Abstract
Introduction
Primary dermal melanoma (PDM) is a recently described clinical entity accounting for less than 1% of all melanomas. Histologically, it is located in the dermis or subcutaneous tissue, and it shows no connections with the overlying epidermis. The differential diagnosis is principally made along with that of metastatic cutaneous melanoma.
Case Report
A 72-year-old Caucasian woman with a history of multiple cancers (metachronous bilateral breast cancer, meningioma, clear cell renal cell carcinoma, uterine fibromatosis and intestinal adenomatous polyposis), came to our attention with a nodular lesion on her back. After removal of the lesion, the histology report indicated malignant PDM or metastatic malignant melanoma. The clinical and instrumental evaluation of the patient did not reveal any other primary tumour, suggesting the primitive nature of the lesion. The absence of an epithelial component argued for a histological diagnosis of PDM. Subsequently, the patient underwent a wide surgical excision with sentinel node biopsy, which was positive for metastatic melanoma. Finally, the mutational status was studied in the main genes that regulate proliferation, apoptosis and cellular senescence. No pathogenetic mutations in CDKN2A, BRAF, NRAS, KRAS, cKIT, TP53 and PTEN genes were observed. This suggests that alternative pathways and low-frequency alterations may be involved.
Conclusions
The differential diagnosis between PDM and isolated metastatic melanoma depends on the negativity of imaging studies and clinical findings for other primary lesions. This distinction is important because 5-year survival rates in such cases are higher than in metastatic cases (80–100 vs. 5–20%, respectively).
Key Words: Dermal melanoma, Sentinel lymph node, Dermis, Skin cancer, Skin nodules, Neoplasia, Tumour
Introduction
Primary dermal melanoma (PDM) has been proposed as a specific subtype of melanoma [1, 2]. Such a skin lesion is confined to the dermis and/or subcutaneous fat, showing no connections with the overlying epidermis and simulating a melanoma metastasis [1]. PDM should be differentiated from primary nodular melanoma, cutaneous metastatic melanoma and clear cell sarcoma, due to its excellent prognosis as compared to that of patients with these three latter diseases [2, 3, 4]. Generally, a diagnosis of PDM should be considered in all patients with a solitary melanoma confined to the dermis and subcutaneous tissue when there is no evidence of a primary tumour or disease at other sites following appropriate staging studies. This uncommon clinical entity is reported in the literature with an incidence ranging between 0.4 and 0.9% [3].
Among the main pathways which have been found to be involved in melanomagenesis, including those inducing cell proliferation (proliferative pathways), overcoming cell senescence (senescence pathway) or affecting apoptosis (apoptotic pathways), molecular characterization of PDM lesions revealed lower expression levels of p53 and cyclin D1 proteins [3] as well as an up-regulation of the cKIT gene [4].
We report a case of PDM in a 72-year-old Caucasian woman who had a peculiar history of multiple previous malignancies. A molecular characterization of the lesion was performed.
Case Report
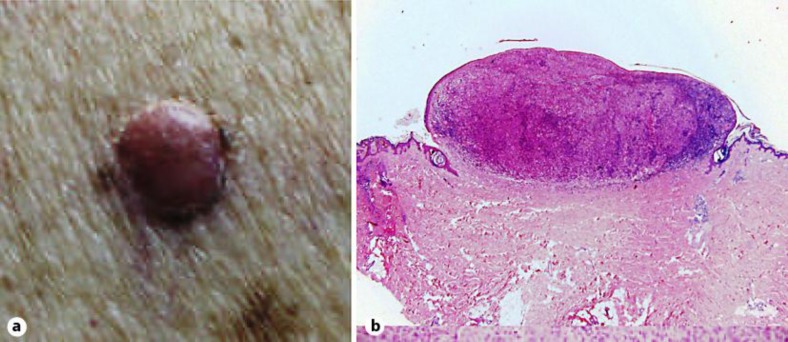
A 72-year-old Caucasian woman came to our attention for a right paravertebral purplish-red nodular lesion on the back, with a few focal pigmentations and a small semilunar perinodular area of brownish pigmentation (fig. 1a). The patient could not report the onset of the lesion. The differential diagnosis included, in this case, basal cell carcinoma, malignant melanoma, vascular lesions, epidermoid carcinoma and dermatofibroma.
Fig. 1.
Dermal PDM nodule of the trunk. a Clinical features. b Haematoxylin-eosin-stained section of the tumour (×20).
The patient's clinical history was characterized by multiple tumours, including bilateral metachronous breast cancer for which she had undergone bilateral mastectomy and axillary lymphadenectomy, clear cell renal cell carcinoma, uterine fibromatosis and intestinal polyposis with low- and high-grade dysplasia. Despite the macroscopic aspect which suggested pigmented basal cell carcinoma, the possibility of the lesion being melanocytic made us opt for an excisional biopsy with a 2-mm margin.
The skin lesion specimen showed a poorly differentiated neoplastic dermal infiltration with morphological aspects of melanocytic proliferation (fig. 1b). The tumour cells displayed round or oval shapes with brisk mitotic activity, and they were arranged in a nodular architecture with rounded margins. Lymphocytic and plasmacellular infiltration of the lesion was observed, without formation of lymphoid nodules. An intraepidermal component, ulceration and regression aspects were not evident, even with an examination of multiple sections; a preexisting nevus, spindle neoplastic cells or bands of mature intercellular collagen were also not found. A distinct grenz zone was appreciable between the dermal neoplasm and the overlying epidermis. The neoplastic cells demonstrated melanocytic-type immunohistochemistry (S100 protein and HMB-45 positive). The proliferative index evaluated with Mib-1 was 25%.
The histopathological report was summarized as ‘malignant melanoma (thickness 2 mm); the absence of intraepidermal atypical melanocytes is compatible with metastatic malignant melanoma or PDM’. Desmoplastic melanoma was excluded on the basis of cytology and because of the lack of a desmoplastic reaction, while nodular melanoma was excluded due to the absence of an intraepidermal component. With the aim of identifying a possible primary lesion, the following tests were performed: dermatological examination; total body CT; ophthalmologic examination; pancolonoscopy; ear, nose and throat examination; esophagogastroduodenoscopy, and gynaecological examination. No evidence of another primary melanoma was found; however, a parallel finding of frontal meningioma was noted. The absence of another primary lesion led us to make the diagnosis of PDM per exclusionem.
The second surgical step included the excision of the biopsy scar with 2-cm margins and sentinel lymph node biopsy. The preoperative lymphoscintigraphy showed 3 areas of uptake − 2 of them involving the soft tissue at the level of the posterior pillar of both the axillae, and the remaining 1 in the left paravertebral area, adjacent to the previous surgical site. However, only a single sentinel node presenting micrometastasis of melanoma was found in the latter area.
A bilateral axillary ultrasound examination was performed to investigate the areas of lymphoscintigraphic uptake; subsequently, the patient underwent an additional surgical procedure for the removal of a lymph node with ambiguous ultrasonographic features found within the residual left axillary fatty tissue (the patient had had ipsilateral axillary lymphadenectomy several years before for breast cancer). The histological examination of the specimen revealed adipose tissue and a lymph node with reactive patterns. The patient is currently alive and free of disease 14 months since her diagnosis.
Mutational Analysis
Different pathways for the development of melanoma have been demonstrated [5]. Melanomas on skin not chronically exposed to the sun – such as the anatomical location of the skin lesion in our case – have been reported to usually carry either a mutated NRAS gene or mutated BRAF gene or concurrently mutated BRAF and PTEN genes [6]. Furthermore, the coexistence of additional tumours in our patient was a strong indication to either investigate the CDKN2A and TP53 genes, which have been demonstrated to be involved in multiple cancer pathogenesis and predisposition [7], or assess the occurrence of the E318K sequence variation in the MITF (microphthalmia-associated transcription factor) oncogene, which has been reported in patients with an association between melanoma and renal cancer [8]. Finally, the mutation analysis was carried out in the cKIT gene, which has been demonstrated to be involved in PDM pathogenesis [4]. Genomic DNA was isolated from a peripheral blood sample and tumour tissue, using standard methods. The full coding sequences and splice junctions of p16CDKN2A (exons 1α, 2 and 3), p14CDKN2A (exon 1β) and PTEN (exons 1–9) genes as well as the most frequently affected exons of NRAS/KRAS (2–3), BRAF (15), cKIT (9, 11, 13, 17 and 18) and TP53 (5–8) genes were screened for mutations on somatic DNA isolated from paraffin-embedded tumour tissue samples by directly sequencing the amplified PCR products through an automated fluorescence-cycle sequencer (ABI PRISM 3130, Applied Biosystems, Foster City, Calif., USA). For p16CDKN2A, p14CDKN2A and MITF genes, mutation analysis was also carried out on germline DNA obtained from peripheral blood of the patient, as previously described [8, 9].
No mutation was identified in such crucial genes involved in melanoma susceptibility and pathogenesis. In particular, alteration of the CDKN2A gene, encoding p16CDKN2A, which is the main component of the CyclinD1-RB pathway, and p14CDKN2A, which has been functionally linked to the MDM2-p53 pathway, have been widely reported as the most common causes of inherited susceptibility to melanoma at the germline level, participating also in melanoma progression at the somatic level [10, 11]. Lack of TP53 mutations in melanoma cells from our patient's tissues is consistent with the absence of involvement of both the p16CDKN2A-CyclinD1-RB and p14CDKN2A-MDM2-TP53 cascades in the pathogenesis of such lesions. Analogously, activation of both the MAPK-ERK pathway (including the cascade of NRAS/KRAS, BRAF, MEK1/2, and ERK1/2 proteins), a major signaling cascade deputed to the control of cell growth and proliferation, and the PTEN-dependent pathway (including the PI3K and AKT effectors), committed to regulate cell survival and apoptosis, were not involved in inducing the malignant transformation in our case. As a further confirmation that genetic alterations in such major pathways played no role in pathogenesis in our PDM case, a wild-type cKIT variant (this gene has been demonstrated to indeed recruit and activate a number of intracellular signalling pathways implicated in melanocytic transformation and progression, including the PI3K-AKT and MAPK pathways [12]) as well as the absence of the MITF E318K variant were observed here. Therefore, one could speculate that the cascade of alterations underlying melanomagenesis may be due to additional low-frequency genetic and molecular events in our patient.
Discussion
Dermal or subcutaneous metastasis of melanoma of unknown primary origin, according to the 2002 AJCC (American Joint Committee on Cancer) staging guidelines, is classified as stage IV M1a disease and has a poor prognosis (5-year survival rate 18.8%). Nevertheless, small series of dermal or subcutaneous solitary lesions have been reported with consistently better recurrence and survival rates, depending on the thickness and presence of ulceration. In 2004, Swetter et al. [1] reported a series of 5 patients with such lesions and speculated that those were cases of a distinct subtype of melanoma and should be referred to by the term primary dermal melanoma. It is, therefore, of utmost importance to differentiate PDM from a solitary dermal or subcutaneous metastasis of melanoma.
Regarding the pathogenesis of PDM, several hypotheses have been proposed. Some authors speculate that the tumour may arise from nonepidermal melanocytes or intradermal nevi, while others believe that it is an epidermally regressed primary [13, 14]. Despite the existence of some evidence in favour of one or the other theory, none has been demonstrated definitely. Given the rarity of PDM and the small number of cases reported, demographic information and data on the anatomic sites most commonly involved are inconsistent. Furthermore, the impact of these factors on prognosis is not known. Lee et al. [15], in a series of 101 solitary dermal melanomas, did not find any site-related differences which might explain the generally better prognosis of these tumours.
From the clinical point of view, a thorough dermatological examination of the skin and of the visible mucosae as well as gynaecological and ophthalmological examinations, a pancolonoscopy and a total body CT should be performed to look for and possibly exclude hidden primaries. The role of sentinel lymph node biopsy in clinical management is not yet clear, but it seems to be useful for the detection of micrometastasis, as occurred in our case. The possibility that such melanomas may show a distinct genetic pattern prompted us to also undertake a genetic analysis. The patient also had a history of multiple previous malignancies that could have been a clue for a genetic predisposition.
The genetic analysis performed at both the germline and somatic levels in our patient revealed that the main genes responsible for the susceptibility and pathogenesis of melanoma, respectively, were not affected. This suggests that alternative, low-frequency altered genes/pathways may be involved. A more comprehensive genetic profiling based on microarray technology will be helpful in making correlations between molecular signatures and clinical features; to date, gene expression arrays have been employed in such a melanoma classification [16]. Nevertheless, the advancements of biotechnology, mostly represented by next-generation sequencing approaches [17], will provide even more reliable tools for detailed gene-based analyses, allowing physicians to better characterize molecular biomarkers in order to achieve a more accurate diagnosis as well as predict prognosis and response to treatment in patients with melanoma.
Conclusions
The differential diagnosis between PDM and isolated metastatic melanoma is generally performed per exclusionem and is based on the negativity of clinical findings and imaging studies for primary lesions. This distinction is important because 5-year survival rates in PDM are consistently higher than in metastatic cases (80–100 vs. 5–20%, respectively).
Disclosure Statement
The authors declare that they have no conflicts of interests.
References
- 1.Swetter SM, Ecker PM, Johnson DL, Harvell JD. Primary dermal melanoma: a distinct subtype of melanoma. Arch Dermatol. 2004;140:99–103. doi: 10.1001/archderm.140.1.99. [DOI] [PubMed] [Google Scholar]
- 2.Chen WC, Hsueh YY. Primary dermal melanoma in a 19-year-old Asian man. Dermatol Surg. 2013;39:662–665. doi: 10.1111/dsu.12114. [DOI] [PubMed] [Google Scholar]
- 3.Cassarino DS, Cabral ES, Kartha RV, Swetter SM. Primary dermal melanoma: distinct immunohistochemical findings and clinical outcome compared with nodular and metastatic melanoma. Arch Dermatol. 2008;144:49–56. doi: 10.1001/archdermatol.2007.16. [DOI] [PubMed] [Google Scholar]
- 4.Hida Y, Kubo Y, Miyajima O, Arase S. Primary dermal melanoma: a case report and molecular characterization. J Dermatol. 2009;36:346–352. doi: 10.1111/j.1346-8138.2009.00650.x. [DOI] [PubMed] [Google Scholar]
- 5.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 6.Haluska FG, Tsao H, Wu H, Haluska FS, Lazar A, Goel V. Genetic alterations in signaling pathways in melanoma. Clin Cancer Res. 2006;12:2301s–2307s. doi: 10.1158/1078-0432.CCR-05-2518. [DOI] [PubMed] [Google Scholar]
- 7.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama S, Woods SL, Boyle GM, et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature. 2011;480:99–103. doi: 10.1038/nature10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casula C, Muggiano A, Cossu A, et al. Role of key-regulator genes in melanoma susceptibility and pathogenesis among patients from South Italy. BMC Cancer. 2009;9:352. doi: 10.1186/1471-2407-9-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharpless E, Chin L. The INK4a/ARF locus and melanoma. Oncogene. 2003;22:3092–3098. doi: 10.1038/sj.onc.1206461. [DOI] [PubMed] [Google Scholar]
- 11.Palmieri G, Capone ME, Ascierto ML, Gentilcore G, Stroncek DF, Casula M, Sini MC, Palla M, Mozzillo N, Ascierto PA. Main roads to melanoma. J Transl Med. 2009;7:86. doi: 10.1186/1479-5876-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol. 2006;126:1102–1110. doi: 10.1038/sj.jid.5700125. [DOI] [PubMed] [Google Scholar]
- 13.Bowen GM, Chang AE, Lowe L, Hamilton T, Patel R, Johnson TM. Solitary melanoma confined to the dermal and/or subcutaneous tissue: evidence for revisiting the staging classification. Arch Dermatol. 2000;136:1397–1399. doi: 10.1001/archderm.136.11.1397. [DOI] [PubMed] [Google Scholar]
- 14.Tansley P, Dewar D, Brown D, Brough M, Cook M, Withey S, Butler P. Eleven-year survival from an intra-dermal melanoma. J Plast Reconstr Aesthet Surg. 2006;59:1355–1358. doi: 10.1016/j.bjps.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 15.Lee CC, Faries MB, Ye X, Morton DL. Solitary dermal melanoma: beginning or end of the metastatic process? Ann Surg Oncol. 2009;16:578–584. doi: 10.1245/s10434-008-0272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaeger J, Koczan D, Thiesen HJ, et al. Gene expression signatures for tumor progression, tumor subtype, and tumor thickness in laser-microdissected melanoma tissues. Clin Cancer Res. 2007;13:806–815. doi: 10.1158/1078-0432.CCR-06-1820. [DOI] [PubMed] [Google Scholar]
- 17.Berger MF, Hodis E, Heffernan TP, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012;485:502–506. doi: 10.1038/nature11071. [DOI] [PMC free article] [PubMed] [Google Scholar]