Abstract
It is now recognized that female carriers of fragile X premutations are at increased risk of premature ovarian failure. We have studied 51 premenopausal women from fragile X families, to determine whether premutation carriers have variations in the hormonal markers of menopause, compared to full mutations and controls. We found a significant increase in serum follicle stimulating hormone in premutation carriers, suggesting that as a group they will enter menopause before full mutation carriers and unaffected controls. These results have important implications for fertility in these women.
Keywords: fragile, X/FSH/premature, ovarian, failure/premutation
Introduction
FRAXA is a polymorphic CGG repeat within the 5′ untranslated region of the FMR1 gene on chromosome Xq27.3 (Verkerk et al., 1991). Dynamic mutation to more than 200 repeats silences the FMR1 gene by hyper-methylation and causes the fragile X syndrome of mental retardation. Repeat lengths smaller than 200 units were long thought to have no obvious phenotype, save the tendency for premutations (61–200 CGG repeats) to increase in size during maternal transmission. Recently, several studies have established that premutations (but not full mutations) are associated with premature ovarian failure, defined as the cessation of menses for a period of >6 months prior to the age of 40 years (Schwartz et al., 1994; Conway et al., 1995; Allingham-Hawkins et al., 1999). Premature ovarian failure is known to have a significant genetic component particularly involving genes on the X chromosome (Vegetti et al., 1998), and the trinucleotide repeat at the fragile X locus is expanded in ~2% of women with premature ovarian failure (Conway et al., 1998).
It has been proposed (Conway et al., 1995) that alleles in the premutation range interfere in some way with FMR1 transcription in the fetal ovary, reducing the number of oocytes at birth. Attrition of the primordial follicle store leads to their depletion at a relatively earlier age and a compensatory rise in follicle stimulating hormone (FSH), presaging ovarian failure. We have examined families selected through fragile X probands to establish whether markers of an early menopause were present.
Materials and methods
All women who had not completed the menopause were defined as premenopausal, and were eligible for this study if they were over the age of 16 years but had not taken fertility treatment, hormone replacement therapy (HRT) or the contraceptive pill within the past month. This gave a sample of 51 women of known FRAXA allele status, 19 premutations (61–200 repeats), nine full mutations (>200 repeats) and 23 controls (<60 repeats). A blood sample was drawn between days 1–10 of their menstrual cycle, and the serum analysed for FSH and oestradiol concentration.
Since levels of hormones vary naturally with age (MacNaughton et al., 1992), regardless of FRAXA allele status, an adjustment for age was needed to ensure that only changes additive to natural fluctuations were considered. A double logarithm transformation of hormone level×(y = lnlnx) was found to stabilize the variance within age better than a single logarithm transformation, but still not completely. We therefore obtained a predicted value (y) from the regression y = a + b(age) + c(age2), and a predicted variance (V) from the regression (y − ŷ)2 = A + B(age) + C(age2), to give the standardized variable (y − ŷ)/√V, which was normalized through rank transformation (Blom, 1958). Stepwise regression (SAS Institute Inc., Cary, NC, USA) was performed on the resulting variable which represented the FSH level x, with a stable mean and variance and a normal distribution within age.
Results
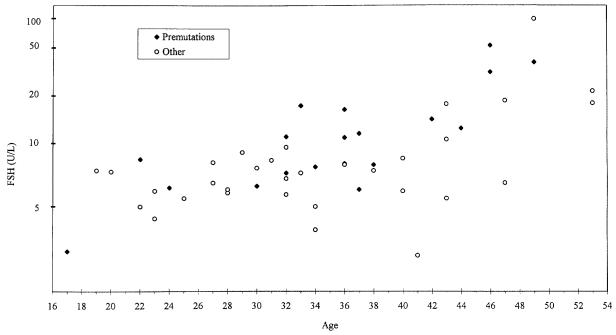
Excluding premutations, there was no significant difference in transformed FSH levels between nine full mutations and the 23 control individuals (P = 0.716). However, the difference between 19 premutations and the remaining 32 subjects was significant (0.555 ± 0.279, one-tailed P = 0.026) (Figure 1). Thirteen families had two or more members and so we were concerned that significance was exaggerated because individuals were considered independent. On the contrary, introducing 13 variables, one for each family comparing that family with the rest of the data, the difference between premutation carriers and other women became more significant (one-tailed P = 0.015). The difference between premutation carriers and other women (full mutations and controls) did not approach significance for oestradiol.
Figure 1.
Follicle stimulating hormone (FSH) concentrations plotted against age.
Discussion
We found that raised serum FSH concentration was associated with FRAXA premutation alleles at a suggestive level (one-tailed P = 0.026); however, oestradiol was not associated. Neither of the hormones was associated with other FRAXA allele size classes (controls or full mutations). The day of cycle on which the serum sample was taken ranged between days 1 and 10, although the majority were taken on days 3, 4 and 5. It is possible that this variation masked subtle differences between the groups, particularly for the oestradiol measurements; however, the distribution of day of cycle was similar for both groups of women (premutations and others) and would therefore not explain the raised serum FSH in the premutation group. It is perhaps not surprising that oestradiol did not vary between groups, as it is almost exclusively released from the ovulatory follicle and all the women were still ovulating. Because of the variation in the day of cycle of our study group, inhibin B testing would not have been a robust enough measure and therefore was not included. The association between FSH levels and premutations is not artificially enhanced by family effects and therefore holds for both sporadic and familial premature ovarian failure.
Women with premutations not only have an increased incidence of premature ovarian failure (Allingham-Hawkins et al., 1999) but our data suggest that those still menstruating have an occult raised serum FSH concentration, suggestive of a depleted ovarian reserve. These data support the hypothesis that premutation carriers as a group have an earlier age of menopause than their unaffected and full mutation carrier relatives (Partington et al., 1996), which manifests as premature ovarian failure in ~23% of premutation carriers, but in others will merely result in menopause at a younger age than they would otherwise have had.
It is rare for women to be fertile with a follicular phase FSH >20 IU/l (O’Herlihy et al., 1980), and therefore the results of this study have important implications for fertility. Female members of fragile X families require combined genetic and fertility counselling. From a practical point of view, women found to have raised FSH concentrations respond poorly to all modes of fertility treatment (Scott and Hofman, 1995). If, however, detected at an early stage (FSH <15 IU/l) conception may still be possible perhaps with the assistance of ovulation induction techniques. The Fragile X Society is aware of the risk of early ovarian failure and is advising women to consider starting their families earlier than they might otherwise have done.
Acknowledgements
We are grateful to the fragile X families in Wessex who took part in the study, for their continued interest in research, and to the Wellcome Trust for financial support.
References
- Allingham Hawkins DJ, Babul-Hirji D, Chitayat D, et al. Fragile X premutation is a significant risk factor for premature ovarian failure. The International Collaborative premature ovarian failure in fragile X study. Am. J. Med. Genet. 1999 in press. [PMC free article] [PubMed] [Google Scholar]
- Blom G. Statistical Elements and Transformed Beta Variables. Wiley; New York: 1958. [Google Scholar]
- Conway GS, Hettiarachchi S, Murray A, Jacobs PA. Fragile X premutations in familial premature ovarian failure. Lancet. 1995;346:309–310. doi: 10.1016/s0140-6736(95)92194-x. [DOI] [PubMed] [Google Scholar]
- Conway GS, Payne NN, Webb J, et al. Fragile X premutation screening in women with premature ovarian failure. Hum. Reprod. 1998;13:1184–1187. doi: 10.1093/humrep/13.5.1184. [DOI] [PubMed] [Google Scholar]
- MacNaughton J, Banah M, McCloud P, et al. Age related changes in follicle stimulating hormone, luteinizing hormone, oestradiol and immunoreactive inhibin in women of reproductive age. Clin. Endocrinol. 1992;36:339–345. doi: 10.1111/j.1365-2265.1992.tb01457.x. [DOI] [PubMed] [Google Scholar]
- O’Herlihy C, Pepperell RJ, Evans JH. The significance of FSH elevation in young women with disorders of ovulation. Br. Med. J. 1980;281:1447. doi: 10.1136/bmj.281.6253.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partington MW, York Moore D, Turner GM. Confirmation of early menopause in fragile X carriers. Am. J. Med. Genet. 1996;64:370–372. doi: 10.1002/(SICI)1096-8628(19960809)64:2<370::AID-AJMG27>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, et al. Obstetrical and gynaecological complications in fragile X carriers: a multicenter study. Am. J. Med. Genet. 1994;51:400–402. doi: 10.1002/ajmg.1320510419. [DOI] [PubMed] [Google Scholar]
- Scott RT, Hofmann GE. Prognostic assessment of ovarian reserve. Fertil. Steril. 1995;63:1–11. [PubMed] [Google Scholar]
- Vegetti W, Tibiletti MG, Testa G, et al. Inheritance in idiopathic premature ovarian failure: analysis of 71 cases. Hum. Reprod. 1998;13:1796–1800. doi: 10.1093/humrep/13.7.1796. [DOI] [PubMed] [Google Scholar]
- Verkerk AJMH, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]