Abstract
Despite ongoing concerns that traumatized children with severe symptoms of emotional dysregulation may be inappropriately receiving a diagnosis of pediatric bipolar-I (BP-I) disorder, this issue has not been adequately examined in the literature. Because both pediatric BP-I disorder and PTSD are familial disorders, if children with both BP-I and PTSD were to be truly affected with BP-I disorder, their relatives would be at high risk for BP-I disorder. To this end, we compared patterns of familial aggregation of BP-I disorder in BP-I children with and without PTSD with age and sex matched controls. Participants were 236 youth with BP-I disorder and 136 controls of both sexes along with their siblings. Participants completed a large battery of measures designed to assess psychiatric disorders, psychosocial, educational, and cognitive parameters. Familial risk analysis revealed that relatives of BP-I probands with and without PTSD had similarly elevated rates of BP-I disorder that significantly differed from those of relatives of controls. Pediatric BP-I disorder is similarly highly familial in probands with and without PTSD indicating that their co-occurrence is not due to diagnostic error.
Keywords: comorbidity, clinical correlates, family risk analysis
1. Introduction
Despite ongoing concerns that traumatized children with severe symptoms of emotional dysregulation may be inappropriately receiving a diagnosis of pediatric bipolar-I (BP-I) disorder (Parens et al., 2010), this issue has not been adequately examined in the extant literature. As proposed by the “traumagenic neurodevelopmental model” (Read et al., 2001), early trauma may negatively affect the developing brain, which could lead to psychiatric disorders. However, the opposite pathway should also be considered; serious psychiatric disorders such as BP-I might increase the risk for exposure to traumatic events or exacerbate a person’s psychological response to such events.
Romero et al. (2009) reported that 20% of a sample of youth with BP spectrum disorders had experienced physical and/or sexual abuse. Likewise, Marchand et al. (2005) reported that 32% of youth treated for BP spectrum disorders in a community clinic had experienced 3 or more adverse life events such as neglect, abuse and foster care placement. That study also found that exposure to adverse events was associated with a poorer prognosis of BP spectrum disorders and a delay in diagnosis, regardless of whether children met criteria for PTSD, suggesting that exposure to early trauma may exacerbate a pre-existing condition in addition to making it more difficult to accurately diagnose a co-morbid disorder. On the other hand, Ford et al. (2000) reported rates of physical or sexual abuse of 36% and 66% in youth with ADHD and ODD, respectively, raising questions about the specificity of the association between trauma, PTSD and BP disorder in the young.
Similarly equivocal are findings regarding the co-occurrence of PTSD and pediatric BP disorder. Havens et al. (2012) found that, in a sample of adolescent inpatients, those with probable PTSD were three times as likely to have a diagnosis of bipolar disorder. However, Strawn et al. (2010) reported that in another sample of adolescent inpatients with a first manic or mixed episode, rates of PTSD were similar to normative data and lower than rates of PTSD in bipolar adults. In addition, the presence of post-traumatic symptoms did not affect recovery or recurrence of manic and depressive symptoms, suggesting a distinct psychopathology. Our group previously reported that pediatric BP-I disorder was a significant antecedent risk factor for the subsequent development of PTSD in clinically referred youth with ADHD (Wozniak et al., 1999), suggesting that the direction of effect may be from BP-I disorder to PTSD. Despite the equivocal nature of these findings, questions remain as to whether children meeting diagnostic criteria for BP-I disorder when they co-occur with PTSD actually suffer from BP-I disorder (Parens et al., 2010).
One way to clarify whether children with BP-I disorder and PTSD suffer from BP-I disorder, PTSD or both disorders is to examine patterns of familial aggregation between these disorders. This well-accepted method has been applied successfully to other disorders and comorbid conditions (Pauls et al., 1993; Pauls et al., 1994; Faraone et al., 2000; Christiansen et al., 2008). Because both pediatric BP-I disorder and PTSD are known to be familial disorders (Faraone et al., 2003; Althoff et al., 2005; Koenen et al., 2005; Koenen et al., 2008; Sartor et al., 2012), if children with both BP-I and PTSD are truly affected with BP-I disorder, we would expect that their relatives would be at high risk for BP-I disorder and that the risk would be similar to the risk imparted to relatives of BP-I children who do not have PTSD. In contrast, if comorbid BP-I+PTSD is a traumatically induced phenocopy of the genetic form of BP-I, then we would expect that relatives of BP-I+PTSD probands would not have an increased risk for BP-I disorder.
Thus, using family study methodology, the main aim of the current study was to examine whether children with BP-I+PTSD suffer from true BP-I disorder. We did this by comparing patterns of familial aggregation of BP-I disorder in BP-I children with and without PTSD with age and sex matched controls. We hypothesized that the familial correlates of BP-I disorder would be similar in children with BP-I disorder irrespective of the comorbidity with PTSD.
2. Methods
Families were recruited through the Clinical and Research Program in Pediatric Psychopharmacology at the Massachusetts General Hospital based on the presence of a diagnosis of bipolar (BP)-I disorder in proband youth 6–17 years of age of both sexes (Wozniak et al., 2005; Wozniak et al., 2010). Comparators were youth without ADHD or BP-I disorder of similar age and sex along with their first-degree relatives that participated in controlled family genetic studies of ADHD (Biederman et al., 1992; Biederman et al., 1999). All studies used the same assessment methodology regardless of the disorder used to classify probands as cases. We recruited 239 BP-I probands and their 726 biologic first-degree (parents and siblings) relatives. From 522 families participating in our case-control ADHD family studies we randomly selected 136 non-bipolar, non-ADHD control probands and their 411 first-degree relatives so that the age and gender distribution were similar to that of the BP-I probands. ADHD probands with co-morbid BP-I disorder were not included in the present analyses. All study procedures were reviewed and approved by the subcommittee for human subjects of our institution. After complete description of the study to the subjects, all subject’s parents or guardians signed written informed consent forms and children older than 7 years of age signed age appropriate written assent forms.
2.1 Ascertainment of BP-I probands
Potential BP-I probands were ascertained from our clinical service, referrals from local clinicians or self-referral in response to advertisements in the local media. To avoid biasing our sample toward familial cases of BP-I disorder, all probands were ascertained blind to the diagnostic status of their relatives. Subjects were first administered a phone screen reviewing symptoms of DSM-IV BP-I disorder and, if criteria were met, were scheduled for a face-to-face structured diagnostic interview. In addition to the structured diagnostic interview, an expert clinician (J.W.) met with each potential proband and his or her parents for a clinical interview in order to confirm the diagnosis of BP-I disorder using the Schedule for Affective Disorders and Schizophrenia for School-Age Children (KSADS) mania module. We have published data on the convergence of these clinical interviews with our structured interview diagnosis on the first 69 cases. We reported a 97% agreement between the structured interview and clinical diagnosis in an analysis of 69 children (Wozniak et al., 2003).
As previously reported (Biederman et al., 1992; Biederman et al., 1999; Wozniak et al., 2010) the controls were ascertained from out-patients referred for routine physical examinations to pediatric medical clinics at each setting, identified from their computerized records as not having ADHD. Screening procedures were similar to those described for the recruitment of the BP-I probands with the exception that we queried about ADHD (and not BP-I disorder) in the initial telephone screening and each proband was not assessed clinically.
2.2 Diagnostic procedures
Psychiatric assessments of subjects younger than 18 years were made with the DSM-IV based Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Epidemiologic Version (KSADS-E-IV) (Orvaschel, 1994) and assessments of adult family members were made with the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1997) supplemented with modules from the KSADS-E-IV to cover childhood disorders. Diagnoses were based on independent interviews with mothers and direct interviews with children older than 12 years of age. Data were combined such that endorsement of a diagnosis by either report resulted in a positive diagnosis.
We computed kappa coefficients of agreement by having experienced clinicians diagnose subjects from audio-taped interviews made by the assessment staff. Based on 500 interviews, the median kappa coefficient between raters and clinicians was 0.99. For individual diagnoses the kappas were ADHD (0.88), conduct disorder (1.0), major depression (1.0), bipolar (0.95), separation anxiety (1.0), agoraphobia (1.0), panic (0.95), substance use disorder (1.0), tics/Tourette’s (0.89) and PTSD (0.8).
Children and adolescents were diagnosed with BP-I disorder according to DSM-IV criteria. The DSM-IV requires subjects to meet criterion A for a distinct period of extreme and persistently elevated, expansive or irritable mood lasting at least 1 week, plus criterion B, manifested by three (four if the mood is irritable only) of seven symptoms during the period of mood disturbance. To ensure that the B criterion symptoms were concurrent with A criterion mood disturbance, subjects were directed to focus on the worst or most impairing episode of mood disturbance while being assessed for the presence of the confirmatory B criterion symptoms. That is, the subject was asked to consider the time during which the screen was at its worst for the purpose of determining whether the remaining symptoms were also evident at the same time as the screening item. Also recorded was the onset of first episode, the number of episodes, offset of last episode, and total duration of illness. Any subject meeting criteria for BP-II or BP-NOS was not included in this study. To gauge a distinct episode, our interviewers asked for ‘a distinct period (of at least 1 week) of extreme and persistently elevated, expansive or irritable mood’ and further required that the irritability endorsed in this module is ‘super’ and ‘extreme.’
Children and adolescents were diagnosed with PTSD disorder according to DSM-IV criteria. The interviewer introduced the module by describing that PTSD is characterized by symptoms following exposure to an extreme trauma. The trauma can include serious threats or actual harm to the individual witnessing such an event. The response to the event is one of intense fear, helplessness, or horror (may include disorganized or agitated behavior). Table 1 describes the specific questions patients were asked to ascertain the presence of trauma and PTSD and the diagnostic algorithms used. For all psychiatric diagnoses, including BP-I disorder and PTSD, we required that the level of symptoms and distress/disability be clinically significant.
Table 1a.
Post-Traumatic Stress Disorder KSADS Module
| A. Traumatic Event |
Have you ever had a terribly frightening experience? For example, were you ever in danger of being killed or badly hurt or have you ever witnessed a loved one in danger of being killed or badly hurt?
|
| INTRODUCTION: Now I’m going to ask you some questions about how you felt after the ___________(___). |
| B. Reexperience of Trauma |
| Did the (__) ever keep coming back to you in some way? For example… |
| 1. Recurrent/Intrusive Recollections Did you ever think about (__) a lot even if you didn’t want to? Did remembering it ever upset you? Did you ever try not to think about (___)? |
| 2. Dreams (Must be Recurrent) Did you ever have bad dreams about (__)?
|
| 3. Reliving/Flashbacks/Reenactment Did you ever feel like (___) was happening again, even when it wasn’t? When you played a game, was what happened ever a part of it sometimes?
|
| 4. Distress at Symbolic Exposure Did you ever get very upset when you saw something that reminded you of (__)?
|
| 5. Physiologic Reactivity to Associative Stimuli When something reminded you of (__), did your heart ever beat really fast or was it ever hard to breathe, or did you ever shake or feel sick?
|
| C. Persistent Avoidance or Numbing |
| 1. Avoids Thoughts or Feelings Did you ever try not to think about (___) or not to get upset about it? |
| 2. Avoids Associative Activities Did you ever stay away from things or people that would remind you of (___) or refuse or avoid talking about (___)?
|
| 3. Psychogenic Amnesia Was there ever some thing about what happened that you couldn’t remember?
|
| 4. Markedly Diminished Interest Were you ever much less interested in things you used to like to do?
|
| 5. Detachment or Estrangement Was it ever harder to care about things or feel close to people?
|
| 6. Restricted Affect Did you ever never feel either really good or really bad after the incident, but persistently just “blah” or numb? Instructions: Code 3 if felt “blah” or numb. |
| 7. Sense of Foreshortened Future Did you ever feel like you had nothing to look forward to in the future?
|
| D. Persistent Increased Arousal |
| 1. Trouble Sleeping Did you ever have trouble falling asleep or staying asleep after (__)?
|
| 2. Irritability/Anger Did you ever feel cranky, grouchy a lot or did you ever lose your temper after (__)? |
| 3. Difficulty Concentrating Was it ever harder to keep your mind on things or harder to concentrate after (__)? |
| 4. Hypervigilance Did you ever feel really sensitive, like you had to be on guard all the time or were you ever always worried that something would happen?
|
| 5. Exaggerated Startle Response Did you ever feel very jumpy, easily startled, or easily scared? |
| E. MARKED DISTRESS |
| Did your feelings about (___) ever make you feel bad… OR IMPAIRMENT Did your feelings about (___) ever keep you from doing things?
|
2.3 Statistical analysis
Differences in demographic and clinical characteristics were assessed using ANOVA for continuous outcomes, Pearson’s χ2 for binary outcomes, and Kruskal Wallis for Socioeconomic status (SES). The Kaplan-Meier cumulative failure function and Cox Proportional Hazard Models were used to calculate survival curves and cumulative, lifetime risk of BP-I and PTSD (including both subthreshold and full diagnoses) in relatives of control children and BP-I children with and without PTSD. Across all Cox models, we used robust estimates of variance to account for the non-independence of the sample resulting from the correlation between family members. We used logistic regression for binary outcomes and linear regression for continuous variables when examining the familial correlates between controls and BP-I children with and without PTSD.
When examining the rates of PTSD in probands and relatives, we restricted the analysis to probands and families where PTSD was assessed. Since PTSD was only assessed at baseline in our study of girls with and without ADHD, we were only able to use data from 36 proband girls without ADHD and their first degree relatives of both sexes. All other analyses were conducted on the full sample. The Hollingshead Four-Factor Index based on occupation and education of the parents was used to assess socioeconomic status (SES) with higher scores on the index representing a lower socioeconomic status (Hollingshead, 1975). Data are expressed as mean ± standard deviation (SD) unless otherwise specified. All tests were two-tailed, and our alpha level was set at 0.05 for all analyses, unless otherwise noted. We calculated all statistics using STATA, version 12.0.
3. Results
Out of the 239 BP-I probands, only 236 had PTSD data available for analysis. Our results were based on three group analysis: 22 BP-I probands with PTSD (full or subthreshold [BP-I+PTSD]; mean age: 11.8 ± 3.4, Table 2), 214 BP-I probands without PTSD (BP-I; mean age: 10.5 ± 3.3) and 136 control probands without BP-I disorder or ADHD (controls; mean age: 10.7 ± 3.0). The rate of PTSD was significantly overrepresented in BP-I probands vs. controls (22 [14 boys and 8 girls]) vs. 1 [girl] overall (P<0.01) as well as for analysis restricted to female only probands (8 vs. 1; P<0.01).
Table 2.
Demographics and Clinical Correlates of BP-1 Disorder
| a. Demographics | ||||
|---|---|---|---|---|
| Controls (n =136) | BP-1 (n =214) | BP-1 + PTSDA (n =22) | Test Statistic, P value | |
| n (%) or Mean ± SD | n (%) or Mean ± SD | n (%) or Mean ± SD | ||
| Age (years) | 10.7 ± 3.0 | 10.5 ± 3.3 | 11.8 ± 3.4 | F (2, 369)=1.58, P=0.21 |
| % Male | 99 (73) | 158 (74) | 14 (64) | χ2 (2)=1.05, P=0.6 |
| SES | 1.5 ± 0.7 | 1.9 ± 0.9* | 1.7 ± 0.8 | χ2 (2)=9.4, P=0.01 |
| Race/Ethnicity | ||||
| Caucasian | 132 (97) | 201 (94) | 20 (91) | χ2 (6)=9.7, P=0.14 |
| African-American | 2 (1.5) | 5 (2) | 1 (4.5) | |
| More than 1 | 0 (0) | 8 (4) | 1 (4.5) | |
| Unknown | 2 (1.5) | 0 (0) | 0 (0) | |
| b. Clinical Correlates | ||||
|---|---|---|---|---|
| Controls (n =136) | BP-I (n =214) | BP-I + PTSD (n =22) | Test Statistic, P value | |
| n (%) or Mean ± SD | n (%) or Mean ± SD | n (%) or Mean ± SD | ||
| BP-I characteristics | ||||
| BP-I age of onset (years) | -- | 6.3 ± 3.5 | 7.0 ± 4.6 | t=−0.9, P=0.4 |
| PTSD age of onset (years) | -- | -- | 8.0 ± 4.3 | |
| BP-I onset before PTSD | -- | -- | 15 (68) | |
| BP-I impairment | ||||
| Mild | -- | 0 (0) | 0 (0) | χ2 (1)=1.1, P=0.8 |
| Moderate | -- | 71 (33) | 8 (36) | |
| Severe | -- | 143 (67) | 14 (64) | |
| BP-I (mania) total symptoms | -- | 5.8 ± 1.1 | 6.02 ± 1.0 | t=−1.0, P=0.3 |
| BP-I (mania) total episodes | -- | 21.4 ± 56.7 | 23.1 ± 54.8 | t=−0.14, P=0.9 |
| Treatment history | ||||
| Hospitalization | 0 (0) | 76 (36)* | 9 (41)* | Exact, P<0.001 |
| Patterns of Psychiatric Comorbidity | ||||
| Major Depression | 9 (7) | 174 (81)* | 21 (95)* | χ2 (2)=231.7, P<0.001 |
| Psychosis | 0 (0) | 76 (36)* | 5 (23)* | Exact, P<0.001 |
| Disruptive Behavior Disorders | ||||
| Conduct Disorder | 2 (1) | 89 (42)* | 14 (64)*ψ | χ2 (2)=106.7, P<0.001 |
| Oppositional Defiant Disorder | 8 (6) | 193 (90)* | 20 (91)* | χ2 (2)=293.2, P<0.001 |
| ADHD | 0 (0) | 173 (81) | 17 (77) | χ2 (2)=2.1, P=0.4 |
| Anxiety Disorders | ||||
| Multiple Anxiety Disorders (≥2) | 7 (5) | 141 (66)* | 20 (91) *ψ | χ2 (2)=169, P<0.001 |
| Avoidant Disorders | 2 (1) | 39 (18)* | 3 (14)* | χ2 (2)=28.9, P<0.001 |
| Agoraphobia | 3 (2) | 79 (37)* | 11 (50)* | χ2 (2)=77.3, P<0.001 |
| Separation Anxiety Disorder | 10 (7) | 121 (55)* | 17 (77)* | χ2 (2)=113.2, P<0.001 |
| Simple Phobia | 8 (6) | 92 (43)* | 13 (59)* | χ2 (2)=74.2, P<0.001 |
| Social Phobia | 3 (2) | 72 (34)* | 10 (45)* | χ2 (2)=67.9, P<0.001 |
| Panic Disorder | 1 (1) | 27 (13)* | 6 (27)* | χ2 (2)=28.7, P<0.001 |
| Generalized Anxiety Disorder | 0 (0) | 92 (43)* | 17 (77) *ψ | Exact, P<0.001 |
| Academic Functioning | ||||
| Repeated grade | 10 (7) | 28 (13) | 4 (18) | χ2 (2)=3.9, P=0.14 |
P<0.05 vs. Controls;
P <0.05 vs. BP-I
PTSD (Full or Subthreshold)
The 22 BP-I + PTSD probands had the following traumas: car accidents (n=6) medical emergencies (n =3), being witness to a family member’s medical emergency (this included the family pet) (n =3), being molested (n =3), being witness to domestic abuse (n =2), being witness to a shooting (n =1), being witness to a robbery (n =1), being assaulted (n =1), being attacked by an animal (n =1), having a father in New York City during September 11, 2001 (n =1). The single control with PTSD had been in car accident.
While there were no significant difference in age, sex, and race, we did find a significant difference across groups in SES with controls having the highest SES status. As a result, all subsequent analyses were adjusted for SES.
3.1 Familial risk analysis
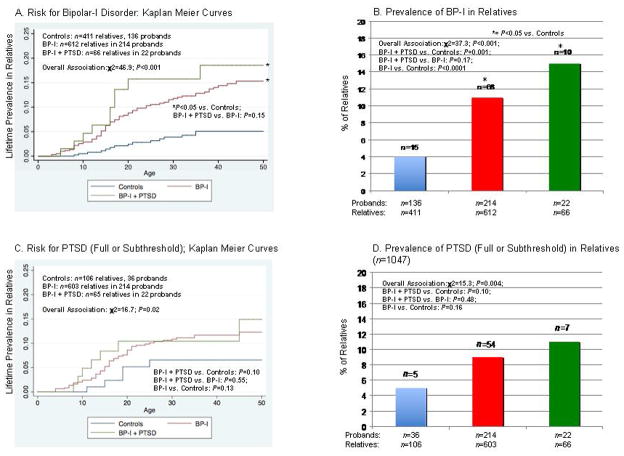
The distribution of age and sex were similar among the parents of controls (mean age: 31.6 ± 14.7; sex (% male): 49%), BP-I (32 ± 15.7; 48%), and BP-I + PTSD (32.1 ± 15.2; 48%) probands. The distribution of age and sex were also similar across the siblings of controls (12.9 ± 5.1; 52%), BP-I (12 ± 4.3; 46%) and BP-I + PTSD (15.3 ± 5.7; 50%) probands. Relatives of both BP-I disorder proband groups had a similarly elevated risk for BP-I irrespective of the comorbidity with PTSD (Panels A and B). Although the risk for PTSD was higher in probands with PTSD, the difference failed to reach our a priori threshold for statistical significance, most likely due to the limited statistical power (Panels C and D).
3.2 Clinical correlates analysis
There were no significant differences between the BP-I probands with and without PTSD in regards to age of onset, BP-I associated impairment, total number of episodes, total number of mania symptom count (Table 2) or individual mania symptoms (Figure 2). As shown in Figure 2, there were no significant differences in individual BP-I symptoms between the BP-I proband groups with and without PTSD. Both BP-I proband groups were more likely than controls to have been psychiatrically hospitalized (Table 2).
Figure 2.
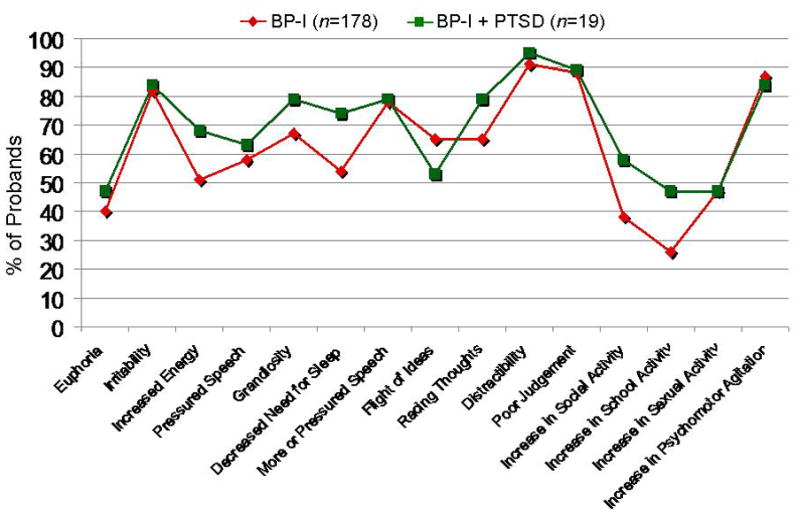
Individual Symptoms of Mania in BP-I Probands (n=197)*
*39 BP-I probands had missing symptom data
3.3 Patterns of psychiatry comorbidity
Rates of almost all disorders assessed were significantly elevated in BP-I probands with and without PTSD than in controls (Table 2). The BP-I + PTSD proband group was more likely to manifest conduct disorder, multiple anxiety disorders, and generalized anxiety disorder compared to the BP-I probands (Figure 3).
Figure 3.
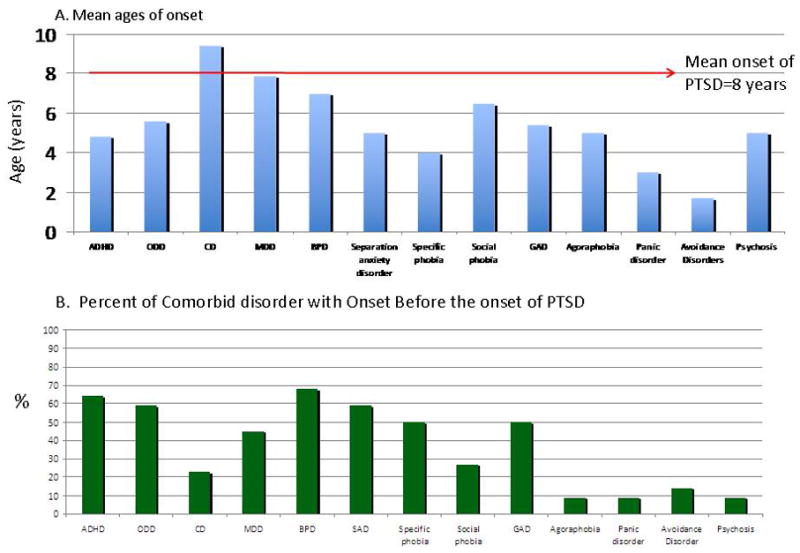
Ages of Onset of Comorbid Disorders in Relation to PTSD
3.4 Measures of functioning
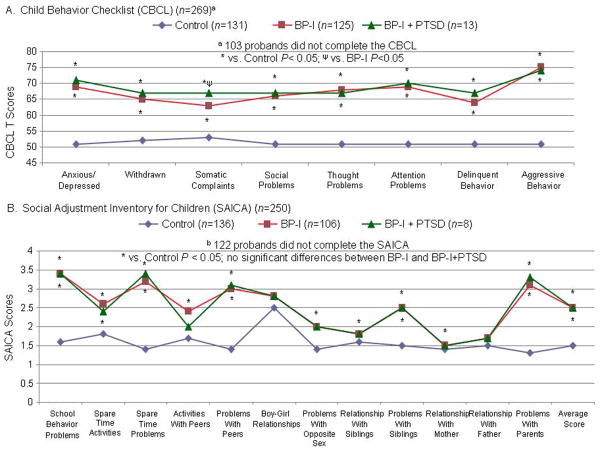
With few exceptions, both BP-I groups were similarly more likely than controls to require placement in special classes, to have received extra help in school, to have a more impaired GAF (Table 2), Child Behavior Check List (CBCL) and the Social Adjustment Inventory for Children and Adolescents (SAICA) scores (Figure 4) and more likely than controls to have lower scores on all measures of cognitive functioning assessed.
Figure 4.
Interpersonal and Emotional Functioning
4. Discussion
Our results from this large, controlled family study of youth with BP-I disorder with and without PTSD comorbidity showed that the clinical features of pediatric BP-I disorder were very similar in BP-I probands irrespective of the presence or the absence of comorbidity with PTSD in terms of mean age of onset, mean number of episodes, mean number and individual symptoms of mania, patterns of psychiatric comorbidity and measures of cognitive and psychosocial functioning. Likewise, familial risk analysis showed that BP-I disorder was equally robustly familial in BP-I probands irrespective of the comorbidity with PTSD. Taken together, these findings support the hypothesis that children with BP-I disorder with PTSD are afflicted by both disorders.
Our finding of a 20-fold increased risk for PTSD in pediatric BP-I probands suggests that pediatric BP-I disorder may be risk factor for PTSD. Considering the severity and morbidity associated with PTSD, confirmation of this hypothesis could have large clinical, scientific and public health relevance. While the reason for this increased risk remain unclear in light of anxiety disorders being significantly higher in the BP-I+PTSD group as compared to the BP-I/no PTSD and Control groups, perhaps having an antecedent anxiety disorder could be a risk factor for the development of PTSD in youth with BP-I disorder. More work, however, is needed to further investigate the etiology of this risk.
Irrespective of the comorbidity with PTSD, BP-I disorder significantly increased the risk for BP-I disorder in first-degree relatives when compared to findings in controls. This finding provides compelling evidence that our subjects with BP-I disorder comorbidly occurring with PTSD had, in fact, BP-I disorder. This familial transmission data directly address the idea that BP-I disorder symptoms in PTSD patients are misdiagnosed due to clinical features of PTSD that might confound a BP-I diagnosis such as hyperarousal and emotional dysregulation (Glod and Teicher, 1996; Ford et al., 2000; Weinstein et al., 2000). If PTSD caused a BP-I-like syndrome that is misdiagnosed as BP-I disorder, we would not have expected to find an elevated risk for BP-I disorder among relatives of BP-I+PTSD probands. The idea that BP-I symptoms among PTSD patients mimic BP-I disorder is further contradicted by our finding that that age at onset of PTSD was typically subsequent to the age at onset of BP-I disorder. This finding indicates that pediatric BP-I disorder is an antecedent risk factor for PTSD and that the converse is not true.
Although not reaching our a priori threshold for statistically significance, most likely due to limited statistical power, it is of note that the risk for PTSD was substantially higher in relatives of BP-I probands with and without comorbid PTSD relative to findings in controls. If these numerical differences could be confirmed statistically in better powered studies, they would support the hypothesis that putative genetic influences may underlie the risk for PTSD in youth with BP-I disorder considering that genetic influences have been suggested as being operant in PTSD. Although prior family-genetic studies of PTSD have not assessed pediatric BP-I disorder, twin studies by Koenen et al. (2005; 2008) and Sartor et al. (2012) found shared heritability between PTSD and major depression. More work is clearly needed with larger samples to re-examine the familial transmission of BP-I disorder and PTSD in youth. Twin studies are also needed to determine if the familial co-transmission of BP-I disorder and PTSD can be attributed to genetic or environmental familial risk factors.
Our findings need to be viewed in light of important methodological limitations. Firstly, the number of PTSD subjects and their relatives was relatively small. Although this would not have caused spurious findings of statistical significance, it did limit our power to detect some effects. Additionally, we lacked a PTSD only comparison group that would have been ideal for establishing the co-transmission of the two disorders. Data on PTSD was only available in a subsample of control girl probands without ADHD and their first-degree relatives. Since both PTSD and BP-I disorder have been associated with executive function deficits (Biederman et al., 2011; Lindstrom et al., 2011), it is possible that our findings could be accounted by such deficits. Future studies could benefit from the examination of executive function deficits in children with BP-I disorder comorbid with PTSD. Also, since our study was cross sectional, the data do not allow us to test potential causal relationships between these two phenomena.
Since we lacked detailed information on exposure to trauma outside the context of PTSD, we could not determine whether the increased rate of PTSD in BP-I probands was due to an increased rate of conversion from exposure to trauma to PTSD, or to a higher exposure to traumatic experiences. While more research is clearly needed to address these important issues, irrespective of clear etiology, our findings still document that children with BP-I disorder are at a significantly higher risk to develop full or subthreshold PTSD than controls. However, the higher rate of PTSD in the BP-I group could be also accounted for by other factors such as living in families who have higher rates of mood disorders which could increase the risk of trauma exposure, and comorbid disorders that increase exposure to trauma. More work is needed to further evaluate these issues.
The majority of the PTSD diagnoses stemmed from single traumatic events. Thus, our findings may not generalize to children who had severe, recurrent trauma or severe abuse and neglect. Our assessment of PTSD was categorical and not continuous. Future research could benefit from using PTSD specific rating scales. Given the young age of our subjects and the fact that they are still transitioning through the age of risk for exposure to traumatic experiences and the development of PTSD, our findings may underrepresent the true risk for trauma and PTSD. Although the DSM-IV criteria are widely used and have substantial validity when applied to youth, there are facets of the diagnosis that lack developmental sensitivity (Blom and Oberink, 2012). Although this may have reduced power for some analyses, it would not have caused spurious associations. Furthermore, because of the number of subjects with full threshold PTSD was so small, we included subjects with subthreshold PTSD. Whether subjects with subthreshold forms of PTSD are on a continuum with those with a full disorder remain unknown, their inclusion in our analysis can be viewed as conservative. Because the sample was largely male with an age of onset of BP-I disorder in early childhood, with very high rates of comorbid ADHD, our results may not generalize to all samples of pediatric BP-I disorder. Also because familiality findings relied on findings in siblings, our familiality results should be viewed as conservative since the siblings are still in the age of risk for PTSD. Since our sample was referred and overwhelmingly Caucasian, our findings may not generalize to community samples or other ethnic groups.
Despite these limitations, our study supports the hypothesis that youth manifesting symptoms of both BP-I disorder and PTSD are afflicted with both disorders. It also suggests that BP-I disorder may be a risk factor for PTSD in some youth.
Supplementary Material
Figure 1.
Risk for Disorders in First-Degree Relatives
Table 1b.
Post-Traumatic Stress Disorder SCID Module
| A. | A. Criteria for PTSD |
1. Have you ever had a terribly frightening experience? For example, were you ever in danger of being killed or badly hurt or have you ever witnessed a loved one in danger of being killed or badly hurt?
|
1) The person experienced, witnessed, or was confronted with an event or events that involved actual or threatened death or serious injury, or a threat to the physical integrity of self or others |
| 2. Sometimes those things keep coming back in nightmares, flashbacks, or thoughts that you can’t get rid of. Has that ever happened to you? Instructions: If this is a follow-up interview, ask #2 within follow-up period. If No: What about ever being very upset when you were in a situation that reminded you of these terrible things? Instructions: If this is a follow-up interview, ask #2 within follow-up period. Instructions: If no to #2, skip out. |
|
| 3. How did you react when [TRAUMA] happened, were you very afraid or did you ever feel terrified or helpless? Instructions: Even in follow-up interviews, ask lifetime for #3 |
2) The person’s response involved intense fear, helplessness, or horror. Note: In children, this may be expressed instead by disorganized or agitated behavior |
| Now I would like to ask a few questions about specific ways that it may have affected you. For example… | |
| B. | B. The traumatic event is persistently reexperienced in one or more of the following ways: |
| 1. Did you ever think about [TRAUMA] when you did not want to or did thoughts about [TRAUMA] ever come to you suddenly when you didn’t want them to? | 1) Recurrent and intrusive distressing recollections of the event, including images, thought, or perceptions. Note: in young children, repetitive play may occur in which themes or aspects of the trauma are expressed |
2. What about ever having dreams about [TRAUMA]?
|
2) Recurrent distressing dreams of the event Note: in children, there may be frightening dreams without recognizable content |
3. What about ever finding yourself acting or feeling as if you were back in the situation?
|
3) Acting or feeling as if the traumatic event were recurring (included a sense or reliving the experience, illusions, hallucinations, and dissociative flashback episodes, including those that occur on awakening or when intoxicated) |
4. What about ever getting very upset when something reminded you of [TRAUMA]?
|
4) Intense psychological distress at exposure to internal or external cues that symbolize or resemble an aspect of the traumatic event Note: in young children, trauma- specific reenactment may occur |
5. What about having physical symptoms such as breaking out in a sweat, breathing heavy or irregularly, or your heart pounding or racing?
|
5) Physiological reactivity on exposure to internal or external cues that symbolize or resemble an aspect of the traumatic event |
| C. Since [TRAUMA]… | C. Persistent avoidance of stimuli associated with the trauma and numbing of general responsiveness (not present before the trauma), as indicated by three (or more) of the following: |
1. Have you ever made a special effort to avoid thinking or talking about what happened?
|
1) Efforts to avoid thought, feelings, or conversations associated with the trauma |
2. Have you ever stayed away from things or people that remind you of [TRAUMA]?
|
2) Efforts to avoid activities, places, or people that arouse recollections of the trauma |
| 3. Have you ever been unable to remember some important part of what happened? | 3) Inability to recall an important aspect of the trauma |
4. Have you ever been much less interested in doing things that used to be important to you, such as seeing friends, reading books, or watching TV?
|
4) Markedly diminished interest or participation in significant activities |
| 5. Have you ever felt distant or cut off from others? | 5) Feeling of detachment or estrangement from others |
| 6. Have you ever felt “numb” or as if you no longer had strong feelings about anything or loving feelings for anyone? | 6) Restricted range of affect (e.g., unable to have loving feelings) |
| 7. Did you ever notice a change in the way you thought about or planned for the future? [Clarification: This would be like not having any hope.] Instructions: If no symptoms endorsed, skip out. |
7) Sense of a foreshortened future (e.g., does not expect to have a career, marriage, children, or a normal life span) |
| D. Since [TRAUMA]… | D. Persistent symptoms of increased arousal (not present before the trauma), as indicated by two (or more) of the following: |
1. After the event, have you ever had trouble sleeping?
|
1) Difficulty falling or staying asleep |
| 2. After the event, have you ever been unusually irritable or what about outbursts of anger? | 2) Irritability or outbursts of anger |
| 3. After the event, have you ever had trouble concentrating? | 3) Difficulty concentrating |
| 4. Have you ever been watchful or on guard even when there was no reason to be? | 4) Hypervigilance |
| 5. Have you ever been jumpy or easily startled, such as by sudden noises? | 5) Exaggerated startle response |
| E. About how long did those problems, such as [PTSD Symptoms], last? | E. Duration of the disturbance (symptoms in criteria B, C, and D) is more than 1 month |
| Instructions: Code 3 if more than 1 month | |
| F. Would you say that those feelings have ever been a very real problem for you? | F. The disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning |
|
Table 1c.
Post-Traumatic Stress Disorder SCID and KSADS Coding Algorithm
| # Positive Symptoms from Section B | # Positive Symptoms from Section C | # Positive Symptoms from Section D | Duration | |
|---|---|---|---|---|
| Full Diagnosis of PTSD | 1 | 3 | 2 | ≥ 1 month |
| Subthreshold Diagnosis of PTSD* | 1 | 2 | 1.5 | ≥ 1 month |
OR Full Criteria needs to be met for two of the three sections (B, C, or D), to meet subthreshold diagnosis
Acknowledgments
This work was supported by NIH grants K08MH001503 and R01MH066237 to Dr Wozniak and R01MH050657 and R01HD036317 to Dr. Biederman. This work was also supported by a grant from the Heinz C. Prechter Bipolar Research Fund, the support of members of the MGH Pediatric Psychopharmacology Council, and the Susan G. Berk Endowed Fund for Juvenile Bipolar Disorder.
Footnotes
Financial Disclosure
Dr. Joseph Biederman is currently receiving research support from the following sources: APSARD, The Department of Defense, ElMindA, Janssen, McNeil, Shire, and Vaya Pharma/Enzymotec.
Janet Wozniak MD receives research support from McNeil, Shire, Janssen, and Johnson & Johnson. Her spouse, John Winkelman MD, PhD, is a consultant/advisory board for Impax Laboratories, Pfizer, UCB, Zeo Inc., Sunovion, and receives research support from GlaxoSmithKline.
In the last three years Dr. Thomas Spencer has received research support from or has been an Advisor or on an Advisory Board of the following sources: Alcobra, Shire Laboratories, Inc, Eli Lilly & Company, Janssen Pharmaceutical, McNeil Pharmaceutical, Novartis Pharmaceuticals, Cephalon, the National Institute of Mental Health and the Department of Defense.
Gagan Joshi, MD is the sub-investigator for clinical trials sponsored by Shire, Johnson & Johnson, Pfizer, Merck, Cephalon, McNeil, Eli-Lily, Abbott, Novartis, Bristol Myers Squibb, Organon, Otsuka, Takeda, & New River Pharmaceuticals. He is the Site PI for a multi-centered trial sponsored by Glaxo Smith Kline, as well as for Bristol Myers Squibb. He is a member of the national advisory board for Shire.
In the past year, Dr. Faraone received consulting income and/or research support from Shire, Akili Interactive Labs and Alcobra and research support from the National Institutes of Health (NIH).
MaryKate Martelon, Andrea Spencer, Mai Uchida, Amelia Kotte, and K. Yvonne Woodworth report no financial disclosures.
For information about additional financial disclosures not directly related to this research, the reader is referred to Supplementary material.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Althoff RR, Faraone SV, Rettew DC, Morley CP, Hudziak JJ. Family, twin, adoption, and molecular genetic studies of juvenile bipolar disorder. Bipolar Disorders. 2005;7:598–609. doi: 10.1111/j.1399-5618.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Keenan K, Benjamin J, Krifcher B, Moore C, Sprich-Buckminster S, Ugaglia K, Jellinek MS, Steingard R, Spencer T, Norman D, Kolodny R, Kraus I, Perrin J, Keller MB, Tsuang MT. Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder. Patterns of comorbidity in probands and relatives in psychiatrically and pediatrically referred samples. Archives of General Psychiatry. 1992;49:728–738. doi: 10.1001/archpsyc.1992.01820090056010. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Mick E, Williamson S, Wilens TE, Spencer TJ, Weber W, Jetton J, Kraus I, Pert J, Zallen B. Clinical correlates of ADHD in females: findings from a large group of girls ascertained from pediatric and psychiatric referral sources. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:966–975. doi: 10.1097/00004583-199908000-00012. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Wozniak J, Wilens TE, Fried R, Doyle A, Henin A, Bateman C, Evans M, Faraone SV. Impact of executive function deficits in youth with bipolar I disorder: A controlled study. Psychiatry Research. 2011;186:58–64. doi: 10.1016/j.psychres.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom M, Oberink R. The validity of the DSM-IV PTSD criteria in children and adolescents: a review. Clinical Child Psychology and Psychiatry. 2012;17:571–601. doi: 10.1177/1359104511426408. [DOI] [PubMed] [Google Scholar]
- Christiansen H, Chen W, Oades RD, Asherson P, Taylor EA, Lasky-Su J, Zhou K, Banaschewski T, Buschgens C, Franke B, Gabriels I, Manor I, Marco R, Muller UC, Mulligan A, Psychogiou L, Rommelse NN, Uebel H, Buitelaar J, Ebstein RP, Eisenberg J, Gill M, Miranda A, Mulas F, Roeyers H, Rothenberger A, Sergeant JA, Sonuga-Barke EJ, Steinhausen HC, Thompson M, Faraone SV. Co-transmission of conduct problems with attention-deficit/hyperactivity disorder: familial evidence for a distinct disorder. Journal of Neural Transmission. 2008;115:163–175. doi: 10.1007/s00702-007-0837-y. [DOI] [PubMed] [Google Scholar]
- Faraone S, Glatt S, Tsuang M. The genetics of pediatric onset bipolar disorder. Biological Psychiatry. 2003;53:970–977. doi: 10.1016/s0006-3223(02)01893-0. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Monuteaux MC. Attention-deficit disorder and conduct disorder in girls: evidence for a familial subtype. Biological Psychiatry. 2000;48:21–29. doi: 10.1016/s0006-3223(00)00230-4. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Ford JD, Racusin R, Ellis CG, Daviss WB, Reiser J, Fleischer A, Thomas J. Child maltreatment, other trauma exposure, and posttraumatic symptomatology among children with oppositional defiant and attention deficit hyperactivity disorders. Child Maltreatment. 2000;5:205–217. doi: 10.1177/1077559500005003001. [DOI] [PubMed] [Google Scholar]
- Glod CA, Teicher MH. Relationship between early abuse, posttraumatic stress disorder, and activity levels in prepbertal children. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;34:1384–1393. doi: 10.1097/00004583-199610000-00026. [DOI] [PubMed] [Google Scholar]
- Havens JF, Gudino OG, Biggs EA, Diamond UN, Weis JR, Cloitre M. Identification of trauma exposure and PTSD in adolescent psychiatric inpatients: an exploratory study. Journal of Traumatic Stress. 2012;25:171–178. doi: 10.1002/jts.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale Press; New Haven: 1975. [Google Scholar]
- Koenen KC, Fu QJ, Ertel K, Lyons MJ, Eisen SA, True WR, Goldberg J, Tsuang MT. Common genetic liability to major depression and posttraumatic stress disorder in men. Journal of Affective Disorders. 2008;105:109–115. doi: 10.1016/j.jad.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, Eisen SA, True W, Tsuang M. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Archives of General Psychiatry. 2005;62:1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- Lindstrom KM, Mandell DJ, Musa GJ, Britton JC, Sankin LS, Mogg K, Bradley BP, Ernst M, Doan T, Bar-Haim Y, Leibenluft E, Pine DS, Hoven CW. Attention orientation in parents exposed to the 9/11 terrorist attacks and their children. Psychiatry Research. 2011;187:261–266. doi: 10.1016/j.psychres.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Potter MP, Woodworth KY, Yorks DM, Petty CR, Wozniak JR, Faraone SV, Biederman J. Pharmacologic treatments for pediatric bipolar disorder: a review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:749–762. e739. doi: 10.1016/j.jaac.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Marchand WR, Wirth L, Simon C. Adverse life events and pediatric bipolar disorder in a community mental health setting. Community Mental Health Journal. 2005;41:67–75. doi: 10.1007/s10597-005-2600-x. [DOI] [PubMed] [Google Scholar]
- Orvaschel H. Schedule for Affective Disorders and Schizophrenia for School-Age Children Epidemiologic Version. 5. Nova Southeastern University, Center for Psychological Studies; Ft. Lauderdale: 1994. [Google Scholar]
- Parens E, Johnston J, Carlson GA. Pediatric mental health care dysfunction disorder? New England Journal of Medicine. 2010;362:1853–1855. doi: 10.1056/NEJMp1003175. [DOI] [PubMed] [Google Scholar]
- Pauls D, Leckman J, Cohen D. Familial relationship between Gilles de la Tourette’s Syndrome, attention deficit disorder, learning disabilities, speech disorders, and stuttering. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32:1044–1050. doi: 10.1097/00004583-199309000-00025. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Leckman JF, Cohen DJ. Evidence against a genetic relationship between Tourette’s syndrome and anxiety, depression, panic and phobic disorders. British Journal of Psychiatry. 1994;164:215–221. doi: 10.1192/bjp.164.2.215. [DOI] [PubMed] [Google Scholar]
- Read J, Perry BD, Moskowitz A, Connolly J. The contribution of early traumatic events to schizophrenia in some patients: a traumagenic neurodevelopmental model. Psychiatry. 2001;64:319–345. doi: 10.1521/psyc.64.4.319.18602. [DOI] [PubMed] [Google Scholar]
- Romero S, Birmaher B, Axelson D, Goldstein T, Goldstein BI, Gill MK, Iosif AM, Strober MA, Hunt J, Esposito-Smythers C, Ryan ND, Leonard H, Keller M. Prevalence and correlates of physical and sexual abuse in children and adolescents with bipolar disorder. Journal of Affective Disorders. 2009;112:144–150. doi: 10.1016/j.jad.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Grant JD, Lynskey MT, McCutcheon VV, Waldron M, Statham DJ, Bucholz KK, Madden PA, Heath AC, Martin NG, Nelson EC. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Archives of General Psychiatry. 2012;69:293–299. doi: 10.1001/archgenpsychiatry.2011.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Adler CM, Fleck DE, Hanseman D, Maue DK, Bitter S, Kraft EM, Geracioti TD, Strakowski SM, DelBello MP. Post-traumatic stress symptoms and trauma exposure in youth with first episode bipolar disorder. Early Intervention in Psychiatry. 2010;4:169–173. doi: 10.1111/j.1751-7893.2010.00173.x. [DOI] [PubMed] [Google Scholar]
- Weinstein D, Staffelbach D, Biaggio M. Attention-deficit hyperactivity disorder and posttraumatic stress disorder: differential diagnosis in childhood sexual abuse. Clinical Psychology Review. 2000;20:359–378. doi: 10.1016/s0272-7358(98)00107-x. [DOI] [PubMed] [Google Scholar]
- Wozniak J, Biederman J, Kwon A, Mick E, Faraone S, Orlovsky K, Schnare L, Cargol C, van Grondelle A. How cardinal are cardinal symptoms in pediatric bipolar disorder? An examination of clinical correlates. Biological Psychiatry. 2005;58:583–588. doi: 10.1016/j.biopsych.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Wozniak J, Crawford MH, Biederman J, Faraone SV, Spencer TJ, Taylor A, Blier HK. Antecedents and complications of trauma in boys with ADHD: findings from a longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:48–55. doi: 10.1097/00004583-199901000-00019. [DOI] [PubMed] [Google Scholar]
- Wozniak J, Faraone SV, Mick E, Monuteaux M, Coville A, Biederman J. A controlled family study of children with DSM-IV bipolar-I disorder and psychiatric co-morbidity. Psychological Medicine. 2010;40:1079–1088. doi: 10.1017/S0033291709991437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J, Monuteaux M, Richards J, Lail K, Faraone SV, Biederman J. Convergence between structured diagnostic interviews and clinical assessment on the diagnosis of pediatric-onset bipolar disorder. Biological Psychiatry. 2003;53:938–944. doi: 10.1016/s0006-3223(03)00344-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.