Abstract
The preliminary results of an international collaborative study examining premature menopause in fragile X carriers are presented. A total of 760 women from fragile X families was surveyed about their fragile X carrier status and their menstrual and reproductive histories. Among the subjects, 395 carried a premutation, 128 carried a full mutation, and 237 were noncarriers. Sixty-three (16%) of the premutation carriers had experienced menopause prior to the age of 40 compared with none of the full mutation carriers and one (0.4%) of the controls. Based on these preliminary data, there is a significant association between fragile X premutation carrier status and premature menopause.
Keywords: fragile X syndrome, premature ovarian failure, premature menopause, fragile X premutation
INTRODUCTION
There have been several reports of an increased incidence of premature ovarian failure (POF), or premature menopause, among carriers of the fragile X syndrome (FraX) [Cronister et al., 1991; Schwartz et al., 1994; Partington et al., 1996; Vianna-Morgante et al., 1996]. Cronister et al. [1991] reported that 13% of carriers experienced POF, whereas Schwartz et al. [1994] reported a larger, multicentre study in which 24% of premutation carriers were found to experience POF and 14% and 6% of full mutation carriers and noncarriers, respectively, did. Finally, Partington et al. [1996] reported that fragile X carriers experience menopause six to eight years earlier, on average, than women in the general population of the United Kingdom. Because of the relatively small numbers in these studies and questions about appropriate control populations, an international collaboration was formed at the 7th International Workshop on the Fragile X and X-Linked Mental Retardation held in Tromsö, Norway, in 1995. The preliminary data from that collaboration are presented here.
MATERIALS AND METHODS
Each of the nine participating centres (Toronto, Canada; Kingston, Canada; Staten Island, New York; Fairfax, Virginia; Sao Paolo, Brazil; Salisbury, England; Ioannina, Greece; Nicosia, Cyprus; and Florence, Italy) collected menstrual and reproductive histories on women from fragile X families who had undergone carrier testing using molecular methodologies. The control population was first- or second-degree relatives of carriers who were known by molecular testing to be noncarriers. All women age 18 and older were eligible for the study. Premature ovarian failure was considered to be complete cessation of periods prior to the age of 40. Pairwise chi-square analysis was used to compare the number of women in each of the three groups (premutation, full mutation, noncarrier) who did or did not experience POF.
RESULTS
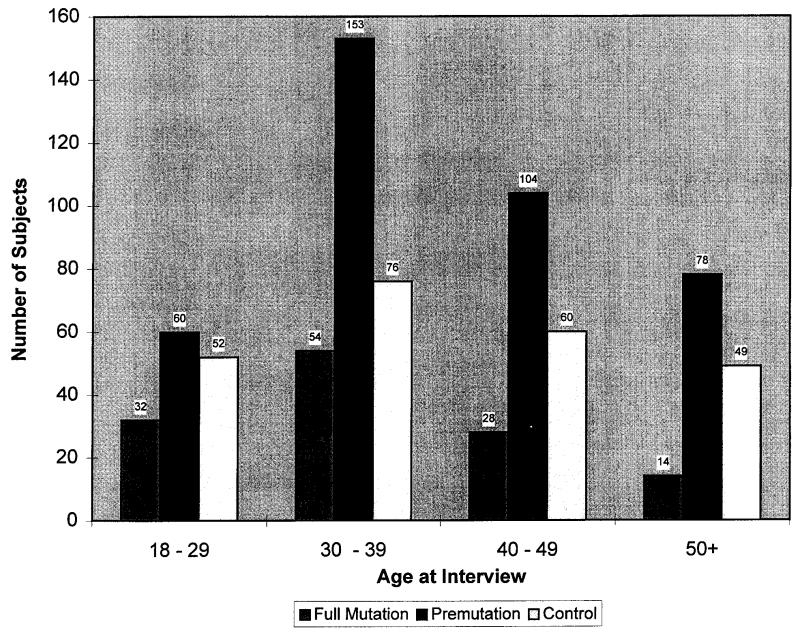
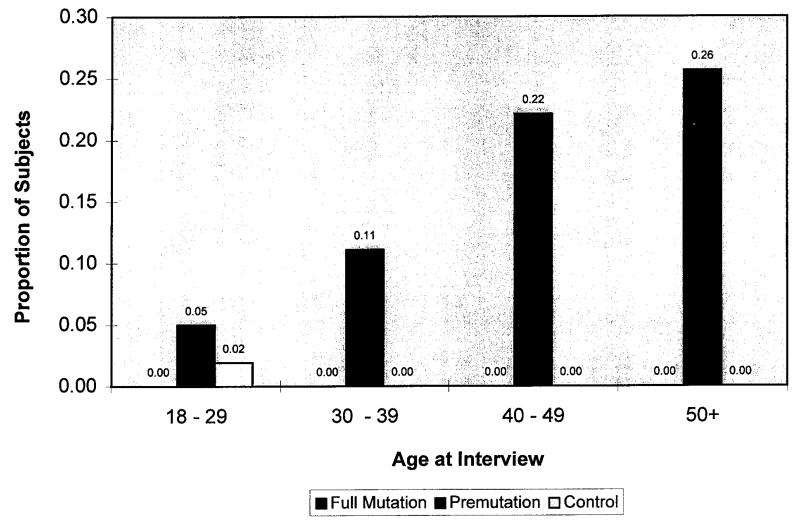
A total of 770 subjects was surveyed by the nine centres. For 10 subjects, mutation status was not known and these subjects were excluded from the study. Of the remaining 760 subjects, 395 were premutation carriers, 128 were full mutation carriers, and 237 were noncarriers. Figure 1 shows the distribution of subjects among four age categories: 18 to 29 years (144 subjects), 30 to 39 years (283 subjects), 40 to 49 years (192 subjects), and 50 years or older (141 subjects). Figure 2 shows the proportion of subjects who experienced POF in each mutation and age category. Overall, 16% (63 subjects; 5 to 26% in each age category) of premutation carriers experienced POF compared with 0% of full mutation carriers (no subjects; p <0.001) and 0.4% (one subject; 0-2%; p <0.001) of noncarriers. The number of full mutation carriers and noncarriers experiencing POF were not significantly different from each other (p = 0.46).
Fig. 1.
Distribution of subjects among the age and mutation categories (number of subjects is indicated above each bar).
Fig. 2.
Proportion of subjects in each of the age and mutation categories that experienced premature ovarian failure (proportion of subjects with POF is indicated above each bar).
DISCUSSION
Women of known mutation status from FraX families were surveyed about their menstrual and reproductive histories. A significantly higher proportion of premutation carriers experienced menopause prior to 40 years of age compared with full mutation carriers and noncarriers in the same families. These findings are consistent with the studies by Cronister et al. [1991] and Schwartz et al. [1994] although, in the former study, no distinction was made between full mutation and premutation carriers. The slightly lower level of POF observed in control samples (0.4%) compared to published estimates (1%; Coulam et al., 1986) is most likely because the majority of controls were under the age of 40 at the time of interview and thus, had not yet experienced menopause. However, given that the age distribution of subjects in each of the three mutation categories is similar (Fig. 1), this is unlikely to skew the results.
The finding that premutations in the FMR1 gene appear to affect ovarian function while full mutations do not is an interesting one. Schwartz et al. [1994] suggested that this discrepancy might be due to the small number of full mutation carriers in their study; to the decreased mental capacity of the full mutation carriers, which might affect their ability to complete the self-administered questionnaire; or to the relatively young age of the full mutation carriers. In the present study, more than threefold more full mutation carriers were surveyed with the same result, suggesting that sample size does not account for the differences. In addition, the distribution of subjects among the age categories was similar for each of the three groups of subjects, indicating that age of subjects is unlikely to be an issue either. Finally, for most of the subjects in the present study, the questionnaire was not self-administered thus reducing the possibility that inability of full mutation carriers to understand the questionnaire affected the results. Given that the results of the present study have confirmed the results of the Schwartz et al. [1994] study, the difference between premutation and full mutation carriers with respect to POF appears to be a real biological phenomenon. Since it is known that the FMR1 protein, FMRP, is expressed from premutation alleles but not full mutation alleles [Feng et al., 1995], it does not seem likely that decreased production of FMRP can result in POF. It is more likely that the premutation results in a gain of function for FMRP in the ovaries, which then leads to POF; however, more research is needed to confirm this hypothesis.
The question of why POF affects only a proportion of premutation carriers has not been addressed in this preliminary analysis; it is possible that the size of the premutation has an effect on the incidence of POF. In addition, for the purposes of the present study, a fairly strict definition of premature menopause was used: complete cessation of periods prior to the age of 40. It is possible that a much higher proportion of premutation carriers exhibit more subtle signs of early menopause (i.e., endocrinological signs) that could only be detected by more in-depth studies. Finally, it is possible that although the majority of premutation carriers do not undergo “premature” menopause, they do undergo menopause earlier, on average, than noncarriers, as was suggested by Partington et al. [1996]. This is a question that may be addressed at a later stage.
It is clear from the present and other studies that carriers of premutations in the FMR1 gene are at significantly increased risk of experiencing premature menopause compared with full mutation carriers and noncarriers in their families. It is important that this information is conveyed to women in FraX families to assist them in making reproductive decisions and to be aware of health issues that most women and their doctors only consider when the women are in their late forties and fifties (i.e., risk for osteoporosis and other complications of menopause). More research is needed to understand why all premutation carriers are not affected with POF as well as to understand the exact mechanism operating in the ovarian tissue that leads to POF.
ACKNOWLEDGMENT
The authors are indebted to Gerry Dimnik, Department of Genetics, North York General Hospital for performing the statistical analyses.
Contract grant sponsor: New York State Office of Mental Retardation and Developmental Disabilities; Contract grant sponsor: National Institutes of Health; Contract grant number: MCJ360587; Contract grant sponsor: Ministry of Development, General Secretariat for Research and Technology (Greece); Contract grant sponsor: FAPESP, FINEP, and CNPq (Brazil).
REFERENCES
- Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- Cronister A, Schreiner R, Wittenberger M, Amiri K, Harris K, Hagerman RJ. Heterozygous fragile X females: historical, physical, cognitive, and cytogenetic features. Am J Med Genet. 1991;38:269–274. doi: 10.1002/ajmg.1320380221. [DOI] [PubMed] [Google Scholar]
- Feng Y, Lakkis L, Devys D, Warren ST. Quantitative comparison of FMR1 gene expression in normal and premutation alleles. Am J Hum Genet. 1995;56:106–113. [PMC free article] [PubMed] [Google Scholar]
- Partington MW, Moore DY, Turner GM. Confirmation of early menopause in fragile X carriers. Am J Med Genet. 1996;64:370–372. doi: 10.1002/(SICI)1096-8628(19960809)64:2<370::AID-AJMG27>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Dean J, Howard-Peebles PN, Bugge M, Mikkelsen M, Tommerup N, Hull C, Hagerman R, Holden JJA, Stevenson RE. Obstetrical and gynecological complications in fragile X carriers: a multicenter study. Am J Med Genet. 1994;51:400–402. doi: 10.1002/ajmg.1320510419. [DOI] [PubMed] [Google Scholar]
- Vianna-Morgante AM, Costa SS, Pares AS, Verreschi ITN. FRAXA premutation associated with premature ovarian failure. Am J Med Genet. 1996;64:373–375. doi: 10.1002/(SICI)1096-8628(19960809)64:2<373::AID-AJMG28>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]