Solid tumors contain regions of oxygen (O2) and nutrient limitation due to their structurally and functionally abnormal vasculature and the rapid proliferation of cancer cells. The major transcriptional response to hypoxia is mediated by hypoxia inducible factors (HIFs), which stimulate the expression of hundreds of genes that promote cellular adaptation to low O2. Some of the HIF target genes regulate processes involved in tumorigenesis, such as increased glucose uptake and glycolysis, angiogenesis, invasion, metastasis and radiation resistance. Furthermore, a number of common oncogenic drivers, such as mTORC1 and Ras, enhance HIF activity.
HIFs function as heterodimers, composed of stable β-subunits and O2-sensitive α-subunits, which accumulate upon O2 deprivation. In normal O2 conditions, HIFαs are modified by metabolically-regulated prolyl hydroxylases (PHDs), leading to physical interactions between HIFα subunits and the von Hippel-Lindau (pVHL)-associated E3-ubiquitin ligase, resulting in rapid HIFα turnover. Two well characterized isoforms of the α-subunit, HIF-1α and HIF-2α, have both overlapping functions, as well as divergent and even antagonistic roles in hypoxic responses1. Increased HIF-1α and HIF-2α protein levels in diagnostic tumor biopsies are correlated with poor prognosis in cancers of the bladder, brain, breast, colon, cervix, endometrium, head/neck, lung, ovary, pancreas, prostrate, rectum and stomach2 These studies strongly support the development of pharmacological HIF inhibitors for cancer treatment.
Two recent studies, however, suggest that HIFs are not universally tumor-promoting3,4. In clear cell renal carcinoma, the most common form of kidney cancer, the VHL tumor suppressor gene is often inactivated, leading to constitutive HIF-1α and/or HIF-2α expression. Considerable evidence shows a primary role for HIF-2α in promoting VHL-deficient renal cancers1; moreover, HIF-2α polymorphisms are linked to renal carcinoma5. However, loss of chromosome 14q, which spans the HIF-1α locus, is a frequent event in clear cell carcinoma and a poor diagnostic indicator3. Furthermore, HIF-1α, but not neighboring genes on chromosome 14q, is subject to focal deletions. These data suggest that VHL mutations lead to the initial activation of both oncogenic HlF-2α and tumor suppressive HIF-1α, but subsequent loss of HIF-1α activity through 14q deletion or HIF-1α truncations leads to a more aggressive form of VHL-deficient renal cell carcinoma (RCC)3. The divergent effects HIF-1α and HIF-2α have on RCC have been linked to their opposing effects on c-Myc transcriptional activity; HIF-2α promotes hypoxic cell cycle progression, while HIF-1α antogizes c-Myc-mediated RCC cell cycle progression1.
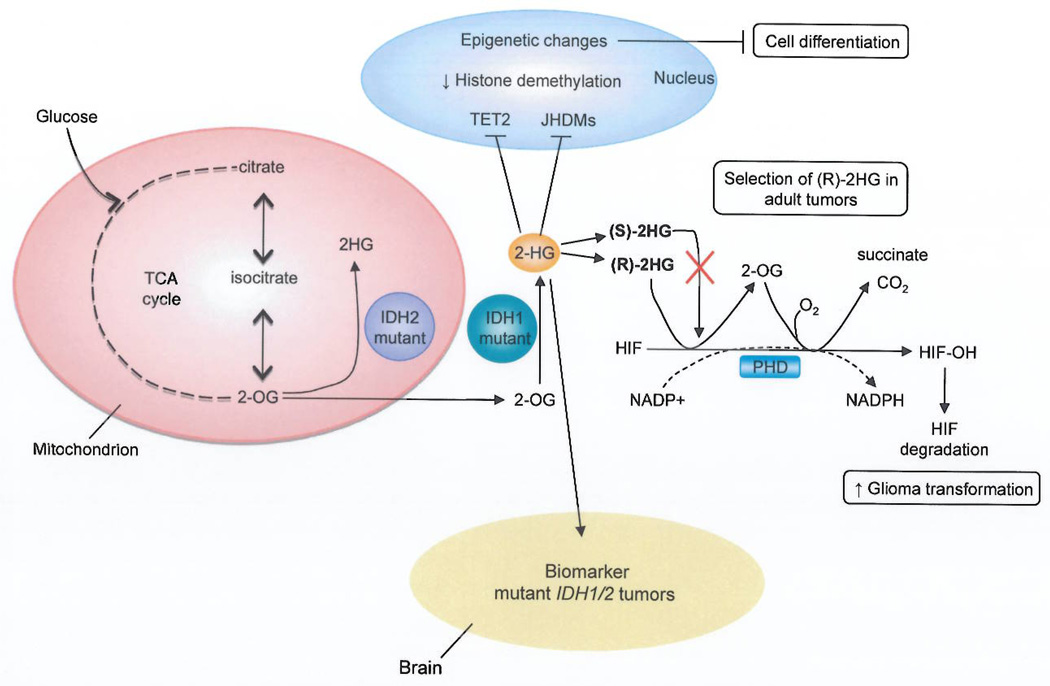
HlF-1α also seems to have a tumor suppressive role in gliomas with mutations in isocitrate dehydrogenase 1/2 (IDH1)IIDH24. These enzymes, which catalyze the conversion of isocitrate to α-ketoglutarate (also known as 2-oxoglutarate [2-OG]), are frequently mutated in lower grade gliomas (more than 70%, grade II and III), and less often in glioblastomas, leukemias and chondrosacrcomas. The most common IDH mutation in glioma results in an amino acid substitution at arginine 132 (R132H), which resides in the active site of the enzyme. IDHRI32H produces the neomorphic metabolite 2-hydroxyglutarate (2HG)—normally present in scarce amounts—through the NADPH-dependent reduction of 2-OG to the (R)-enantiomer of 2HG6 (Fig. 1). This oncometabolite is supposed to exert its effect by influencing several dioxygenases that use 2-OG as a substrate, which include the HIF PHDs7.
Figure 1.
A model for how 2-hydroxyglutarate (2HG) promotes glioma transformation. Tumors bearing mutations in IDH1/2 accumulate high amounts of the metabolite (R)-2HG. The roie of this oncometabolite in cellular transformation may be linked to its ability to substitute for 2-oxoglutarate (2-OG) as a cosubstrate for HIF prolyl 4-hydroxylases (PHDs) with the production of succinate through a 2-OG intermediate. High levels of cellular 2HG are hypothesized to drive the hydroxylation of HIFα, leading to decreased HIF expression and increased glioma transformation. This reaction is specific for the (R)-2HG enantiomer, which may explain the selection for (R)-2HG as opposed to (S)-2HG in adult tumors. In addition, the build up of 2HG, in IDH mutant cells has been linked to epigenetic changes through the inhibition of methylcytosine dioxygenase 2 (TET2) and Jumonji-C domain histone demethylases (JHDMs). Finally, increased levels of 2HG can be detected noninvasively by magnetic resonance spectroscopy (MRS). Detection of the biomarker, 2HG, by MRS has important prognostic value, since IDH mutant tumors are associated with improved survival in glioma bearing patients.
Three papers in a recent issue of Nature explore in more detail how the build up of 2HG resulting from IDH1/2 mutations contributes to glioma4,8,9. Two studies provide further support for the role of IDH1/2 mutations, and the consequential accumulation of 2HG, in aberrant methylation8'9. Lu et. al. go on to demonstrate that cell permeable 2HG, the neomorphic product of mutant IDH, blocks the differentiation of 3T3-L1 to adipocytes, and increases repressive histone methylation8. Furthermore, this block in adipocyte differentiation can be mimicked by siRNA-mediated repression of Jumonji-C domain histone demethylases(JHDMs). Whereas Turcan et. al. show that the introduction of mutant IDH1 into human primary astrocytes progressively remodeled the methylome to mirror the CpG island methylator phenotype (CIMP), observed in low grade gliomas9. In contrast, Koivunen et al.4 exploited the fact that 2HG accumulation in an enantiomer-specific manner in IDH mutant brain tumors explains how transformation occurs4.
Consistent with previous studies, 2HG accumulation inhibits 2-OG-dependent enzymes in vitro, including the TET1 and TET2 methyl cytosine hydroxylases, JMJD2D histone demethylases and HIF PHDs. But the S enantiomer of 2HG is a more potent inhibitor than (R)-2HG for these enzymes4. In fact, at tumor relevant concentrations, (R)-2HG was not an effective inhibitor of PHD; instead, it promoted PHD activity while sparing other 2-OG dependent enzymes. Koivenen et. al.4 monitored the in vitro hydroxylation of HIF by PHD2 and observed the production of 2-OG and succinate when catalytically-active PHD2 was incubated with (R)-2HG and HIF-1α polypeptide (Fig. 1). Furthermore, modeling studies predict that binding of (S)-2HG to PHD2 would not allow O2 to enter the active site and therefore would block HIF degradation. Collectively these results suggest that (R)-2HG—but not (S)-2HG—can substitute for2-OG as a cosubstrate in the hydroxylation of HIF-1α and HIF-2αand in this manner promote their degradation.
What is the in vivo relevance of this phenotype? Using midpassage immortalized human astrocytes expressing wild-type or mutant IDH1(R132H), HIF1α and HIF2α amounts were shown to decrease upon expression of mutant IDH relative to the wild-type enzyme and HIF1α decreased in mutant IDH1 expressing oligodendroglioma and HCT116 cell lines. In addition, the HIF-responsive gene expression signature in proneural tumors with IDH mutations was attenuated relative to IDH wild-type lesions. Finally, downregulation of HIF-1α or over expression of PHD2 promoted growth of human immortalized astrocytes in soft agar. Conversely, decreased levels of PHD2 inhibited astrocyte proliferation.
Accumulation of (R)-2HG therefore leads to reduced HIF-1α expression and pathogenesis of IDH mutant gliomas. This finding is not inconsistent with (R)-2HG also impacting other 2-OG dependent enzymes, such as TET2 and JmjC-containing histone demethylases, but it might explain the selection for (R)-2HG as opposed to (S)-2HG in human adult tumors. Interestingly, Li et. al. have previously shown that HIF-2α is preferentially expressed in glioma stem cells and high HIF-2α mRNA levels are associated with poor glioma patient survival10. In GSCs, both HIF-1α and HIF-2α, were observed to promote self-renewal, proliferation and survival. Although the results from Li et. al.10 and Koivunen et. al4, appear to be contradictory, they may indicate that distinct neural populations-low grade IDH mutant gliomas and GSC-respond differently to alterations in HIF activity. However, it would be interesting to know whether downregulation of HIF-2α inhibits the soft agar growth of IDH mutant immortalized astrocytes.
Whether HIF is tumor promoting or inhibitory is complex. In some tumor types, the pro or anti tumorigeneic effects of HIFα are isoform-specific; in VHL-deficient RCC, HIF-2α is tumor promoting and HIF-1α is tumor inhibitory3. This is due, in part, to the fact that HIF-1α and HIF-2α can regulate unique downstream targets and interact with important oncogenes and tumor suppressors, such as MYC and p53 in an isoform-specific manner1. In addition, inappropriate levels of HIF activity may be an important determinate in tumor growth. For example, whereas HIF-2α overexpression contributes to the progression of non-small cell lung cancer, inhibition of HIF-2α can, paradoxically, promote growth of the same tumor type11. Consequently, any discussion of employing pharmacological inhibitors of the HIF/PHD pathway as a potential therapeutic strategy must be laced with caution.
When and how to employ HIF inhibitors in the treatment of cancer is under intense investigation; however, the accumulation of 2HG-upstream of decreased HIF accumulation- in IDH1/2 mutant cells opens up the potential for new diagnostic tools in the clinical evaluation of individuals with brain tumors. A recent study of glioma patients undergoing magnetic resonance imaging (MRS) revealed that this noninvasive test, routinely used in the clinics, reliably detects the presence or absence of 2HG within brains12. This information has prognostic value, because mutant IDH1/2 tumors are associated with improved patient survival among those harboring gliomas. In addition, it is possible that 2HG levels can be followed over time to monitor glioma progression, detect recurrent disease, and finally evaluate patients’ response to chemotherapy.
Human genetic mutations can reprogram metabolism to promote tumor formation and progression. Notably, there are many examples where genetic alterations in metabolic pathways impact HIFα activity and downstream cellular transformation1,4,7. A deeper understanding of the intersections between tumor-potentiating genetic mutations and their metabolic consequences promises new advances in cancer therapy.
References
- 1.Keith B, Johnson RS, Simon MC. H1F1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen C, et al. Genetic and Functional Studies Implicate HIF1alpha as a 14q Kidney Cancer Suppressor Gene. Cancer Discov. 2012;1:222–235. doi: 10.1158/2159-8290.CD-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koivunen P, et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature. 2012;483:484–488. doi: 10.1038/nature10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purdue MP, et al. Genome-wide association study of renal cell carcinoma identifies two susceptibility loci on 2p21 and 11q13.3. Nat Genet. 2012;43:60–65. doi: 10.1038/ng.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dang L, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Celt. 2012;148:1132–1144. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu C, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turcan S, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazumdar J, et al. HIF-2alpha deletion promotes Kras-driven lung tumor development. Proc Natl Acad Sci USA. 2010;107:14182–14187. doi: 10.1073/pnas.1001296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi C, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18:624–629. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]