Abstract
This systematic study clarified a few interfacial aspects of cancer cell phenotypes on polydimethylsiloxane (PDMS) substrates and indicated that the cell phenotypic equilibrium greatly responds to cell-to-surface interactions. We demonstrated that coatings of fibronectin, bovine serum albumin (BSA), or collagen with or without oxygen-plasma treatments of the PDMS surfaces dramatically impacted the phenotypic equilibrium of breast cancer stem cells, while the variations of the PDMS elastic stiffness had much less such effects. Our results showed that the surface coatings of collagen and fibronectin on PDMS maintained breast cancer cell phenotypes to be nearly identical to the cultures on commercial polystyrene Petri dishes. The surface coating of BSA provided a weak cell-substrate adhesion that stimulated the increase in stem-cell-like subpopulation. Our observations may potentially guide surface modification approaches to obtain specific cell phenotypes.
Micro- and nano-technology based bio-analytical systems (microdevices) have continuously advanced and revolutionized the biomedical research field, because of their many advantages including small structure dimensions and high sample handling throughput1,2,3,4,5. Specifically, polydimethylsiloxane (PDMS) has been increasingly employed for the fabrication of these microdevices used as cell culture platforms. The PDMS substrate possesses a universal appeal over other materials owing to its physical attributes, e.g. simple fabrication, optical transparency, tunable elasticity, gas permeability, biological inertness, and inexpensiveness6,7. Additionally, neither PDMS nor its degraded by-products have harmful effects on living species8. Moreover, PDMS can easily be modified and finely tuned for specific molecular interactions, possessing a highly hydrophobic surface in its native state that can be rendered hydrophilic via oxygen plasma treatment, UV-ozone radiation, self-assembled monolayer coating, or polymer/peptide grafting techniques. All of these strengths make PDMS a robust platform for “cell on a chip” technology, particularly for drug screening/discovery on microfluidic chips or microwell plates5,9,10.
It is important, however, to focus on the potential complications that may arise when using PDMS substrates for these applications. One common concern, often overlooked, is the physicochemical properties of PDMS surfaces may affect proper cell functions. Disparities in the fabrication conditions (such as curing temperature and time), the ratios of base to curing reagent (ranging from 5:1 to 100:1), the oxidation states of the surface (hydrophilicity and hydrophobicity), and surface modifications (active or passive) may greatly influence cell culture results, explicitly for each cell types. For instance, Whitesides and his coworkers demonstrated that the different compositions of PDMS surfaces have altered cell attachment and growth rates for primary human umbilical artery endothelial cells and transformed 3T3 fibroblasts, osteoblast-like MC3T3-E1 cells, and HeLa (transformed epithelial) cells11. Toworfe and his coworkers reported that fibronectin-coated PDMS could enhance and upgrade MC3T3-E1 cellular functions, particularly on its attachment of and spreading on the PDMS surfaces12. Many other studies have also proven that PDMS surfaces, as well as cellular microenvironment, could affect and regulate embryonic and functional stem cell fates13,14,15. The PDMS topography and stiffness also have micro-environmental effects on the differentiation of human epidermal stem cells, mesenchymal stem cells, and others13,14,15. Very recently, a study showed that extracellular-matrix tethering can influence the way stem cells signal feedback to the surrounding cells for collective determination of cell-fate16. Surface properties are known to affect stem cell attachment, proliferation, and differentiation, but few studies have characterized phenotypic equilibrium of cancer cells on PDMS, which becomes an important aspect as the material is widely used in cancer research and medical applications.
Mammalian cells must be attached onto either solid substrates or scaffolds in order to proliferate and function17,18. In the animal body, tumor cells are supported by specific extracellular matrix. The growth, metastasis, migration, chemotherapy survival, and other functions of carcinoma cells are regulated by a combination of surrounding extracellular matrix and mechanical cues. When cancer cells are cultured in vitro, the adequate biochemical and biomechanical support must be provided within the artificial cell culture environment. In turn, the behavior and states of cells are related to physico-chemical properties of the environment. In particular, the cytoadherence, elasticity and topology of surrounding environment may affect cancer cell states. For example, cancer stem cell (CSC) properties of breast cancer cells can be enhanced in 3D collagen scaffolds19. So far, it has been difficult to predict how cancer cells respond to specific surface properties in cell attachment and states. They may be affected directly or indirectly through the elastic stiffness of substrates (e.g. on polyacrylamide hydrogel surface), or via a secondary adsorbed molecule on the substrate (such as extracellular matrix protein). Many adsorbed protein, like fibronectin or collagen, have been identified as affecting cellular attachment. Recent reports have studied the stem-cell differentiation of stem cells cultured on substrates with varying elastic modulus and different pre-adsorbed proteins16,20. However, the examination of the effect of surface properties on cancer cell states (such as the phenotypic equilibrium of cells among stem-like, luminal, and basal states) is complicated and remains unclear.
Herein, we present a set of work to understand how the surface engineering and physicochemical properties of PDMS alter breast cancer cell phenotypes, to guide future on-chip cancer cell study. We have examined the attachment of breast cancer cells and the subsequent phenotypic equilibrium in cell-state proportions on PDMS substrates with varying stiffness and surface modifications. We have cultured breast cancer cell lines SUM159 and MDA-MB-468 on PDMS with different elastic properties and surface coatings of fibronectin, collagen, or BSA, all of which are common surface-modifying molecules in “cell on a chip” research. Additionally, we have monitored the self-renewal and maintenance of CSC population on varying PDMS substrates. This work thus provides a potential approach to promote and maintain CSCs population on PDMS-based microdevices, and thereby facilitate on-chip CSC research21,22,23.
Results
Phenotyping cells by flow cytometry
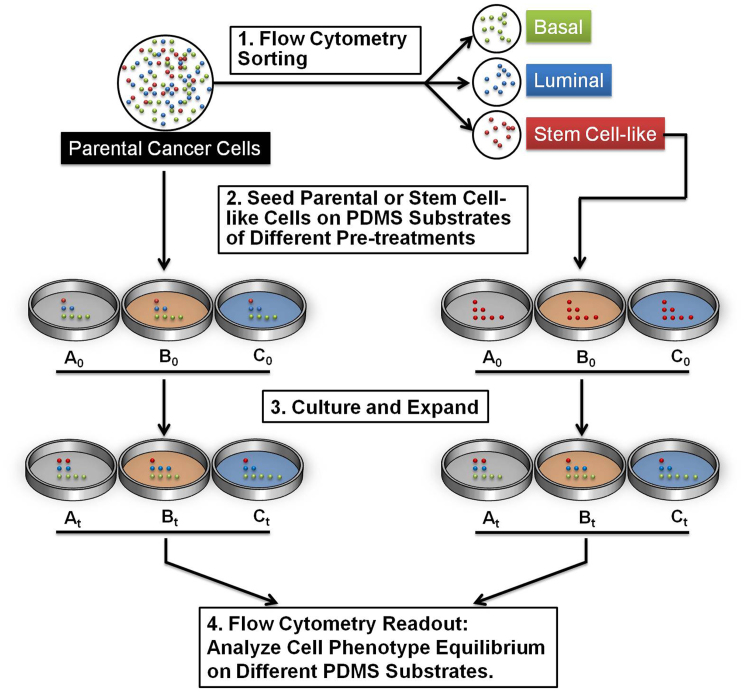
Taking into consideration that inter-conversions between cell-states occur, we have designed our study to carefully observe the changes in cell states under various environmental conditions commonly used in microdevices, as shown in Figure 1. We first characterized the cell-states equilibrium of SUM159 on PDMS substrate. Cell culture plates were coated with PDMS and treated with aqueous solutions of fibronectin, collagen, or BSA, proteins often used in modifying PDMS substrates5,11,16. Then SUM159 parental cells were seeded on these prepared cell culture plates for growth and expansion. Fractions of cell-states (basal, luminal, and stem-cell-like) were analyzed by flow cytometry. The flow cytometry gating strategy for isolating and determining stem-cell-like, basal, and luminal cancer cells is demonstrated in Figure 2 a. In another experiment, the SUM159 CSC populations and basal cells fractions were separately collected by flow cytometry, seeded, and allowed to expand on polystyrene and PDMS substrates. These resulting cell cultures, after a 2-week period, were also fractionated by flow cytometry to further determine the extent of differentiation of these CSCs on various surfaces.
Figure 1. Two examples of the experimental flow for the study of phenotypic equilibrium in cell-state proportions cultured on PDMS substrates.
The left experiment demonstrates how the parental cells were directly cultured onto 3 separate PDMS substrates (A0, B0, and C0), which were then allowed to be cultured and expanded over a 2-day period (At, Bt, and Ct) before flow cytometry analysis. Alternatvely, the right experiment demonstrates the separation of the parental cells into its subpopulations. The stem cells were then specifically isolated, cultured, and analyzed as in the left experiment.
Figure 2.
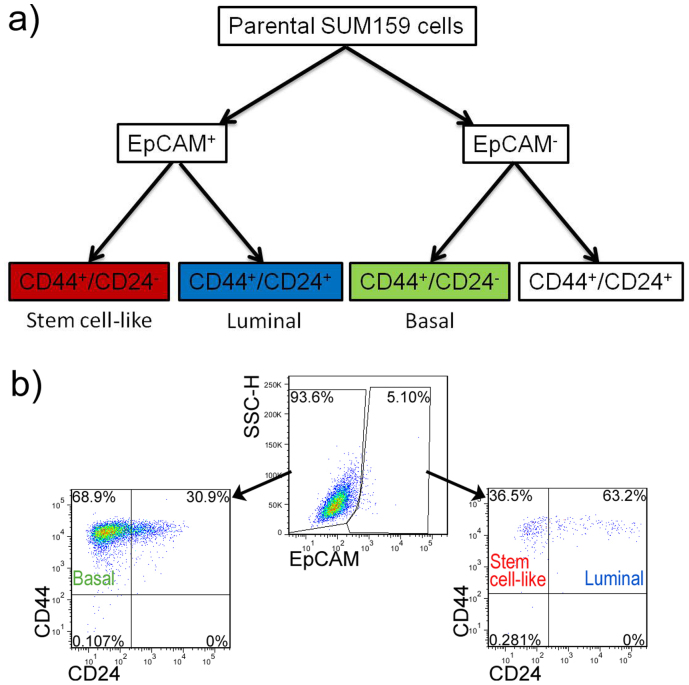
(a) Flow cytometry gating strategy of sorting and analyzing cell states of SUM159 cells. (b) Flow cytometry profiles of EpCAM, CD44, and CD24 expressions in human breast cell lines SUM159 cultured on polystyrene petri dish.
To sort and determine the cell-states of SUM159, we used fluorescence-activated cell sorting (FACS) to analyze and identify the cancer cell states. Here, breast cancer cells can be divided into three types of phenotypic states (subtypes). Three mammary epithelial cell-states were defined by cell-surface biomarkers: stem-cell-like (CD44+CD24−/lowEpCAM+), basal (CD44+CD24−EpCAM−), and luminal (CD44+CD24+EpCAM+)24,25. The dot plots from FACS analysis show the resulting three cell fractions of parental SUM159 cell line cultured on polystyrene petri dish (Figure 2 b). First, cell population was divided into two sub-populations (EpCAM positive and negative) and then was distinguished by biomarkers of CD44 and CD24. Using this approach, SUM159 was found to exhibit predominantly basal differentiation (65%) with minor populations of stem-cell-like and luminal cells (collectively at 5%).
Cell-states equilibrium on planar PDMS and polystyrene substrates
Differences in curing time and temperature as well as the cross-linking degree between the curing agent and the base solution will affect the resulting elasticity of the PDMS substrates, which can influence cell culture attachment and growth26,27. Generally speaking, stiffer PDMS substrates can be achieved with either a higher cross-linking degree, a higher temperature during the curing step, or a longer curing time11. To test the influence of stiffness on breast cancer cell-states, we cultured cells on substrates with a base to curing reagent ratios in the range of 10:1 to 100:1. The Young's modulus of (10:1) and (100:1) PDMS were approximately 2 MPa and 0.1 KPa, respectively11,16. This is orders of magnitude lower than polystyrene substrate, with a Young's modulus of 3–3.5 GPa. To further evaluate surface effects on cell morphology during cultures, the PDMS substrates were modified with fibronectin, collagen, or BSA before cell culture seeding to a density of 2 × 104 cells/cm2 as described in detail in the Experimental Section.
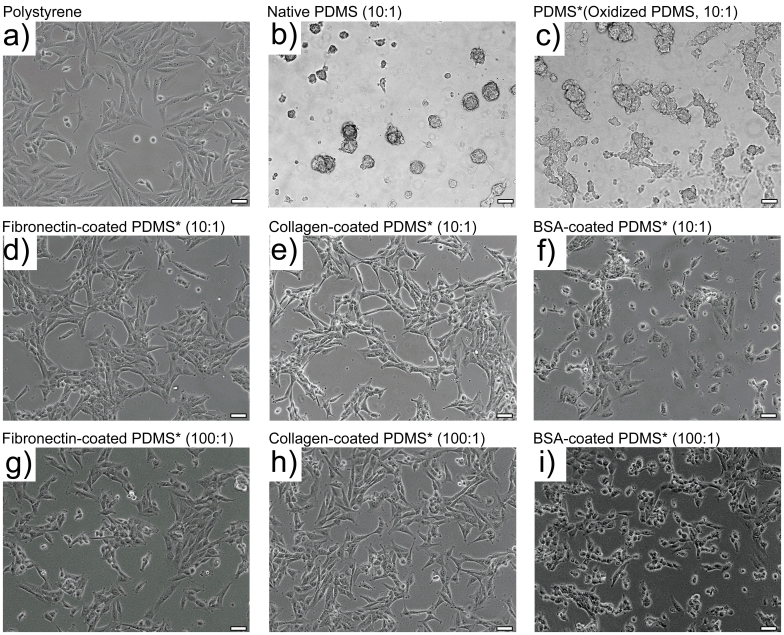
As shown in Figure 3, results indicated that SUM159 cells propagated as a monolayer, spreading on collagen- or fibronectin-coated PDMS substrates as well as on polystyrene substrate. On both of these substrates, SUM159 cells exhibited spindle-like and in general healthy physical appearances, reminiscent of the basal-type classification. Morphologies of cell colonies on softer (100:1) or stiffer (10:1) PDMS were nearly identical (Figure 3 d, e, g and h). In contrast, all cells tended to propagate with non-adherent spherical (cluster) morphology on native hydrophobic PDMS substrates. On naked PDMS substrates that were rendered hydrophilic by plasma-oxidization, only a limited number of cells (approximately 1%) attached and exhibited a well spread morphology; whereas, a majority of the cell population remained suspended in the media and formed mammary spheres instead. This observation was typically resulted from low-attachment cell culture conditions. Without any surface modifications, either hydrophilic or hydrophobic PDMS substrate was unfavorable for the attachment of breast cancer cells. After BSA was incubated to coat plasma-treated PDMS (Figure 3f), the attachment of cells was greatly improved. On the BSA-coated oxidized PDMS, a few cells were observed to aggregate together, growing in small clusters; whereas, most cells (approximately 95%) remained rounded and adherent on the substrates. The resulting morphologies of these cells on the BSA-coated, oxidized PDMS with different stiffnesses were observed to be very similar (Figure 3 f and i).
Figure 3. Morphologies of SUM159 colonies on polystyrene and PDMS substrates.
Tumorigenic breast cancer cell line propagated over 2 days on (a) commercial polystyrene petri dish; (b) native (10:1) PDMS; (c–f) plasma-oxidized (10:1) PDMS that were either unmodified (c), fibronectin coated (d), collagen coated (e), or BSA coated (f); (g–i) plasma-oxidized (100:1) PDMS that were either fibronectin coated (g), collagen coated (h), or BSA coated (i). Scale bar: 20 μm. PDMS substrates that were plasma-oxidized are marked with “*” in the images. All ratios are of base to curing agent.
Since we observed different cellular morphologies on varying PDMS and polystyrene substrates, we next determined the proportions of the individual cell-states by flow cytometry. The SUM159 cells cultured on polystyrene substrates contained 1.9% of stem-cell-like, 71.1% of basal, and 2.2% of luminal populations. As shown in Table 1 and Figure S1–6, stem-cell-like (CD44+CD24−/lowEpCAM+) subpopulation increased on BSA coated PDMS substrates but did not show obvious changes on collagen or fibronectin coated PDMS substrates. SUM159 cells exhibited similar subpopulations regardless of the differences in the stiffness of the culturing substrates. This result indicates that plasma oxidized PDMS with fibronectin or collagen coatings have limited effects on cell-states of SUM159 despite their differences in substrate stiffness. However, cell phenotypic states can be altered on BSA coated PDMS substrates.
Table 1. Phenotypic equilibrium of SUM159 cell-states on polystyrene and PDMS substrates.
| Substrate | Surface coating | Stem-like | Basal | Luminal |
|---|---|---|---|---|
| Polystyrene | None | 1.905 ± 0.710% | 71.056 ± 6.084% | 2.163 ± 0.706% |
| PDMS (10:1) | BSA | 5.719 ± 0.762% | 77.058 ± 9.908% | 3.030 ± 0.939% |
| PDMS (100:1) | BSA | 5.796 ± 1.186% | 76.927 ± 8.079% | 2.520 ± 0.929% |
| PDMS (10:1) | Collagen | 1.738 ± 0.513% | 80.802 ± 5.802% | 2.080 ± 0.788% |
| PDMS (100:1) | Collagen | 2.336 ± 0.344% | 79.377 ± 1.514% | 1.063 ± 0.460% |
| PDMS (10:1) | Fibronectin | 2.469 ± 0.344% | 79.687 ± 5.035% | 1.296 ± 0.673% |
| PDMS (100:1) | Fibronectin | 2.407 ± 0.555% | 83.396 ± 7.991% | 0.927 ± 0.406% |
Many breast cancer cell lines were developed from various sources, including pleural effusions (MDA-MB-468, MDA-MB-231, and MCF7) and primary breast cancers (SUM159). However, only a few cell lines such as SUM159, MDA-MB-468, MDA-MB-231, etc. contains relatively higher CD44+/CD24−/EpCAM+ subpopulation (1–2%). SUM159 with CD44+/CD24−/EpCAM+ has been well characterized with cancer stem cell like features including the abilities to self-renew, constitute the parental cell line, survive chemotherapy, and form tumor with as few as 100 cells28. To further validate the findings in this study, we also cultured another breast cancer cell line MDA-MB-468 on those PDMS surfaces and analyze their states by FACS (Table 2 and Figure S7–10). The identical results showed the increasement of the cancer stem-like subpopulation on BSA-coated PDMS substrates but no changes on collagen or fibronectin coated PDMS substrates.
Table 2. Phenotypic equilibrium of MDA-MB-468 cell-states on polystyrene and PDMS substrates.
| Substrate | Surface coating | Stem-like | Basal | Luminal |
|---|---|---|---|---|
| Polystyrene | None | 2.234 ± 0.285% | 0 | 96.436 ± 0.280% |
| PDMS (10:1) | BSA | 7.349 ± 0.595% | 0.175 ± 0.022% | 81.279 ± 1.217% |
| PDMS (10:1) | Collagen | 2.430 ± 0.219% | 0.008 ± 0.013% | 96.842 ± 0.232% |
| PDMS (10:1) | Fibronectin | 2.705 ± 0.097% | 2.479 ± 0.110% | 94.229 ± 0.116% |
Cell-states inter-conversion on planar PDMS and polystyrene
The ability of self-renewal and differentiation into heterogeneous cell types are two essential characteristics of stem-cells inherited by cancer stem-cells29. Since we observed that the coating of BSA can alter the cell states on PDMS substrates, we next assessed the differentiation abilities of stem-like cells on BSA-coated PDMS. SUM159 cells were sorted on the basis of CD44, CD24 and EpCAM (see the FACS gating in Figure 2), re-seeded, and allowed to expand. By isolating certain sub-populations in a given cell state and allowing them to expand on the polystyrene and BSA-coated PDMS substrates, we can monitor the influences of adsorbed BSA on the reconstitution of parental cells from stem-cell-like and basal subpopulations.
Using FACS, we obtained stem-cell-like and basal cells from the SUM159 line. After sorting and initial seeding on substrates, the two weeks expanded cultures from stem-like cells were analyzed for differentiation (Table 3). The flow cytometry result demonstrated that the cultured stem-like cells expanded back to the cell-state equilibrium that was identical to the unsorted parental cell line on the corresponding substrates, with high efficiency both on polystyrene and BSA-coated oxidized PDMS substrates.
Table 3. Two weeks reconstruction of parental cells of stem-like cells (differentiation) on polystyrene and PDMS substrates with a BSA coating.
| Substrate | Surface coating | Stem-like | Basal | Luminal |
|---|---|---|---|---|
| Polystyrene | None | 2.459 ± 0.4885% | 71.883 ± 5.537% | 1.381 ± 0.512% |
| PDMS (10:1) | BSA | 6.079 ± 1.158% | 77.627 ± 5.792% | 2.165 ± 0.848% |
The reconstitution of parental cell line from basal cells, also known as a dedifferentiation process, was evaluated on polystyrene and BSA-coated oxidized PDMS substrates. Generally, the efficiency of reconstitution from basal cells was lower than the differentiation of stem-cell-like phenotype (Table 4). The dedifferentiation of basal cells on BSA-coated PDMS substrate showed higher efficiency than that of cells on polystyrene. In other words, BSA-coated PDMS substrate is helpful for promoting and maintaining CSC populations, which is consistent with the above results. Our results further support evidence that BSA-modified microdevices greatly enhance CSC population during cancer cell differentiation and propagation, making this substrate ideal for on-chip CSC research.
Table 4. Two weeks reconstruction of parental cells from basal cells (dedifferentiation) on polystyrene and PDMS substrates with a BSA coating.
| Substrate | Surface coating | Stem-like | Basal | Luminal |
|---|---|---|---|---|
| Polystyrene | None | 1.626 ± 0.161% | 92.985 ± 1.721% | 0.315 ± 0.094% |
| PDMS (10:1) | BSA | 4.145 ± 0.439% | 81.972 ± 6.655% | 1.881 ± 0.422% |
Topography of PDMS surfaces
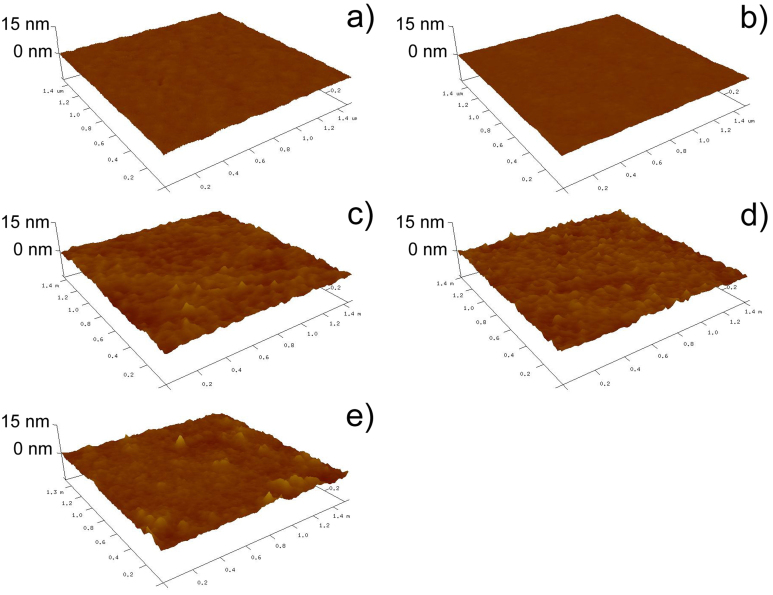
To assess the possible topological effects on cancer cell states, the topology and roughness of modified PDMS surfaces were characterized by Atomic force microscopy (AFM), as shown in Figure 4. The results showed that both native PDMS and plasma treated PDMS substrates have very smooth surfaces (Figure 4 a and b). As shown in Figure 4 c–e, the AFM images demonstrated an increase in surface roughness on fibronectin (Ra = 0.54 nm), collagen (Ra = 0.58 nm) or BSA-coated surfaces (Ra = 0.74 nm). It is likely that the increase in surface roughness was caused by the adsorption of proteins (fibronectin/collagen/BSA). Despite slight differences among the modifications, all substrates were relatively uniform, resulting in favorable surfaces for cancer cell culture. Therefore, we could exclude the possible effect of surface topology from our discussions. However, the maintenance of CSC population on BSA-coated and oxidized PDMS could have resulted from other aspects that are worth further explorations.
Figure 4.
(a) Atomic force microscopy images of PDMS surface: (a) native; (b) plasma-oxidized; (c) plasma-oxidized and fibronectin-coated; (d) plasma-oxidized and collagen-coated; (e) plasma-oxidized and BSA-coated.
Cell adherence on BSA-coated oxidized PDMS
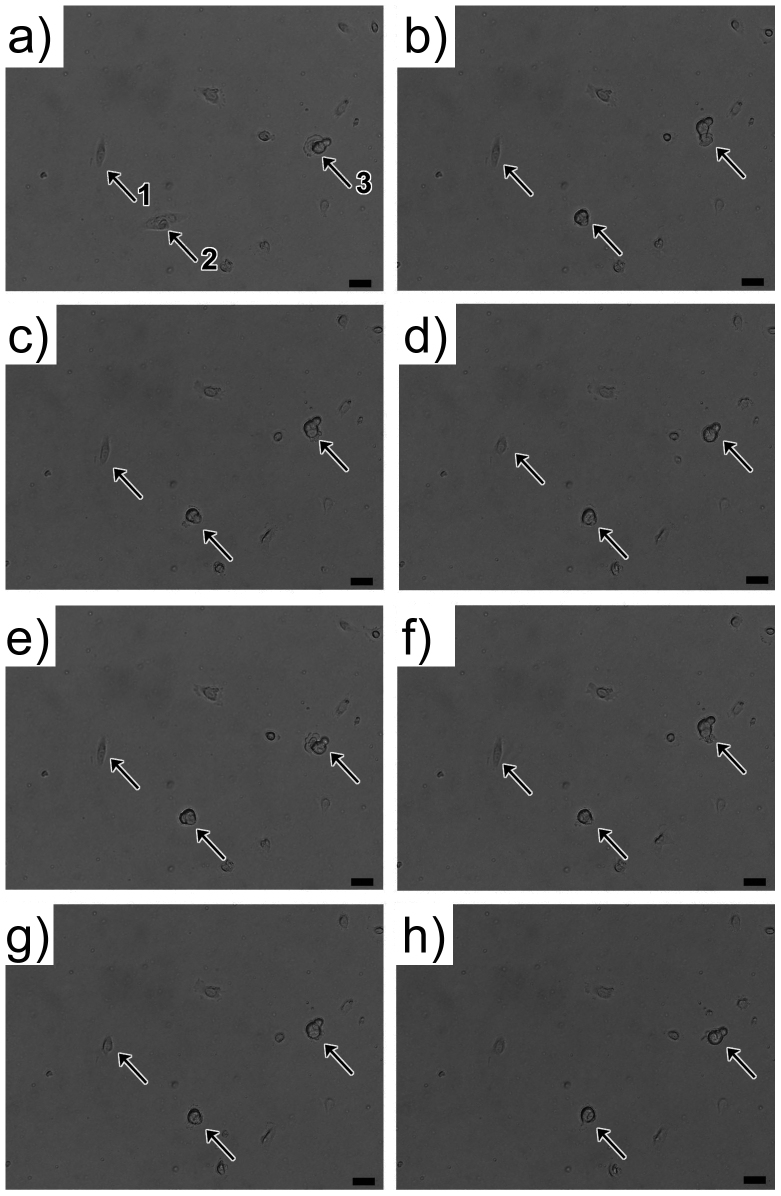
Judging from the morphologies of cells cultured on BSA-coated oxidized PDMS substrates (Figure 3 f and i), we suspected that the different phenotypic equilibrium resulted from the attachment of cancer cells. Though cells can still adhere to BSA-coated oxidized PDMS substrates, they did not show adherence force as strong as that on PS substrate. We observed the cell behaviors on BSA-coated oxidized PDMS substrate in real-time. The supplemental time-lapse video S1 recorded the behaviors of SUM159 cells on BSA-modified PDMS substrates. Figure 5 shows video frames from the supplemental video S1. On the BSA-coated plasma-oxidized PDMS substrate, SUM159 cells could adhere to the surfaces but exhibited a round shape rather than common spindle-like epithelial cell morphology. Even after 12 hours after seeding, many cells that were adhered to the surfaces maintained the round morphology. Due to the poor cell-substrate interaction, cells extended and retracted frequently seeking for a favorable site to spread. The two cells, indicated by arrow 1 and 2 in Figure 5, exhibited a temporal well-spread morphology, but were also unstable on the substrate; these cells tended to revert back to a rounded shape by membrane-cytoskeletal retraction. Arrow 3 indicates another cell that frequently extended its pseudopodia for movement across the substrate. The formation of pseudopodial protrusion and related invadopodia has been previously correlated with cancer cell migration and invasion30. The activity of pseudopodia is also a characteristic of mesenchymal-like cancer cell which is a related concept of CSC31.
Figure 5. Cell movement on BSA-coated plasma oxidized PDMS substrate, 12 hours after cell seeding.
Arrows indicate three individual cells that migrate and change shapes between round and elongated morphology. Cells extended and retracted frequently seeking for a favorable site to spread. For example, arrow 3 indicates a cell that frequently extended its pseudopodia for movement across the substrate. The formation and activity of pseudopodia are characteristics of mesenchymal-like cancer cell. Scale bar: 20 μm.
Yet, cell colonies derived on PDMS with BSA coating exhibit a mixed morphology, which is entirely different from cell colonies derived on polystyrene and PDMS with collagen or fibronectin coatings (Figure 3). The differences in morphology of these cell colonies suggest that the cell states may be affected. The flow cytometry analysis showed that stem cell-like subpopulation significantly increased for both softer and stiffer PDMS substrates. These results should prompt researchers to take caution in choosing BSA as a coating for on-chip cell cultures. In order to achieve results similar to that obtained on conventional petri dishes, it is better to avoid using BSA as a sole surface-modifying molecule. Extracellular matrix proteins such as collagen and fibronectin would serve as better alternatives. On the other hand, if the scientific purpose is to probe various behaviors of CSC, then the BSA-coated PDMS substrate can provide a better environment for promoting and maintaining CSC populations on chip.
Western blot analysis of CSC-related proteins
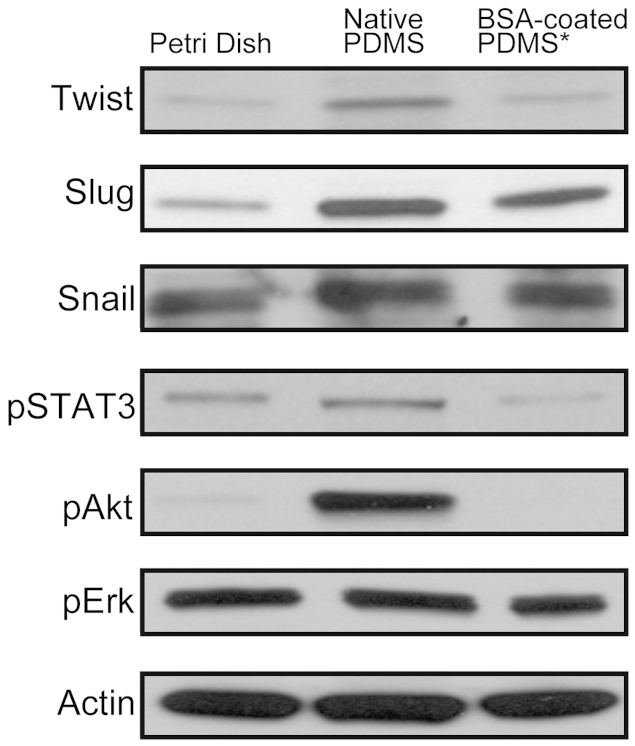
To better understand how BSA-coated PDMS substrates give rise to CSC population, we evaluated the expression level of some tumorigenic proteins in cells cultured on BSA-coated PDMS, native PDMS, and polystyrene substrates. We tested the expression of six proteins (Twist, Slug, Snail, pSTAT3, pAkt, and pErk) and actin as a protein reference in breast cancer cells cultured on native PDMS and BSA-coated PDMS substrates (Figure 6 and Figure S11). Breast cancer stem cells have been correlated with epithelial-mesenchymal transition (EMT), where the expressed proteins Twist, Slug, and Snail are known as EMT-deriving transcription factors32,33. We also took into consideration of Stat3 protein activation, previously proven to be critical for the maintenance of the CSCs34. In our experiments, the cells cultured on native PDMS showed the highest expression of Twist proteins. Native PDMS and BSA-coated PDMS both induced expressions of Slug proteins, with a higher Slug induction by the native PDMS surface. The expression of Snail proteins was not significantly changed by either native PDMS or BSA-coated PDMS. Compared to the control sample on polystyrene Petri dish, pSTAT3 phosphorylation was induced by native PDMS, while inhibited by BSA-coated PDMS. Also, we observed a significant increase in activation of Akt (Akt308) in cells cultured on the native PDMS but inhibited in cells cultured on BSA-coated PDMS. It is important to note that there were no differences in the phosphorylation of Erk ½. Thus, these results suggest the underlying mechanism of the increased CSC population cultured on BSA-coated PDMS substrates compared to other modifications studied here. PDMS substrates may promote CSC population by the activations of EMT, pSTAT3 expression, and Akt signaling pathway.
Figure 6. Western blot analysis for active Twist, Slug, Snail, pSTAT3, pAkt, and pErk.
Actin was used as loading control. Samples derive from the same experiment and gels/blots were processed in parallel. Cropped blots were used here and uncropped images of blots are shown in Supp Figure S11.
Discussion
In the domain of microfluidic cell biology research, PDMS is predominantly used as the device materials. Depending on the cell states, the native, hydrophobic PDMS or hydrophilic, plasma-oxidized PDMS can poorly affect cell attachment or lower colonization efficiency unless pre-adsorbed with extracellular matrix molecules. Thus, the energetic alterations and protein coating treatments are the most common methods for cell adhering, spreading, and proliferating on PDMS-based microdevices. Here, we demonstrated that the surface modification of PDMS impacts cell-substrate interactions and cell states of cancer cells. Prior to the utility in cell biological research, PDMS substrates are commonly plasma-oxidized for surface wetness or pre-adsorbed by fibronectin, collagen, BSA, or other soluble serum proteins to better mimic the natural cellular environment5,35. Adsorption properties of proteins on hydrophobic and hydrophilic surfaces and subsequent cell attachment have been previously studied36,37. In PDMS-based “cell on a chip” technology, BSA and extracellular matrix proteins, such as fibronectin and collagen, are very common surface-modifying agents used to create the appropriate environment for cell cultures5,11,16. Previous studies showed that fibronectin promotes adhesion at cell boundaries whereas collagen enhances extracelluar matrix-cell contact38. Our studies focus on the phenotypic equilibrium of breast cancer cell line SUM159 which is phenotypically heterogeneous. We discovered that both of the hydrophilic, plasma-oxidized surfaces and the adsorption of surface-modifying proteins are more optimal for the subsequent bonding of PDMS to glass slides than the natural hydrophobic state of PDMS substrates. The adsorbed proteins are known to mediate the specific interactions of cell-substrates via cell integrin receptors39.
Here, we tested two types of PDMS with different stiffness and three types of pre-adsorbed proteins for PDMS surface modification. Those proteins include BSA, fibronectin, and collagen. We observed that, in general, the stiffness of PDMS did not significantly affect the cell phenotypic equilibrium. All three surface modifying proteins were biocompatible for cancer cell culture, however, were differently favorable depending on differential research purposes. The flow cytometry results showed that BSA was a favorite coating for the purpose of CSC research, while fibronectin and collagen were preferred by general-purpose on-chip cell culture. We further observed the cell growth on PDMS substrates and analyzed the expression level of tumorigenic proteins in cells cultured on them. The activations of EMT, pSTAT3 expression, and Akt signaling pathway, were proposed to be responsible for the promotion of CSC population on PDMS substrates. Our studies involved the characterization of substrate properties, the maintenance and differentiation of CSC population, and the cell-substrate interaction. The surface coating of collagen and fibronectin on PDMS maintains cancer cell phenotypes nearly identical to the cultures on commercial polystyrene petri dishes. The surface coating of BSA may provide a poor-adhesion cell-substrate interaction that stimulates the increase of stem-cell-like subpopulation. Thus, depending on different research aim, appropriate coating method could be chosen for better experimental support and performance.
In summary, PDMS is a widely used material for cell assay on a chip because it can be easily fabricated to have different dimensions, shapes, and elasticity. Our study clarified certain interfacial aspects of cell states on PDMS substrates and indicated that the cell phenotypic equilibrium possibly respond to cell-to-surface interaction. Overall, PDMS is a suitable substrate for culturing breast cancer cells. Nevertheless, the appropriate surface modification rather than elastic stiffness should be taken into account for maintaining proper cell states on PDMS substrate. Proper pretreatment is critical for on-chip cell culture to recapitulate the same results obtained off-chip.
Methods
Materials and cell lines
Polydimethylsiloxane (GE RTV615), lyophilized bovine serum albumin (BSA), phosphate buffered saline (PBS), trypsin, and penicillin-streptomycin were purchased from Fisher Scientific. Ham's F-12 medium, insulin, hydrocortisone, fetal bovine serum and phosphate-buffered saline were purchased from Life Technologies, Inc. CD326 (EpCAM)-FITC and CD24-PE antibody was purchased from Miltenyi Biotec Inc. CD44-APC antibodies were purchased from BD Bioscience Inc. Bovine collagen type I was purchased from Advanced Biomatrix Inc. The SUM159 cell line was purchased from Asterand PLC. SUM159 cells were grown in Ham's F-12 medium supplemented with 5% fetal bovine serum (FBS), 1% penicillin-streptomycin, 5 μg/mL insulin, and 1 μg/mL hydrocortisone in an incubator with a humidified atmosphere containing 5% CO2 at 37°C. MBA-MD-468 cells were cultured with DMEM medium supplemented with 5% FBS under the same condition. Cells were seeded at density of 2 × 104 cells per cm2. Cells were washed three times with phosphate-buffered saline and harvested by trypsinization. Only adherently cultured cells were collected for FACS analysis.
Preparation of PDMS substrate
PDMS base and curing agent were mixed at 10:1 and 100:1 separately. After a degas process, PDMS layers with height of approximate 1 mm were cast on cell culture plates and then cured at 70°C for 24 h. The PDMS layer was then cleaned by briefly rinsing with isopropyl alcohol and deionized water followed by drying with nitrogen gas. After subsequent treatment with oxygen plasma, the PDMS layer was incubated with BSA, fibronectin or collagen solution for 1 h at 37°C. The excess solution was aspirated and the substrate was washed with PBS.
Analytical flow cytometry
Cells were harvested and re-suspended in cold HBSS with 2% FBS (HBSS+), and cell numbers were adjusted to 50,000 cells/ml for antibodies staining. After the antibody staining on ice, the cells were washed twice with the cold HBSS+ and kept on ice in HBSS+ until FACS analysis. Isotype control antibodies were used as the gating control.
Western blot analysis
Cells were harvested by trypsinization and lysed using a lysis buffer in PBS with 1% TritonX-100 and cocktails of protease and phosphatase inhibitors (Thermo Scientific Inc., Rockford, IL) at 4°C. After centrifugation at 12,000 g for 10 minutes, the supernatants were used as the whole cell lysate for the western blot assay. To normalize the protein loading, the concentration of proteins was measured using BCA Protein Assay Kit (Thermo Scientific Inc.). All primary and secondary antibodies were purchased from Cell Signaling Technology, Boston, MA. All electrophoresis running buffer and gradient gels (4–20%) were purchased from Bio-Rad Laboratories, Hercules, CA.
Author Contributions
W.Z. did fabrication and characterization of PDMS substrates. D.C. did the FACS and western blot analysis. L.Q. guided the research direction. W.Z., D.C., L.Q., Y.N. and J.C. optimized the experimental protocol and prepared the manuscript, and all authors discussed the results and commented on the manuscript.
Supplementary Material
Supplementary Information
Video S1
Acknowledgments
This study is funded by the Cancer Prevention and Research Institute of Texas (CPRIT-R1007), Emily Herman Research Fund, and Golfers Against Cancer Foundation.
References
- Whitesides G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006). [DOI] [PubMed] [Google Scholar]
- Fan R. et al. Integrated barcode chips for rapid, multiplexed analysis of proteins in microliter quantities of blood. Nat. Biotechnol. 26, 1373–1378 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin J. & Quake S. R. Microfluidic Large-Scale Integration: The Evolution of Design Rules for Biological Automation. Annu. Rev. Biophys. Biomol. Struct. 36, 213–231 (2007). [DOI] [PubMed] [Google Scholar]
- Weibel D. B. & Whitesides G. M. Applications of microfluidics in chemical biology. Curr. Opin. Chem. Biol. 10, 584–591 (2006). [DOI] [PubMed] [Google Scholar]
- Love J. C., Ronan J. L., Grotenbreg G. M., van der Veen A. G. & Ploegh H. L. A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nat. Biotechnol. 24, 703–707 (2006). [DOI] [PubMed] [Google Scholar]
- McDonald J. C. & Whitesides G. M. Poly(dimethylsiloxane) as a Material for Fabricating Microfluidic Devices. Acc. Chem. Res. 35, 491–499 (2002). [DOI] [PubMed] [Google Scholar]
- Sia S. K. & Whitesides G. M. Microfluidic devices fabricated in Poly(dimethylsiloxane) for biological studies. Electrophoresis 24, 3563–3576 (2003). [DOI] [PubMed] [Google Scholar]
- Tolle D. A., Frye C. L., Lehmann R. G. & Zwick T. C. Ecological effects of PDMS-augmented sludge amended to agricultural microcosms. Sci. Total Environ. 162, 193–207 (1995). [Google Scholar]
- El-Ali J., Sorger P. K. & Jensen K. F. Cells on chips. Nature 442, 403–411 (2006). [DOI] [PubMed] [Google Scholar]
- Dittrich P. S. & Manz A. Lab-on-a-chip: microfluidics in drug discovery. Nat. Rev. Drug Discov. 5, 210–218 (2006). [DOI] [PubMed] [Google Scholar]
- Lee J. N., Jiang X., Ryan D. & Whitesides G. M. Compatibility of Mammalian Cells on Surfaces of Poly(dimethylsiloxane). Langmuir 20, 11684–11691 (2004). [DOI] [PubMed] [Google Scholar]
- Toworfe G. K., Composto R. J., Adams C. S., Shapiro I. M. & Ducheyne P. Fibronectin adsorption on surface-activated poly(dimethylsiloxane) and its effect on cellular function. J. Biomed. Mater. Res., Part A 71A, 449–461 (2004). [DOI] [PubMed] [Google Scholar]
- Kurpinski K., Chu J., Hashi C. & Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc. Natl. Acad. Sci. U. S. A. 103, 16095–16100 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valamehr B. et al. Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proc. Natl. Acad. Sci. U. S. A. 105, 14459–14464 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F. et al. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell stem cell 5, 17–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappmann B. et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 11, 642–649 (2012). [DOI] [PubMed] [Google Scholar]
- Saltzman W. M., Parsons-Wingerter P., Leong K. W. & Lin S. Fibroblast and hepatocyte behavior on synthetic polymer surfaces. J. Biomed. Mater. Res. 25, 741–759 (1991). [DOI] [PubMed] [Google Scholar]
- Folkman J. & Moscona A. Role of cell shape in growth control. Nature 273, 345–349 (1978). [DOI] [PubMed] [Google Scholar]
- Chen L. et al. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials 33, 1437–1444 (2012). [DOI] [PubMed] [Google Scholar]
- Prager-Khoutorsky M. et al. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat. Cell Biol. 13, 1457–1465 (2011). [DOI] [PubMed] [Google Scholar]
- Hsiao A. Y. et al. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials 30, 3020–3027 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran S. & DeLouise L. A. Enriching and characterizing cancer stem cell sub-populations in the WM115 melanoma cell line. Biomaterials 32, 9316–9327 (2011). [DOI] [PubMed] [Google Scholar]
- Stott S. L. et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. U. S. A. 107, 18392–18397 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore C. & Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 10, R25 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipitsin M. et al. Molecular Definition of Breast Tumor Heterogeneity. Cancer Cell 11, 259–273 (2007). [DOI] [PubMed] [Google Scholar]
- Gray D. S., Tien J. & Chen C. S. Repositioning of cells by mechanotaxis on surfaces with micropatterned Young's modulus. J. Biomed. Mater. Res., Part A 66A, 605–614 (2003). [DOI] [PubMed] [Google Scholar]
- Brodbeck W. G. et al. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc. Natl. Acad. Sci. U. S. A. 99, 10287–10292 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore C. & Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 10, R25 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R., Clarke M. F. & Morrison S. J. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer 3, 895–902 (2003). [DOI] [PubMed] [Google Scholar]
- Guirguis R., Margulies I., Taraboletti G., Schiffmann E. & Liotta L. Cytokine-induced pseudopodial protrusion is coupled to tumour cell migration. Nature 329, 261–263 (1987). [DOI] [PubMed] [Google Scholar]
- Shankar J. et al. Pseudopodial Actin Dynamics Control Epithelial-Mesenchymal Transition in Metastatic Cancer Cells. Cancer Res. 70, 3780–3790 (2010). [DOI] [PubMed] [Google Scholar]
- Kang Y. & Massagué J. Epithelial-Mesenchymal Transitions: Twist in Development and Metastasis. Cell 118, 277–279 (2004). [DOI] [PubMed] [Google Scholar]
- Sarrió D. et al. Epithelial-Mesenchymal Transition in Breast Cancer Relates to the Basal-like Phenotype. Cancer Res. 68, 989–997 (2008). [DOI] [PubMed] [Google Scholar]
- Zhou J. et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc. Natl. Acad. Sci. U. S. A. 104, 16158–16163 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. et al. Microfluidics separation reveals the stem-cell–like deformability of tumor-initiating cells. Proc. Natl. Acad. Sci. U. S. A. 109, 18707–18712 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon M. J., Minett T. W. & Tighe B. J. Cellular interactions with synthetic polymer surfaces in culture. Biomaterials 6, 396–402 (1985). [DOI] [PubMed] [Google Scholar]
- Webb K., Hlady V. & Tresco P. A. Relationships among cell attachment, spreading, cytoskeletal organization, and migration rate for anchorage-dependent cells on model surfaces. J. Biomed. Mater. Res. 49, 362–368 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima S., Chu J. S. F., Li S. & Komvopoulos K. Differential regulation of endothelial cell adhesion, spreading, and cytoskeleton on low-density polyethylene by nanotopography and surface chemistry modification induced by argon plasma treatment. J. Biomed. Mater. Res., Part A 84A, 828–836 (2008). [DOI] [PubMed] [Google Scholar]
- Koenig A. L., Gambillara V. & Grainger D. W. Correlating fibronectin adsorption with endothelial cell adhesion and signaling on polymer substrates. J. Biomed. Mater. Res., Part A 64A, 20–37 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Video S1