Abstract
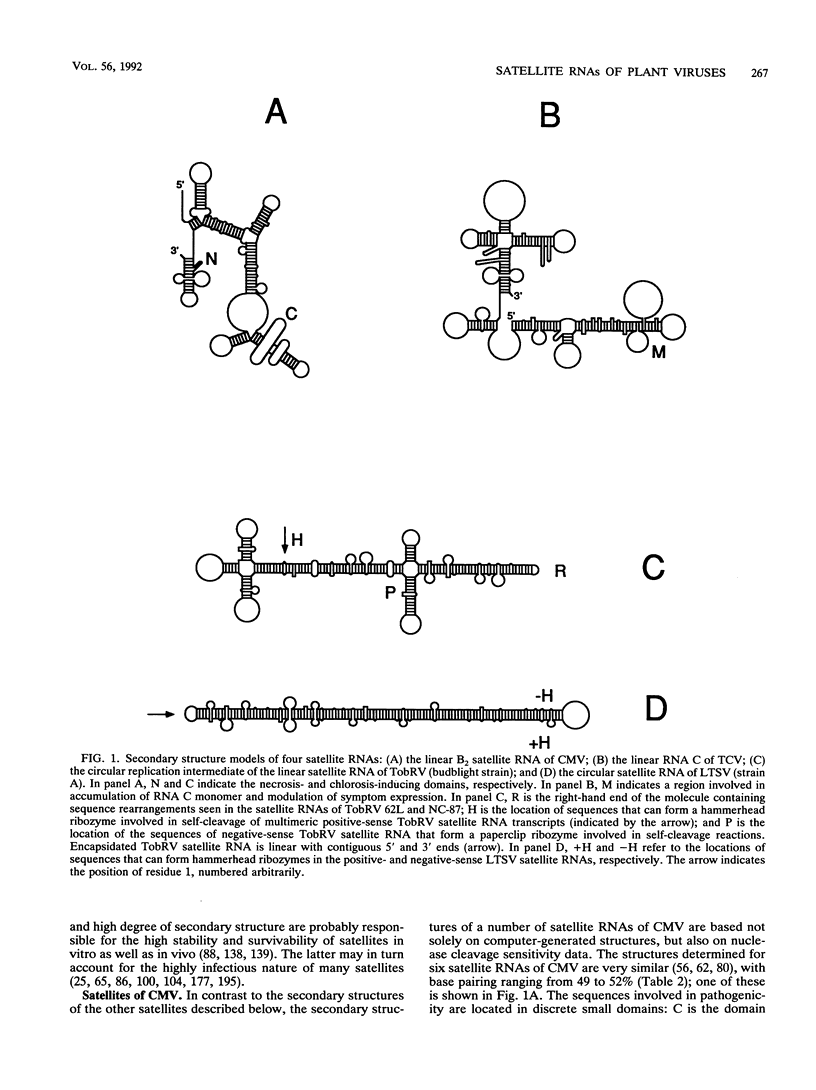
Plant viruses often contain parasites of their own, referred to as satellites. Satellite RNAs are dependent on their associated (helper) virus for both replication and encapsidation. Satellite RNAs vary from 194 to approximately 1,500 nucleotides (nt). The larger satellites (900 to 1,500 nt) contain open reading frames and express proteins in vitro and in vivo, whereas the smaller satellites (194 to 700 nt) do not appear to produce functional proteins. The smaller satellites contain a high degree of secondary structure involving 49 to 73% of their sequences, with the circular satellites containing more base pairing than the linear satellites. Many of the smaller satellites produce multimeric forms during replication. There are various models to account for their formation and role in satellite replication. Some of these smaller satellites encode ribozymes and are able to undergo autocatalytic cleavage. The enzymology of satellite replication is poorly understood, as is the replication of their helper viruses. In many cases the coreplication of satellites suppresses the replication of the helper virus genome. This is usually paralleled by a reduction in the disease induced by the helper virus; however, there are notable exceptions in which the satellite exacerbates the pathogenicity of the helper virus, albeit on only a limited number of hosts. The ameliorative satellites are being assessed as biocontrol agents of virus-induced disease. In greenhouse studies, satellites have been known to "spontaneously" appear in virus cultures. The possible origin of satellites will be briefly considered.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila-Rincon M. J., Collmer C. W., Kaper J. M. In vitro translation of cucumoviral satellites. II. CARNA 5 from cucumber mosaic virus strain S and SP6 transcripts of cloned (S)CARNA 5 cDNA produce electrophoretically comigrating protein products. Virology. 1986 Jul 30;152(2):455–458. doi: 10.1016/0042-6822(86)90147-9. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Buckley B., Bruening G. Effect of actinomycin D on replication of satellite tobacco ringspot virus RNA in plant protoplasts. Virology. 1990 Jul;177(1):298–304. doi: 10.1016/0042-6822(90)90483-8. [DOI] [PubMed] [Google Scholar]
- Buzayan J. M., Gerlach W. L., Bruening G. Satellite tobacco ringspot virus RNA: A subset of the RNA sequence is sufficient for autolytic processing. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8859–8862. doi: 10.1073/pnas.83.23.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzayan J. M., Hampel A., Bruening G. Nucleotide sequence and newly formed phosphodiester bond of spontaneously ligated satellite tobacco ringspot virus RNA. Nucleic Acids Res. 1986 Dec 22;14(24):9729–9743. doi: 10.1093/nar/14.24.9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzayan J. M., McNinch J. S., Schneider I. R., Bruening G. A nucleotide sequence rearrangement distinguishes two isolates of satellite tobacco ringspot virus RNA. Virology. 1987 Sep;160(1):95–99. doi: 10.1016/0042-6822(87)90049-3. [DOI] [PubMed] [Google Scholar]
- Carpenter C. D., Cascone P. J., Simon A. E. Formation of multimers of linear satellite RNAs. Virology. 1991 Aug;183(2):586–594. doi: 10.1016/0042-6822(91)90987-m. [DOI] [PubMed] [Google Scholar]
- Carpenter C. D., Cascone P. J., Simon A. E. Mutations in a satellite RNA of turnip crinkle virus result in addition of poly(U) in vivo. Virology. 1991 Aug;183(2):595–601. doi: 10.1016/0042-6822(91)90988-n. [DOI] [PubMed] [Google Scholar]
- Chen P. J., Kalpana G., Goldberg J., Mason W., Werner B., Gerin J., Taylor J. Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8774–8778. doi: 10.1073/pnas.83.22.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collmer C. W., Hadidi A., Kaper J. M. Nucleotide sequence of the satellite of peanut stunt virus reveals structural homologies with viroids and certain nuclear and mitochondrial introns. Proc Natl Acad Sci U S A. 1985 May;82(10):3110–3114. doi: 10.1073/pnas.82.10.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collmer C. W., Kaper J. M. Infectious RNA transcripts from cloned cDNAs of cucumber mosaic viral satellites. Biochem Biophys Res Commun. 1986 Feb 26;135(1):290–296. doi: 10.1016/0006-291x(86)90975-7. [DOI] [PubMed] [Google Scholar]
- Collmer C. W., Kaper J. M. Site-directed mutagenesis of potential protein-coding regions in expressible cloned cDNAs of cucumber mosaic viral satellites. Virology. 1988 Apr;163(2):293–298. doi: 10.1016/0042-6822(88)90269-3. [DOI] [PubMed] [Google Scholar]
- Collmer C. W., Stenzler L., Fay N., Howell S. H. Nonmutant forms of the avirulent satellite D of turnip crinkle virus are produced following inoculation of plants with mutant forms synthesized in vitro. Virology. 1991 Jul;183(1):251–259. doi: 10.1016/0042-6822(91)90137-z. [DOI] [PubMed] [Google Scholar]
- Dall D. J., Graddon D. J., Randles J. W., Francki R. I. Isolation of a subterranean clover mottle virus-like satellite RNA from lucerne infected with lucerne transient streak virus. J Gen Virol. 1990 Aug;71(Pt 8):1873–1875. doi: 10.1099/0022-1317-71-8-1873. [DOI] [PubMed] [Google Scholar]
- Davies C., Haseloff J., Symons R. H. Structure, self-cleavage, and replication of two viroid-like satellite RNAs (virusoids) of subterranean clover mottle virus. Virology. 1990 Jul;177(1):216–224. doi: 10.1016/0042-6822(90)90475-7. [DOI] [PubMed] [Google Scholar]
- Devic M., Jaegle M., Baulcombe D. Cucumber mosaic virus satellite RNA (strain Y): analysis of sequences which affect systemic necrosis on tomato. J Gen Virol. 1990 Jul;71(Pt 7):1443–1449. doi: 10.1099/0022-1317-71-7-1443. [DOI] [PubMed] [Google Scholar]
- Devic M., Jaegle M., Baulcombe D. Symptom production on tobacco and tomato is determined by two distinct domains of the satellite RNA of cucumber mosaic virus (strain Y). J Gen Virol. 1989 Oct;70(Pt 10):2765–2774. doi: 10.1099/0022-1317-70-10-2765. [DOI] [PubMed] [Google Scholar]
- Feldstein P. A., Buzayan J. M., Bruening G. Two sequences participating in the autolytic processing of satellite tobacco ringspot virus complementary RNA. Gene. 1989 Oct 15;82(1):53–61. doi: 10.1016/0378-1119(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Davies C., Sheldon C. C., Jeffries A. C., Symons R. H. Self-cleaving viroid and newt RNAs may only be active as dimers. Nature. 1988 Jul 21;334(6179):265–267. doi: 10.1038/334265a0. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Francki R. I. Plant virus satellites. Annu Rev Microbiol. 1985;39:151–174. doi: 10.1146/annurev.mi.39.100185.001055. [DOI] [PubMed] [Google Scholar]
- Fuchs M., Pinck M., Serghini M. A., Ravelonandro M., Walter B., Pinck L. The nucleotide sequence of satellite RNA in grapevine fanleaf virus, strain F13. J Gen Virol. 1989 Apr;70(Pt 4):955–962. doi: 10.1099/0022-1317-70-4-955. [DOI] [PubMed] [Google Scholar]
- Gordon K. H., Symons R. H. Satellite RNA of cucumber mosaic virus forms a secondary structure with partial 3'-terminal homology to genomal RNAs. Nucleic Acids Res. 1983 Feb 25;11(4):947–960. doi: 10.1093/nar/11.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A. R., Palukaitis P., Symons R. H., Mossop D. W. Characterization of a satellite RNA associated with cucumber mosaic virus. Virology. 1978 Feb;84(2):443–455. doi: 10.1016/0042-6822(78)90261-1. [DOI] [PubMed] [Google Scholar]
- Greif C., Hemmer O., Demangeat G., Fritsch C. In vitro synthesis of biologically active transcripts of tomato black ring virus satellite RNA. J Gen Virol. 1990 Apr;71(Pt 4):907–915. doi: 10.1099/0022-1317-71-4-907. [DOI] [PubMed] [Google Scholar]
- Hampel A., Tritz R. RNA catalytic properties of the minimum (-)sTRSV sequence. Biochemistry. 1989 Jun 13;28(12):4929–4933. doi: 10.1021/bi00438a002. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Sequences required for self-catalysed cleavage of the satellite RNA of tobacco ringspot virus. Gene. 1989 Oct 15;82(1):43–52. doi: 10.1016/0378-1119(89)90028-0. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Symons R. H. Comparative sequence and structure of viroid-like RNAs of two plant viruses. Nucleic Acids Res. 1982 Jun 25;10(12):3681–3691. doi: 10.1093/nar/10.12.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes R. J., Buck K. W. Complete replication of a eukaryotic virus RNA in vitro by a purified RNA-dependent RNA polymerase. Cell. 1990 Oct 19;63(2):363–368. doi: 10.1016/0092-8674(90)90169-f. [DOI] [PubMed] [Google Scholar]
- Hayes R. J., Buck K. W. Infectious cucumber mosaic virus RNA transcribed in vitro from clones obtained from cDNA amplified using the polymerase chain reaction. J Gen Virol. 1990 Nov;71(Pt 11):2503–2508. doi: 10.1099/0022-1317-71-11-2503. [DOI] [PubMed] [Google Scholar]
- Heaton L. A., Carrington J. C., Morris T. J. Turnip crinkle virus infection from RNA synthesized in vitro. Virology. 1989 May;170(1):214–218. doi: 10.1016/0042-6822(89)90368-1. [DOI] [PubMed] [Google Scholar]
- Hidaka S., Hanada K., Ishikawa K. In vitro messenger properties of a satellite RNA of cucumber mosaic virus. J Gen Virol. 1990 Feb;71(Pt 2):439–442. doi: 10.1099/0022-1317-71-2-439. [DOI] [PubMed] [Google Scholar]
- Hidaka S., Hanada K., Ishikawa K., Miura K. Complete nucleotide sequence of two new satellite RNAs associated with cucumber mosaic virus. Virology. 1988 Jun;164(2):326–333. doi: 10.1016/0042-6822(88)90545-4. [DOI] [PubMed] [Google Scholar]
- Jacquemond M., Amselem J., Tepfer M. A gene coding for a monomeric form of cucumber mosaic virus satellite RNA confers tolerance to CMV. Mol Plant Microbe Interact. 1988 Nov-Dec;1(8):311–316. doi: 10.1094/mpmi-1-311. [DOI] [PubMed] [Google Scholar]
- Jacquemond M., Lauquin G. J. The cDNA of cucumber mosaic virus-associated satellite RNA has in vivo biological properties. Biochem Biophys Res Commun. 1988 Feb 29;151(1):388–395. doi: 10.1016/0006-291x(88)90605-5. [DOI] [PubMed] [Google Scholar]
- Jaegle M., Devic M., Longstaff M., Baulcombe D. Cucumber mosaic virus satellite RNA (Y strain): analysis of sequences which affect yellow mosaic symptoms on tobacco. J Gen Virol. 1990 Sep;71(Pt 9):1905–1912. doi: 10.1099/0022-1317-71-9-1905. [DOI] [PubMed] [Google Scholar]
- Jupin I., Bouzoubaa S., Richards K., Jonard G., Guilley H. Multiplication of beet necrotic yellow vein virus RNA 3 lacking a 3' poly(A) tail is accompanied by reappearance of the poly(A) tail and a novel short U-rich tract preceding it. Virology. 1990 Sep;178(1):281–284. doi: 10.1016/0042-6822(90)90404-f. [DOI] [PubMed] [Google Scholar]
- KASSANIS B. Properties and behaviour of a virus depending for its multiplication on another. J Gen Microbiol. 1962 Mar;27:477–488. doi: 10.1099/00221287-27-3-477. [DOI] [PubMed] [Google Scholar]
- Kaper J. M. Rapid synthesis of double-stranded cucumber mosaic virus-associated RNA 5: mechanism controlling viral pathogenesis? Biochem Biophys Res Commun. 1982 Apr 14;105(3):1014–1022. doi: 10.1016/0006-291x(82)91071-3. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E. Cucumber mosaic virus-associating RNA 5. I. Role of host plant and helper strain in determining amount of associated RNA 5 with virions. Virology. 1977 Jul 1;80(1):186–195. doi: 10.1016/0042-6822(77)90391-9. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E., Diaz-Ruiz J. R., Tolin S. A. Peanut stunt virus-associated RNA 5: second tripartite genome virus with an associated satellite-like replicating RNA. Virology. 1978 Jul 1;88(1):166–170. doi: 10.1016/0042-6822(78)90119-8. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E., Geletka L. M. Cucumber-mosaic-virus-associated RNA-5. XII. Symptom-modulating effect is codetermined by the helper virus satellite replication support function. Res Virol. 1990 Sep-Oct;141(5):487–503. doi: 10.1016/0923-2516(90)90082-t. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E., Lot H. A low molecular weight replicating RNA associated with a divided genome plant virus: defective or satellite RNA? Biochem Biophys Res Commun. 1976 Oct 18;72(4):1237–1243. [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E., Steen M. T. Cucumber mosaic virus-associated RNA 5. XI. Comparison of 14 CARNA 5 variants relates ability to induce tomato necrosis to a conserved nucleotide sequence. Virology. 1988 Apr;163(2):284–292. doi: 10.1016/0042-6822(88)90268-1. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E., Steger G. Nucleotide sequence predicts circularity and self-cleavage of 300-ribonucleotide satellite of arabis mosaic virus. Biochem Biophys Res Commun. 1988 Jul 15;154(1):318–325. doi: 10.1016/0006-291x(88)90687-0. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E. Viral satellites: parasitic nucleic acids capable of modulating disease expression. Endeavour. 1984;8(4):194–200. doi: 10.1016/0160-9327(84)90084-x. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Waterworth H. E. Cucumber mosaic virus associated RNA 5: causal agent for tomato necrosis. Science. 1977 Apr 22;196(4288):429–431. doi: 10.1126/science.196.4288.429. [DOI] [PubMed] [Google Scholar]
- Kiberstis P. A., Zimmern D. Translational strategy of Solanum nodiflorum mottle virus RNA: synthesis of a coat protein precursor in vitro and in vivo. Nucleic Acids Res. 1984 Jan 25;12(2):933–943. doi: 10.1093/nar/12.2.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Domdey H., Gross H. J. Binding of ribosomes to linear and circular forms of the 5'-terminal leader fragment of tobacco-mosaic-virus RNA. Eur J Biochem. 1981 Feb;114(2):221–227. doi: 10.1111/j.1432-1033.1981.tb05139.x. [DOI] [PubMed] [Google Scholar]
- Kurath G., Palukaitis P. RNA sequence heterogeneity in natural populations of three satellite RNAs of cucumber mosaic virus. Virology. 1989 Nov;173(1):231–240. doi: 10.1016/0042-6822(89)90239-0. [DOI] [PubMed] [Google Scholar]
- Kurath G., Palukaitis P. Serial passage of infectious transcripts of a cucumber mosaic virus satellite RNA clone results in sequence heterogeneity. Virology. 1990 May;176(1):8–15. doi: 10.1016/0042-6822(90)90224-f. [DOI] [PubMed] [Google Scholar]
- Lemaire O., Merdinoglu D., Valentin P., Putz C., Ziegler-Graff V., Guilley H., Jonard G., Richards K. Effect of beet necrotic yellow vein virus RNA composition on transmission by Polymyxa betae. Virology. 1988 Jan;162(1):232–235. doi: 10.1016/0042-6822(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Linthorst H. J., Kaper J. M. Replication of peanut stunt virus and its associated RNA 5 in cowpea protoplasts. Virology. 1984 Dec;139(2):317–329. doi: 10.1016/0042-6822(84)90377-5. [DOI] [PubMed] [Google Scholar]
- Liu Y. Y., Hellen C. U., Cooper J. I., Bertioli D. J., Coates D., Bauer G. The nucleotide sequence of a satellite RNA associated with arabis mosaic nepovirus. J Gen Virol. 1990 Jun;71(Pt 6):1259–1263. doi: 10.1099/0022-1317-71-6-1259. [DOI] [PubMed] [Google Scholar]
- Masuta C., Kuwata S., Takanami Y. Effects of extra 5' non-viral bases on the infectivity of transcripts from a cDNA clone of satellite RNA (strain Y) of cucumber mosaic virus. J Biochem. 1988 Nov;104(5):841–846. doi: 10.1093/oxfordjournals.jbchem.a122560. [DOI] [PubMed] [Google Scholar]
- Masuta C., Takanami Y. Determination of sequence and structural requirements for pathogenicity of a cucumber mosaic virus satellite RNA (Y-satRNA). Plant Cell. 1989 Dec;1(12):1165–1173. doi: 10.1105/tpc.1.12.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H. Y., Kaaret T. W., Bruice T. C. A computational approach to the mechanism of self-cleavage of hammerhead RNA. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9727–9731. doi: 10.1073/pnas.86.24.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. A., Hercus T., Waterhouse P. M., Gerlach W. L. A satellite RNA of barley yellow dwarf virus contains a novel hammerhead structure in the self-cleavage domain. Virology. 1991 Aug;183(2):711–720. doi: 10.1016/0042-6822(91)91000-7. [DOI] [PubMed] [Google Scholar]
- Mossop D. W., Francki R. I. Survival of a satellite RNA in vivo and its dependence on cucumber mosaic virus for replication. Virology. 1978 May 15;86(2):562–566. doi: 10.1016/0042-6822(78)90095-8. [DOI] [PubMed] [Google Scholar]
- Murant A. F. Dependence of groundnut rosette virus on its satellite RNA as well as on groundnut rosette assistor luteovirus for transmission by Aphis craccivora. J Gen Virol. 1990 Sep;71(Pt 9):2163–2166. doi: 10.1099/0022-1317-71-9-2163. [DOI] [PubMed] [Google Scholar]
- Naidu R. A., Collins G. B., Ghabrial S. A. Symptom-modulating properties of peanut stunt virus satellite RNA sequence variants. Mol Plant Microbe Interact. 1991 May-Jun;4(3):268–275. doi: 10.1094/mpmi-4-268. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Kaper J. M. Cucumber mosaic virus-associated RNA 5. II. In vitro translation in a wheat germ protein-synthesis system. Virology. 1977 Jul 1;80(1):196–203. doi: 10.1016/0042-6822(77)90392-0. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Schneider I. R. Satellite of tobacco ringspot virus RNA lacks detectable mRNA activity. Virology. 1977 Jul 1;80(1):222–224. doi: 10.1016/0042-6822(77)90396-8. [DOI] [PubMed] [Google Scholar]
- Palukaitis P. Pathogenicity regulation by satellite RNAs of cucumber mosaic virus: minor nucleotide sequence changes alter host responses. Mol Plant Microbe Interact. 1988 Apr;1(4):175–181. doi: 10.1094/mpmi-1-175. [DOI] [PubMed] [Google Scholar]
- Prody G. A., Bakos J. T., Buzayan J. M., Schneider I. R., Bruening G. Autolytic processing of dimeric plant virus satellite RNA. Science. 1986 Mar 28;231(4745):1577–1580. doi: 10.1126/science.231.4745.1577. [DOI] [PubMed] [Google Scholar]
- Quadt R., Jaspars E. M. Characterization of cucumber mosaic virus RNA-dependent RNA polymerase. FEBS Lett. 1991 Feb 25;279(2):273–276. doi: 10.1016/0014-5793(91)80166-z. [DOI] [PubMed] [Google Scholar]
- Quillet L., Guilley H., Jonard G., Richards K. In vitro synthesis of biologically active beet necrotic yellow vein virus RNA. Virology. 1989 Sep;172(1):293–301. doi: 10.1016/0042-6822(89)90131-1. [DOI] [PubMed] [Google Scholar]
- Rezaian M. A., Symons R. H. Anti-sense regions in satellite RNA of cucumber mosaic virus form stable complexes with the viral coat protein gene. Nucleic Acids Res. 1986 Apr 25;14(8):3229–3239. doi: 10.1093/nar/14.8.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaian M. A., Williams R. H., Symons R. H. Nucleotide sequence of cucumber mosaic virus RNA. 1. Presence of a sequence complementary to part of the viral satellite RNA and homologies with other viral RNAs. Eur J Biochem. 1985 Jul 15;150(2):331–339. doi: 10.1111/j.1432-1033.1985.tb09025.x. [DOI] [PubMed] [Google Scholar]
- Riesner D., Kaper J. M., Randles J. W. Stiffness of viroids and viroid-like RNA in solution. Nucleic Acids Res. 1982 Sep 25;10(18):5587–5598. doi: 10.1093/nar/10.18.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon D. M., Johnston J. C. Infectious transcripts from cloned cucumber necrosis virus cDNA: evidence for a bifunctional subgenomic mRNA. Virology. 1991 Apr;181(2):656–665. doi: 10.1016/0042-6822(91)90899-m. [DOI] [PubMed] [Google Scholar]
- Roossinck M. J., Palukaitis P. Differential replication in zucchini squash of a cucumber mosaic virus satellite RNA maps to RNA 1 of the helper virus. Virology. 1991 Mar;181(1):371–373. doi: 10.1016/0042-6822(91)90506-7. [DOI] [PubMed] [Google Scholar]
- Rubino L., Burgyan J., Grieco F., Russo M. Sequence analysis of cymbidium ringspot virus satellite and defective interfering RNAs. J Gen Virol. 1990 Aug;71(Pt 8):1655–1660. doi: 10.1099/0022-1317-71-8-1655. [DOI] [PubMed] [Google Scholar]
- Rubino L., Tousignant M. E., Steger G., Kaper J. M. Nucleotide sequence and structural analysis of two satellite RNAs associated with chicory yellow mottle virus. J Gen Virol. 1990 Sep;71(Pt 9):1897–1903. doi: 10.1099/0022-1317-71-9-1897. [DOI] [PubMed] [Google Scholar]
- Ruffner D. E., Dahm S. C., Uhlenbeck O. C. Studies on the hammerhead RNA self-cleaving domain. Gene. 1989 Oct 15;82(1):31–41. doi: 10.1016/0378-1119(89)90027-9. [DOI] [PubMed] [Google Scholar]
- Schneider I. R. Satellite-like particle of tobacco ringspot virus that resembles tobacco ringspot virus. Science. 1969 Dec 26;166(3913):1627–1629. doi: 10.1126/science.166.3913.1627. [DOI] [PubMed] [Google Scholar]
- Schneider I. R., White R. M., Gooding G. V., Jr Two new isolates of the satellite of tobacco ringspot virus. Virology. 1972 Dec;50(3):902–905. doi: 10.1016/0042-6822(72)90444-8. [DOI] [PubMed] [Google Scholar]
- Sheldon C. C., Symons R. H. RNA stem stability in the formation of a self-cleaving hammerhead structure. Nucleic Acids Res. 1989 Jul 25;17(14):5665–5677. doi: 10.1093/nar/17.14.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A. E., Engel H., Johnson R. P., Howell S. H. Identification of regions affecting virulence, RNA processing and infectivity in the virulent satellite of turnip crinkle virus. EMBO J. 1988 Sep;7(9):2645–2651. doi: 10.1002/j.1460-2075.1988.tb03117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A. E., Howell S. H. Synthesis in vitro of infectious RNA copies of the virulent satellite of turnip crinkle virus. Virology. 1987 Jan;156(1):146–152. doi: 10.1016/0042-6822(87)90445-4. [DOI] [PubMed] [Google Scholar]
- Simon A. E., Howell S. H. The virulent satellite RNA of turnip crinkle virus has a major domain homologous to the 3' end of the helper virus genome. EMBO J. 1986 Dec 20;5(13):3423–3428. doi: 10.1002/j.1460-2075.1986.tb04664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat D. E. Nucleotide sequence of a new satellite RNA of cucumber mosaic virus. Nucleic Acids Res. 1990 Jun 11;18(11):3416–3416. doi: 10.1093/nar/18.11.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat D. E., Palukaitis P. Induction of tobacco chlorosis by certain cucumber mosaic virus satellite RNAs is specific to subgroup II helper strains. Virology. 1990 May;176(1):292–295. doi: 10.1016/0042-6822(90)90256-q. [DOI] [PubMed] [Google Scholar]
- Sleat D. E., Palukaitis P. Site-directed mutagenesis of a plant viral satellite RNA changes its phenotype from ameliorative to necrogenic. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2946–2950. doi: 10.1073/pnas.87.8.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Kuwata S., Kataoka J., Masuta C., Nitta N., Takanami Y. Functional analysis of deletion mutants of cucumber mosaic virus RNA3 using an in vitro transcription system. Virology. 1991 Jul;183(1):106–113. doi: 10.1016/0042-6822(91)90123-s. [DOI] [PubMed] [Google Scholar]
- Tien P., Wu G. S. Satellite RNA for the biocontrol of plant disease. Adv Virus Res. 1991;39:321–339. doi: 10.1016/s0065-3527(08)60799-x. [DOI] [PubMed] [Google Scholar]
- Van Tol H., Buzayan J. M., Bruening G. Evidence for spontaneous circle formation in the replication of the satellite RNA of tobacco ringspot virus. Virology. 1991 Jan;180(1):23–30. doi: 10.1016/0042-6822(91)90005-v. [DOI] [PubMed] [Google Scholar]
- Wang K. S., Choo Q. L., Weiner A. J., Ou J. H., Najarian R. C., Thayer R. M., Mullenbach G. T., Denniston K. J., Gerin J. L., Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986 Oct 9;323(6088):508–514. doi: 10.1038/323508a0. [DOI] [PubMed] [Google Scholar]
- Waterworth H. E., Kaper J. M., Tousignant M. E. CARNA 5, the Small Cucumber Mosaic Virus--Dependent Replicating RNA, Regulates Disease Expression. Science. 1979 May 25;204(4395):845–847. doi: 10.1126/science.204.4395.845. [DOI] [PubMed] [Google Scholar]



