Abstract
DNA-looping mechanisms are part of networks that regulate all aspects of DNA metabolism, including transcription, replication, and recombination. DNA looping is involved in regulation of transcriptional initiation in prokaryotic operons, including ara, gal, lac, and deo, and in phage systems. Similarly, in eukaryotic organisms, the effects of enhancers appear to be mediated at least in part by loop formation, and examples of DNA looping by hormone receptor proteins and developmental regulatory proteins have been found. In addition, instances of looped structures have been found in replication and in recombination in both prokaryotes and eukaryotes. DNA loop formation may have different functions in different cellular contexts; in some cases, the loop itself is requisite for regulation, while in others the increase in the effective local concentration of protein may account for the effects observed. The ability of DNA to form loops is affected by the distance between binding sites; by the DNA sequence, which determines deformability and bendability; and by the presence of other proteins that exert an influence on the conformation of a particular sequence. Alteration of the stability of DNA loops and/or protein-DNA binding by extra- or intracellular signals provides responsivity to changing metabolic or environmental conditions. The fundamental property of site-specific protein binding to DNA can be combined with protein-protein and protein-ligand interaction to generate a broad range of physiological states.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S. Multipartite genetic control elements: communication by DNA loop. Annu Rev Genet. 1989;23:227–250. doi: 10.1146/annurev.ge.23.120189.001303. [DOI] [PubMed] [Google Scholar]
- Adzuma K., Mizuuchi K. Interaction of proteins located at a distance along DNA: mechanism of target immunity in the Mu DNA strand-transfer reaction. Cell. 1989 Apr 7;57(1):41–47. doi: 10.1016/0092-8674(89)90170-0. [DOI] [PubMed] [Google Scholar]
- Almer A., Rudolph H., Hinnen A., Hörz W. Removal of positioned nucleosomes from the yeast PHO5 promoter upon PHO5 induction releases additional upstream activating DNA elements. EMBO J. 1986 Oct;5(10):2689–2696. doi: 10.1002/j.1460-2075.1986.tb04552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amouyal M., Mortensen L., Buc H., Hammer K. Single and double loop formation when deoR repressor binds to its natural operator sites. Cell. 1989 Aug 11;58(3):545–551. doi: 10.1016/0092-8674(89)90435-2. [DOI] [PubMed] [Google Scholar]
- Beachy P. A. A molecular view of the Ultrabithorax homeotic gene of Drosophila. Trends Genet. 1990 Feb;6(2):46–51. doi: 10.1016/0168-9525(90)90073-f. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Beato M. Transcriptional control by nuclear receptors. FASEB J. 1991 Apr;5(7):2044–2051. doi: 10.1096/fasebj.5.7.2010057. [DOI] [PubMed] [Google Scholar]
- Bellomy G. R., Mossing M. C., Record M. T., Jr Physical properties of DNA in vivo as probed by the length dependence of the lac operator looping process. Biochemistry. 1988 May 31;27(11):3900–3906. doi: 10.1021/bi00411a002. [DOI] [PubMed] [Google Scholar]
- Bellomy G. R., Record M. T., Jr Stable DNA loops in vivo and in vitro: roles in gene regulation at a distance and in biophysical characterization of DNA. Prog Nucleic Acid Res Mol Biol. 1990;39:81–128. doi: 10.1016/s0079-6603(08)60624-8. [DOI] [PubMed] [Google Scholar]
- Besse M., von Wilcken-Bergmann B., Müller-Hill B. Synthetic lac operator mediates repression through lac repressor when introduced upstream and downstream from lac promoter. EMBO J. 1986 Jun;5(6):1377–1381. doi: 10.1002/j.1460-2075.1986.tb04370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Zhang L., Sasse-Dwight S., Gralla J. D. DNA supercoiling promotes formation of a bent repression loop in lac DNA. J Mol Biol. 1987 Jul 5;196(1):101–111. doi: 10.1016/0022-2836(87)90513-4. [DOI] [PubMed] [Google Scholar]
- Brenowitz M., Jamison E., Majumdar A., Adhya S. Interaction of the Escherichia coli Gal repressor protein with its DNA operators in vitro. Biochemistry. 1990 Apr 3;29(13):3374–3383. doi: 10.1021/bi00465a033. [DOI] [PubMed] [Google Scholar]
- Brenowitz M., Mandal N., Pickar A., Jamison E., Adhya S. DNA-binding properties of a lac repressor mutant incapable of forming tetramers. J Biol Chem. 1991 Jan 15;266(2):1281–1288. [PubMed] [Google Scholar]
- Cann J. R. Analysis of the gel electrophoresis of looped protein-DNA complexes by computer simulation. J Mol Biol. 1990 Dec 20;216(4):1067–1075. doi: 10.1016/S0022-2836(99)80020-5. [DOI] [PubMed] [Google Scholar]
- Carey M., Leatherwood J., Ptashne M. A potent GAL4 derivative activates transcription at a distance in vitro. Science. 1990 Feb 9;247(4943):710–712. doi: 10.1126/science.2405489. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Mason R. J., Wickner S. H. Mini-P1 plasmid replication: the autoregulation-sequestration paradox. Cell. 1988 Feb 26;52(4):551–557. doi: 10.1016/0092-8674(88)90468-0. [DOI] [PubMed] [Google Scholar]
- Culard F., Maurizot J. C. Binding of lac repressor induces different conformational changes on operator and non-operator DNAs. FEBS Lett. 1982 Sep 6;146(1):153–156. doi: 10.1016/0014-5793(82)80724-2. [DOI] [PubMed] [Google Scholar]
- Culard F., Maurizot J. C. Lac repressor - lac operator interaction. Circular dichroism study. Nucleic Acids Res. 1981 Oct 10;9(19):5175–5184. doi: 10.1093/nar/9.19.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandanell G., Hammer K. Two operator sites separated by 599 base pairs are required for deoR repression of the deo operon of Escherichia coli. EMBO J. 1985 Dec 1;4(12):3333–3338. doi: 10.1002/j.1460-2075.1985.tb04085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandanell G., Valentin-Hansen P., Larsen J. E., Hammer K. Long-range cooperativity between gene regulatory sequences in a prokaryote. 1987 Feb 26-Mar 4Nature. 325(6107):823–826. doi: 10.1038/325823a0. [DOI] [PubMed] [Google Scholar]
- Dripps D., Wartell R. M. DNA bending induced by the catabolite activator protein allows ring formation of a 144 bp DNA. J Biomol Struct Dyn. 1987 Aug;5(1):1–13. doi: 10.1080/07391102.1987.10506370. [DOI] [PubMed] [Google Scholar]
- Dunaway M., Dröge P. Transactivation of the Xenopus rRNA gene promoter by its enhancer. Nature. 1989 Oct 19;341(6243):657–659. doi: 10.1038/341657a0. [DOI] [PubMed] [Google Scholar]
- Dunn T. M., Hahn S., Ogden S., Schleif R. F. An operator at -280 base pairs that is required for repression of araBAD operon promoter: addition of DNA helical turns between the operator and promoter cyclically hinders repression. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5017–5020. doi: 10.1073/pnas.81.16.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eismann E. R., Müller-Hill B. lac repressor forms stable loops in vitro with supercoiled wild-type lac DNA containing all three natural lac operators. J Mol Biol. 1990 Jun 20;213(4):763–775. doi: 10.1016/S0022-2836(05)80262-1. [DOI] [PubMed] [Google Scholar]
- Eismann E., von Wilcken-Bergmann B., Müller-Hill B. Specific destruction of the second lac operator decreases repression of the lac operon in Escherichia coli fivefold. J Mol Biol. 1987 Jun 20;195(4):949–952. doi: 10.1016/0022-2836(87)90499-2. [DOI] [PubMed] [Google Scholar]
- Englesberg E., Wilcox G. Regulation: positive control. Annu Rev Genet. 1974;8:219–242. doi: 10.1146/annurev.ge.08.120174.001251. [DOI] [PubMed] [Google Scholar]
- Flashner Y., Gralla J. D. Dual mechanism of repression at a distance in the lac operon. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8968–8972. doi: 10.1073/pnas.85.23.8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman K., Göransson M., Uhlin B. E. Autoregulation and multiple DNA interactions by a transcriptional regulatory protein in E. coli pili biogenesis. EMBO J. 1989 Apr;8(4):1271–1277. doi: 10.1002/j.1460-2075.1989.tb03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H. J., Bicknäse H., Gleumes B., Heibach C., Rosahl S., Ehring R. Characterization of two mutations in the Escherichia coli galE gene inactivating the second galactose operator and comparative studies of repressor binding. EMBO J. 1983;2(12):2129–2135. doi: 10.1002/j.1460-2075.1983.tb01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E. Multipurpose role for RAP1, a yeast DNA looping protein. Res Microbiol. 1989 Sep;140(7):433–438. doi: 10.1016/0923-2508(89)90063-6. [DOI] [PubMed] [Google Scholar]
- Gralla J. D. Bacterial gene regulation from distant DNA sites. Cell. 1989 Apr 21;57(2):193–195. doi: 10.1016/0092-8674(89)90955-0. [DOI] [PubMed] [Google Scholar]
- Griffith J., Hochschild A., Ptashne M. DNA loops induced by cooperative binding of lambda repressor. Nature. 1986 Aug 21;322(6081):750–752. doi: 10.1038/322750a0. [DOI] [PubMed] [Google Scholar]
- Haber R., Adhya S. Interaction of spatially separated protein-DNA complexes for control of gene expression: operator conversions. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9683–9687. doi: 10.1073/pnas.85.24.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Dunn T., Schleif R. Upstream repression and CRP stimulation of the Escherichia coli L-arabinose operon. J Mol Biol. 1984 Nov 25;180(1):61–72. doi: 10.1016/0022-2836(84)90430-3. [DOI] [PubMed] [Google Scholar]
- Hahn S., Hendrickson W., Schleif R. Transcription of Escherichia coli ara in vitro. The cyclic AMP receptor protein requirement for PBAD induction that depends on the presence and orientation of the araO2 site. J Mol Biol. 1986 Apr 5;188(3):355–367. doi: 10.1016/0022-2836(86)90160-9. [DOI] [PubMed] [Google Scholar]
- Hamilton E. P., Lee N. Three binding sites for AraC protein are required for autoregulation of araC in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1749–1753. doi: 10.1073/pnas.85.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Scott M. P. What determines the specificity of action of Drosophila homeodomain proteins? Cell. 1990 Nov 30;63(5):883–894. doi: 10.1016/0092-8674(90)90492-w. [DOI] [PubMed] [Google Scholar]
- Heichman K. A., Johnson R. C. The Hin invertasome: protein-mediated joining of distant recombination sites at the enhancer. Science. 1990 Aug 3;249(4968):511–517. doi: 10.1126/science.2166334. [DOI] [PubMed] [Google Scholar]
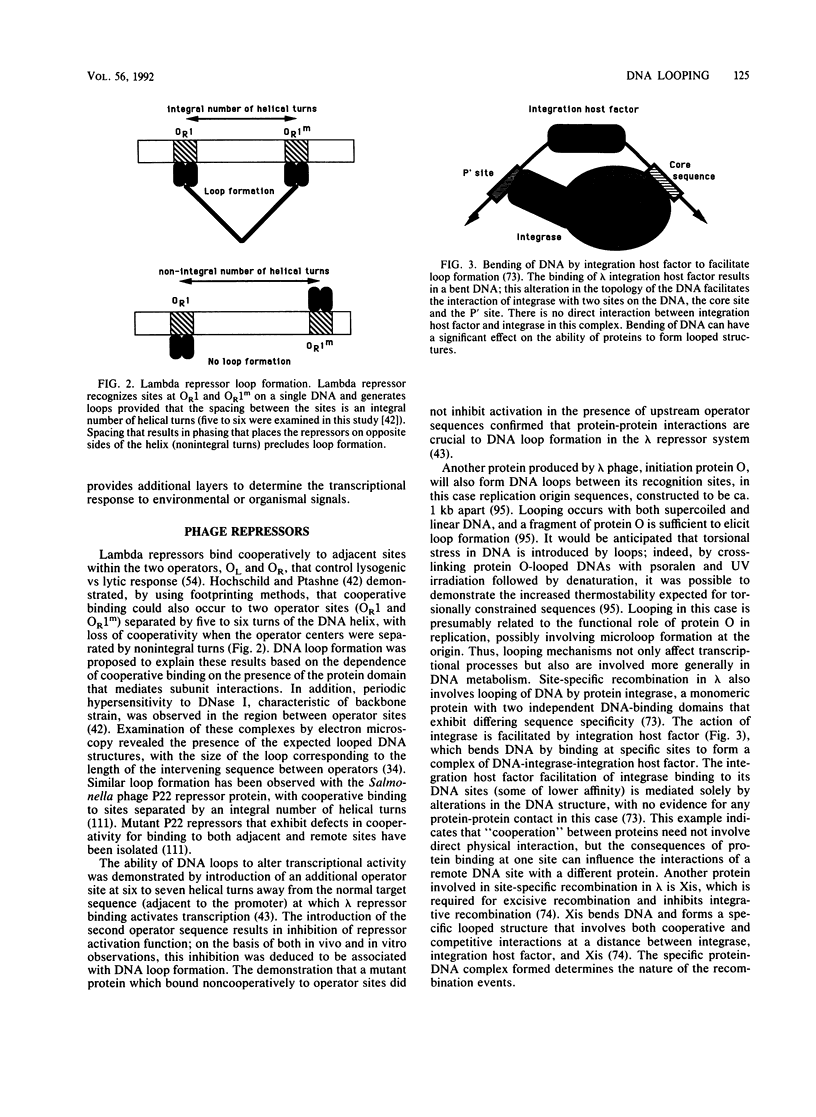
- Hochschild A., Ptashne M. Cooperative binding of lambda repressors to sites separated by integral turns of the DNA helix. Cell. 1986 Mar 14;44(5):681–687. doi: 10.1016/0092-8674(86)90833-0. [DOI] [PubMed] [Google Scholar]
- Hochschild A., Ptashne M. Interaction at a distance between lambda repressors disrupts gene activation. Nature. 1988 Nov 24;336(6197):353–357. doi: 10.1038/336353a0. [DOI] [PubMed] [Google Scholar]
- Hodges-Garcia Y., Hagerman P. J., Pettijohn D. E. DNA ring closure mediated by protein HU. J Biol Chem. 1989 Sep 5;264(25):14621–14623. [PubMed] [Google Scholar]
- Hofmann J. F., Laroche T., Brand A. H., Gasser S. M. RAP-1 factor is necessary for DNA loop formation in vitro at the silent mating type locus HML. Cell. 1989 Jun 2;57(5):725–737. doi: 10.1016/0092-8674(89)90788-5. [DOI] [PubMed] [Google Scholar]
- Hsieh W. T., Whitson P. A., Matthews K. S., Wells R. D. Influence of sequence and distance between two operators on interaction with the lac repressor. J Biol Chem. 1987 Oct 25;262(30):14583–14591. [PubMed] [Google Scholar]
- Hudson J. M., Fried M. G. Co-operative interactions between the catabolite gene activator protein and the lac repressor at the lactose promoter. J Mol Biol. 1990 Jul 20;214(2):381–396. doi: 10.1016/0022-2836(90)90188-R. [DOI] [PubMed] [Google Scholar]
- Huo L., Martin K. J., Schleif R. Alternative DNA loops regulate the arabinose operon in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5444–5448. doi: 10.1073/pnas.85.15.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Gomada M., Sangodkar U. M., Nakazawa A., Nakazawa T. Upstream regulatory sequence for transcriptional activator XylR in the first operon of xylene metabolism on the TOL plasmid. J Mol Biol. 1990 Nov 20;216(2):251–260. doi: 10.1016/S0022-2836(05)80317-1. [DOI] [PubMed] [Google Scholar]
- Irani M. H., Orosz L., Adhya S. A control element within a structural gene: the gal operon of Escherichia coli. Cell. 1983 Mar;32(3):783–788. doi: 10.1016/0092-8674(83)90064-8. [DOI] [PubMed] [Google Scholar]
- Iwasato T., Shimizu A., Honjo T., Yamagishi H. Circular DNA is excised by immunoglobulin class switch recombination. Cell. 1990 Jul 13;62(1):143–149. doi: 10.1016/0092-8674(90)90248-d. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Johnson A. D., Poteete A. R., Lauer G., Sauer R. T., Ackers G. K., Ptashne M. lambda Repressor and cro--components of an efficient molecular switch. Nature. 1981 Nov 19;294(5838):217–223. doi: 10.1038/294217a0. [DOI] [PubMed] [Google Scholar]
- Jäck H. M., McDowell M., Steinberg C. M., Wabl M. Looping out and deletion mechanism for the immunoglobulin heavy-chain class switch. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1581–1585. doi: 10.1073/pnas.85.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania J., Müller-Hill B. Construction, isolation and implications of repressor-galactosidase - beta-galactosidase hybrid molecules. Eur J Biochem. 1977 Oct 3;79(2):381–386. doi: 10.1111/j.1432-1033.1977.tb11819.x. [DOI] [PubMed] [Google Scholar]
- Knight J. D., Li R., Botchan M. The activation domain of the bovine papillomavirus E2 protein mediates association of DNA-bound dimers to form DNA loops. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3204–3208. doi: 10.1073/pnas.88.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer H., Amouyal M., Nordheim A., Müller-Hill B. DNA supercoiling changes the spacing requirement of two lac operators for DNA loop formation with lac repressor. EMBO J. 1988 Feb;7(2):547–556. doi: 10.1002/j.1460-2075.1988.tb02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer H., Niemöller M., Amouyal M., Revet B., von Wilcken-Bergmann B., Müller-Hill B. lac repressor forms loops with linear DNA carrying two suitably spaced lac operators. EMBO J. 1987 May;6(5):1481–1491. doi: 10.1002/j.1460-2075.1987.tb02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnke G., Fritz H. J., Ehring R. Unusual properties of promoter-up mutations in the Escherichia coli galactose operon and evidence suggesting RNA polymerase-induced DNA bending. EMBO J. 1987 Feb;6(2):507–513. doi: 10.1002/j.1460-2075.1987.tb04782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnke G., Krause A., Heibach C., Gieske U., Fritz H. J., Ehring R. The upstream operator of the Escherichia coli galactose operon is sufficient for repression of transcription initiated at the cyclic AMP-stimulated promoter. EMBO J. 1986 Jan;5(1):167–173. doi: 10.1002/j.1460-2075.1986.tb04192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnke G., Theres C., Fritz H. J., Ehring R. RNA polymerase and gal repressor bind simultaneously and with DNA bending to the control region of the Escherichia coli galactose operon. EMBO J. 1989 Apr;8(4):1247–1255. doi: 10.1002/j.1460-2075.1989.tb03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. H., Schleif R. F. In vivo DNA loops in araCBAD: size limits and helical repeat. Proc Natl Acad Sci U S A. 1989 Jan;86(2):476–480. doi: 10.1073/pnas.86.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N. E., Davis M. M. T cell receptor beta-chain genes in BW5147 and other AKR tumors. Deletion order of murine V beta gene segments and possible 5' regulatory regions. J Immunol. 1988 Mar 1;140(5):1665–1675. [PubMed] [Google Scholar]
- Lee N., Francklyn C., Hamilton E. P. Arabinose-induced binding of AraC protein to araI2 activates the araBAD operon promoter. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8814–8818. doi: 10.1073/pnas.84.24.8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobell R. B., Schleif R. F. AraC-DNA looping: orientation and distance-dependent loop breaking by the cyclic AMP receptor protein. J Mol Biol. 1991 Mar 5;218(1):45–54. doi: 10.1016/0022-2836(91)90872-4. [DOI] [PubMed] [Google Scholar]
- Lobell R. B., Schleif R. F. DNA looping and unlooping by AraC protein. Science. 1990 Oct 26;250(4980):528–532. doi: 10.1126/science.2237403. [DOI] [PubMed] [Google Scholar]
- Majumdar A., Adhya S. Demonstration of two operator elements in gal: in vitro repressor binding studies. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6100–6104. doi: 10.1073/pnas.81.19.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A., Adhya S. Probing the structure of gal operator-repressor complexes. Conformation change in DNA. J Biol Chem. 1987 Sep 25;262(27):13258–13262. [PubMed] [Google Scholar]
- Mandal N., Su W., Haber R., Adhya S., Echols H. DNA looping in cellular repression of transcription of the galactose operon. Genes Dev. 1990 Mar;4(3):410–418. doi: 10.1101/gad.4.3.410. [DOI] [PubMed] [Google Scholar]
- Martin K., Huo L., Schleif R. F. The DNA loop model for ara repression: AraC protein occupies the proposed loop sites in vivo and repression-negative mutations lie in these same sites. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3654–3658. doi: 10.1073/pnas.83.11.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Yoshida K., Maeda T., Usuda S., Sakano H. Switch circular DNA formed in cytokine-treated mouse splenocytes: evidence for intramolecular DNA deletion in immunoglobulin class switching. Cell. 1990 Jul 13;62(1):135–142. doi: 10.1016/0092-8674(90)90247-c. [DOI] [PubMed] [Google Scholar]
- Menon K. P., Lee N. L. Activation of ara operons by a truncated AraC protein does not require inducer. Proc Natl Acad Sci U S A. 1990 May;87(10):3708–3712. doi: 10.1073/pnas.87.10.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
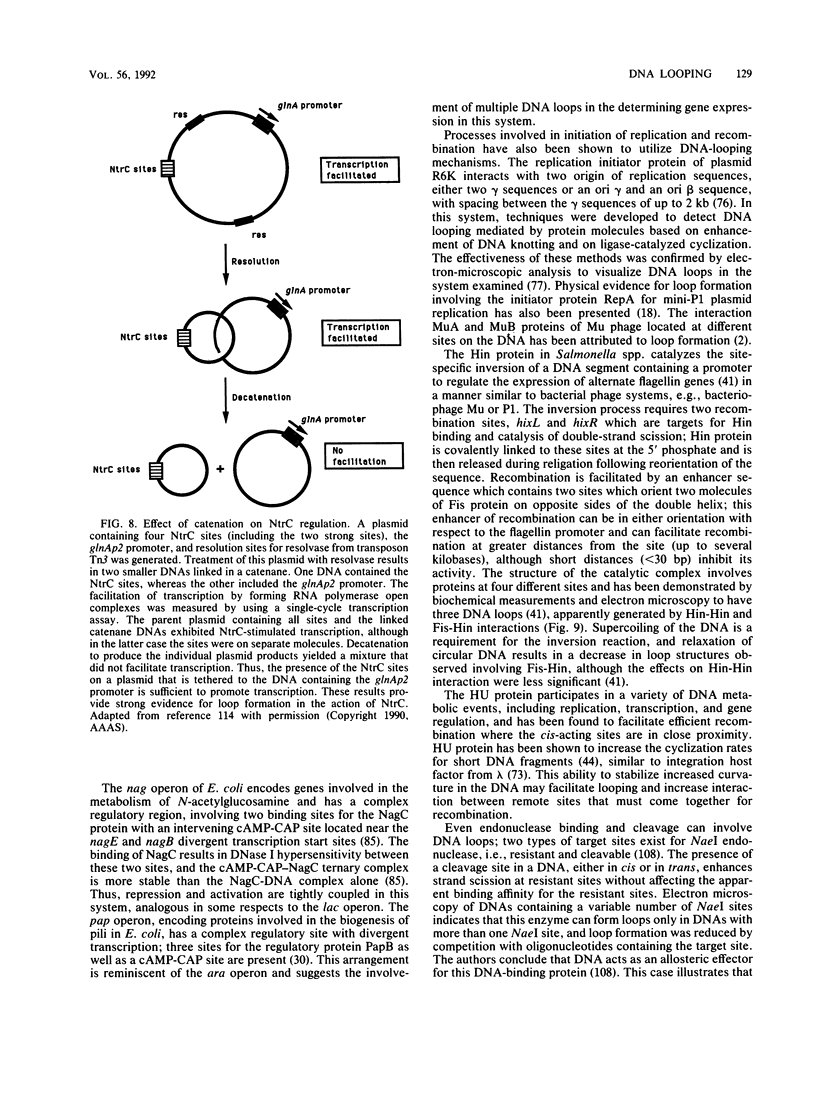
- Moitoso de Vargas L., Kim S., Landy A. DNA looping generated by DNA bending protein IHF and the two domains of lambda integrase. Science. 1989 Jun 23;244(4911):1457–1461. doi: 10.1126/science.2544029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitoso de Vargas L., Landy A. A switch in the formation of alternative DNA loops modulates lambda site-specific recombination. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):588–592. doi: 10.1073/pnas.88.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossing M. C., Record M. T., Jr Upstream operators enhance repression of the lac promoter. Science. 1986 Aug 22;233(4766):889–892. doi: 10.1126/science.3090685. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Erickson H., Bastia D. Detection of DNA looping due to simultaneous interaction of a DNA-binding protein with two spatially separated binding sites on DNA. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6287–6291. doi: 10.1073/pnas.85.17.6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Erickson H., Bastia D. Enhancer-origin interaction in plasmid R6K involves a DNA loop mediated by initiator protein. Cell. 1988 Feb 12;52(3):375–383. doi: 10.1016/s0092-8674(88)80030-8. [DOI] [PubMed] [Google Scholar]
- Müeller-Storm H. P., Sogo J. M., Schaffner W. An enhancer stimulates transcription in trans when attached to the promoter via a protein bridge. Cell. 1989 Aug 25;58(4):767–777. doi: 10.1016/0092-8674(89)90110-4. [DOI] [PubMed] [Google Scholar]
- Müller H. P., Matthias P., Schaffner W. A transcriptional terminator between enhancer and promoter does not affect remote transcriptional control. Somat Cell Mol Genet. 1990 Jul;16(4):351–360. doi: 10.1007/BF01232463. [DOI] [PubMed] [Google Scholar]
- Müller M. M., Gerster T., Schaffner W. Enhancer sequences and the regulation of gene transcription. Eur J Biochem. 1988 Oct 1;176(3):485–495. doi: 10.1111/j.1432-1033.1988.tb14306.x. [DOI] [PubMed] [Google Scholar]
- O'Gorman R. B., Dunaway M., Matthews K. S. DNA binding characteristics of lactose repressor and the trypsin-resistant core repressor. J Biol Chem. 1980 Nov 10;255(21):10100–10106. [PubMed] [Google Scholar]
- Oehler S., Eismann E. R., Krämer H., Müller-Hill B. The three operators of the lac operon cooperate in repression. EMBO J. 1990 Apr;9(4):973–979. doi: 10.1002/j.1460-2075.1990.tb08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfahl M., Gulde V., Bourgeois S. "Second" and "third operator" of the lac operon: an investigation of their role in the regulatory mechanism. J Mol Biol. 1979 Jan 25;127(3):339–344. doi: 10.1016/0022-2836(79)90333-4. [DOI] [PubMed] [Google Scholar]
- Piña B., Brüggemeier U., Beato M. Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell. 1990 Mar 9;60(5):719–731. doi: 10.1016/0092-8674(90)90087-u. [DOI] [PubMed] [Google Scholar]
- Plumbridge J., Kolb A. CAP and Nag repressor binding to the regulatory regions of the nagE-B and manX genes of Escherichia coli. J Mol Biol. 1991 Feb 20;217(4):661–679. doi: 10.1016/0022-2836(91)90524-a. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell. 1986 Jun 20;45(6):785–792. doi: 10.1016/0092-8674(86)90553-2. [DOI] [PubMed] [Google Scholar]
- Reznikoff W. S., Winter R. B., Hurley C. K. The location of the repressor binding sites in the lac operon. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2314–2318. doi: 10.1073/pnas.71.6.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C., Riggs K., Merrell K., Calame K. Activator proteins which regulate immunoglobulin heavy chain gene transcription in B lymphocytes. Prog Clin Biol Res. 1990;352:241–248. [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. Probing co-operative DNA-binding in vivo. The lac O1:O3 interaction. J Mol Biol. 1988 Jul 5;202(1):107–119. doi: 10.1016/0022-2836(88)90523-2. [DOI] [PubMed] [Google Scholar]
- Schiedner G., Wessel R., Scheffner M., Stahl H. Renaturation and DNA looping promoted by the SV40 large tumour antigen. EMBO J. 1990 Sep;9(9):2937–2943. doi: 10.1002/j.1460-2075.1990.tb07485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. DNA looping. Science. 1988 Apr 8;240(4849):127–128. doi: 10.1126/science.3353710. [DOI] [PubMed] [Google Scholar]
- Schleif R. Gene regulation: why should DNA loop? Nature. 1987 Jun 4;327(6121):369–370. doi: 10.1038/327369a0. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Schaffner W. Long-range activation of transcription by SV40 enhancer is affected by "inhibitory" or "permissive" DNA sequences between enhancer and promoter. Somat Cell Mol Genet. 1989 Nov;15(6):591–603. doi: 10.1007/BF01534920. [DOI] [PubMed] [Google Scholar]
- Schultz P., Marzouki N., Marck C., Ruet A., Oudet P., Sentenac A. The two DNA-binding domains of yeast transcription factor tau as observed by scanning transmission electron microscopy. EMBO J. 1989 Dec 1;8(12):3815–3824. doi: 10.1002/j.1460-2075.1989.tb08559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D., Baldwin R. L. Energetics of DNA twisting. I. Relation between twist and cyclization probability. J Mol Biol. 1983 Nov 15;170(4):957–981. doi: 10.1016/s0022-2836(83)80198-3. [DOI] [PubMed] [Google Scholar]
- Shore D., Langowski J., Baldwin R. L. DNA flexibility studied by covalent closure of short fragments into circles. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4833–4837. doi: 10.1073/pnas.78.8.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz T. A., Richmond T. J., Wise D., Engelman D. The lac repressor protein: molecular shape, subunit structure, and proposed model for operator interaction based on structural studies of microcrystals. Proc Natl Acad Sci U S A. 1974 Mar;71(3):593–597. doi: 10.1073/pnas.71.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus L., Wilcox G. Effect of mutations in the cyclic AMP receptor protein-binding site on araBAD and araC expression. J Bacteriol. 1989 Feb;171(2):1178–1184. doi: 10.1128/jb.171.2.1178-1184.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straney S. B., Crothers D. M. Lac repressor is a transient gene-activating protein. Cell. 1987 Dec 4;51(5):699–707. doi: 10.1016/0092-8674(87)90093-6. [DOI] [PubMed] [Google Scholar]
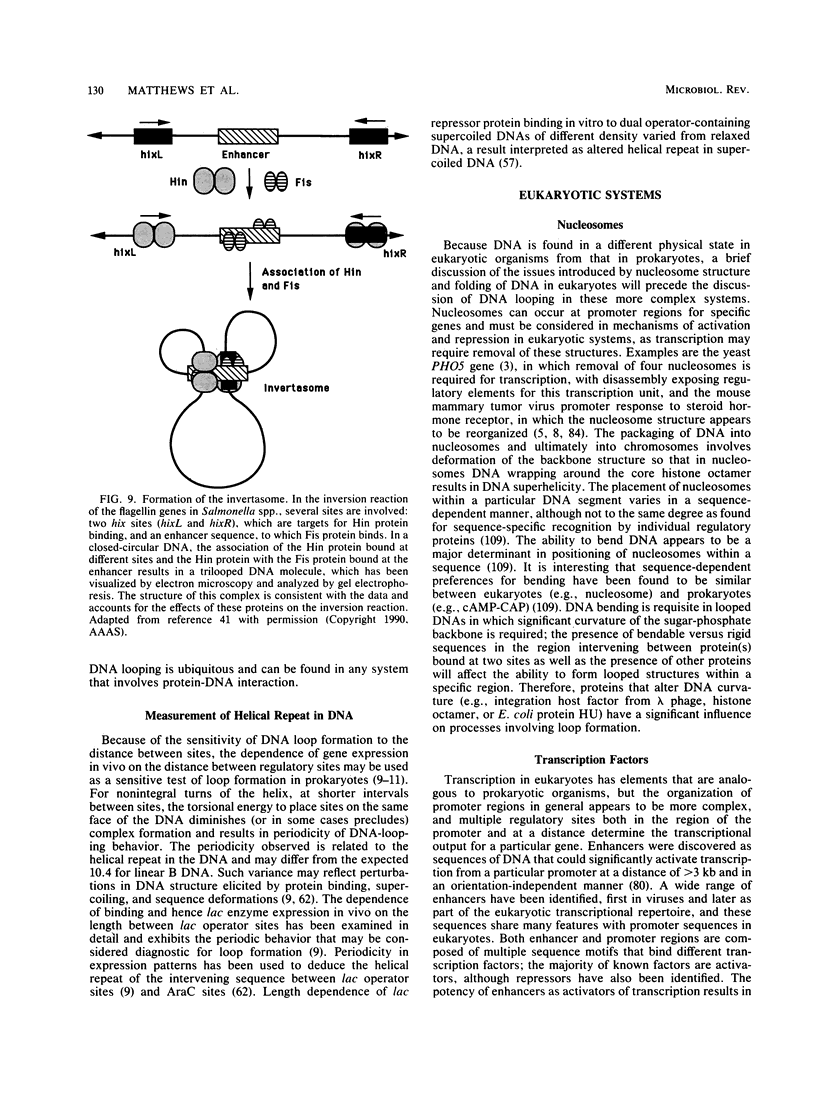
- Su W., Porter S., Kustu S., Echols H. DNA-looping and enhancer activity: association between DNA-bound NtrC activator and RNA polymerase at the bacterial glnA promoter. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5504–5508. doi: 10.1073/pnas.87.14.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry F., Dostatni N., Arnos F., Yaniv M. Cooperative activation of transcription by bovine papillomavirus type 1 E2 can occur over a large distance. Mol Cell Biol. 1990 Aug;10(8):4431–4437. doi: 10.1128/mcb.10.8.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théveny B., Bailly A., Rauch C., Rauch M., Delain E., Milgrom E. Association of DNA-bound progesterone receptors. Nature. 1987 Sep 3;329(6134):79–81. doi: 10.1038/329079a0. [DOI] [PubMed] [Google Scholar]
- Toda M., Fujimoto S., Iwasato T., Takeshita S., Tezuka K., Ohbayashi T., Yamagishi H. Structure of extrachromosomal circular DNAs excised from T-cell antigen receptor alpha and delta-chain loci. J Mol Biol. 1988 Jul 20;202(2):219–231. doi: 10.1016/0022-2836(88)90453-6. [DOI] [PubMed] [Google Scholar]
- Toda M., Hirama T., Takeshita S., Yamagishi H. Excision products of immunoglobulin gene rearrangements. Immunol Lett. 1989 Jun 15;21(4):311–316. doi: 10.1016/0165-2478(89)90025-4. [DOI] [PubMed] [Google Scholar]
- Topal M. D., Thresher R. J., Conrad M., Griffith J. NaeI endonuclease binding to pBR322 DNA induces looping. Biochemistry. 1991 Feb 19;30(7):2006–2010. doi: 10.1021/bi00221a038. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Klug A. The bending of DNA in nucleosomes and its wider implications. Philos Trans R Soc Lond B Biol Sci. 1987 Dec 15;317(1187):537–561. doi: 10.1098/rstb.1987.0080. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P., Albrechtsen B., Løve Larsen J. E. DNA-protein recognition: demonstration of three genetically separated operator elements that are required for repression of the Escherichia coli deoCABD promoters by the DeoR repressor. EMBO J. 1986 Aug;5(8):2015–2021. doi: 10.1002/j.1460-2075.1986.tb04458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela D., Ptashne M. P22 repressor mutants deficient in co-operative binding and DNA loop formation. EMBO J. 1989 Dec 20;8(13):4345–4350. doi: 10.1002/j.1460-2075.1989.tb08621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C., Giaever G. N. Action at a distance along a DNA. Science. 1988 Apr 15;240(4850):300–304. doi: 10.1126/science.3281259. [DOI] [PubMed] [Google Scholar]
- Wedel A., Weiss D. S., Popham D., Dröge P., Kustu S. A bacterial enhancer functions to tether a transcriptional activator near a promoter. Science. 1990 Apr 27;248(4954):486–490. doi: 10.1126/science.1970441. [DOI] [PubMed] [Google Scholar]
- Whitson P. A., Hsieh W. T., Wells R. D., Matthews K. S. Influence of supercoiling and sequence context on operator DNA binding with lac repressor. J Biol Chem. 1987 Oct 25;262(30):14592–14599. [PubMed] [Google Scholar]
- Whitson P. A., Hsieh W. T., Wells R. D., Matthews K. S. Supercoiling facilitates lac operator-repressor-pseudooperator interactions. J Biol Chem. 1987 Apr 15;262(11):4943–4946. [PubMed] [Google Scholar]
- Whitson P. A., Matthews K. S. Dissociation of the lactose repressor-operator DNA complex: effects of size and sequence context of operator-containing DNA. Biochemistry. 1986 Jul 1;25(13):3845–3852. doi: 10.1021/bi00361a016. [DOI] [PubMed] [Google Scholar]
- Whitson P. A., Olson J. S., Matthews K. S. Thermodynamic analysis of the lactose repressor-operator DNA interaction. Biochemistry. 1986 Jul 1;25(13):3852–3858. doi: 10.1021/bi00361a017. [DOI] [PubMed] [Google Scholar]
- Winter R. B., von Hippel P. H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 2. The Escherichia coli repressor--operator interaction: equilibrium measurements. Biochemistry. 1981 Nov 24;20(24):6948–6960. doi: 10.1021/bi00527a029. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- von Schwedler U., Jäck H. M., Wabl M. Circular DNA is a product of the immunoglobulin class switch rearrangement. Nature. 1990 May 31;345(6274):452–456. doi: 10.1038/345452a0. [DOI] [PubMed] [Google Scholar]



