Abstract
Background & Aims
Administration of terlipressin plus albumin is effective in reversing type 1 HRS as compared to albumin alone. However, only about 1/3 of patients respond to treatment, therefore, predictors of response and survival would help identify the patients most likely to benefit from treatment.
Methods
We analyzed our controlled trial of terlipressin vs. placebo (Gastroenterology 2008;134:1360) to define factors predictive of a response and to correlate hemodynamic changes to changes in renal function.
Results
Single variant analysis showed treatment with terlipressin, MELD score, and baseline serum creatinine to be predictive of HRS reversal. Alcoholic hepatitis, baseline serum creatinine, and MELD score were predictive of survival. When treatment was not considered as a variable, only baseline serum creatinine predicted HRS reversal. Baseline serum creatinine, presence of alcoholic hepatitis, and Child-Pugh score were also predictive of survival on multivariate analysis. The rise in mean arterial pressure (MAP) following terlipressin administration was not predictive of HRS reversal. However, in those who achieved HRS reversal from terlipressin, there was a significant rise in MAP from beginning to end of treatment.
Conclusions
The most consistent predictor of response to terlipressin and of survival is the baseline serum creatinine. Patients most likely to benefit from terlipressin have earlier onset renal failure (i.e. serum creatinine <5.0 mg/dl). A sustained rise in MAP is required for HRS reversal. As MAP is a surrogate marker for the hyperdynamic circulation, it is only with improvement in the hyperdynamic circulation that HRS reversal is observed.
Introduction
Hepatorenal syndrome (HRS) type 1 is a rapidly progressive but potentially reversible form of renal failure that occurs in patients with cirrhosis and ascites and is associated with high mortality [1–3]. The main pathophysiological basis for the development of HRS type 1 is the progressive systemic arterial vasodilation. Arterial vasodilation, especially in the splanchnic bed, leads to a decrease in effective arterial blood volume with subsequent activation of renal sodium-retentive mechanisms and intrarenal arterial vasoconstriction. As the hyperdynamic circulation worsens, there is a progressive intrarenal arterial vasoconstriction leading to renal failure in the absence of intrinsic kidney disease [4–7].
Appreciation of the central role of arterial vasodilation in the pathogenesis of HRS has led to the use of arterial vasoconstrictors for its treatment. A number of vasoconstrictors including terlipressin, ornipressin, midodrine plus octreotide, and norepinephrine have been used in HRS type 1 [2,3,7–10]. Terlipressin, a 12 amino acid synthetic analog of lysine-vasopressin, is the most widely used drug for the treatment of HRS and three randomized controlled trials have compared terlipressin plus albumin to albumin alone [8–10]. In the largest and only placebo-controlled multicenter trial, HRS reversal was observed in 34% of the terlipressin treated patients and 13% of those receiving placebo (p = 0.008) [8]. In a recent systematic analysis of vasoconstrictors for HRS, terlipressin plus albumin was significantly more likely to reverse HRS and also to improve short term survival as compared to albumin alone [11].
Given the fact that terlipressin has the side-effects expected of a V1-mediated vasoconstrictor, it would be preferable to restrict exposure to the patient group most likely to respond to treatment. We examined the variables predictive of a response to terlipressin plus albumin and albumin alone in the treatment of HRS type 1 in our previously published randomized controlled trial [8]. We wanted to better define the population of patients most likely to benefit from treatment with terlipressin. We also hypothesized that mean arterial pressure (MAP)was a surrogate marker of the hyperdynamic circulation, and if terlipressin caused a consistent rise in MAP and that rise was associated with an improvement in renal function, then terlipressin would be working by improving the hyperdynamic circulation. Therefore, by examining the hemodynamic response to terlipressin plus albumin vs. albumin alone in the same patients, we hoped to gain a better understanding of how terlipressin reverses renal failure in patients with type 1 HRS. The results of these analyses are the subject of this report.
Materials and methods
Patients and study design
Adult subjects (≥18 years of age) with acute or chronic liver disease and HRS type 1, as defined by the International Ascites Club criteria (rapidly progressive reduction in renal function, e.g., doubling of serum creatinine (SCr) to ≥2.5 mg/dl in less than two weeks, and failure of renal function to improve following diuretic withdrawal and plasma volume expansion) [12] were included in this trial which has been described in detail [8]. The study was a prospective, randomized, double-blind, placebo-controlled, multicenter clinical trial conducted at 35 medical centers across the United States (n = 30), Germany (n = 2), and Russia (n = 3) from 2004 through 2006. The study was registered in the national clinical trials database (ClinicalTrials.gov identifier NCT00089570) and approved by the institutional review boards at each center. All data were recorded on standardized case report forms that were entered into a database at a data coordinating center.
Subjects with acute or chronic liver disease and acute worsening of renal function were initially screened for the study. The diagnosis of alcoholic hepatitis was made by the investigator at the site at the time of enrollment and was based on clinical criteria as a liver biopsy was not required. Following randomization, patients received blinded study medication either terlipressin at a dose of 1 mg administered by slow intravenous (IV) push every 6 h or matching placebo. Most of the patients (88%) also received intravenous albumin [8]. Serum electrolytes, BUN, and creatinine were evaluated daily during treatment. Concomitant medications were recorded. MAP was measured immediately before and 2 h after each dose of the study drug, to correspond with peak effect of terlipressin, and the mean value for each day was calculated.
Statistical analysis
All analyses conducted for this report used all patients in the ITT population who received at least one dose of the study drug (56 patients on terlipressin and 55 on placebo) unless otherwise stated. When analyses were conducted by treatment arm, this was explicitly mentioned. Key analyses presented were prospectively planned, with additional exploratory analyses performed retrospectively.
Univariate and multivariate logistic regression analyses were conducted to determine baseline patient characteristics that were predictive of HRS reversal (defined as serum creatinine on treatment ≤1.5 mg/dl) and overall survival up to day 180. For HRS reversal Wald Chi-Square Tests and relative risks from a single logistic regression were used, both with and without treatment as a factor. For survival, log-rank tests for association were used. The following factors were used for univariate analysis and a subgroup of these for multivariate analysis: age group (<65, ≥65), gender (M vs. F), race group (non-white, white), alcoholic hepatitis (present vs. not), baseline MELD score, Child-Pugh score, MAP, serum sodium, bilirubin, and creatinine (SCr). Median values and confidence intervals (CI) were determined as appropriate. SAS® software version 8.2 was used to perform all statistical analyses and to prepare summary tables and data listings. The protocol provided for descriptive subgroup and correlative analyses, included the univariate analysis and in-depth examination of the MAP data. There was also a provision for unspecified exploratory analyses. The multivariate analysis was thus performed retrospectively, as was the plotting of MAP vs. serum creatinine by response group, as well as the beta-blocker and the renal function subgroup analyses.
Results
Predictors of HRS reversal and survival
HRS reversal was seen in 19/56 terlipressin treated patients and 7/56 placebo patients. The only variables that were significantly different by single variant analysis between those with and without HRS reversal were MELD score, treatment group, and baseline SCr. For overall survival, univariate predictors of outcome were alcoholic hepatitis, SCr and MELD, with Child-Pugh and baseline bilirubin showing non-significant trends (Table 1). We conducted multivariate analyses to verify the independent influence of these factors on HRS reversal. When treatment was considered in the analysis, due to the strength of this effect on HRS reversal, treatment with terlipressin was the only significant factor (p = 0.002), with terlipressin patients more than six times as likely to respond than placebo patients (RR 6.053). When treatment was not considered, SCr became the only significant predictor of HRS reversal (p = 0.029) (Table 2). The likelihood of HRS reversal in either treatment arm was greatest in those with serum creatinines of <3.0 mg/dl, 50% (CI 26–74) terlipressin vs. 33% (CI 10–65) placebo, less so in those with serum creatinines of 3.0–5.0 mg/dl, 31% terlipressin (CI 15–51) vs. 9% (CI 2–24) placebo and least in those with serum creatinines >5.0 mg/dl, 11% (CI 0–48) terlipressin vs. 0% (CI 0–31) placebo. However, the treatment effect of terlipressin vs. placebo appeared to be greatest in the 3–5 mg/dl baseline SCr group, with a 22% absolute difference in HRS reversal compared to a difference of 17% with baseline SCr <3 mg/dl and 11% for patients with SCr >5 mg/dl.
Table 1.
Summary of the effects of baseline characteristics on HRS reversal and survival (univariate analysis, ITT population).
| Baseline parameter | HRS Reversal
|
Survival
|
||||
|---|---|---|---|---|---|---|
| RR | 95% CI | p value | RR | 95% CI | p value | |
| Treatment group | 2.71 | 1.24–5.94 | 0.009 | 0.93 | 0.58–1.51 | 0.782 |
|
| ||||||
| Alcoholic hepatitis | 0.97 | 0.49–1.92 | 0.890 | 2.29 | 1.41–3.72 | <0.001 |
|
| ||||||
| Gender | 0.57 | 0.31–1.08 | 0.055 | 1.00 | 0.59–1.69 | 0.963 |
|
| ||||||
| MELD score | 0.95 | 0.91–0.99 | 0.017 | 1.05 | 1.01–1.10 | 0.030 |
|
| ||||||
| Child-Pugh score | 0.87 | 0.75–1.02 | 0.065 | 1.15 | 1.00–1.32 | 0.051 |
|
| ||||||
| Serum creatinine | 0.65 | 0.46–0.93 | 0.021 | 1.40 | 1.22–1.60 | <0.001 |
|
| ||||||
| Bilirubin | 1.00 | 0.97–1.02 | 0.805 | 1.01 | 1.00–1.03 | 0.087 |
|
| ||||||
| MAP | 0.99 | 0.96–1.02 | 0.459 | 1.02 | 0.99–1.04 | 0.216 |
|
| ||||||
| Serum sodium | 0.99 | 0.95–1.04 | 0.730 | 0.99 | 0.95–1.03 | 0.519 |
RR: relative risk; 95% CI: 95% confidence intervals.
Table 2.
Summary of the effects of baseline characteristics on HRS reversal (multivariate analysis, ITT population).
| Baseline parameter | RR | 95% CI | p value |
|---|---|---|---|
| Alcoholic Hepatitis | 0.98 | 0.32–2.94 | 0.965 |
| Gender | 0.68 | 0.23–1.96 | 0.472 |
| MELD Score | 0.92 | 0.80–1.05 | 0.223 |
| Child-Pugh Score | 0.89 | 0.62–1.27 | 0.513 |
| Serum Creatinine | 0.51 | 0.28–0.93 | 0.029 |
| Bilirubin | 1.02 | 0.97–1.08 | 0.374 |
| Mean Arterial Pressure | 0.98 | 0.94–1.02 | 0.348 |
RR: relative risk; 95% CI: 95% confidence intervals.
Baseline serum creatinine was also predictive of survival in the multivariate analysis (p <0.001), with a 50% increase in risk of dying for every mg/dl increase in creatinine. Alcoholic hepatitis more than doubled the risk of death, and baseline Child-Pugh score also had a significant influence on survival (Table 3). The concomitant use of beta-blockers did not appear to negatively impact either HRS reversal or survival, as detailed in Table 4.
Table 3.
Summary of the effects of baseline characteristics on overall survival at day 180 (multivariate analysis, ITT population).
| Baseline parameter | RR | 95% CI | p value |
|---|---|---|---|
| Alcoholic Hepatitis | 2.28 | 1.31–3.97 | 0.003 |
| MELD Score | 0.99 | 0.92–1.06 | 0.769 |
| Child-Pugh Score | 1.25 | 1.05–1.50 | 0.014 |
| Serum Creatinine | 1.50 | 1.27–1.76 | <0.001 |
| Bilirubin | 1.01 | 0.98–1.03 | 0.632 |
| Mean Arterial Pressure | 1.02 | 0.99–1.04 | 0.215 |
RR: relative risk; 95% CI: 95% confidence intervals.
Table 4.
Summary of HRS reversal and survival by concomitant beta-blocker use.
| Terlipressin (n = 56)
|
Placebo (n = 56)
|
|||||
|---|---|---|---|---|---|---|
| n | % | 95% CI | n | % | 95% CI | |
| Received concomitant beta-blockers | ||||||
| HRS reversal | 8/16 | 50 | 25–75 | 2/23 | 9 | 1–28 |
| 180-day survival estimate | - | 56 | - | - | 35 | - |
|
| ||||||
| Did not receive concomitant beta-blockers | ||||||
| HRS reversal | 11/40 | 28 | 15–44 | 5/33 | 15 | 5–32 |
| 180-day survival estimate | - | 38 | - | - | 39 | - |
95% CI: 95% confidence intervals.
Response of MAP to terlipressin and correlation with renal function
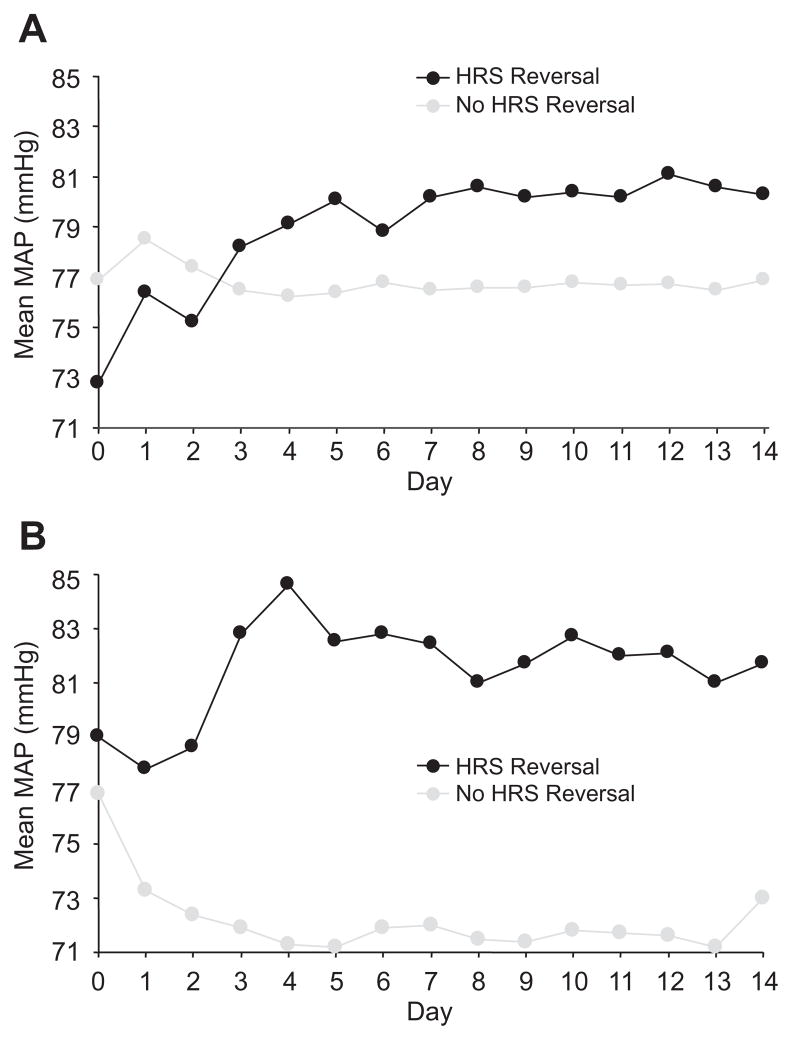
Following the administration of terlipressin there was a similar immediate increase in MAP in both those who achieved HRS reversal and those who did not. The daily MAP increased over time in those receiving terlipressin who achieved HRS reversal whereas in those who failed to respond the MAP did not change (Fig. 1A). In the placebo-treated patients the MAP rose in those who had HRS reversal and fell in those who did not respond (Fig. 1B). Shown in Table 5 is the MAP at initiation of treatment and at end of treatment (EOT). There was a significant decrease in MAP at EOT in the placebo group and a small rise in MAP in the terlipressin group. We further examined the change in MAP from the beginning to end of treatment in both groups comparing those who achieved HRS reversal and those who did not (Table 6). The difference in MAP at the beginning and end of treatment was significantly greater in the terlipressin responders vs. non-responders. In the placebo group, the fall in MAP was significant in the non-responders but the difference between the responders and non-responders was not significant.
Fig. 1. Mean arterial pressure in patients treated with (A) terlipressin or (B) placebo.
Each data point represents the MAP averaged for 4 injections/day measured just before and 2 h after terlipressin/placebo, presented using last observation carried forward. (A) Mean MAP over time on terlipressin. (B) Mean MAP over time on placebo.
Table 5.
Mean MAP at end of treatment (EOT) vs baseline for terlipressin and placebo.
| Treatment | n | Baseline | EOT | Change from baseline
|
|||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean change* (SE) | p vs. baseline | Difference (SE) | p vs. placebo | ||
| Terlipressin | 56 | 75.5 (11.4) | 77.9 (11.2) | +1.8 (1.8) | 0.333 | 6.2 (2.5) | 0.017 |
|
| |||||||
| Placebo | 55 | 77.2 (13.7) | 73.5 (11.4) | −4.4 (1.9) | 0.020 | - | - |
Least squares mean.
Analysis using last observation carried forward. SD: standard deviation, SE: standard error, difference = terlipressin minus placebo.
Table 6.
Change in MAP from baseline to EOT for terlipressin and placebo non-responders and responders.
| Treatment | n | Baseline | EOT | Change from baseline
|
|||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean change* (SE) | p vs. baseline | Difference (SE) | p vs. non-responders | ||
| Terlipressin | |||||||
| Responders | 19 | 72.8 (11.6) | 80.7 (7.9) | 7.3 (3.0) | 0.017 | 8.3 (3.6) | 0.025 |
| Non-responders | 37 | 76.9 (11.3) | 76.5 (12.4) | −1.0 (2.2) | 0.641 | ||
|
| |||||||
| Placebo | |||||||
| Responders | 7 | 79.0 (13.9) | 83.1 (6.7) | 3.1 (5.1) | 0.547 | 8.8 (5.4) | 0.110 |
| Non-responders | 48 | 77.0 (13.8) | 71.9 (11.2) | −5.7 (2.0) | 0.006 | ||
Least squares mean.
EOT: end of treatment, SD: standard deviation, SE: standard error.
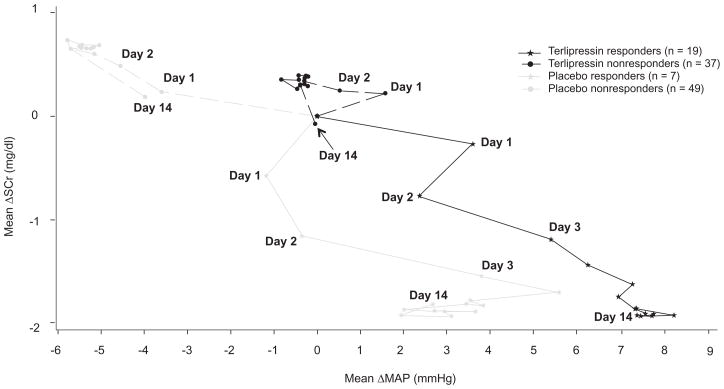
In order to better understand the relationship between the change in MAP and creatinine, we plotted the change in the two variables each day for the four groups of patients (Fig. 2). In the placebo non-responders the progressive fall in MAP was associated with a decline in renal function. In the terlipressin non-responders, MAP did not change and renal function either did not improve or declined slightly. The placebo responders showed an improvement in renal function followed by a rise in MAP. The terlipressin responders showed a rise in MAP followed by an improvement in renal function.
Fig. 2. Relationship between daily change in MAP and serum creatinine over time.
Each data point represents the daily change in MAP averaged for 4 injections/day measured just before and 2 h after terlipressin, presented using last observation carried forward.
Discussion
The only variable predictive of HRS reversal was the baseline serum creatinine. The patient who is most likely to benefit from treatment with terlipressin and/or albumin is the one with early onset moderate renal failure (SCr <3.0 mg/dl). As their renal function worsens their likelihood of benefiting from terlipressin or albumin declines, although the treatment effect of terlipressin vs. placebo appears most dominant in patients with baseline SCr 3–5 mg/dl. The highest baseline serum creatinine in a terlipressin responder was 5.6 mg/dl. Once the creatinine exceeded 7 mg/dl the likelihood of response was negligible and one can question the utility of using this drug in patients with this advanced form of disease. However, in the literature the highest reported baseline SCr in a patient responding to terlipressin was 8.3 mg/dl [13].
Other studies have examined predictors of response to terlipressin. The other, smaller, controlled trial comparing terlipressin plus albumin to albumin alone found that baseline urine volume, SCr, and use of terlipressin were predictive of HRS reversal [9]. Another study of 39 patients with HRS type 1 treated with terlipressin plus albumin found that baseline bilirubin was predictive of a response to terlipressin [14]. However, we found no relationship between bilirubin levels and HRS reversal (Table 1). In another report, norepinephrine was compared to terlipressin in the treatment of HRS type 1, and predictors of a response to vasoconstrictor therapy were baseline serum creatinine, MAP, and plasma renin activity [15]. Univariate analysis of a retrospective series of 18 consecutive patients with HRS type 1 suggested that only Child-Pugh score was predictive of HRS reversal, whereas treatment with ≥3 mg terlipressin per day, absence of precipitating factors, and response to terlipressin were predictive of survival [16]. In the current study, Child-Pugh score and treatment with terlipressin also appear to have predictive value. Based on this and previous reports, it would appear that baseline SCr is consistently the best predictor of HRS reversal. Terlipressin or other vasoconstrictors are likely to be most effective in those with early onset and moderate to severe renal failure. More advanced renal failure (SCr >7.0 mg/dl) is less likely to benefit from vasoconstrictor therapy.
Baseline SCr, Child-Pugh Score, and presence of alcoholic hepatitis were predictive of survival at 180 days (Table 2). The negative effect of alcoholic hepatitis could reflect the lack of transplantation as an option and the rapidly progressive nature of this type of liver disease. Increases in baseline Child-Pugh score or SCr were associated with an increasing risk of death. In another controlled trial, patients with MELD scores of >28 had a significantly worse prognosis as compared to those with lower scores [9]. However, the median MELD score of responding patients on both arms of our trial was 32, with 10/19 terlipressin responders and 5/7 albumin responders having MELD scores >30 at baseline. As we did not find serum bilirubin or MELD to be independent predictors of survival, the most important factor driving the association with MELD is likely to be the SCr. Based on our own results, we do not believe that a high MELD score should preclude the use of terlipressin, as if the patient achieves HRS reversal then their prognosis improves [8,9].
A recent, large, single-center, non-randomized investigation concluded that the use of beta-blockers was detrimental to survival in patients with late-stage liver disease and refractory ascites [17]. Our study did not randomize for beta-blocker use, but a substantial proportion of patients were receiving concomitant beta-blockers before and during the study. We, therefore, performed a subgroup analysis of patients by their beta-blocker status (either receiving or not). We did not find a detrimental effect of beta-blocker use on HRS reversal nor on survival (Table 4).
This study also demonstrates that a progressive rise in MAP is essential for patients to have HRS reversal irrespective of whether they receive albumin or terlipressin. However, the initial rise seen immediately after the administration of terlipressin is not predictive of HRS reversal. It is only the sustained increased in MAP seen after terlipressin administration that is associated with HRS reversal (Table 5 and Fig. 1). This finding may suggest that the baseline MAP in the terlipressin responders is reset at a higher level as they continue to receive the drug, reflecting an improvement in the hyperdynamic circulation. In support of this suggestion are the results presented in Fig. 2 showing that as the MAP improved so did the patients’ renal function. In contrast, in the non-responders, the MAP fell back to baseline preceding the next dose of the drug and thus MAP did not rise over time. The rise in MAP in those who responded to terlipressin was significantly greater than in the non-responders (7.3 ± 3 mmHg vs. −1 ± 2.2 mmHg respectively, p <0.025). A previous report suggested that a rise in MAP at day 3 of ≥5 mmHg was necessary for HRS reversal [14]. Our data, although supportive of the idea that a sustained rise in MAP is required for HRS reversal, does not support the use of an absolute change in MAP to decide whether or not treatment with terlipressin is effective. We believe there is too much variation in the values we obtained, to make this measurement useful for predicting a response (Tables 5 and 6).
In those responding to terlipressin, the rise in MAP preceded the fall in serum creatinine (Fig. 2). The relationship between the changes in MAP and renal function is supportive of the idea that terlipressin works by reducing splanchnic vasodilation leading to an improvement in renal perfusion. Further support of this hypothesis is the finding that plasma renin activity and plasma aldosterone levels fall in patients who achieve HRS reversal [13]. In contrast, in the placebo responders serum creatinine improved before a rise in MAP, suggesting they were still somehow volume depleted, despite administration of fluids preceding randomization, and the additional albumin they received corrected this deficiency. However, for the placebo patients to achieve HRS reversal there had to be a sustained improvement in the hyperdynamic circulation as manifested by a later rise in MAP. These observations suggest that irrespective of how the hyperdynamic circulation is improved, the improvement leads to increased renal perfusion and a sustained fall in SCr. Once this improvement has occurred, the effect is durable as demonstrated by the low rates of relapse in the responders in our and other studies [8–10].
Those who received placebo and did not have HRS reversal demonstrate how this disease progresses. The hyperdynamic circulation worsens (progressive fall in MAP) and renal function continues to decline. Those who received terlipressin and did not respond show that, although MAP stabilized, the hyperdynamic circulation had not improved and, therefore, neither did renal function. Unfortunately, there is no measure available to determine how much improvement in the hyperdynamic circulation is required to reverse renal failure.
In conclusion, use of terlipressin plus albumin in the treatment of type 1 HRS is most likely to be of benefit if the renal failure is moderate (creatinine <3.0 mg/dl) or severe (creatinine 3.0–5.0 mg/dl). The use of terlipressin in patients with more advanced renal failure (SCr >7.0 mg/dl) is less likely to be of benefit or to improve patient survival. The risk of unnecessary exposure to terlipressin in patients unlikely to respond may also be mitigated by stopping treatment at day 4 in those whose SCr has not begun to decrease. In our trial, patients, whose SCr had not decreased by day 4, did not subsequently respond to treatment [8,18]. Patients responding to terlipressin will show a sustained improvement in MAP during therapy. However, the change in MAP immediately following the administration of terlipressin is not predictive of a response. The finding that terlipressin and perhaps other vasoconstrictors improve renal function by improving the hyperdynamic circulation should lead to a search for new agents that might be effective in the patient groups that are resistant to treatment with terlipressin or other vasoconstrictors. Ultimately, our efforts should be directed at preventing the development of the hyperdynamic circulation in order to reduce the risk of developing HRS-type 1 [19].
Acknowledgments
Financial support
This work was supported in part by a grant from the FDA (Office of Orphan Products Grant 1R01FD003024-01) and in part by Orphan Therapeutics.
The authors are grateful for the secretarial assistance of Mr. L.T. Tucker.
Terlipressin study group and Study Sites: Listed are site principal investigators.
United States (87 Patients): St. Luke’s Texas Liver Institute, Houston, TX: V. Ankoma-Sey; Albert Einstein Medical Center, Philadelphia, PA: V. Araya; Cleveland Clinic, Cleveland, OH: D. Barnes; Northwestern Memorial Hospital, Chicago, IL: A. Blei; University of Arizona, Tucson, AZ: T. Boyer; Washington University, St. Louis, MO: J. Crippin; University of Colorado Hospital, Denver, CO: G. Everson; California Pacific Medical Center, San Francisco, CA: T. Frederick; Yale University School of Medicine, New Haven, CT: G. Garcia-Tsao; Lahey Clinic Medical Center, Burlington, MA: F. Gordon; Mayo Clinic Transplant Center, Jacksonville, FL: A. Keaveny; University of Washington Medical Center, Seattle, WA:K, Kowdley; San Diego VA, San Diego, CA: D. Kravetz; UT Southwestern, Dallas, TX: M. Mayo; University of Nebraska, Omaha, NE: T. McCashland; University of Pennsylvania Hospital - Div. of GI, Philadelphia, PA: KR. Reddy; Tulane University Health Sciences Center, New Orleans, LA: F. Regenstein; Medical University of South Carolina, Charleston, SC: A. Reuben University of California Davis Medical Center, Sacramento, CA: L. Rossaro; UT Memphis, Memphis, TN: M. Sachdev; University Hospital-UMDNJ, Newark, NJ: A. Samanta; Medical College of Virginia, Richmond, VA: A. Sanyal; Mount Sinai Medical Center, NewYork, NY: T. Schiano; Georgetown, Washington, DC: K. Shetty; Weill Cornell Medical Center & Columbia, New York, NY: S. Sigal; Johns Hopkins Hospital, Baltimore, MD: P. Thuluvath; Mayo Clinic, Scottsdale, Phoenix, AZ: H. Vargas; N. Carolina Memorial Hospital/UNC Hospital, Chapel Hill, NC: S. Zacks; Oregon Health and Science University, Portland, OR: A. Zaman.
Russia (13 Patients): Russian People’s Friendship University, Moscow: V. Dvornikov; Centre of Applied Pharmacology, Moscow: V. Moiseev; Russian State Medical University, Moscow: G. Storozhakov.
Germany (12 Patients): University Clinic Munich, Munich: V. Gulberg; University Clinic Bonn, Bonn: T. Sauerbruch/B. Appenrodt.
Abbreviations
- HRS
hepatorenal syndrome
- MELD
model for end-stage liver disease
- MAP
mean arterial pressure
- SCr
serum creatinine
- CI
confidence intervals
- EOT
end of treatment
Footnotes
Conflict of interest
The authors have declared that they received funding from Orphan Therapeutics in order to carry out their research in this manuscript.
Contributor Information
Thomas D. Boyer, Email: tboyer@deptofmed.arizona.edu.
Arun J. Sanyal, Email: asanyal@mcvh-vcu.edu.
Guadalupe Garcia-Tsao, Email: guadalupe.garcia-tsao@yale.edu.
Daniel Carl, Email: dcarl@mcvh-vcu.edu.
Peter Teuber, Email: peter.teuber@orphantherapeutics.com.
References
- 1.Gines A, Escorsell A, Gines P, Salo J, Jimenez W, Inglada L, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105 (1):229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 2.Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of the hepatorenal syndrome in cirrhosis. A consensus workshop of the international ascites club. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064–2077. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 4.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodes J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 5.Arroyo V, Guevara M, Gines P. Hepatorenal syndrome in cirrhosis: pathogenesis and treatment. Gastroenterology. 2002 May;122(6):1658–1676. doi: 10.1053/gast.2002.33575. [DOI] [PubMed] [Google Scholar]
- 6.Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46 (5):935–946. doi: 10.1016/j.jhep.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279–1290. doi: 10.1056/NEJMra0809139. [DOI] [PubMed] [Google Scholar]
- 8.Sanyal AJ, Boyer T, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, et al. A randomized prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–1368. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-Llahi M, Pepin MN, Guevara M, Diaz F, Torre A, Monescillo A, et al. Terlipressin and albumin vs. albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352–1359. doi: 10.1053/j.gastro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Neri S, Pulvirenti D, Malaguarnera M, Cosimo BM, Bertino G, Ignaccolo L, et al. Terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Dig Dis Sci. 2008;52:830–835. doi: 10.1007/s10620-007-9919-9. [DOI] [PubMed] [Google Scholar]
- 11.Gluud LL, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51:576–584. doi: 10.1002/hep.23286. [DOI] [PubMed] [Google Scholar]
- 12.Arroyo V, Gines P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Int Ascites Club Hepatol. 1996 Jan;23(1):164–176. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 13.Uriz J, Gines P, Cardenas A, et al. Terlipressin plus albumin infusion: an effective and safe therapy of hepatorenal syndrome. J Hepatol. 2000;33:43–48. doi: 10.1016/s0168-8278(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 14.Nazar A, Pereira GH, Guevara M, Martin-Llahi M, Pepin M-N, Marinelli M, et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219–226. doi: 10.1002/hep.23283. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, Kumar A, Sharma BC, Sarin SK. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol. 2008;103:1689–1697. doi: 10.1111/j.1572-0241.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 16.Colle I, Durand F, Pessione F, Rassiat E, Bernuau J, Barriere E, et al. Clinical course, predictive factors and prognosis in patients with cirrhosis and type 1 hepatorenal syndrome treated with terlipressin: a retrospective analysis. J Gastroenterol Hepatol. 2002;17:882–888. doi: 10.1046/j.1440-1746.2002.02816.x. [DOI] [PubMed] [Google Scholar]
- 17.Serste’ T, Melot C, Francoz C, Durand F, Rautou P, Valla D, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017–1022. doi: 10.1002/hep.23775. [DOI] [PubMed] [Google Scholar]
- 18.Keaveny A, Garcia-Tsao G, Bexon A, Sanyal A. Early indicators of outcome in type 1 hepatorenal syndrome: focus on serum creatinine in the pivotal terlipressin trial. Am J Transplant. 2009;9:327. (abstract) [Google Scholar]
- 19.Fernandez J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818–824. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]