Abstract
Background/Aims
The prevalence of the risk factors for atherosclerosis, other than diabetes mellitus, among type 2 diabetic patients with different stages of chronic kidney disease (CKD) determined by glomerular filtration rate (GFR) was investigated.
Methods
The prevalence of ten risk factors (age ≥65 years, history of smoking, male gender, obesity, albuminuria, hypertension, hypercholesterolemia, hypo-HDL-cholesterolemia, hyperuricemia and anemia) was determined in 2,107 Japanese type 2 diabetic patients with different stages of CKD (six stages according to GFR).
Results
The risk factors for age ≥65 years and male gender were found in 49 and 62% of the study subjects, respectively. The percentages of subjects with a current history of smoking, obesity, albuminuria, hypertension, hypercholesterolemia, hypo-HDL-cholesterolemia, hyperuricemia and anemia were 35, 44, 47, 70, 61, 13, 21 and 26%, respectively. The prevalence of age ≥65 years, male gender, albuminuria, hypertension, hypo-HDL-cholesterolemia, hyperuricemia and anemia was greater in the later stages of GFR, whereas the prevalence of hypercholesterolemia and obesity did not differ between stages. The prevalence of a current history of smoking was lower in the later stages of GFR. The cumulative number of risk factors increased from 3.1 to 6.8 in the later stages of GFR.
Conclusion
Among type 2 diabetic patients with CKD, the total number of risk factors increases with the progression of renal dysfunction. It is important to pay attention to newly recognized risk factors for hyperuricemia and anemia, in addition to hypertension, albuminuria and hypo-HDL-cholesterolemia, in monitoring diabetic patients with later stages of CKD.
Key Words : Chronic kidney disease, Hyperuricemia, Atherosclerosis, Anemia, Diabetic nephropathy
Introduction
Atherosclerotic diseases are commonly found in patients with type 2 diabetes mellitus. It is well-known that some traditional risk factors for atherosclerosis, such as obesity, hypertension and dyslipidemia, are frequently complicated by type 2 diabetes mellitus. Chronic kidney disease (CKD), which is defined by a reduced glomerular filtration rate (GFR) and/or proteinuria, also causes atherosclerotic diseases [1,2]. Anemia and hyperuricemia, which are common in CKD patients [3,4], are also risk factors for diabetic macroangiopathies in patients with type 2 diabetes complicated by CKD [5,6]. A greater level of an intima-media thickening, a surrogate marker for atherosclerosis [7], and a high incidence of diabetic macroangiopathies are found in patients with later stages of diabetic nephropathy [8]. Therefore, an accumulation of the risk factors for cardiovascular events might be present in patients with diabetes mellitus and CKD.
In the present study, the prevalence of risk factors for atherosclerosis among type 2 diabetic patients with different stages of CKD was cross-sectionally investigated. The results highlight the importance of ranking the risk factors for atherosclerosis among type 2 diabetic patients with CKD.
Patients and Methods
A total of 2,107 patients diagnosed with type 2 diabetes mellitus who underwent consecutive evaluations, including urinalysis and measurements of the serum creatinine levels, at the Department of Diabetes, Metabolism and Kidney Disease of the Edogawa Hospital, Tokyo, Japan between April 2008 and March 2011, were recruited for this study.
Obese individuals were defined as those with a BMI ≥25. Hypertension was defined as a systolic blood pressure ≥140 mm Hg and/or a diastolic blood pressure ≥90 mm Hg. Patients currently using antihypertensive medications were also classified as positive for hypertension.
Hypercholesterolemia was defined as either a serum concentration of total cholesterol ≥5.69 mmol/l, an LDL cholesterol level ≥3.62 mmol/l, or the current use of lipid-lowering agents. Hypo-HDL-cholesterolemia was defined by serum HDL cholesterol concentrations <1.03 mmol/l. Hyperuricemia was defined either as a serum uric acid level >416 μmol/l or the use of allopurinol according to the guidelines proposed by the Japanese Society of Gout and Nucleic Acid Metabolism [9]. Anemia was defined as a hemoglobin level <135 g/l in males and <120 g/l in females according to the guidelines of the European Renal Association – European Dialysis and Transplantation Association [10] and the National Kidney Foundation [11].
The estimated GFR (eGFR) was calculated using the formula reported by Matsuo et al. [12]. This equation originated from the MDRD study group [13] and was adapted for Japanese individuals; it is recommended by the Japanese Society of Nephrology: eGFR (ml/min/ 1.73 m2) = 194 × Scr−1.094 × age−0.287 × 0.739 (if female). The CKD stage was classified according to the levels of the eGFR and the urinary albumin to creatinine rate based on the new classification of CKD proposed by the Kidney Disease: Improving Global Outcomes (KDIGO) in 2011 [14].
Ethics Statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. The Ethics Committees of the Edogawa Hospital approved the protocol of this study and waived the need for written informed consent because this cross-sectional study analyzed anonymized data stored in the hospital database.
Statistical Methods
All data are shown as means ± SD. The χ2 test was used to determine the differences in the prevalence of the risk factors among the subjects with different stages of GFR. An analysis of variance (ANOVA) was used to compare the number of risk factors and the HbA1c levels among the patients with different stages of GFR. Differences of p < 0.05 (two-tailed) were considered to be statistically significant. The statistical software package JMP, version 8.0 (SAS Institute, Cary, N.C., USA), was used for all analyses.
Results
Table 1 shows the clinical characteristics and laboratory parameters of the patients. The mean patient age was 63 ± 13 years, and 49% of the patients were ≥65 years. Sixty-two percent of the patients were male. The percentages of subjects with a current history of smoking, obesity, hypertension, hypercholesterolemia, hypo-HDL-cholesterolemia, hyperuricemia and anemia were 35, 44, 70, 61, 13, 21 and 26%, respectively. The prevalence of diabetic micro- and macroangiopathies was significantly greater in the patients with later stages of GFR (table 2).
Table 1.
Clinical characteristics of the patients
| Mean±SD | Evaluated patients, n | |
|---|---|---|
| Age, years | 63±13 | 2,107 |
| Males, % | 62 | 2,107 |
| Duration of diabetes mellitus, years | 9±10 | 1,794 |
| Current smokers, % | 35 | 1,647 |
| Treatment for diabetes mellitus | ||
| Diet only/OHA/insulin, % | 17/56/27 | 2,107 |
| Body mass index | 24.9±4.3 | 2,078 |
| Obesity, % | 44 | 2,078 |
| HbA1c, % | 8.4±2.2 | 1,964 |
| Hypertension, % | 70 | 2,107 |
| Systolic blood pressure, mm Hg | 138±21 | 2,107 |
| Diastolic blood pressure, mm Hg | 80±14 | 2,107 |
| Hypercholesterolemia, % | 61 | 2,102 |
| Total cholesterol, mmol/l | 5.2±1.2 | 1,800 |
| LDL cholesterol, mmol/l | 3.2±1.0 | 1,427 |
| Hypo-HDL-cholesterolemia, % | 13 | 1,598 |
| HDL cholesterol, mmol/1 | 1.5±0.4 | 1,598 |
| Serum creatinine, μmol/l | 86±51 | 2,107 |
| Estimated GFR, ml/min/1.73 m2 | 55±20 | 2,107 |
| GFR stage, % G1/G2/G3a/G3b/G4/G5 | 5/32/34/20/7/2 | 2,107 |
| Albuminuria stage, % A1/A2/A3 | 53/24/23 | 2,027 |
| Hyperuricemia, % | 21 | 2,096 |
| Serum uric acid, μmol/l | 307±89 | 2,096 |
| Anemia, % | 26 | 2,101 |
| Hemoglobin, g/dl | 135±17 | 2,101 |
| Diabetic retinopathy1, % | 40 | 1,588 |
| Diabetic neuropathy, % | 68 | 1,575 |
| Cerebrovascular disease, % | 13 | 2,106 |
| Coronary heart disease, % | 19 | 2,105 |
| Peripheral arterial disease, % | 5 | 2,107 |
OHA = Oral hypoglycemic agents.
Diabetic retinopathy includes simple, preproliferative and proliferative retinopathies.
Table 2.
Prevalence of diabetic micro- and macroangiopathies between the GFR stages
| GFR stage | Gl (105) | G2 (668) | G3a (716) | G3b (411) | G4 (157) | G5 (50) | p |
|---|---|---|---|---|---|---|---|
| Retinopathy1 | 36 | 34 | 33 | 45 | 70 | 85 | <0.001 |
| Nephropathy2 | 46 | 35 | 41 | 53 | 93 | 100 | <0.001 |
| Neuropathy | 64 | 60 | 66 | 75 | 84 | 87 | <0.001 |
| Cerebrovascular disease | 3 | 9 | 14 | 18 | 17 | 26 | <0.001 |
| Coronary heart disease | 9 | 10 | 18 | 29 | 34 | 28 | <0.001 |
| Peripheral artery disease | 5 | 2 | 5 | 8 | 9 | 10 | <0.001 |
Values are given as percentages (values in parentheses represent patient numbers).
Diabetic retinopathy includes simple, preproliferative and proliferative retinopathies.
Diabetic nephropathy includes stages A2 and A3.
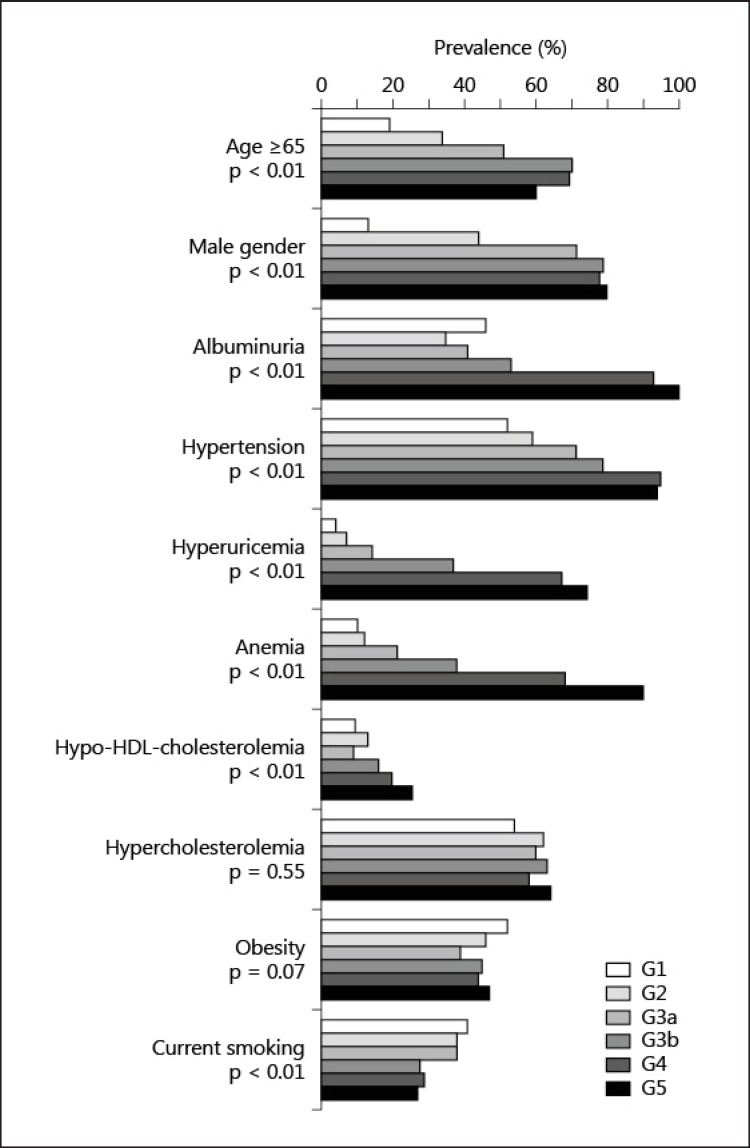
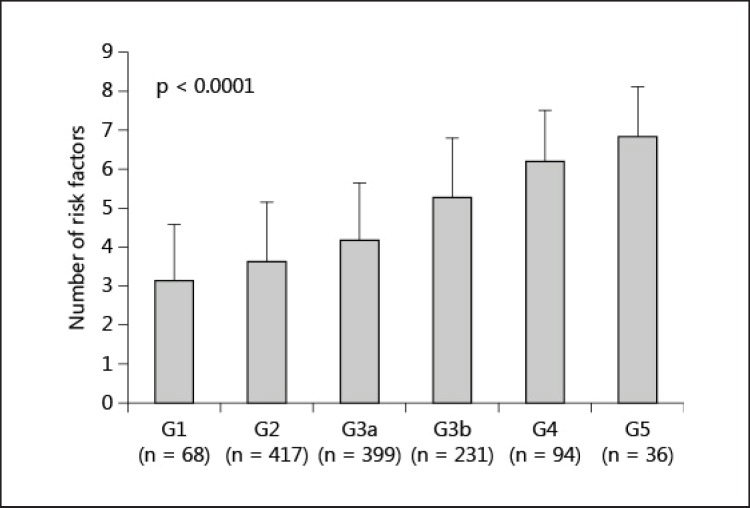
The prevalence of each risk factor for atherosclerosis is shown in figure 1. The prevalence of age ≥65 years, male gender, albuminuria (stages A2 and A3), hypertension, hypo-HDL-cholesterolemia, hyperuricemia and anemia was greater in the patients with later stages of GFR, whereas the prevalence of hypercholesterolemia and obesity did not differ between stages. The prevalence of a current history of smoking was lower in the patients with later stages of GFR. The cumulative numbers of risk factors for atherosclerosis were higher in the patients with later stages of GFR (3.1 ± 1.4, 3.6 ± 1.5, 4.1 ± 1.4, 5.2 ± 1.5, 6.1 ± 1.3 and 6.8 ± 1.3 in G1, G2, G3a, G3b, G4 and G5, respectively) when age ≥65 years, male gender, albuminuria, hypertension, hyperuricemia, anemia, hypo-HDL-cholesterolemia, hypercholesterolemia, obesity and a current history of smoking were selected as the risk factors (fig. 2).
Fig. 1.
The prevalence of risk factors for atherosclerosis among type 2 diabetic patients with different stages of GFR. Albuminuria includes stages A2 and A3. The χ2 test was used to determine the differences in the prevalence of the risk factors among the subjects with different stages of GFR.
Fig. 2.
The cumulative number of risk factors for atherosclerosis among type 2 diabetic patients with different stages of GFR. Age ≥65 years, male gender, albuminuria, hypertension, hyperuricemia, anemia, hypo-HDL-cholesterolemia, hypercholesterolemia, obesity and a current history of smoking were selected as the risk factors. ANOVA was used to compare the numbers of risk factors between the GFR stages.
The levels of HbA1c gradually decreased with the progression of GFR stages (10.0 ± 2.7, 8.9 ± 2.3, 8.2 ± 2.0, 7.9 ± 1.9, 7.7 ± 1.8 and 7.4 ± 1.6% in G1, G2, G3a, G3b, G4 and G5, respectively, p < 0.001).
Discussion
This study showed that risk factors for atherosclerosis, a condition that should be treated intensively in patients with type 2 diabetes mellitus and/or CKD, increased in patients with later stages of GFR and that the prevalence of risk factors differs between GFR stages. It has been established that aging, male gender, obesity, hypertension, hypercholesterolemia, hypo-HDL-cholesterolemia and a history of smoking are important risk factors for the development of cardiovascular events. It has also recently been recognized that albuminuria, anemia and hyperuricemia are associated with atherosclerotic diseases. In the present study, the prevalence of many risk factors was higher in type 2 diabetic patients with later stages of GFR. We have previously reported that micro- and macroangiopathies occur more frequently in type 2 diabetic patients with GFR stages 3-5 than in those with GFR stage 1 or 2, even if albuminuria is not found [15]. The higher prevalence of micro- and macroangiopathies might be associated with the accumulation of other risk factors in the progressive stages of GFR. In the present study, overt proteinuria (10 vs. 4%), hypertension (64% in males and 50% in females vs. 60% in males and 44.6% in females), hypercholesterolemia (48 vs. 22.3% in males and 69 vs.17.7% in females), and obesity (44 vs. 31.4% in males and 49 vs. 22.2% in females) were more frequently found even in patients with GFR stage 1 and 2 compared with the Japanese adult population according to recent statistics from the Ministry of Health, Labour and Welfare [16,17]. Furthermore, twice the number of risk factors was observed in type 2 diabetic patients with GFR stage 4 or later. We must pay attention to the increases in hyperuricemia and anemia in the progressive stages of GFR; however, the prevalence of these risk factors in the earlier stages of GFR does not differ from that seen in the general population [5,6].
Tanaka et al. [18] have recently reported that the prevalence of macrovascular disease was found to be increased from the GFR stage 3 in 1,493 Japanese diabetic CKD patients. In this study, the prevalence of older age, male gender, hypertension, a past (but no current) history of smoking and hyperuricemia increased with the progression of GFR stages, although the prevalence of hyperlipidemia did not change. The levels of HbA1c and hemoglobin decreased in later stages of GFR, similar to the results of our study. Nakagawa et al. [19] showed that the prevalence of dyslipidemia and hypertension increased with the progression of GFR stages in the general population (n = 647), including patients with type 2 diabetes mellitus. The reason for the difference in the prevalence of dyslipidemia observed between the study conducted by Nakagawa et al. [19], the study conducted by Tanaka et al. [18] and our study is unclear; however, relatively small numbers of subjects with GFR stages 4 and 5 (n = 12 and 5, respectively) were included in the study conducted by Nakagawa et al. [19], which might have resulted in a high frequency of dyslipidemia and a prevalence of obesity and smoking in the later stages of GFR. Iseki et al. [20] have demonstrated that the prevalence of hypertension and proteinuria and the frequencies of obesity and smoking are decreased in the progressive stages of GFR in the general population. However, the relationship between dyslipidemia and the GFR stage was not described.
Recently, Dzau et al. [21] have proposed the concept of the cardiorenal continuum. Various mechanisms are assumed to be present in these cardiovascular and kidney diseases, such as reduced NO production, activation of the tissue renin-angiotensin system and inflammation [22]. The association between these mechanisms is considered to be simply expressed as an increased number of risk factors for atherosclerosis in the clinical assessment of type 2 diabetic patients with CKD.
The present study has limitations that should be kept in mind when considering the results. First, our study used a cross-sectional design and was therefore unable to show any causal effects between the GFR stage and the prevalence of the risk factors for atherosclerosis. This study only demonstrated an association between the GFR stage and the prevalence of risk factors in a large number of subjects. Second, we did not examine other risk factors, such as family/previous history of vascular events or serum triglyceride concentrations. Sone et al. [23] showed that the serum triglyceride level is an important risk factor for coronary heart disease using a longstanding observational study of a larger number of Japanese patients with type 2 diabetes mellitus.
In conclusion, it is noteworthy that risk factors for atherosclerosis differ between the stages of GFR. In this study, the total number for atherosclerosis in patients with type 2 diabetes mellitus increased with the progression of renal dysfunction. It is important to pay attention to hyperuricemia and anemia, which are newly recognized risk factors for atherosclerosis, in addition to hypertension, albuminuria and hypo-HDL-cholesterolemia. A study investigating the effects of comprehensive interventions for these risk factors is expected in the future.
Disclosure Statement
None declared.
Acknowledgements
The authors thank Ms. Tomoko Koyanagi of the secretarial section of the Edogawa Hospital for her valuable help in the data collection.
References
- 1.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg DS, Wexler D, Iaina A. The importance of anemia and its correction in the management of severe congestive heart failure. Eur J Heart Fail. 2002;4:681–686. doi: 10.1016/s1388-9842(02)00115-0. [DOI] [PubMed] [Google Scholar]
- 4.Madero M, Sarnak MJ, Wang X, et al. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53:796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito H, Takeuchi Y, Ishida H, et al. Mild anemia is frequent and associated with micro- and macroangiopathies in patients with type 2 diabetes mellitus. J Diabetes Invest. 2010;1:273–278. doi: 10.1111/j.2040-1124.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito H, Abe M, Mifune M, et al. Hyperuricemia is independently associated with coronary heart disease and renal dysfunction in patients with type 2 diabetes mellitus. PLoS One. 2011;6:e27817. doi: 10.1371/journal.pone.0027817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito H, Komatsu Y, Mifune M, et al. The estimated GFR, but not the stage of diabetic nephropathy graded by the urinary albumin excretion, is associated with the carotid intima-media thickness in patients with type 2 diabetes mellitus: a cross-sectional study. Cardiovasc Diabetol. 2010;9:18. doi: 10.1186/1475-2840-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito H, Oshikiri K, Mifune M, et al. The usefulness of the revised classification for chronic kidney disease by the KDIGO for determining the frequency of diabetic micro- and macroangiopathies in patients with type 2 diabetes mellitus. J Diabetes Complications. 2012;26:286–290. doi: 10.1016/j.jdiacomp.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Japanese Society of Gout and Nucleic Acid Metabolism . Guideline for the Management of Hyperuricemia and Gout. ed 2. Tokyo: Medical Review; 2010. [Google Scholar]
- 10.Locatelli F, Covic A, Eckardt KU, et al. Anaemia management in patients with chronic kidney disease. A position statement by the Anaemia Working Group of European Renal Best Practice (ERBP) Nephrol Dial Transplant. 2009;24:348–354. doi: 10.1093/ndt/gfn653. [DOI] [PubMed] [Google Scholar]
- 11.KDOQI; National Kidney Foundation KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis. 2006;47(5 suppl 3):S11–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 15.Ito H, Takeuchi Y, Ishida H, et al. High frequencies of diabetic micro- and macroangiopathies in patients with type 2 diabetes mellitus with decreased estimated glomerular filtration rate and normoalbuminuria. Nephrol Dial Transplant. 2010;25:1161–1167. doi: 10.1093/ndt/gfp579. [DOI] [PubMed] [Google Scholar]
- 16.http://www.mhlw.go.jp/stf/shingi/2r98520000023zzr-att/2r9852000002402d.pdf
- 17.http://www.mhlw.go.jp/stf/houdou/2r98520000020qbb-att/2r98520000021c1n.pdf
- 18.Tanaka K, Hara S, Kushiyama A, et al. Risk of macrovascular disease stratified by stage of chronic kidney disease in type 2 diabetic patients: critical level of the estimated glomerular filtration rate and the significance of hyperuricemia. Clin Exp Nephrol. 2011;15:391–397. doi: 10.1007/s10157-011-0420-6. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa N, Takahashi F, Chinda J, et al. A newly estimated glomerular filtration rate is independently associated with arterial stiffness in Japanese patients. Hypertens Res. 2008;31:193–201. doi: 10.1291/hypres.31.193. [DOI] [PubMed] [Google Scholar]
- 20.Iseki K, Asahi K, Moriyama T, et al. Risk factor profiles based on estimated glomerular filtration rate and dipstick proteinuria among participants of the Specific Health Check and Guidance System in Japan 2008. Clin Exp Nephrol. 2012;16:244–249. doi: 10.1007/s10157-011-0551-9. [DOI] [PubMed] [Google Scholar]
- 21.Dzau VJ, Antman EM, Black HR, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes. II. Clinical trial evidence (acute coronary syndromes through renal disease) and future directions. Circulation. 2006;114:2871–2891. doi: 10.1161/CIRCULATIONAHA.106.655761. [DOI] [PubMed] [Google Scholar]
- 22.Dzau VJ, Antman EM, Black HR, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes. I. Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease) Circulation. 2006;114:2850–2870. doi: 10.1161/CIRCULATIONAHA.106.655688. [DOI] [PubMed] [Google Scholar]
- 23.Sone H, Tanaka S, Tanaka S, et al. Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS) J Clin Endocrinol Metab. 2011;96:3448–3456. doi: 10.1210/jc.2011-0622. [DOI] [PubMed] [Google Scholar]