-bound state in tissue (35, 36). It is possible, though, that some TG2 associates with fibronectin through crosslinking rather than the specific non-covalent interaction. In this regard, TG2 has been described to target specific glutamines in fibronectin and is also able to crosslink itself (37). Interestingly, when TG2 was added to coated fibronectin in the presence of Ca2+ to allow crosslinking, the resulting complexes were recognized by mAbs that otherwise did not react with fibronectin-bound TG2 in ELISA (Supplemental Fig. 2A), thus showing that TG2 associated with fibronectin through crosslinking can be targeted by antibodies. However, crosslinking between TG2 and fibronectin cannot be the only possible explanation for the EMA positivity of mAbs targeting epitope 1 or epitope 4, since addition of recombinant enzyme to tissue sections from TG2 knockout mice in the absence of Ca2+ allowed staining with VH5-51 mAbs targeting epitope 1 (Supplemental Fig. 2B). This would indicate that TG2 can associate with other structures in the tissue through interactions that do not involve the fibronectin binding site. Potential binding partners include proteoglycans in the ECM, as TG2 binding to heparan sulfate has earlier been described (38).
The reactivity of polyclonal IgA antibodies partly reflects the epitope distribution among TG2-specific mAbs
In agreement with the findings recently reported by Korponay-Szabo and coworkers (19) we saw that introduction of the R19S E153S M659S triple mutation in TG2 reduces the reactivity of serum IgA as well as IgA secreted by small intestinal biopsies obtained from celiac disease patients (Fig. 5A). Importantly, however, remaining reactivity could be detected in all cases, indicating that more epitopes are targeted in polyclonal anti-TG2 responses. Mutation of the catalytic triad residues in TG2 did not lead to reduced binding of polyclonal IgA from celiac disease patients (Fig. 5A), thus supporting our findings with TG2-specific mAbs and strongly suggesting that the TG2 active site is not targeted by autoantibodies in celiac disease. Association of TG2 with fibronectin was found to give equal or better binding of polyclonal IgA when comparing the reactivity against TG2 on coated fibronectin to that against directly coated TG2 in ELISA (Fig. 5B). This was somewhat unexpected, as our results with TG2-specific mAbs indicate that around half of the antibodies target an epitope that overlaps with the fibronectin binding site. However, the association with fibronectin might increase binding of antibodies targeting other epitopes as seems to be the case for the mAb 679-14-D04, for instance (Fig. 4C). This could be due to increased accessibility of some epitopes, maybe as a result of partial shielding and/or loss of structure induced by direct coating of TG2 to plastic. It could also be that the association with fibronectin leads to structural changes that increase the binding affinity for some antibodies.
FIGURE 5.
Reactivity of polyclonal IgA from celiac disease patients. (A and B) Saturation binding curves showing the reactivity of polyclonal IgA antibodies with TG2 in ELISA. Celiac disease patient serum and supernatants (SN) from cultured small intestinal biopsies were used as sources of polyclonal IgA. (A) Comparison of reactivity to wild-type (WT) and mutant TG2. The tested mutants were the R19S E153S M659S (REM) triple mutant and the C277A H335A D358A (CHD) catalytic triad mutant. (B) Comparison of reactivity to directly coated TG2 (Free) and TG2 captured on the 45 kDa gelatin-binding fragment of human fibronectin (Fn-bound). (C) Detection of TG2-bound IgA in ELISA after incubation of coated TG2 with a single concentration of serum (n=3) or biopsy supernatant (n=2) in the presence or absence of three pooled IgG mAbs: 679-14-E06, 693-1-A03 and 763-4-A06, representing epitope 1, 2 and 3, respectively.
To see if the identified epitopes targeted by our panel of mAbs are truly dominating the anti-TG2 response in celiac disease, we tested if mAbs representing epitope 1, 2 and 3 compete with serum IgA or IgA secreted from small intestinal biopsies for binding to TG2 (Fig. 5C). In all cases, pre-incubation of TG2 with the mAbs resulted in loss of binding capacity for polyclonal IgA antibodies, indicating that the epitopes we have identified are indeed important in the anti-TG2 response in celiac disease. For one high-titer serum, however, a substantial amount of TG2-specific IgA was not outcompeted by the mAbs, possibly because the mAbs are inefficient in quantitatively blocking the epitopes. Alternatively, additional epitopes may exist in some patients.
Antibodies do not target cell surface-associated TG2
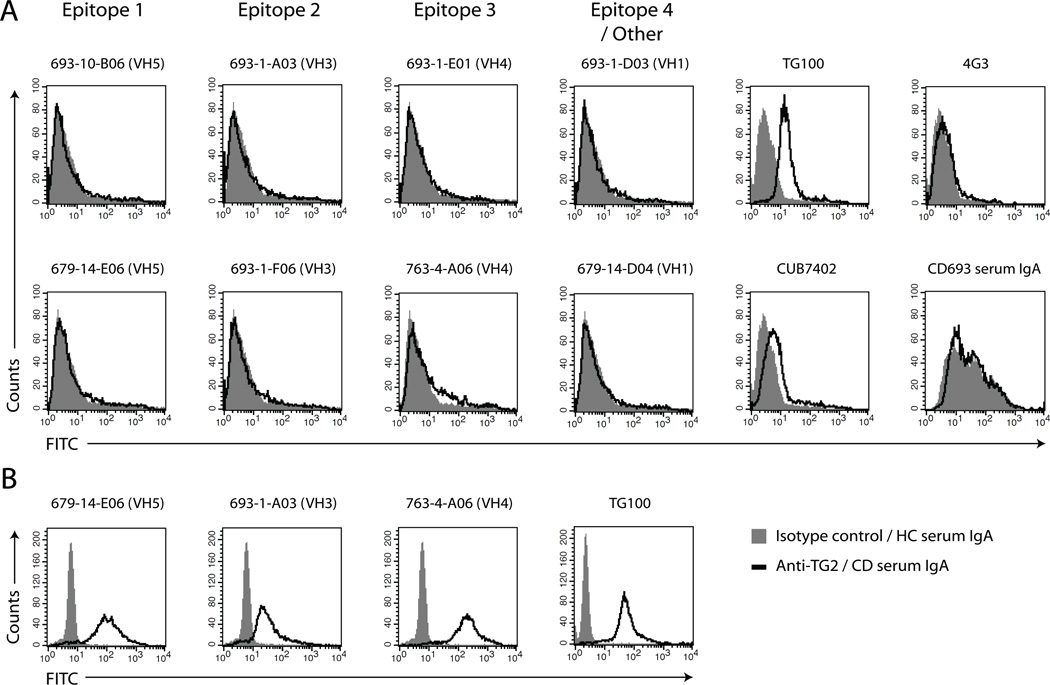
TG2 has been found on the surface of various cell types where it presumably interacts with fibronectin and integrins of the β1 and β3 subfamilies or heparan sulfate proteoglycans (35, 38, 39). It has also been shown that TG2 can be detected on the surface of dendritic cells and macrophages with the mouse mAb TG100 (40). To see if our TG2-specific human mAbs recognize cell surface TG2 we tested the ability of antibodies from each epitope group to stain immature dendritic cells (iDCs) in flow cytometry. None of the tested antibodies stained TG2 on the cells, suggesting that the assigned epitopes are hidden when TG2 is surface-bound (Fig. 6A). In agreement with this, IgA antibodies purified from serum from a celiac disease patient did not stain cell surface TG2 either. As expected, surface-bound TG2 could be detected with TG100, whereas two other mouse mAbs, CUB7402 and 4G3, gave weak and no staining, respectively.
FIGURE 6.
Reactivity of antibodies with TG2 on iDCs. (A and B) Representative flow cytometry histograms showing staining of iDCs with human and mouse TG2-specific mAbs or purified serum IgA from a patient with celiac disease (CD) followed by FITC-conjugated secondary antibody. The signals obtained with irrelevant isotype control antibody or serum IgA from a healthy control (HC) individual are shown in gray. For the human mAbs, a rotavirus-specific antibody cloned in the same way as the TG2-specific mAbs (41) was used as isotype control. (A) Surface staining. (B) Intracellular staining.
Since the majority of TG2 is found in the cytosol, we also tested if the mAbs bind TG2 inside iDCs. The three tested human mAbs and TG100 all stained intracellular TG2 after permeabilization (Fig. 6B). This shows that TG2 inside cells can be recognized by autoantibodies and, thus, exists in a state that differs from the one found on cell surfaces.
DISCUSSION
We have earlier described the generation of a panel of TG2-reactive mAbs obtained by cloning of the expressed variable region genes in single plasma cells from celiac disease patients (20). Here we have extended the characterization of these mAbs and show that most of the antibodies target one of four distinct regions based on their ability to compete with each other for TG2 binding. For simplicity we have called these regions epitope 1–4, although they do not represent single structural motifs recognized by the mAbs. Instead, each of them most likely comprises to a selection of individual overlapping epitopes. The competition pattern shows that some of these epitope regions are partly overlapping and suggests that all four are clustered closely together (Fig. 1A).
The mAbs were highly specific to TG2 and did not cross-react with TG3 and TG6 (Fig. 2C). This indicates that celiac disease autoantibodies against other transglutaminases are generated in an independent immune response and are not a result of cross-reactivity of anti-TG2 autoantibodies. The results are in agreement with previously reported findings with polyclonal serum IgA showing that TG2 and TG6 are targeted by discrete antibody populations (28). While being specific to TG2, the mAbs were found to discriminate between different sources of recombinant enzyme, as TG2 produced in E. coli gave poorer signals in ELISA than TG2 produced in Sf9 insect cells for a number of mAbs. This could indicate that producing the enzyme in a eukaryotic cell line results in more optimal folding and a conformation which is recognized better by TG2-specific antibodies compared to the standard recombinant expression system using E. coli. The importance of protein folding is in line with our own and others’ previous findings stating that the targeted epitopes are conformational (20, 42, 43). The binding strength of the antibodies was found to reflect the distribution between an open and a closed TG2 conformation. Hence, forcing the enzyme into the open conformation increased the reactivity with both mAbs and polyclonal IgA from celiac disease patients (Fig. 3B–D, Supplemental Fig. 1B). Preferred binding of an open TG2 conformation has earlier been described for serum antibodies (44). Our results with mAbs show that some antibodies bind equally well to the open and closed conformations but many bind with increased affinity to the open state. None of the mAbs, on the other hand, bound preferentially to the closed state. These findings strongly suggest that the open, Ca2+ activated TG2 conformation is the autoantigen that is targeted in celiac disease. This could be expected since TG2 found outside of cells is believed to take an open conformation due to high Ca2+ and low GDP/GTP concentrations in the extracellular environment.
The recently described TG2 epitope made up of Arg19, Glu153 and Met659 (19) was found to be targeted by the antibodies we have assigned to epitope 2 as well as some epitope 3 antibodies (Fig. 4A, 4D). The data presented here is in agreement with the previous report saying that this is a main epitope targeted by autoantibodies in celiac disease, but our results indicate that it is not the single most important epitope at the cellular level as it is targeted by relatively few mAbs. Furthermore, for Met659 to be part of an epitope together with Arg19 and Glu153, it is required that TG2 takes a closed conformation, and since the antibodies preferentially target an open TG2 conformation, all three residues are not likely to be part of the same epitope.
The mAbs we have assigned to epitope 1 and 4 as well as three mAbs not assigned to any of the epitopes were found to compete with fibronectin for binding to TG2 (Fig. 4C, 4D). Although we cannot rule out that the observed effect is due to conformational changes in TG2 induced by fibronectin binding, it suggests that the epitopes overlap with the fibronectin binding site. In addition, the results suggest a possible pathogenic role for TG2-reactive antibodies in celiac disease as competition with fibronectin could lead to antibody-induced displacement of TG2 from the fibronectin meshwork in the ECM. The resulting release of immobilized TG2 could possibly lead to increased enzymatic activity as the enzyme is brought into solution. This might explain previously reported findings of increased TG2 activity upon addition of celiac disease antibodies to endothelial cell cultures and the suggested role of the TG2-specific antibodies in increasing endothelial permeability (45). Increased enzymatic activity in the celiac lesion could also lead to more gluten deamidation, thereby enhancing the pathogenic T-cell response against deamidated gluten peptides.
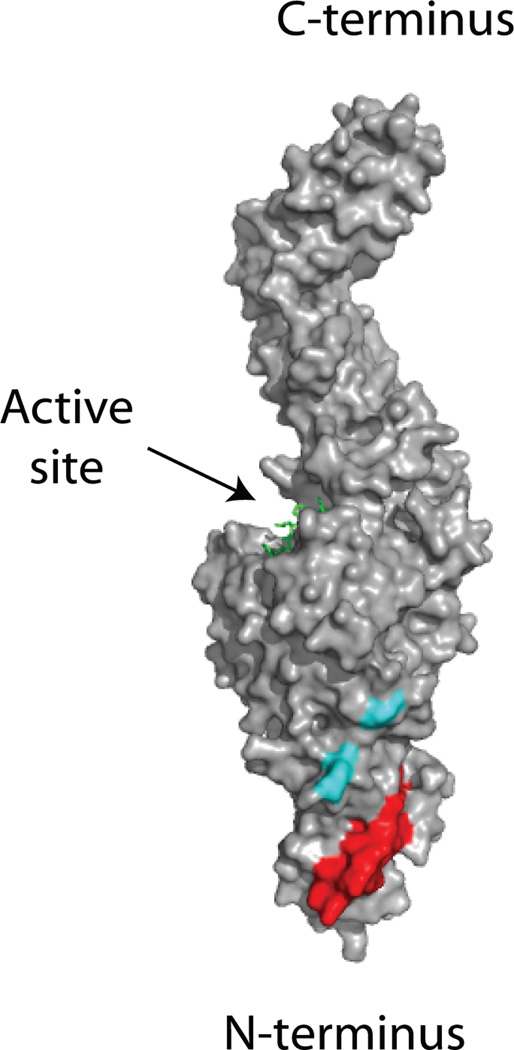
The fibronectin binding site in the N-terminal domain is located close to Arg19 and Glu153 in the three-dimensional structure of open TG2 (Fig. 7). As these two regions represent epitope 1/4 and 2/3, respectively, their location supports our assumption that the assigned epitopes are clustered closely together. In addition, we found that all mAbs, including the ones not assigned to an epitope, were reactive with a truncated version of TG2 lacking the two C-terminal domains (aa 466–687), thus confirming that none of the antibodies targets C-terminal epitopes (not shown). A possible explanation for why the epitopes seem to cluster in the N-terminal end of TG2 is that B cells recognizing epitopes in other parts of TG2 are negatively selected during development. To further explore this possibility we tested if the mAbs are reactive with cell surface-associated TG2 since surface-bound self antigens have been shown to induce deletion or anergy in cognate B cell populations in mice (17, 18). None of the tested mAbs was reactive with TG2 on dendritic cells in contrast to the mouse mAb TG100, which targets a C-terminal epitope in the region made up by aa 447–538 (Fig. 6A). This suggests that the assigned, N-terminal epitopes are not accessible when TG2 is found on cell surfaces, possibly due to the association of TG2 with integrins and fibronectin or other interaction partners. The mouse mAb 4G3, which is generated against the N-terminal β-sandwich domain in TG2 (39), also did not react with TG2 on the surface of iDCs. Interestingly, an intact N-terminal domain has been shown to be crucial for cell surface expression, thus indicating that the N-terminal part is involved in binding to factors on the surface (35). A third mouse mAb, CUB7402, which binds to a region in the C-terminal end of TG2 between aa 447 and 478, only gave weak staining of iDCs, suggesting that regions in the C-terminal end of the protein can also be partly shielded on the surface of cells. As shielding appears to be incomplete in this region, B cells reactive with all C-terminal epitopes may be negatively selected. An alternative explanation for the clustering of epitopes is that antibodies binding to other parts of TG2 are more likely to interfere with the enzyme’s catalytic activity. We have earlier hypothesized that the enzymatic activity of TG2 is directly involved in the activation of autoreactive B cells through the generation of isopeptide-bonded BCRs (20). Such a mechanism would selectively recruit B cells with specificities that do not inhibit the enzymatic activity as it requires BCR-bound TG2 to remain active. In this regard, it is noteworthy that the N-terminal domain is the only of the four structural domains in TG2 that does not participate directly in the activity-regulating switch between an open and a closed conformation.
FIGURE 7.
Regions targeted by TG2-specific autoantibodies. Surface representation of the open TG2 structure with a bound active site inhibitor shown in green (PDB code 2Q3Z). The region in the N-terminal domain that has been implicated in binding to fibronectin (aa 88–106) is shown in red, whereas Arg19 in the N-terminal domain and Glu153 in the core domain are shown in cyan. These two regions represent epitope 1/4 and epitope 2/3, respectively.
Specific targeting of epitopes in the N-terminal half of TG2 has earlier been described for serum antibodies and cloned scFv antibody fragments from phage display libraries based on their ability to bind truncated versions of TG2 (46, 47). The recognition pattern of scFvs using the VH5-51 segment suggested that the targeted epitope is located in the core domain of TG2 (47). This is in apparent contrast to our data, which suggest that epitope 1 is in the N-terminal domain based on competition of VH5-51 mAbs with fibronectin. It is possible, though, that epitope 1, similarly to epitope 2, includes residues in the core domain together with residues of the N-terminal domain. It has also been suggested that serum IgA targets the active site of TG2 since mutation of the catalytic triad residues Cys277, His335 and Asp358 resulted in loss of antibody reactivity (31). This is in contrast to our results with both mAbs and polyclonal IgA, showing that antibody binding is not markedly affected by the mutations (Fig. 4A, Fig. 5A). As human antibodies are sensitive to conformational changes in TG2, one reason for this discrepancy could be the use of different recombinant proteins that are not conformationally equal and may behave differently upon introduction of mutations. Hence, in the present study we have used His-tagged protein whereas a GST-TG2 fusion protein was used in the previous study. Our conclusion that the antibodies do not target the active site of TG2 agrees with them not having an inhibitory effect on the activity of the enzyme. It therefore supports our previously suggested model in which the enzymatic activity of BCR-bound TG2 is involved in B-cell uptake of TG2-gluten complexes followed by processing and presentation of gluten-derived peptides to CD4+ T cells (20).
In both our mAb panel (20) and an earlier reported panel of TG2-reactive antibody fragments isolated from phage display libraries (48), the anti-TG2 response was dominated by antibodies using the VH5-51 gene segment. We have shown here that most of the VH5-51 antibodies recognize the same epitope and that this epitope overlaps with the binding site for fibronectin. But despite the overrepresentation of VH5-51 at the single-cell level, polyclonal IgA did not display decreased binding to fibronectin-associated TG2 (Fig. 5B). These seemingly contradicting results might be explained by increased binding of antibodies recognizing other epitopes when TG2 is complexed with fibronectin. In addition, we have earlier shown that the VH5-51 mAbs bind TG2 with lower affinity than mAbs using other VH gene segments (20). Hence, in a polyclonal response, the contribution of VH5-51 antibodies to the overall binding strength will not be proportional to their number. This could also explain the surprisingly large effect of the R19S E153S M659S triple mutation on TG2 reactivity with polyclonal IgA even though the mutation does not disrupt the epitopes recognized by the majority of the mAbs, in particular those using the VH5-51 gene segment.
In summary, by analyzing a panel of 57 TG2-specific mAbs from four adult patients with celiac disease we have found that at least four autoantigenic epitopes in TG2 are clustered in the N-terminal half of the enzyme. We propose that this clustering of epitopes can be explained by a model in which B cells recognizing this region escape from negative selection because the epitopes are not accessible on cell surface-associated TG2. In general, it is likely that B cells reactive with soluble self-antigens can escape negative selection, whereas B cells reactive with cell-surface antigens are deleted or rendered anergic during B cell development. Thus, our findings provide insight into the mechanisms that govern tolerance and autoimmunity by focusing on the targeted epitopes in an ongoing human autoimmune response.
Supplementary Material
ACKNOWLEDGMENTS
We thank Chaitan Khosla for providing the KCC009 TG2 inhibitor and Gerry Melino for the generous gift of TG2 knockout mice. We also wish to thank Marie K. Johannesen and Bjørg Simonsen for excellent technical assistance and Luka Mesin for culturing of small intestinal biopsies.
Footnotes
This work was supported by grants from the Research Council of Norway, the South-Eastern Norway Regional Health Authority and the European Commission (MRTN-CT-2006-036032 and ERC-2010-Ad-268541) to L.M.S and the National Institutes of Health/National Institute of Allergy and Infectious Diseases (U19 AI082724-01, R01 AI76585-03 and HHSN266200500026C) to P.C.W. R.I. was funded by the University of Oslo.
Abbreviations used in this article: ECM, extracellular matrix; iDC, immature dendritic cell; TG2, transglutaminase 2.
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 2.Qiao SW, Iversen R, Raki M, Sollid LM. The adaptive immune response in celiac disease. Semin. Immunopathol. 2012;34:523–540. doi: 10.1007/s00281-012-0314-z. [DOI] [PubMed] [Google Scholar]
- 3.Leffler DA, Schuppan D. Update on serologic testing in celiac disease. Am. J. Gastroenterol. 2010;105:2520–2524. doi: 10.1038/ajg.2010.276. [DOI] [PubMed] [Google Scholar]
- 4.Husby S, Koletzko S, Korponay-Szabo IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, Lelgeman M, Maki M, Ribes-Koninckx C, Ventura A, Zimmer KP. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 5.Molberg O, McAdam SN, Korner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Noren O, Roepstorff P, Lundin KE, Sjostrom H, Sollid LM. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 1998;4:713–717. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 6.van de Wal Y, Kooy Y, van Veelen P, Pena S, Mearin L, Papadopoulos G, Koning F. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 1998;161:1585–1588. [PubMed] [Google Scholar]
- 7.Sollid LM, Molberg O, McAdam S, Lundin KE. Autoantibodies in coeliac disease: tissue transglutaminase--guilt by association? Gut. 1997;41:851–852. doi: 10.1136/gut.41.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maki M. Autoantibodies as markers of autoimmunity in coeliac disease pathogenesis. In: Feighery C, O'Farelly C, editors. Proceedings of the Sixth International Symposium on Coeliac Disease Held at Trinity College, Dublin, in July 1992. Ireland: Oak Tree Press, Dublin; 1994. pp. 246–252. [Google Scholar]
- 9.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 10.Nakaoka H, Perez DM, Baek KJ, Das T, Husain A, Misono K, Im MJ, Graham RM. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science. 1994;264:1593–1596. doi: 10.1126/science.7911253. [DOI] [PubMed] [Google Scholar]
- 11.Achyuthan KE, Greenberg CS. Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity. J. Biol. Chem. 1987;262:1901–1906. [PubMed] [Google Scholar]
- 12.Di Venere A, Rossi A, De Matteis F, Rosato N, Agro AF, Mei G. Opposite effects of Ca(2+) and GTP binding on tissue transglutaminase tertiary structure. J. Biol. Chem. 2000;275:3915–3921. doi: 10.1074/jbc.275.6.3915. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Cerione RA, Clardy J. Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc. Natl. Acad. Sci. USA. 2002;99:2743–2747. doi: 10.1073/pnas.042454899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. doi: 10.1371/journal.pbio.0050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zemskov EA, Mikhailenko I, Hsia RC, Zaritskaya L, Belkin AM. Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. PLoS One. 2011;6:e19414. doi: 10.1371/journal.pone.0019414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J. Cell Biol. 2000;148:825–838. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 18.Taylor JJ, Martinez RJ, Titcombe PJ, Barsness LO, Thomas SR, Zhang N, Katzman SD, Jenkins MK, Mueller DL. Deletion and anergy of polyclonal B cells specific for ubiquitous membrane-bound self-antigen. J. Exp. Med. 2012;209:2065–2077. doi: 10.1084/jem.20112272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon-Vecsei Z, Kiraly R, Bagossi P, Toth B, Dahlbom I, Caja S, Csosz E, Lindfors K, Sblattero D, Nemes E, Maki M, Fesus L, Korponay-Szabo IR. A single conformational transglutaminase 2 epitope contributed by three domains is critical for celiac antibody binding and effects. Proc. Natl. Acad. Sci. USA. 2012;109:431–436. doi: 10.1073/pnas.1107811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Niro R, Mesin L, Zheng NY, Stamnaes J, Morrissey M, Lee JH, Huang M, Iversen R, du Pre MF, Qiao SW, Lundin KE, Wilson PC, Sollid LM. High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat. Med. 2012;18:441–445. doi: 10.1038/nm.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Laurenzi V, Melino G. Gene disruption of tissue transglutaminase. Mol. Cell. Biol. 2001;21:148–155. doi: 10.1128/MCB.21.1.148-155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamnaes J, Pinkas DM, Fleckenstein B, Khosla C, Sollid LM. Redox regulation of transglutaminase 2 activity. J. Biol. Chem. 2010;285:25402–25409. doi: 10.1074/jbc.M109.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piper JL, Gray GM, Khosla C. High selectivity of human tissue transglutaminase for immunoactive gliadin peptides: implications for celiac sprue. Biochemistry. 2002;41:386–393. doi: 10.1021/bi011715x. [DOI] [PubMed] [Google Scholar]
- 24.Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, Wilson PC. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat. Protoc. 2009;4:372–384. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi K, Siegel M, Piper JL, Yuan L, Cho E, Strnad P, Omary B, Rich KM, Khosla C. Chemistry and biology of dihydroisoxazole derivatives: selective inhibitors of human transglutaminase 2. Chem. Biol. 2005;12:469–475. doi: 10.1016/j.chembiol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Raki M, Schjetne KW, Stamnaes J, Molberg O, Jahnsen FL, Issekutz TB, Bogen B, Sollid LM. Surface expression of transglutaminase 2 by dendritic cells and its potential role for uptake and presentation of gluten peptides to T cells. Scand. J. Immunol. 2007;65:213–220. doi: 10.1111/j.1365-3083.2006.01881.x. [DOI] [PubMed] [Google Scholar]
- 27.Sardy M, Karpati S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J. Exp. Med. 2002;195:747–757. doi: 10.1084/jem.20011299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Aeschlimann D. Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann. Neurol. 2008;64:332–343. doi: 10.1002/ana.21450. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein RM, Morgan SH, Chapman J, Bunn CC, Mathews MB, Turner-Warwick M, Hughes GR. Anti-Jo-1 antibody: a marker for myositis with interstitial lung disease. Br. Med. J. (Clin. Res. Ed.) 1984;289:151–152. doi: 10.1136/bmj.289.6438.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casadio R, Polverini E, Mariani P, Spinozzi F, Carsughi F, Fontana A, Polverino de Laureto P, Matteucci G, Bergamini CM. The structural basis for the regulation of tissue transglutaminase by calcium ions. Eur. J. Biochem. 1999;262:672–679. doi: 10.1046/j.1432-1327.1999.00437.x. [DOI] [PubMed] [Google Scholar]
- 31.Byrne G, Ryan F, Jackson J, Feighery C, Kelly J. Mutagenesis of the catalytic triad of tissue transglutaminase abrogates coeliac disease serum IgA autoantibody binding. Gut. 2007;56:336–341. doi: 10.1136/gut.2006.092908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radek JT, Jeong JM, Murthy SN, Ingham KC, Lorand L. Affinity of human erythrocyte transglutaminase for a 42-kDa gelatin-binding fragment of human plasma fibronectin. Proc. Natl. Acad. Sci. USA. 1993;90:3152–3156. doi: 10.1073/pnas.90.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeong JM, Murthy SN, Radek JT, Lorand L. The fibronectin-binding domain of transglutaminase. J. Biol. Chem. 1995;270:5654–5658. doi: 10.1074/jbc.270.10.5654. [DOI] [PubMed] [Google Scholar]
- 34.Hang J, Zemskov EA, Lorand L, Belkin AM. Identification of a novel recognition sequence for fibronectin within the NH2-terminal beta-sandwich domain of tissue transglutaminase. J. Biol. Chem. 2005;280:23675–23683. doi: 10.1074/jbc.M503323200. [DOI] [PubMed] [Google Scholar]
- 35.Gaudry CA, Verderio E, Aeschlimann D, Cox A, Smith C, Griffin M. Cell surface localization of tissue transglutaminase is dependent on a fibronectin-binding site in its N-terminal beta-sandwich domain. J. Biol. Chem. 1999;274:30707–30714. doi: 10.1074/jbc.274.43.30707. [DOI] [PubMed] [Google Scholar]
- 36.Korponay-Szabo IR, Laurila K, Szondy Z, Halttunen T, Szalai Z, Dahlbom I, Rantala I, Kovacs JB, Fesus L, Maki M. Missing endomysial and reticulin binding of coeliac antibodies in transglutaminase 2 knockout tissues. Gut. 2003;52:199–204. doi: 10.1136/gut.52.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann BR, Annis DS, Mosher DF. Reactivity of the N-terminal region of fibronectin protein to transglutaminase 2 and factor XIIIA. J. Biol. Chem. 2011;286:32220–32230. doi: 10.1074/jbc.M111.255562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scarpellini A, Germack R, Lortat-Jacob H, Muramatsu T, Billett E, Johnson T, Verderio EA. Heparan sulfate proteoglycans are receptors for the cell-surface trafficking and biological activity of transglutaminase-2. J. Biol. Chem. 2009;284:18411–18423. doi: 10.1074/jbc.M109.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akimov SS, Belkin AM. Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fibronectin. Blood. 2001;98:1567–1576. doi: 10.1182/blood.v98.5.1567. [DOI] [PubMed] [Google Scholar]
- 40.Hodrea J, Demeny MA, Majai G, Sarang Z, Korponay-Szabo IR, Fesus L. Transglutaminase 2 is expressed and active on the surface of human monocyte-derived dendritic cells and macrophages. Immunol. Lett. 2010;130:74–81. doi: 10.1016/j.imlet.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Di Niro R, Mesin L, Raki M, Zheng NY, Lund-Johansen F, Lundin KE, Charpilienne A, Poncet D, Wilson PC, Sollid LM. Rapid generation of rotavirus-specific human monoclonal antibodies from small-intestinal mucosa. J. Immunol. 2010;185:5377–5383. doi: 10.4049/jimmunol.1001587. [DOI] [PubMed] [Google Scholar]
- 42.Seissler J, Wohlrab U, Wuensche C, Scherbaum WA, Boehm BO. Autoantibodies from patients with coeliac disease recognize distinct functional domains of the autoantigen tissue transglutaminase. Clin. Exp. Immunol. 2001;125:216–221. doi: 10.1046/j.1365-2249.2001.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sulkanen S, Halttunen T, Laurila K, Kolho KL, Korponay-Szabo IR, Sarnesto A, Savilahti E, Collin P, Maki M. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology. 1998;115:1322–1328. doi: 10.1016/s0016-5085(98)70008-3. [DOI] [PubMed] [Google Scholar]
- 44.Lindfors K, Koskinen O, Kurppa K, Laurila K, Collin P, Haimila K, Partanen J, Saavalainen P, Maki M, Kaukinen K. Serodiagnostic assays for celiac disease based on the open or closed conformation of the autoantigen, transglutaminase 2. J. Clin. Immunol. 2011;31:436–442. doi: 10.1007/s10875-011-9514-x. [DOI] [PubMed] [Google Scholar]
- 45.Myrsky E, Caja S, Simon-Vecsei Z, Korponay-Szabo IR, Nadalutti C, Collighan R, Mongeot A, Griffin M, Maki M, Kaukinen K, Lindfors K. Celiac disease IgA modulates vascular permeability in vitro through the activity of transglutaminase 2 and RhoA. Cell. Mol. Life Sci. 2009;66:3375–3385. doi: 10.1007/s00018-009-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakachi K, Powell M, Swift G, Amoroso MA, Ananieva-Jordanova R, Arnold C, Sanders J, Furmaniak J, Rees Smith B. Epitopes recognised by tissue transglutaminase antibodies in coeliac disease. J. Autoimmun. 2004;22:53–63. doi: 10.1016/j.jaut.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Sblattero D, Florian F, Azzoni E, Zyla T, Park M, Baldas V, Not T, Ventura A, Bradbury A, Marzari R. The analysis of the fine specificity of celiac disease antibodies using tissue transglutaminase fragments. Eur. J. Biochem. 2002;269:5175–5181. doi: 10.1046/j.1432-1033.2002.03215.x. [DOI] [PubMed] [Google Scholar]
- 48.Marzari R, Sblattero D, Florian F, Tongiorgi E, Not T, Tommasini A, Ventura A, Bradbury A. Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J. Immunol. 2001;166:4170–4176. doi: 10.4049/jimmunol.166.6.4170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.