Abstract
Aim
We have previously found that chronic endothelin-1 (ET-1) infusion in Sprague-Dawley rats increases glomerular permeability to albumin (Palb) as assessed in vitro independent of blood pressure with no observed albuminuria. In this study, we hypothesized that ET-1 increases glomerular albumin filtration with accompanied increase in albumin uptake via the proximal tubule, which masks the expected increase in urinary albumin excretion.
Main methods
Nonfasting Munich-Wistar Fromter rats were surgically prepared for in vivo imaging (n=6). Rats were placed on the microscope stage with the exposed kidney placed in a cover slip-bottomed dish bathed in warm isotonic saline. Rats were then injected i.v. with rat serum albumin conjugated to Texas Red that was observed to enter capillary loops of superficial glomeruli, move into Bowman’s space, bind to the proximal tubular cell brush border and reabsorbed across the apical membrane. Glomerular sieving coefficient (GSC) was calculated as the ratio of conjugated albumin within the glomerular capillary versus that in Bowman’s space. Rats were again studied after 2 wk of chronic ET-1 (2 pmol/kg/min; i.v. osmotic minipump).
Key findings
Glomerular sieving coefficient was significantly increased in rats following chronic ET-1 infusion (0.025± 0.005 vs. 0.017 ± 0.003, p<0.05). Mean fluorescence intensity for conjugated albumin within proximal tubules was increased by ET-1 infusion: 118.40 ± 6.34 vs. 74.27 ± 4.45 pixel intensity (p<0.01).
Significance
These data provide in vivo evidence that ET-1 directly increases glomerular permeability to albumin and that albuminuria is prevented by increased PT albumin uptake in the rat.
Keywords: Endothelin, intravital 2-photon microscopy, glomerular sieving coefficient, albumin reuptake, proximal tubule, kidney, rat
Introduction
Proteinuria and albuminuria represent early signs of glomerular injury, and their presence predicts not only an elevated risk for nephropathy, but also cardiovascular disease in general (Perkins et al., 2007). The mechanistic pathways of albuminuria in chronic kidney disease (CKD) have not been resolved. Concerning the proteinuric role of ET-1, a recent phase III clinical trial in patients with diabetic nephropathy demonstrated that avosentan, a modestly selective endothelin-A (ETA) antagonist, decreased urinary albumin excretion rate after 12 weeks of treatment (Wenzel et al., 2009). These studies have been confirmed with a more selective ETA blocker (Kohan et al., 2011). Furthermore, most of the subjects in these studies were already receiving treatment with angiotensin receptor blockers and/or angiotensin converting enzyme inhibitors indicating an independent anti-proteinuric effect of endothelin receptor antagonism. Despite these observations in both animals and humans, little is known about the specific mechanisms of ET-1 action in CKDs and many of the beneficial effects of ET antagonists have not been distinguished from their blood pressure lowering effect.
We have recently reported that a non-pressor 2-week infusion of ET-1 increases glomerular permeability to albumin (Palb), however, this dose does not induce proteinuria or albuminuria in normal Sprague-Dawley rats (Saleh et al., 2010a). The absence of albuminuria suggests that the changes in permeability determined in isolated glomeruli are not sufficient to translate into measurable albuminuria. Recent studies have renewed interest in the role of proximal tubular uptake of albumin in protecting against albuminuria (Russo et al., 2007). In addition, changes in Palb on the order of magnitude that we observed in the chronic ET-1 model, 0.4 (Saleh et al., 2010a), are much less than those observed in rats displaying overt proteinuria associated with hyperglycemia, >0.8 (Fabris et al., 2001; Saleh et al., 2010b; Saleh et al., 2011). Several studies have established that an increase in glomerular permeability typically occurs prior to the development of overt proteinuria (Melnick et al., 1981; Bjorn et al., 1995; Sharma et al., 2002; Doublier et al., 2003).
The current study was designed to 1) confirm whether chronic ET-1 causes glomerular albumin leakage in an intact kidney, and 2) whether proximal tubular re-uptake of albumin could account for a lack of proteinuria following chronic ET-1 infusion in the rat. Our approach used intravital 2-photon microscopy to visualize glomerular permeability in vivo. Although the clinical relevance of proteinuria (especially albuminuria) has been well documented, the quantitative and mechanistic role of each “barrier” component, including the proximal tubule, in albuminuria remains an area of considerable excitement and debate (Molitoris, 2010). Over the past 5 years, the long-standing controversy regarding the quantitative role of the proximal tubule in the development of albuminuria has reached new heights, with supporting evidence coming from many different genetic, molecular, and technical approaches. The use of two-photon microscopy offers a unique approach to improving the understanding of the roles of glomerular filtration barrier and proximal tubule in albumin filtration and reabsorption. It allows for direct visualization and quantification of the glomerular filtration and its associated S1 proximal tubule segment in Munich Wistar rats with surface glomeruli. It also permits quantification and relating of changes in structure and function longitudinally over the course of a disease process within the same glomerulus, and the corresponding S1 proximal tubule segment. Because the final composition of albumin in the urine is affected by both glomerular filtration and proximal tubule reabsorption, both processes must be considered (Sandoval et al., 2012). Our hypothesis predicts that chronic ET-1 increases glomerular albumin filtration with an accompanied increase in albumin uptake via the proximal tubule, which masks the expected increase in urinary albumin excretion.
Methods
Chronic ET-1 infusion
All animal studies were conducted in compliance with the American Physiological Society guidelines with all protocols and procedures being reviewed and approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. Male Munich-Wistar rats 200 to 250 g (Simonsen Laboratories, Gilroy, CA) were used in this study. To facilitate ET-1 or vehicle infusion, rats were anesthetized with sodium pentobarbital 50 mg/kg, i.p. (Ovation Pharmaceutical, Deerfield, IL) and a catheter (PE-90) was placed in the jugular vein and then tunneled subcutaneously to an osmotic minipump (model 2ML2; Alza Scientific, Palo Atlo, CA) placed subcutaneously at the dorsum of the neck. The minipump contained ET-1 (American Peptide Inc., CA) dissolved in 0.9% NaCl infused at a rate of 2 pmol/kg/min for a period of 14 days (Saleh et al., 2010a). Only one group of rats were studied (n= 6 rats) which glomerular sieving coefficient and fluorescent albumin proximal reuptake were evaluated before starting ET-1 infusion and after two weeks of the continuous infusion of ET-1.
Animal preparation for imaging
Nonfasting Munich Wistar Fromter rats were anesthetized with 50 mg/kg; i.p. sodium pentobarbital (Ovation Pharmaceutical, Deerfield, IL). First a catheter was inserted in the femoral vein to enable the delivery of fluorescent conjugated albumin. To expose the kidney for intravital imaging an incision was made on the left flank. The rat was placed on the microscope stage with the exposed kidney in contact with the inner surface of 50-mm glass-bottom dish (Willco Wells BV, Netherlands). Animals were covered by a water-circulating warming blanket in order to stabilize the temperature during imaging, In addition, the microscope stage was warmed using 2 ReptiTherm heating pads (Zoo Med Laboratories, San Luis Obispo, CA) one under the upper torso/head and one under the lower abdomen. The temperature of the animal was maintained between 36.5 and 37.5°C. The x60 water immersion objective (Nikon Plan Apo, NA 1.2) was heated using an objective heater (Warner Instruments, Hamden, CT). The mean arterial blood pressure was from 80 to 112 mmHg as measured via a femoral arterial line using Lab Chart 6 (AD Instruments, Colorado Springs, CO).
Two-photon microscopy
Unless specified otherwise, two-photon microscopy was conducted on the Bio-Rad MRC 1024 confocal/two-photon microscope as described previously (Dunn et al., 2002; Yu et al., 2007). The illumination source was a tunable Tsunami Ti:Sapphire laser from Spectra-physics (Mountain View, CA). The microscope was an inverted Nikon Eclipes TE200. The band pass filters used for the red and green channels were 605/90 and 525/50, respectively. The excitation wavelength was set at 800 nm for all experiments. The anesthetized rat was placed on the microscope stage with the exposed kidney in direct contact with the cover glass (see Animal preparation for imaging). A 60X Nikon Plan Apo water immersion objective (NA 1.2) was used for imaging. All images were collected at a constant pixel dwell time, which yielded a 1.1-s frame time for the frame size of 512 × 512. All images for a given study were collected at approximately the same focal plane at a depth of approximately 10–20 um.
Proteins and Probes
Rat serum albumin (Fraction V; Sigma-Aldrich) was conjugated to Texas Red Sulfonyl Chloride (Invitrogen, Carlsbad, CA) to yield a stoichiometric ratio of 4:1 moles dye:albumin, according to the manufacturer’s instructions for amine-reactive probes as previously reported (Russo et al., 2007).
Glomerular sieving coefficient (GSC) determination
Images were analyzed using MetaMorph v.7.1 (Molecular Devices, Downingtown, PA), and calculations were done on a Microsoft Excel spreadsheet. The fluorescence intensity in the plasma was recorded in a capillary loop, which had the brightest fluorescence in the field.
Plasma fluorescence was measured at the outer margins of the capillary lumen, a red blood cell free zone. We selected 3 areas in the urinary space of Bowman’s capsule where there were no blood vessels and where fluorescence intensity was minimal. The average of the 3 areas was calculated. We recorded the number of pixels (area) and average fluorescence intensities. Calculations of GSCs were based on average intensity values after subtracting background readings for plasma and Bowman’s space.
Albumin proximal reuptake measurement
Still frames from two-photon movies at 45 min post injection taken with a 60X water immersion lens with a 1.2 numerical aperture were captured. Mean pixel intensities were generated following background subtraction, thresholding of the endosomal pool and quantitative analysis on ten random fields per rat, as previously reported(Sandoval and Molitoris, 2008).
Statistical analysis
All data are presented as mean ± SEM. Data was compared using paired Student’s t-test. Differences were considered statistically significant with p<0.05. Analysis was performed using GraphPad Prism Version 5.0 software (GraphPad Software Inc, La Jolla, CA).
Results
GSC was calculated as the intensity of fluorescent albumin in Bowman’s space divided by the intensity of fluorescent albumin in glomerular capillaries. The background intensity for all locations was subtracted prior to calculating GSC. As shown in Figure 1, GSC was significantly increased in rats following chronic ET-1 infusion (0.025 ± 0.005 vs. 0.017 ± 0.003, p<0.05). Intensity of the fluorescent signal was also quantified for proximal tubular re-uptake of albumin by measurement of the fluorescence intensity within the S1 segment. As depicted in Figure 2, mean fluorescence intensity for conjugated albumin with Texas Red in proximal tubules was increased by ET-1 infusion: 118.4 ± 6.3 vs. 74.3 ± 4.4 pixel intensity (p<0.01).
Figure 1. Long term ET-1 treatment increases glomerular albumin permeability.
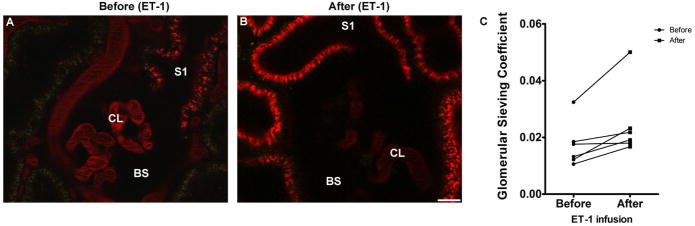
Representative images of Munich Wistar Frömter glomeruli using 2-photon intravital microscopy before (panel A) and after ET-1 treatment (panel B). Although it is not possible to visually distinguish relative differences in Bowman’s space (BS) albumin content by the naked eye, the fluorescence intensity ratio between BS and capillary loop (CL) can be readily determined. Despite having been given an identical amount of Texas Red rat serum albumin initially, and imaged at the same time post injection, plasma levels of albumin following ET-1 treatment decreased more rapidly and yielded a higher fluorescence ratio, i.e., glomerular sieving coeficients (GSC). Panel C shows average GSC for individual rats taken before and after chronic ET-1 infusion. For the 6 rats, a total of 28 glomeruli were studied before and after ET-1 infusion, (Bar=15μm; CL=capillary loops).
Figure 2. ET-1 exposure increases albumin reabsorption by proximal tubule (PT) segments in ET-1 treated rats.
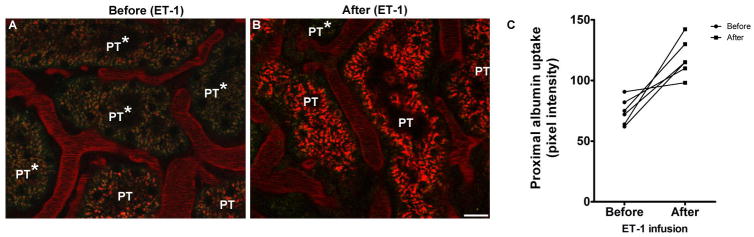
Representative images of proximal tubule (PT) segments from Munich Wistar Frömter rats observed via 2-photon intravital microscopy before (panel A) and after (panel B) ET-1 treatment. Images taken before ET-1 exposure show some heterogenous accumulation of filtered albumin in PT segments; with some segments exhibiting decreased accumulation (PT*). In images taken after ET-1 infusion, accumulation of albumin was seen in a greater percentage of PT segments, with the number of PT segments showing little or no albumin accumulation (although still present) decreasing in number. Average pixel intensity values for background-subtracted images for individual rats before and after ET-1 treatment in are presented in panel C. (Bar=15μm).
Discussion
The current study provides mechanistic insight into our recent observations that chronic infusion of exogenous ET-1 increases glomerular permeability to albumin, but has no effect on proteinuria. The explanation for these apparent contradictory findings appear to be the result of a limited, yet significant increase in glomerular permeability to albumin while the proximal tubules have the capacity to take up the additional filtered albumin. Thus, our current study substantiates previous findings that attribute a greater role to the proximal tubules in maintaining albumin homeostasis under both physiologic and pathologic conditions.
Over the past 21 years since ET-1 discovery, a prominent role of ET-1 in CKD has been increasingly established. In various hypertensive animal models, there is considerable evidence that ET-1 contributes to renal inflammation, cell proliferation, and fibrosis mainly dependent of its hypertensive effects mediated by the ETA receptor. Furthermore, a series of studies originating from the Benigni lab (Benigni et al., 1993; Bruzzi and Benigni, 1996) and more recently, our own (Saleh et al., 2010b; Saleh et al., 2010a; Saleh et al., 2011), provide compelling evidence that ET-1 contributes to glomerular podocyte dysfunction as a means of producing proteinuria. Recent clinical trials also demonstrate that ETA receptor blockade can produce effective anti-proteinuric effects. However, the degree to which this occurs independent of blood pressure lowering is not clear. To address this question, we have used the chronic ET-1 infusion model in the rat where blood pressure is unchanged, yet there is an increase in glomerular permeability to albumin (Palb) in isolated glomeruli as well as increases in early inflammatory markers such as plasma and glomerular monocyte chemoattractant protein-1 (MCP-1) and soluble intercellular adhesion molecule-1 (sICAM-1) (Saleh et al., 2010a).
We believe that there is a strong relationship between ET-1 and podocytes either functionally or morphologically. We have found that ET-1 infusion appeared to cause some detachment of podocytes and foot process effacement in glomerular tufts relative to saline-infused control rats via using transmission electron micrographs suggesting that ET-1 mediates podocyte injury and increases glomerular permeability (Saleh et al., 2010a). We also observed that ET-1 infusion increases nephrin shedding from the glomeruli into the urine (nephrinuria). Nephrin is a filtration slit protein expressed in podocytes (Ruotsalainen et al., 1999) and acts as a scaffold of the glomerular filtration barrier (Garg et al., 2007). Shedding of nephrin into the tubular fluid is a clear marker of glomerular injury and hence increase in glomerular permeability. We found that nephrin excretion was significantly increased in ET-1-infused rats compared to saline-infused control rats (Saleh et al., 2010a). In addition, we noticed that chronic ETA receptor activation increased the excretion of nephrin into the urine, again suggesting that ETA receptors influence podocyte function and Palb. Morphologically, others have shown that up- regulation of ET-1 production by podocytes is induced by protein overload, resulting in cytoskeletal changes associated with foot-process effacement, a hallmark of chronic glomerular disease (Morigi et al., 2005). Moreover, it has been reported that inhibition of Rho kinases in podocytes, which are important for the formation of stress fibers, resulted in a significant inhibition of F-actin rearrangement in response to ET-1 (Morigi et al., 2006).
Results of the present study along with those of our previous investigation demonstrate that ET-1 has important effects on glomerular permeability independent of blood pressure. We have also observed that ETA receptor blockade reduced aluminuria and proteinuria in rats with diabetes (type 1 model) (Saleh et al., 2010b). Importantly, these previous studies also included experiments where isolated glomeruli were incubated with ETA receptor antagonists ex vivo, and we were able to observe reductions in glomerular albumin permeability. These experiments also provide further evidence for actions of ET-1 within the glomerulus independent of blood pressure lowering. Guo et al demonstrated that ablation of as few as 20% of podocytes resulted in albuminuria that resolved over 1–2 weeks after the re-establishment of normal podocyte morphology (Guo et al., 2012). They also showed that immediately after the onset of albuminuria, proximal tubule cells underwent a transient burst of proliferation without evidence of tubular damage or increased apoptosis, resulting in an increase in total tubular cell numbers which may explain the increased proximal uptake of albumin in our study (Guo et al., 2012).
In a model of streptozotocin induced diabetes, Gagliardini and colleagues used fractional clearance of graded-size Ficoll molecules to determine the effects of ET receptor blockade on glomerular dynamics. Avosentan, a modestly selective ETA antagonist, had no effect on the ultrafiltration coefficient, but was able to prevent podocyte loss and partially lowered proteinuria and albuminuria. This could be due to the effect of the ETA receptor antagonist on glomerular hemodynamics as opposed to a direct effect on permselectivity. In addition, avosentan increased peritubular capillary perfusion, which could enhance proximal tubular protein reabsorption (Gagliardini et al., 2009). While it is possible that ET-1 could have a direct effect to enhance proximal tubular uptake of filtered albumin, it is possible that the increased filtered load of albumin is still below the capacity of the proximal tubule to reabsorb albumin, but this will need to be further investigated.
Infusion of ET-1 for longer time periods and/or at higher concentrations in the rat could potentially have a more profound impact on glomerular permeability that could result in significant albuminuria and/or proteinuria. The level at which glomeruli are exposed to ET-1 in our model versus models of overt proteinuria are not known. Future studies will have to provide more detailed characterization of this model.
Acknowledgments
This work was supported by an NIH George M. O’Brien Center Award NIH P-30 and DK 079312 (B. Molitoris) and Fellowship (M. Saleh), American Heart Association Fellowship (M. Saleh), and NIH Program Project Grant HL HL95499 (D. Pollock).
References
- Benigni A, Zoja C, Corna D, Orisio S, Longaretti L, Bertani T, Remuzzi G. A specific endothelin subtype A receptor antagonist protects against injury in renal disease progression. Kidney Int. 1993;44:440–444. doi: 10.1038/ki.1993.263. [DOI] [PubMed] [Google Scholar]
- Bjorn SF, Bangstad HJ, Hanssen KF, Nyberg G, Walker JD, Viberti GC, Osterby R. Glomerular epithelial foot processes and filtration slits in IDDM patients. Diabetologia. 1995;38:1197–1204. doi: 10.1007/BF00422369. [DOI] [PubMed] [Google Scholar]
- Bruzzi I, Benigni A. Endothelin is a key modulator of progressive renal injury: experimental data and novel therapeutic strategies. Clin Exp Pharmacol Physiol. 1996;23:349–353. doi: 10.1111/j.1440-1681.1996.tb02836.x. [DOI] [PubMed] [Google Scholar]
- Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G. Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes. 2003;52:1023–1030. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol. 2002;283:C905–916. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- Fabris B, Candido R, Carraro M, Fior F, Artero M, Zennaro C, Cattin MR, Fiorotto A, Bortoletto M, Millevoi C, Bardelli M, Faccini L, Carretta R. Modulation of incipient glomerular lesions in experimental diabetic nephropathy by hypotensive and subhypotensive dosages of an ACE inhibitor. Diabetes. 2001;50:2619–2624. doi: 10.2337/diabetes.50.11.2619. [DOI] [PubMed] [Google Scholar]
- Gagliardini E, Corna D, Zoja C, Sangalli F, Carrara F, Rossi M, Conti S, Rottoli D, Longaretti L, Remuzzi A, Remuzzi G, Benigni A. Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am J Physiol Renal Physiol. 2009;297:F1448–1456. doi: 10.1152/ajprenal.00340.2009. [DOI] [PubMed] [Google Scholar]
- Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol. 2007;27:8698–8712. doi: 10.1128/MCB.00948-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JK, Marlier A, Shi H, Shan A, Ardito TA, Du ZP, Kashgarian M, Krause DS, Biemesderfer D, Cantley LG. Increased tubular proliferation as an adaptive response to glomerular albuminuria. J Am Soc Nephrol. 2012;23:429–437. doi: 10.1681/ASN.2011040396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan DE, Pritchett Y, Molitch M, Wen S, Garimella T, Audhya P, Andress DL. Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy. J Am Soc Nephrol. 2011;22:763–772. doi: 10.1681/ASN.2010080869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick GF, Ladoulis CT, Cavallo T. Decreased anionic groups and increased permeability precedes deposition of immune complexes in the glomerular capillary wall. Am J Pathol. 1981;105:114–120. [PMC free article] [PubMed] [Google Scholar]
- Molitoris BA. Yet another advance in understanding albuminuria? J Am Soc Nephrol. 2010;21:2013–2015. doi: 10.1681/ASN.2010101075. [DOI] [PubMed] [Google Scholar]
- Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, Zoja C, Galbusera M, Gastoldi S, Mundel P, Remuzzi G, Benigni A. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies. Am J Pathol. 2005;166:1309–1320. doi: 10.1016/S0002-9440(10)62350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morigi M, Buelli S, Zanchi C, Longaretti L, Macconi D, Benigni A, Moioli D, Remuzzi G, Zoja C. Shigatoxin-induced endothelin-1 expression in cultured podocytes autocrinally mediates actin remodeling. Am J Pathol. 2006;169:1965–1975. doi: 10.2353/ajpath.2006.051331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18:1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 2007;71:504–513. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension. 2010a;56:942–949. doi: 10.1161/HYPERTENSIONAHA.110.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. Endothelin receptor A-specific stimulation of glomerular inflammation and injury in a streptozotocin-induced rat model of diabetes. Diabetologia. 2010b;54:979–988. doi: 10.1007/s00125-010-2021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MA, Pollock JS, Pollock DM. Distinct actions of endothelin A-selective versus combined endothelin A/B receptor antagonists in early diabetic kidney disease. J Pharmacol Exp Ther. 2011;338:263–270. doi: 10.1124/jpet.111.178988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval RM, Molitoris BA. Quantifying endocytosis in vivo using intravital two-photon microscopy. Methods Mol Biol. 2008;440:389–402. doi: 10.1007/978-1-59745-178-9_28. [DOI] [PubMed] [Google Scholar]
- Sandoval RM, Wagner CM, Patel M, Campos-Bilderback SB, Rhodes GJ, Wang E, Wean SE, Clendenon SS, Molitoris BA. Factors influencing glomerular albumin permeability in the rat. J Am Soc Nephrol. 2012 doi: 10.1681/ASN.2011070666. In-Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Sharma R, Reddy SR, McCarthy ET, Savin VJ. Proteinuria after injection of human focal segmental glomerulosclerosis factor. Transplantation. 2002;73:366–372. doi: 10.1097/00007890-200202150-00009. [DOI] [PubMed] [Google Scholar]
- Wenzel RR, Littke T, Kuranoff S, Jurgens C, Bruck H, Ritz E, Philipp T, Mitchell A. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol. 2009;20:655–664. doi: 10.1681/ASN.2008050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Sandoval RM, Molitoris BA. Rapid determination of renal filtration function using an optical ratiometric imaging approach. Am J Physiol Renal Physiol. 2007;292:F1873–1880. doi: 10.1152/ajprenal.00218.2006. [DOI] [PubMed] [Google Scholar]


