Abstract
Background and Objectives
Chronic rejection leads to kidney allograft failure and develops in many kidney transplant recipients. One cause of chronic rejection, chronic antibody mediated rejection (CAMR), is attributed to alloantibodies. Maintenance immunosuppression including prednisone, mycophenolate mofetil (MMF) and calcineurin inhibitors may limit alloantibody production in some patients, but many maintain or develop alloantibody production, leading to CAMR. Therefore, no efficacious therapy to treat CAMR is presently available to prevent the progression of CAMR to kidney allograft failure.
Design, Setting, Participants, and Measurements
We performed a retrospective review of 31 subjects with CAMR, of which 14 received Rituximab and 17 subjects did not. Response to Rituximab was defined as decline or stabilization of serum creatinine for at least one year. Data reviewed included demographic, clinical, allograft, post-transplant, and pathological variables. Pathological variables in the diagnostic allograft biopsy were scored according to Banff criteria.
Results
The median survival time (MST) for allografts in the control group was 439 days, and for the Rituximab treated group was 685 days. The Rituximab group was dichotomous with 8 subjects showing a medial survival time of 1180 days, and 6 subjects having a median survival time of 431 days. The MST for the responders was statistically significant from the non-responders and controls. No pathological parameter distinguished any subset of subjects.
Conclusions
These data show that Rituximab followed by standard maintenance immunosuppression shows a therapeutic effect in the treatment of CAMR, which is confined to a subset of treated subjects, not identifiable a priori.
Keywords: Chronic humoral rejection, Donor specific antibodies, Rituximab, Renal allograft survival
1. Introduction
Although short term survival of kidney allografts has improved, chronic rejection leading to kidney allograft failure develops in a significant number of kidney transplant recipients [4,5] and accounts for kidney failure in 50 to 80% of subjects [2]. Chronic antibody mediated rejection (CAMR), one cause of chronic rejection, is characterized by chronic glomerular and capillary endothelial injury [1–3], is usually associated with proteinuria [6–9] and pathological hallmarks including transplant glomerulopathy (duplication and laminations of the glomerular basement membrane) plus excess laminations of the peritubular capillaries. CAMR correlates with alloantibodies [2,9–14]. The presence of donor specific de novo anti-HLA antibodies (DSA) also associates with a poorer kidney graft survival as compared to subjects without de novo anti-HLA antibodies [15–19]. In animal studies, monkeys with kidney allografts and alloantibodies but off immunosuppression have identical pathology to humans and universally progress to kidney allograft failure [20,21]. Although mycophenolate mofetil (MMF) and calcineurin inhibitors are common in maintenance immunosuppression and may limit alloantibody production in some transplant patients, many maintain or develop alloantibodies.
Rituximab, a monoclonal anti-CD20 antibody, a possible drug to treat CAMR, depletes B cells, which later can develop into alloanti-body secreting plasma cells. The potential efficacy of Rituximab is demonstrated in other antibody mediated conditions, e.g., acute humoral rejection in kidney transplant recipients [22], ANCA-associated vasculitis [23], idiopathic membranous glomerulonephritis [24], and resistant cases of rheumatoid arthritis [25].
Currently, no data exist standardizing a successful treatment for kidney allografts with CAMR. Therefore, we performed a retrospective analysis of subjects diagnosed with CAMR at Massachusetts General Hospital (MGH) between 1997 and 2007 and compared the outcomes of those who received Rituximab. Our goal is to determine if combined therapy of Rituximab followed by maintenance MMF and tacrolimus improves long term kidney allograft function.
2. Materials and methods
2.1. Subjects
Subjects diagnosed with CAMR at the Massachusetts General Hospital (MGH) had kidney allograft biopsies from 1997 to 2007 were retrospectively reviewed under an institutional investigational review board approval and. All biopsies were for cause, elevated creatinine with or without proteinuria. Criteria for this diagnosis included transplant glomerulopathy (glomerular basement duplication), C4d staining of the peritubular capillaries by immunofluorescence, and presence of DSA (Table 1). Exclusion criteria included diagnoses of acute cellular rejection, acute antibody mediated rejection, de novo or recurrent glomerular disease, and thrombotic microangiopathy. One subject with de novo membranous nephropathy, considered a variant of CAMR [26–29], was also included. Data for proteinuria were too limited to evaluate. Of these 31 subjects identified, 14 subjects (Rituximab study group) received Rituximab alone and/or in combination with other therapies, including solumedrol, Thymoglobulin (ATG), plasmapheresis, intravenous human immunoglobulin (IVIG) or actinomycin with or without a change in their baseline immunosuppression (Table 1). The control group of 17 subjects was treated with therapies other than Rituximab or received no change in therapy (Table 1). Clinical data were obtained from electronic medical records and clinical databases included subject demographics (age at kidney biopsy, gender), allograft variables (number of cadaveric donors, living donors and previous transplants), and post-transplant variables (episodes of rejection, time from transplantation to kidney allograft biopsy, and serum creatinine). Response to Rituximab was defined as decline or stabilization of serum creatinine for at least one year. Sequential creatinines were followed, and the endpoint was either date of the creatinine nearest to the time of death (four subjects), the date of initiation of dialysis, or the date of the most recent creatinine in those subjects who responded to Rituximab. A total of 590 serum creatinines for all subjects was available (mean=19, range 4–47).
Table 1.
Patient demographics.
| Subject | Group | Immunosuppressions at biopsy | Immunosuppressions at post-biopsy | Patient age (years) at biopsy | Patient age (years) at Transplant | Death | Donor | Original disease | Race | Sex |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Control | C, A, P | C, MMF, P | 49.4 | 45.4 | No | CD | Diabetes | Caucasian | M |
| 2 | Control | C, P | C, MMF, P | 51.3 | 33.2 | No | CD | Diabetes | Caucasian | M |
| 3 | Control | FK, P, MMF | FK, P, MMF | 46.9 | 39.0 | No | CD | Obstruction | AfroAmerican | M |
| 4 | Control | FK, P, MMF | FK, P, MMF, IVIG | 59.5 | 56.9 | Yes | CD | ADPKD | Hispanic | M |
| 5 | Control | MMF, S | MMF, P | 68.8 | 65.5 | Yes | CD | Diabetes | Caucasian | M |
| 6 | Control | C, MMF, P | FK, P, MMF | 45.2 | 38.3 | No | LRD | HTN | Caucasian | F |
| 7 | Control | C, A | C, MMF | 51.2 | 44.2 | No | LRD | HTN | Caucasian | M |
| 8 | Control | C, A, P | C, MMF, P | 17.8 | 7.6 | No | CD | Dysplasia | Caucasian | M |
| 9 | Control | C, MMF | C, MMF | 66.6 | 46.0 | No | CD | Alport’s | Caucasian | M |
| 10 | Control | C, MMF, P | C, MMF, P | 40.4 | 31.8 | No | CD | IgA | Caucasian | M |
| 11 | Control | C, A | C, MMF, P | 70.5 | 58.4 | No | LRD | IgA | Caucasian | M |
| 12 | Control | C, MMF | FK, MMF | 48.4 | 39.1 | Yes | LRD | Diabetes | Caucasian | M |
| 13 | Control | C, A, P | C, MMF, P | 33.8 | 27.6 | No | CD | IgA | Hispanic | M |
| 14 | Control | C, A, P | C, MMF, P | 36.1 | 30.8 | No | LRD | Post Strep GN | Caucasian | F |
| 15 | Control | FK, P, MMF | FK, P, MMF | 55.8 | 49.0 | No | CD | ADPKD | Caucasian | M |
| 16 | Control | FK, P, MMF | FK, P, MMF | 50.3 | 43.0 | No | CD | FSGS | Caucasian | M |
| 17 | Control | A, P | MMF, P | 37.4 | 21.6 | No | CD | FSGS | Caucasian | M |
| 18 | RNR | C, A, P | FK, P, MMF, R | 32.7 | 18.5 | No | LRD | Obstruction | Caucasian | M |
| 19 | RNR | A, P | FK, P, MMF, R | 42.4 | 22.5 | No | LRD | GN | Caucasian | F |
| 20 | RNR | C, A | FK, P, MMF, R | 38.5 | 16.9 | No | LRD | IgA | Caucasian | M |
| 21 | RNR | FK, P, MMF | FK, P, MMF, R | 58.8 | 52.5 | No | CD | Lupus | AfroAmerican | F |
| 22 | RNR | FK, P, MMF | FK, P, MMF, R | 48.7 | 43.0 | No | LURD | Lupus | Caucasian | M |
| 23 | RNR | FK, MMK | FK, P, MMF, R | 45.2 | 40.0 | No | LRD | Diabetes | Caucasian | M |
| 24 | RR | C, A | FK, P, MMF, R | 63.6 | 48.6 | No | CD | Chronic Pyelonephritis | Caucasian | M |
| 25 | RR | FK, MMF | FK, P, MMF, R | 63.6 | 52.6 | No | CD | Dysplasia | Caucasian | F |
| 26 | RR | FK, P | FK, P, MMF, R | 76.6 | 64.3 | No | CD | Analgesic Nephropathy | Caucasian | M |
| 27 | RR | FK, P, MMF | FK, P, MMF, R | 59.2 | 56.6 | No | LRD | ADPKD | Caucasian | M |
| 28 | RR | FK, P, MMF | FK, P, MMF, R | 20.8 | 19.1 | No | CD | FSGS | AfroAmerican | M |
| 29 | RR | FK, P, MMF | FK, P, MMF, R | 11.3 | 7.9 | No | LURD | MCD | Caucasian | M |
| 30 | RR | P, MMF, S | FK, P, MMF, R | 69.7 | 63.2 | No | LURD | Diabetes | Hispanic | M |
| 31 | RR | FK, P, MMF | FK, P, MMF, R | 29.9 | 25.0 | Yes | LURD | Alport’s | Caucasian | M |
| Control | 48.8+/−13.5* | 39.9+/−14.1* | ||||||||
| RNR | 44.4+/−9.0* | 32.2+/−14.9* | ||||||||
| RNR | 49.3+/−24.8* | 42.2+/−21.7* |
There was an excess of cyclosporine over FK treatment in the non-Rituximab treated control subjects vs the Rituximab treated subjects before biopsy: Pearson=0.01. This likely reflects historical treatment protocols.
There was no statistical difference in the usage of Azathioprine vs MMF in non-Rituximab treated control subjects vs the Rituximab treated subjects.
There was statistical no difference between Rituximab treated vs Non-Rituximab treated subjects or between responder vs non-responder subjects for 1) death, 2) original disease, 3) race, 4) gender, or 5) living/deceased donation.
Abbreviations: C: cyclosporine; A: azathioprine; P: prednisone; MMF: mycophenolate; FK: tacrolimus; S: sirolimus; R: Rituximab; IVIG: intravenous human immunoglobulin; DD: deceased donor; LRD: living related donor; LURD: living unrelated donor; MCD: medullary cystic disease; RNR: Rituximab non-responder; RR: Rituximab responder. ADPKD: autosomal dominant polycystic kidney disease. FSGS: focal and segmental glomerulosclerosis. IgA: IgA nephropathy. GN: glomerulonephritis not specified. ESRD, end stage renal disease, not specified. HTN: hypertension.
Age: no statistical significance between Rituximab treated and control groups; no statistical significance among control, responder, or non-responder subjects.
2.2. Pathology
Biopsies of 31 subjects were analyzed for the presence of C4d by immunofluorescence [30] and were scored according to Banff criteria [3,31], including interstitial fibrosis and tubular atrophy, supplemented by a score for hyaline arteriolopathy [32] and peritubular capillary inflammation [33]. Peritubular laminations identified by electron microscopy (EM) were scored according to Ivanyi [34]. Insufficient paraffin tissue was available to perform immunohistochemistry for CD20.
2.3. Anti-HLA antibodies and DSA
Pre- and post-biopsy anti-HLA antibodies and or donor specific antibodies (DSA) were identified in all 31 subjects as previously described [2].
2.4. Immunosuppression management
Control subjects received minor modifications in immunosuppression after their diagnosis of CAMR, which mostly commonly was a change from azathioprine to mycophenolate (MMF) (41%). Control subject #4 also received intravenous human immunoglobulin (IVIG). Fourteen subjects received Rituximab (375 mg/m2) with a total of 3 to 5 doses. Eight of 14 subjects received Rituximab as the only therapy for CAMR while 6 subjects received Rituximab plus other therapy. In addition to Rituximab, subject #18 received ATG and plasmapheresis; subject #21 received ATG, plasmapheresis, and IVIG; subject #24 received prednisone and IVIG; subject #27 received actinomycin; subject #28 received ATG and IVIG; and subject #31 received plasmapheresis and IVIG. After the diagnosis of CAMR, the baseline immunosuppression was changed to mycophenolate (Cellcept), and tacrolimus (Prograf) in those who previously had received cyclosporine and azathioprine. Three of 14 subjects who were not on prednisone prior to diagnosis of CAMR were placed on prednisone after diagnosis.
2.5. Statistical review
Kidney transplant survival estimates were evaluated by linear regression and survival by Kaplan–Meier (JMP 8.0.2, SAS Institute Inc.). Other data were analyzed by ANOVA (JMP 8.0.2, SAS Institute Inc.). Linear regression and comparison of slopes were performed on all available creatinines for each subject (total 590 for all subjects, mean 19 for all subjects with a minimum 4 and maximum 47 for individual subjects) (JMP 8.0.2, SAS Institute Inc., StatsitiXL 1.8; http://www.statistixl.com).
3. Results
3.1. All subjects
The 31 subjects identified in this study were diagnosed with CAMR (Tables 1 and 2). Biopsies were for cause, either an increase in creatinine or proteinuria. The median time of diagnosis after transplant was 7.0 years (range 1.7 to 21.6 years). The mean creatinine at diagnosis was 2.9 +/− 1.1 mg/dl (range 1.6 to 6.1). Native kidney disease and other demographics are listed in Tables 1 and 2 along with baseline immunosuppression at the time of biopsy. Control subjects were receiving maintenance cyclosporine (76%) or FK (24%). In the Rituxan group, pre-Rituxan maintenance immunosuppression include cyclosporine (21%), FK (64%), or no calcineurin (14%). There is no statistical significance (P=.47) in Azathioprine vs MMF maintenance therapy at the time of biopsy. 29% of controls received a living donor transplant as compared to 64% of the Rituximab treated subjects. In the Rituximab non-responder group, 83% of the subjects received a living donor kidney as compared to 50% of the Rituximab responders. There was no statistical significance to the variation in race or sex of the control or Rituximab treated subjects.
Table 2.
Subject transplant data.
| Patient | Group | Duration (years) at Bx | Total duration (years) Tx | Creatinine mg/dl at biopsy | Intercept | Slope/year | R2 | Renal survival (days) after biopsy |
|---|---|---|---|---|---|---|---|---|
| 1 | Control | 4.0 | 6.0 | 3.3 | 2.9 | 2.0 | 0.8 | 727 |
| 2 | Control | 18.1 | 21.5 | 2.2 | 2.0 | 1.3 | 1.0 | 1252 |
| 3 | Control | 8.0 | 9.0 | 2.4 | 1.5 | 3.6 | 0.6 | 365 |
| 4 | Control | 2.6 | 3.3 | 2.3 | 2.2 | 4.3 | 0.9 | 249 |
| 5 | Control | 3.2 | 3.4 | 3.6 | 3.7 | 3.7 | 0.9 | 54 |
| 6 | Control | 6.9 | 8.4 | 1.7 | 1.5 | 2.3 | 0.8 | 536 |
| 7 | Control | 7.0 | 8.7 | 2.5 | 2.3 | 1.6 | 0.6 | 637 |
| 8 | Control | 10.2 | 15.1 | 1.7 | 1.2 | 0.8 | 0.8 | 1775 |
| 9 | Control | 20.5 | 22.1 | 3.3 | 3.3 | 1.4 | 1.0 | 565 |
| 10 | Control | 8.6 | 9.2 | 6.1 | 5.0 | 1.8 | 0.5 | 248 |
| 11 | Control | 12.1 | 12.4 | 4.0 | 4.0 | 10.8 | 1.0 | 101 |
| 12 | Control | 9.3 | 10.5 | 2.2 | 2.1 | 0.2 | 0.8 | 424 |
| 13 | Control | 6.2 | 6.8 | 5.9 | 4.3 | 0.9 | 0.6 | 213 |
| 14 | Control | 5.3 | 5.8 | 3.9 | 3.9 | 3.8 | 0.6 | 164 |
| 15 | Control | 6.8 | 8.1 | 1.6 | 1.8 | 2.9 | 0.9 | 463 |
| 16 | Control | 7.3 | 8.5 | 1.9 | 1.5 | 1.6 | 0.6 | 439 |
| 17 | Control | 15.8 | 18.5 | 3.1 | 3.0 | 1.0 | 0.6 | 1014 |
| 18 | RNR | 14.2 | 14.4 | 3.4 | 3.5 | 5.7 | 0.7 | 62 |
| 19 | RNR | 19.9 | 20.5 | 2.4 | 2.4 | 9.2 | 0.8 | 196 |
| 20 | RNR | 21.6 | 22.8 | 2.9 | 2.7 | 1.3 | 0.9 | 428 |
| 21 | RNR | 6.3 | 7.8 | 3.3 | 3.6 | 1.0 | 0.6 | 540 |
| 22 | RNR | 5.7 | 6.9 | 2.4 | 2.1 | 3.5 | 0.9 | 434 |
| 23 | RNR | 5.2 | 6.4 | 3.0 | 3.2 | 0.9 | 0.6 | 448 |
| 24 | RR | 15.0 | 19.4 | 2.5 | 1.9 | −0.2 | 0.5 | >1706 |
| 25 | RR | 10.9 | 14.2 | 2.6 | 2.4 | 0.1 | 0.6 | >1268 |
| 26 | RR | 12.3 | 16.4 | 2.1 | 2.1 | 0.1 | 0.7 | 1067 |
| 27 | RR | 2.5 | 8.0 | 2.2 | 2.6 | −0.1 | 0.6 | >2063 |
| 28 | RR | 1.7 | 6.3 | 3.5 | 2.3 | −0.1 | 0.7 | >1723 |
| 29 | RR | 4.6 | 5.6 | 2.0 | 1.7 | 1.0 | 0.5 | 831 |
| 30 | RR | 6.5 | 10.2 | 2.8 | 1.9 | 0.8 | 0.8 | 1092 |
| 31 | RR | 4.9 | 8.7 | 2.2 | 2.1 | −0.4 | 0.4 | 411 |
| Control | Mean | 8.9 | 10.4 | 3.0 | 2.7 | 2.6 | 0.7 | 542.0 |
| Control | SD | 5.1 | 5.7 | 1.4 | 1.2 | 2.4 | 0.2 | 447.0 |
| Control | Median | 7.3 | 439.0 | |||||
| Rituximab | Mean | 9.4 | 12.0 | 2.7 | 2.5 | 1.6 | 0.7 | 876.0 |
| Rituximab | SD | 6.4 | 5.9 | 0.5 | 0.6 | 2.7 | 0.1 | 625.0 |
| Rituximab | Median | 6.4 | 685.5 | |||||
| RNR | Mean | 12.2 | 13.1 | 2.9 | 2.9 | 3.6 | 0.7 | 351.0 |
| RNR | SD | 7.5 | 7.2 | 0.4 | 0.6 | 3.3 | 0.1 | 182.0 |
| RNR | Median | 431.0 | ||||||
| RR | Mean | 7.3 | 11.1 | 2.5 | 2.1 | 0.2 | 0.6 | 1270.0 |
| RR | SD | 4.9 | 5.0 | 0.5 | 0.3 | 0.5 | 0.1 | 538.0 |
| RR | Median | 1180.0 |
Control: no Rituximab; RNR: Rituximab non-responder; RR: Rituximab responder; Rituximab: all subjects given Rituximab.
In addition to the diagnosis of CAMR, these subjects had variable degrees of hyaline arteriolopathy, tubular atrophy, and interstitial fibrosis, which correlated with the age of the transplant at the time of diagnosis (P<0.001), but no statistical association was identified for these parameters between or among any of the three groups at the time of biopsy. Potential causes for chronic rejection such as non-compliance, dose reduction or change in immunosuppression due to cutaneous squamous cell carcinoma, PTLD, or presumed chronic calcineurin inhibitor toxicity were present in 18 of the 31 subjects (58%), 7 of 17 in the control group (41%) and 11 of 14 (79%) subjects in the Rituximab group.
Diffuse staining of the PTC for C4d was seen in 77.4% by immunofluorescence (Banff C4d3, >50%), and 22.6% had focal staining (Banff C4d2, 10–50%). By electron microscopy (EM) peritubular capillary laminations were identified with 64% (IV2) and 36% (IV1)[34]. Glomerular basement membrane (GBM) duplication was focal in 81.5% of the cases and circumferential in 18.5%. GBM laminations were identified focally in 34.6%, circumferential in 19.2%, and absent in 46.2% of the cases. There was no statistical significance for these findings between groups. Deposits were identified in one subject with de novo membranous, (Ehrenreich and Churg stage three), and interpreted as a variant of CAMR.
Of all the subjects, 55% had only class II alloantibodies, 24% had only class I alloantibodies, and 21% had both class I and class II alloantibodies. In the control groups, 67% had only class II, 13% had only class I, and 20% had both class I and class II alloantibodies. In the Rituximab treated group 43% had only class II; 36% had only class I, and 22% had both.
3.2. Control group
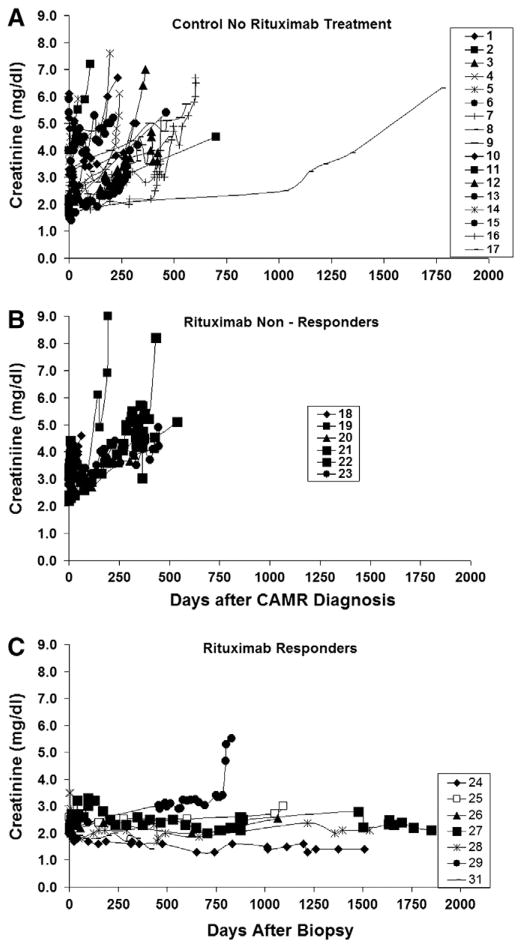
In the control group, the median time diagnosis of CAMR was 7.3 years (range 2.6 to 20.5 years) after transplantation with mean creatinines at diagnostic biopsy of 3.0 mg/dl +/− 1.4 (range 1.6 to 6.1), Table 2. The slope of the regression line for sequential creatinines vs time after biopsy in the control group was 0.6 × years with an interpolated creatinine intercept of 3.3, (P<.001), (Fig. 1A). Day zero is the day of biopsy, and all available creatinines are plotted. Table 2 shows summaries of individual regressions with the annual creatinine rise per year (slope) and the interpolated creatinine at day zero, which is close to the actual initial creatinine. R2 verify a good model fit for the regressions of individual creatinines. The median allograft survival time for control subjects (Fig. 1A) was 439 days after the date of diagnosis (range 54 to 1775 days). One subject (#8) had a more protracted course as compared to other subjects, suggesting that a long latency may occur in some subjects before the creatinine rises significantly. This subject (#8) had a lower initial creatinine of 1.7 mg/dl as compared to the mean for the group of 2.9 mg/dl. However, a lower initial creatinine at diagnosis does not guarantee a long latency because subject #15 also had an initial creatinine of 1.6 mg/dl but developed allograft failure by 462 days. While on dialysis, three deaths occurred in the control group, subject numbers 9, 15, and 17.
Fig. 1.
Sequential creatinines for all patients from the time of biopsy (day zero) until endpoint (death, dialysis, or most currently available date for those with surviving kidney transplants). A: control subjects. B: subjects with a clinical response to Rituximab. C: subjects without a clinical response to Rituximab.
3.3. Rituximab group
In the Rituximab group (n=14), the median time of diagnosis of CAMR was 6.4 years (range 1.7 to 21.6 years) after kidney transplantation (Table 1). The mean creatinine at biopsy was 2.7 +/− 0.5 mg/dl (range 2.0 to 3.5). Table 2 shows summaries of individual regressions with the annual creatinine rise per year (slope) and the interpolated creatinine at day zero, which is close to the actual initial creatinine. R2 also verify a good model fit for the individual creatinine regressions, Table 2. Day zero is the day of biopsy, and all available creatinines are plotted. The linear regression of time after biopsy vs sequential creatinines for the Rituximab group showed markedly heterogeneous data with a lack of model fit for the regression, R2 was 0.06, (P= 0.16). The poor model fix indicates that the response of the Rituximab treated group is markedly heterogeneous. The median survival time for the Rituximab group was 685 days (range 62 to 2063 days) as compared to 439 days for the control group, which were not statistically significant comparing median or mean survival times.
The clinical response in Rituximab treated subjects clearly was dichotomous with some subjects showing stabilization or lowering of creatinine while others showed no response. Therefore, the two groups of Rituximab treated patients were analyzed separately, (Fig. 1B and C). Sequential creatinines vs time after renal biopsy for the Rituximab treated group are plotted in Fig. 1B and C as non-responders in Fig. 1B, and responders in Fig. 1C.
3.4. No response to Rituximab—non-responders
Fig. 1B shows that six of the 14 subjects treated with Rituximab had a deterioration of allograft function with a median renal survival time 431 days (range 62 to 540 days) (Table 2). The slope of the regression line for creatinines in the non-responder group was 1.38 × years with an interpolated creatinine intercept of 3.2, which was close to the actual mean creatinine of 2.9 +/− 0.4 mg/dl (range 2.4 to 3.4). Table 2 shows individual regressions with the annual creatinine rise per year (slope) and the interpolated creatinine at day zero, which is close to the actual initial creatinine. R2 also verify a good model fit for the individual creatinine regressions. No deaths occurred in the non-responder group.
3.5. Clinical response to Rituximab—responders
Eight of the 14 subjects in the Rituximab group showed a response by a decline or stabilization in serum creatinine at 1 year or greater of follow-up, Fig. 1B. The median renal survival time was 1180 days (range 411 to 2063 days). The slope of the regression line for creatinines in the responder group was 0.08 × years with an interpolated creatinine intercept of 2.3, and a mean initial creatinine of 2.5 +/− 0.5 mg/dl. Table 2 shows summaries of individual regressions with the annual creatinine rise per year (slope) and the interpolated creatinine at day zero, which is close to the actual initial creatinine. R2 also verify a good model fit for the individual creatinine regressions. Five of the eight responders (#24, #25, #27 and #28) had sustained remissions greater than 1000 days. Two subjects (#29 and #30) had responses greater than 750 days but then rapidly developed allograft failure. The durability of the response in subject #31, whose creatinine has stabilized to 1.4 from an initial creatinine of 2.2, is unknown because the last included creatinine is day 411, after which non-compliance occurred. Subject #26 died with a functioning kidney and a creatinine of 2.6 mg/dl 1067 days after the diagnosis of CAMR.
3.6. Comparison of creatinine slopes, initial creatinines, and survival times
Comparing the slopes of the three groups (control, responders, and non-responders) using all 590 creatinines showed statistical significance (P<0.001) for the responder slope as compared to the control or the non-responder groups. There was no statistical significance between the slopes for the control and non-responder groups (P=0.07). There was no statistical significance by ANOVA for the initial creatinines (creatinines at the time of biopsy) by post-hoc tests for the three groups or for the treatment and control groups. Intercepts (interpolated day zero creatinines) were indistinguishable between the three groups. Comparing allograft survival for the Rituximab group, including both responders and non-responders, there is no statistical significance in post-treatment survival as compared to controls for mean or median survival times. Comparing allograft survival after biopsy, only the Rituximab responders are statistically significant to all other groups by post-hoc ANOVA (means), (P<0.002) or non-parametric medians, Kruskal–Wallis, (P<0.007).
3.7. Survival analysis
Fig. 2A shows the Kaplan–Meier allograft survival curve for the Rituximab treated and the control group, which for censored data is statistically significant (log rank P= 0.05 and Wilcoxon P=0.05) but is not statistically significant for uncensored data. Fig. 2B shows the Kaplan–Meier allograft survival curves for the three groups with a statistical significance of P<.001 (log rank and Wilcoxon) for the censored data and P=0.001 for uncensored data, which is due to the prolonged kidney allograft survival of the responder group. There was no statistical significance in survival between the controls and non-responders, P=0.23. Fig. 2C shows a probability estimate for the Rituximab treated and control groups, which is not statistically significant. Fig. 2D shows a probability estimate for survival times for the three groups showing that the responders had about three fold increase in 50% allograft survival, which is statistically significant, P=0.005.
Fig. 2.
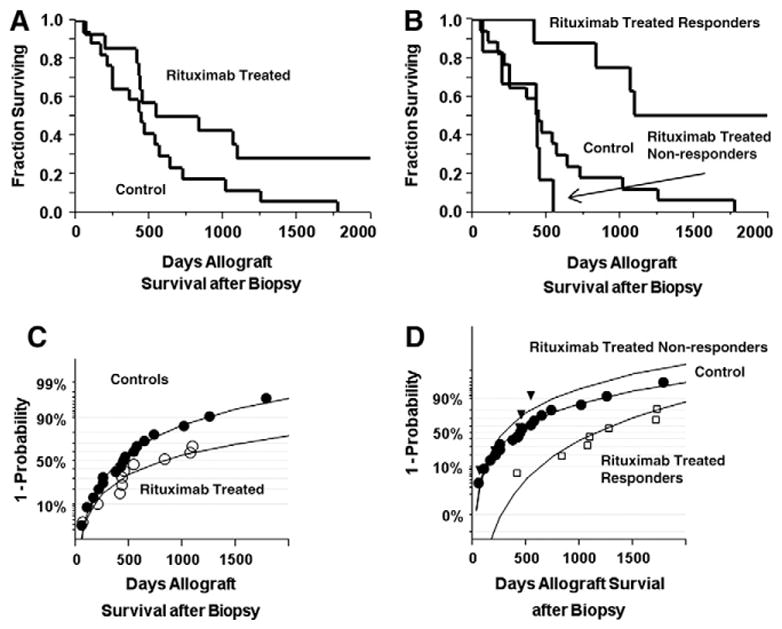
Survival analyses. A: Kaplan–Meier plot of kidney transplant survival for control and Rituximab treated patients. B: Kaplan–Meier plot of kidney transplant survival for control and Rituximab responder and non-responder subjects. C: probability of kidney transplant survival for control and Rituximab treated subjects. D: probability of kidney transplant survival for control, responder, and non-responder subjects.
3.8. Effect of Rituximab on alloantibody titers
Alloantibodies remained unchanged in 92% of control subjects, in 64% of Rituximab treated subjects, in 67% in the Rituximab non-responders, and 60% of the Rituximab responders.
3.9. Adverse events to Rituximab
There were no reported side-effects to Rituximab such as rash, fever, hypotension, or anaphylaxis. There were no significant changes in hemoglobin, platelet or white cell counts. Despite valganciclovir prophylaxis, one subject developed CMV viremia after ATG and a second dose of Rituximab. There were no documented cases of CMV or BKV.
3.10. Pathology
To try to identify pathological correlates that might explain the variability of the response to Rituximab therapy, we performed ANOVA, ordinal logistic regression and multivariate linear regression on nine Banff scores (Table 3): Post-hoc tests by ANOVA failed to identify any pathological correlations within subject groups, including interstitial fibrosis and tubular atrophy (IFTA). Logistic or linear regression also failed to identify any pathological factor or initial creatinine as a predictor of any group. Tubular atrophy and interstitial fibrosis correlated by multivariate and principal component analyses with the duration of the transplant but did not partition among subject groups.
Table 3.
Tranplant biopsy pathological data.
| Patient | Group | Hyaline | Glomerulitis | Glomerulopathy | Gibson PTC* | Mesangial expansion | Percent fibrosis | Percent infiltrate | Tubular atrophy | Tubulitis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Control | 2 | 1 | 2 | 1 | 0 | 2 | 2 | 2 | 1 |
| 2 | Control | 2 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 1 |
| 3 | Control | 1 | 2 | 3 | 1 | 1 | 1 | 0 | 0 | 1 |
| 4 | Control | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 |
| 5 | Control | 3 | 2 | 3 | 1 | 2 | 3 | 1 | 3 | 0 |
| 6 | Control | 3 | 1 | 2 | 2 | 1 | 2 | 1 | 2 | 0 |
| 7 | Control | 2 | 1 | 2 | 1 | 0 | 2 | 0 | 2 | 1 |
| 8 | Control | 1 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 0 |
| 9 | Control | 0 | 1 | 2 | 1 | 0 | 3 | 1 | 2 | 0 |
| 10 | Control | 2 | 2 | 3 | 1 | 1 | 2 | 1 | 2 | 0 |
| 11 | Control | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 0 |
| 12 | Control | 0 | 1 | 2 | 2 | 0 | 1 | 0 | 1 | 1 |
| 13 | Control | 2 | 2 | 3 | 1 | 1 | 1 | 0 | 1 | 0 |
| 14 | Control | 2 | 1 | 3 | 2 | 1 | 1 | 0 | 1 | 0 |
| 15 | Control | 0 | 1 | 2 | 1 | 1 | 2 | 0 | 2 | 0 |
| 16 | Control | 3 | 1 | 3 | 2 | 1 | 0 | 0 | 0 | 0 |
| 17 | Control | 1 | 1 | 2 | 1 | 1 | 2 | 0 | 2 | 0 |
| 18 | RNR | 3 | 1 | 3 | 2 | 2 | 2 | 1 | 1 | 0 |
| 19 | RNR | 3 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 0 |
| 20 | RNR | 2 | 1 | 2 | 2 | 1 | 1 | 0 | 1 | 1 |
| 21 | RNR | 1 | 2 | 3 | 2 | 1 | 1 | 1 | 1 | 0 |
| 22 | RNR | 1 | 1 | 3 | 2 | 1 | 1 | 1 | 1 | 0 |
| 23 | RNR | 3 | 2 | 2 | 1 | 2 | 1 | 0 | 1 | 1 |
| 24 | RR | 1 | 2 | 2 | 2 | 0 | 1 | 2 | 1 | 0 |
| 25 | RR | 3 | 1 | 3 | 1 | 2 | 2 | 0 | 2 | 1 |
| 26 | RR | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 1 | 0 |
| 27 | RR | 3 | 1 | 3 | 1 | 1 | 0 | 1 | 0 | 0 |
| 28 | RR | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 |
| 29 | RR | 1 | 2 | 2 | 2 | 1 | 2 | 0 | 2 | 0 |
| 30 | RR | 1 | 2 | 3 | 1 | 3 | 1 | 0 | 1 | 0 |
| 31 | RR | 0 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 0 |
Control: subjects not given Rituximab; RNR: Rituximab non-responders; RR: Rituximab responders;
peritubular capillaries.
4. Discussion
Previous studies have shown decreased kidney allograft survival in patients with CAMR (6–9). This study confirms those findings with control and Rituximab non-responder group having a median allograft survival post diagnosis of 439 days and 431 days, respectively. In the Rituximab responder group, median kidney survival is 1180 days. The variable response in kidney allograft survival between the Rituximab treated responder and Rituximab treated non-responder groups is striking, and quite dichotomous.
Kidney biopsy was done for cause in these 31 subjects, either elevated serum creatinine or proteinuria. It is likely that the diagnosis CAMR was made late in the disease process in most subjects. Many of these subjects show a slow rise in creatinine, but when the creatinine approaches ~3 mg/dl, a tipping point occurs with a precipitous decline in GFR with creatinines rising about 1 mg/dl per year. This was also true for two subjects (#24, #30) in the responder group, who had a remission with Rituximab for over 750 days but then developed allograft failure when the creatinine approached ~3 mg/dl. It is unknown in these subjects when the alloantibodies were first developed and how long the interval was between the time of the first appearance of alloantibodies and the diagnostic biopsy. The latency interval is, therefore, unknown between the first appearance of alloantibodies and the later development of proteinuria, rising creatinine, and diagnostic pathology. The interval is likely to be years for most subjects, and subject #8 in the control group may represent a more typical example of the natural history of human chronic alloantibody mediated rejection.
Limitations of the study include those associated with any retrospective study including observer bias. This study also has a small sample size of treated subjects which can cause the heterogeneity of the Rituximab response.
Rituximab rapidly depletes B cells by apoptotic, complement-dependent, antibody dependent cytoxic mechanisms [35,36]. The reductions in antibodies were at best modest and inconsistent [36,37] so that the clinical efficacy of Rituximab in some subjects cannot be attributed to a decrease in titer. In Rituximab treated ANCA-associated vasculitis efficacious clinical end points often occur before the predicted reduction in antibody titers [23]. Our data are consistent with this pattern because stabilization of creatinine occurred well before an expected decrease in DSA, ~2 months (Fig. 1B). In addition, 60% of the subjects responded to Rituximab without a reduction in DSA. The efficacy of Rituximab could be related to the loss of B cells and their antigen presenting function [38,39] or their capacity to secrete proinflammatory cytokines. If the doses of Rituximab were sufficient to deplete B cells within the allograft, their elimination might improve graft function assuming intragraft B cells are deleterious to graft function [40,41].
In summary, these data show that Rituximab followed by standard maintenance immunosuppression shows a therapeutic effect in the treatment of CAMR, which is confined to a subset of treated subjects, which cannot be identified a priori.
Footnotes
R.N.S., F.M., N.G., A.B.F., E.Z., S.S., N.T.-R., S.P., and W.W. participated in data collection. R.N.S., F.M., N.G., and N.T.-R. participated in the research design. R.N.S., F.M., N.G., A.B.F., N.T.-R., E.Z. and W.W. participated in preparation of the manuscript. F.M., N.G., and N.T.-R. participated in clinical care. R.N.S. performed statistical analyses.
References
- 1.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, et al. Banff ‘05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’) Am J Transplant. 2007;7:518–26. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 2.Mauiyyedi S, Pelle PD, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, et al. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12:574–82. doi: 10.1681/ASN.V123574. [DOI] [PubMed] [Google Scholar]
- 3.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, et al. Antibody-mediated rejection criteria—An addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–14. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 4.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–12. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol. 2009;5:513–9. doi: 10.1038/nrneph.2009.113. [DOI] [PubMed] [Google Scholar]
- 6.Aita K, Yamaguchi Y, Shimizu T, Horita S, Furusawa M, Tanabe K, et al. Histological analysis of late renal allografts of antidonor antibody positive patients with C4d deposits in peritubular capillaries. Clin Transplant. 2004;18(Suppl 11):7–12. doi: 10.1111/j.1399-0012.2004.00240. [DOI] [PubMed] [Google Scholar]
- 7.Cosio FG, Gloor JM, Sethi S, Stegall MD. Transplant glomerulopathy. Am J Transplant. 2008;8:492–6. doi: 10.1111/j.1600-6143.2007.02104.x. [DOI] [PubMed] [Google Scholar]
- 8.Hidalgo LG, Campbell PM, Sis B, Einecke G, Mengel M, Chang J, et al. De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am J Transplant. 2009;9:2532–41. doi: 10.1111/j.1600-6143.2009.02800.x. [DOI] [PubMed] [Google Scholar]
- 9.Kieran N, Wang X, Perkins J, Davis C, Kendrick E, Bakthavatsalam R, et al. Combination of peritubular c4d and transplant glomerulopathy predicts late renal allograft failure. J Am Soc Nephrol. 2009;20:2260–8. doi: 10.1681/ASN.2009020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520–31. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 11.Gloor JM, Sethi S, Stegall MD, Park WD, Moore SB, DeGoey S, et al. Transplant glomerulopathy: subclinical incidence and association with alloantibody. Am J Transplant. 2007;7:2124–32. doi: 10.1111/j.1600-6143.2007.01895.x. [DOI] [PubMed] [Google Scholar]
- 12.Ishii Y, Sawada T, Kubota K, Fuchinoue S, Teraoka S, Shimizu A. Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int. 2005;67:321–32. doi: 10.1111/j.1523-1755.2005.00085.x. [DOI] [PubMed] [Google Scholar]
- 13.Sis B, Campbell PM, Mueller T, Hunter C, Cockfield SM, Cruz J, et al. Transplant glomerulopathy, late antibody-mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. Am J Transplant. 2007;7:1743–52. doi: 10.1111/j.1600-6143.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 14.Regele H, Bohmig GA, Habicht A, Gollowitzer D, Schillinger M, Rockenschaub S, et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002;13:2371–80. doi: 10.1097/01.asn.0000025780.03790.0f. [DOI] [PubMed] [Google Scholar]
- 15.Opelz G. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005;365:1570–6. doi: 10.1016/S0140-6736(05)66458-6. [DOI] [PubMed] [Google Scholar]
- 16.Piazza A, Poggi E, Borrelli L, Servetti S, Monaco PI, Buonomo O, et al. Impact of donor-specific antibodies on chronic rejection occurrence and graft loss in renal transplantation: posttransplant analysis using flow cytometric techniques. Transplantation. 2001;71:1106–12. doi: 10.1097/00007890-200104270-00017. [DOI] [PubMed] [Google Scholar]
- 17.Suciu-Foca N, Reed E, D’Agati VD, Ho E, Cohen DJ, Benvenisty AI, et al. Soluble HLA antigens, anti-HLA antibodies, and antiidiotypic antibodies in the circulation of renal transplant recipients. Transplantation. 1991;51:593–601. doi: 10.1097/00007890-199103000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3:665–73. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 19.Worthington JE, McEwen A, McWilliam LJ, Picton ML, Martin S. Association between C4d staining in renal transplant biopsies, production of donor-specific HLA antibodies, and graft outcome. Transplantation. 2007;83:398–403. doi: 10.1097/01.tp.0000251430.11723.b6. [DOI] [PubMed] [Google Scholar]
- 20.Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, et al. Chronic antibody mediated rejection of renal allografts: pathological, serological and immunologic features in nonhuman primates. Am J Transplant. 2006;6:1790–8. doi: 10.1111/j.1600-6143.2006.01351.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith RN, Kawai T, Boskovic S, Nadazdin O, Sachs DH, Cosimi AB, et al. Four stages and lack of stable accommodation in chronic alloantibody-mediated renal allograft rejection in Cynomolgus monkeys. Am J Transplant. 2008;8:1662–72. doi: 10.1111/j.1600-6143.2008.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faguer S, Kamar N, Guilbeaud-Frugier C, Fort M, Modesto A, Mari A, et al. Rituximab therapy for acute humoral rejection after kidney transplantation. Transplantation. 2007;83:1277–80. doi: 10.1097/01.tp.0000261113.30757.d1. [DOI] [PubMed] [Google Scholar]
- 23.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggenenti P, Chiurchiu C, Abbate M, Perna A, Cravedi P, Bontempelli M, et al. Rituximab for idiopathic membranous nephropathy: who can benefit? Clin J Am Soc Nephrol. 2006;1:738–48. doi: 10.2215/CJN.01080905. [DOI] [PubMed] [Google Scholar]
- 25.Bokarewa M, Lindholm C, Zendjanchi K, Nadali M, Tarkowski A. Efficacy of anti-CD20 treatment in patients with rheumatoid arthritis resistant to a combination of methotrexate/anti-TNF therapy. Scand J Immunol. 2007;66:476–83. doi: 10.1111/j.1365-3083.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- 26.Heidet L, Gagnadoux ME, Beziau A, Niaudet P, Broyer M, Habib R. Recurrence of de novo membranous glomerulonephritis on renal grafts. Clin Nephrol. 1994;41:314–8. [PubMed] [Google Scholar]
- 27.Monga G, Mazzucco G, Basolo B, Quaranta S, Motta M, Segoloni G, et al. Membranous glomerulonephritis (MGN) in transplanted kidneys: morphologic investigation on 256 renal allografts. Mod Pathol. 1993;6:249–58. [PubMed] [Google Scholar]
- 28.Truong L, Gelfand J, D’Agati V, Tomaszewski J, Appel G, Hardy M, et al. De novo membranous glomerulonephropathy in renal allografts: a report of ten cases and review of the literature. Am J Kidney Dis. 1989;14:131–44. doi: 10.1016/s0272-6386(89)80189-1. [DOI] [PubMed] [Google Scholar]
- 29.Honda K, Horita S, Toki D, Taneda S, Nitta K, Hattori M, et al. De novo membranous nephropathy and antibody-mediated rejection in transplanted kidney. Clin Transplant. 2011;25:191–200. doi: 10.1111/j.1399-0012.2010.01213.x. [DOI] [PubMed] [Google Scholar]
- 30.Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, et al. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10:2208–14. doi: 10.1681/ASN.V10102208. [DOI] [PubMed] [Google Scholar]
- 31.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–23. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 32.Sis B, Dadras F, Khoshjou F, Cockfield S, Mihatsch MJ, Solez K. Reproducibility studies on arteriolar hyaline thickening scoring in calcineurin inhibitor-treated renal allograft recipients. Am J Transplant. 2006;6:1444–50. doi: 10.1111/j.1600-6143.2006.01302.x. [DOI] [PubMed] [Google Scholar]
- 33.Gibson IW, Gwinner W, Brocker V, Sis B, Riopel J, Roberts IS, et al. Peritubular capillaritis in renal allografts: prevalence, scoring system, reproducibility and clinicopathological correlates. Am J Transplant. 2008;8:819–25. doi: 10.1111/j.1600-6143.2007.02137.x. [DOI] [PubMed] [Google Scholar]
- 34.Ivanyi B, Fahmy H, Brown H, Szenohradszky P, Halloran PF, Solez K. Peritubular capillaries in chronic renal allograft rejection: a quantitative ultrastructural study. Hum Pathol. 2000;31:1129–38. doi: 10.1053/hupa.2000.16677. [DOI] [PubMed] [Google Scholar]
- 35.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45. [PubMed] [Google Scholar]
- 36.Pescovitz MD. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006;6:859–66. doi: 10.1111/j.1600-6143.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 38.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836–43. [PubMed] [Google Scholar]
- 39.Rivera A, Chen CC, Ron N, Dougherty JP, Ron Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol. 2001;13:1583–93. doi: 10.1093/intimm/13.12.1583. [DOI] [PubMed] [Google Scholar]
- 40.Hippen BE, DeMattos A, Cook WJ, Kew CE, II, Gaston RS. Association of CD20+ infiltrates with poorer clinical outcomes in acute cellular rejection of renal allografts. Am J Transplant. 2005;5:2248–52. doi: 10.1111/j.1600-6143.2005.01009.x. [DOI] [PubMed] [Google Scholar]
- 41.Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, et al. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349:125–38. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]