Abstract
Here we report the first example of the use of supramolecular hydrogels to discover the protein targets of aggregates of small molecules.
Driven by noncovalent interactions, molecular self-assembly—that is, the spontaneous association of molecules results in structurally well-defined aggregates (e.g., in the form of nanofibers, nanoparticles, or micelles)—is ubiquitous in biology.1 For example, the self-assembly of proteins generates aggregates that are crucial for cellular functions (e.g., actins and tubulins to form F-actins and microtubules as cytoskeletons2) or associate with certain diseases (e.g., fibrillar aggregates of aberrant proteins as hallmarks of neurodegenerative diseases3), the self-assembly of lipids forms the cell membrane that compartmentalizes subcellular organelles and controls the substance in and out cells,2 and the development of small molecule hydrogels (e.g., lanreotide autogel) for treating acromegaly.4 These essential processes of cells have led to intensive studies on the aggregates of proteins for elucidating cellular functions and mechanisms of certain diseases,5 as well as exploration on the aggregates of small molecules for understanding the origin of life.6
Because one of essential features of molecular self-assembly is to exhibit emergent properties that drastically differ from those of the individual molecules,1 the above well-established cases of molecular self-assembly, thus, also raise an intriguing question about the biological functions of the aggregates of small molecules other than lipids, a question, however, remains unanswered. Three major factors likely contribute to the lack of study on the biological functions of the aggregates of small molecules: First, most of the studies of the aggregates of small molecules, except the research of lipids, focused crystalline or liquid crystalline aggregates formed via molecular self-assembly in non-aqueous media.8 Despite generating considerable physicochemical principles in the context of supramolecular chemistry,9 those studies offer little insights for the aggregates in biological processes that, obviously, occur in a complex aqueous phase. Second, except the case of lipids, there are few examples of the aggregates of small molecules that directly associate with cellular functions or find applications in biomedicine. Third, there is no general assay for evaluating the aggregates of small molecules and their corresponding biological targets.
Since most cells are soft and their interiors are highly viscous and crowded, we use supramolecular hydrogels to mimic the cellular environment and have developed supramolecular hydrogel protein binding assay for discovering the protein targets of the nanofibers of small molecules. Supramolecular hydrogels10,11 are one kind of heterogeneous, viscoelastic materials that can immobilize a large amount of water (up to 99% by weight) by the small molecules (normally less than 1000 Dalton) that self-assemble in water to form nanofibers. Unlike polymeric hydrogels12 that consist of random networks with small pore sizes, supramolecular hydrogels consist of only water and the networks formed by the self-assembly of small molecules. Having high water content and being viscoelastic, supramolecular hydrogels closely resemble the crowded cellular environment and have emerged as a new class of materials for tissue engineering.13 More importantly, Hamachi et al. have demonstrate that supramolecular hydrogels minimize the denature of proteins.14 Thus, supramolecular hydrogel can serve as an equivalent of the aggregates of small molecules in cellular environments for the binding of the protein targets. Most importantly, the diameters of the molecular nanofibers are in nanometer scale, which is comparable to the sizes of protein complexes. Such finite sizes minimize the area of hydrophobic patches, thus reducing protein denature caused by nonspecific interactions. In addition, it is easy to separate the hydrogel particles from aqueous solution by centrifugation after they interact with the proteins.
As shown in Scheme 1, this assay consists of following major steps: (i) incubation of the hydrogel with cell lysate; (ii) photo activation for fixing the proteins in the hydrogels; (iii) separation of the hydrogel and removal of the non-specific proteins; (iv) analysis and identification of the proteins (by SDS-PAGE, gel staining, tandem mass spectrometry, and Western blot, etc.). Our results shows that the nanofibers of a hydrophobic small peptide derivative (1) exhibit rather selective interactions with tubulins and several other proteins in the lysate of HeLa cells, which indicates that this supramolecular hydrogel based protein binding assay can offer a unique starting point for discovering interactions between the molecular nanofibers and the proteins.
Scheme 1.
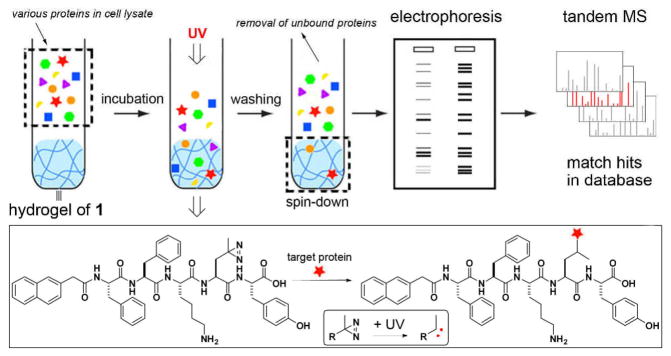
The illustration of the hydrogel protein pull-down assay coupled with electrophoresis and tandem mass spectrometry for the identification of the cytosolic proteins that bind to molecular nanofibers. The expand box shows the photo reaction between the hydrogelator and the proteins bound to the molecular nanofibers.
To achieve the design in Scheme 1, we incorporate photoleucine, a diazirine based photo reactive analog of leucine, into the hydrogelator backbone because photo affinity is a successful direct method to identify the interaction between a specific protein with its biological target.15 The noncovalent interactions between the hydrogelators allow the dissociation of the supramolecular hydrogels, thus easily releasing the proteins bound to the molecular nanofibers. We chose 1, which contains naphthalene-diphenylalanine (NapFF) motif, because the NapFF motif is able to enable the self-assembly of many other bioactive molecules.16 Starting with naphthalene acetic acid, we connect it with Phe, Phe, Lys(Boc), photo-Leu, and Tyr(P) sequentially via the cycle of NHS activation and ester-amide exchange reaction and obtain the precursor of the hydrogelator 1 after the deprotection of the lysine via the cleavage of the Boc group (Scheme S1). After dissolving the precursor in water at the concentration of 0.6 wt% (pH 7.4, adjusted by 1M NaOH), we add 10 U/mL of alkaline phosphatase (ALP) to the precursor solution and obtain a transparent hydrogel of 1 (Fig. 1A, left panel) after two hours of dephosphorylation. The use of enzyme-instructed formation of hydrogelation16 readily ensures the hydrogels to form at the condition that is compatible with cell lysate.
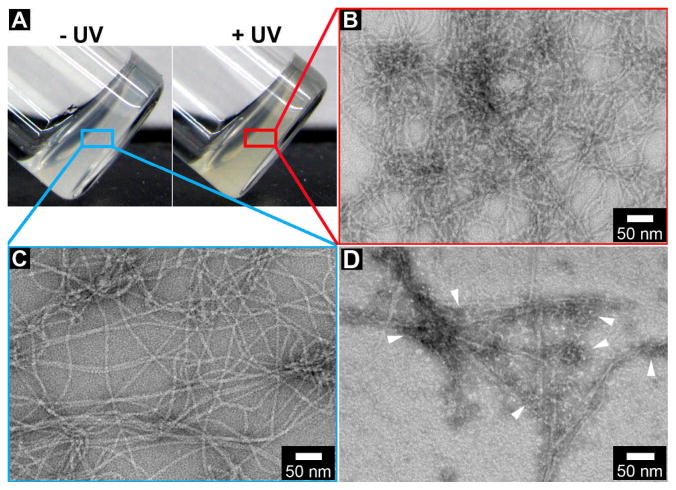
Figure 1.
(A) The optical images of the hydrogel of 1 (0.6 wt%, pH 7.4) without UV irradiation (left) and with UV irradiation (right). Typical TEM images of the nanofibers in (B) the hydrogel of 1 with UV irradiation; (C) the hydrogel of 1 without UV irradiation; (D) the hydrogel of 1 incubated with cell lysate under UV irradiation and then thoroughly washed. White arrow heads indicate the proteins on the nanofibers.
The negative staining transmission electron microscopy (TEM) image reveals that the hydrogel of 1 contains the network of uniform nanofibers that have the diameter of 10±2 nm (Fig. 1C). To confirm the photo reactivity of the hydrogelator of 1, we use UV light (365 nm) to irradiate the hydrogel. After UV irradiation, the hydrogel becomes slightly yellowish (Fig. 1A, right panel). TEM reveals that this light yellow hydrogel, though containing denser nanofiber networks, consists of the nanofibers with the diameters of 10±2 nm (Fig. 1B). Rheological test indicates that the storage moduli (G′) and loss moduli (G″) increase modestly after the UV irradiation (Fig. S1). After using UV to irradiate the hydrogel of 1 incubated with the cell lysate and thoroughly washing the irradiated hydrogel by PBS buffer containing 0.6% of Triton and 250 mM of NaCl, we use TEM to examine the hydrogel, and find protein particles and nanofibers co-exisit in the hydrogel. These results suggest that (i) the hydrogelator of 1 is photo reactive; (ii) the interaction between the hydrogelators is largely noncovalent despite the UV irradiation; (iii) UV irradiation is able to retain the proteins on the nanofibers of 1.
We perform the hydrogel-based protein pull-down assay outlined in Scheme 1 and confirm that the nanofibers of 1 are able to interact selectively with proteins. To find the proper conditions to remove the non-specifically-absorbed proteins on the molecular nanofibers, we use a series of concentration of Triton surfactant (as indicated on the top of the gel) together with 250 mM of NaCl solution for washing the hydrogels after the photoreaction. The purpose of the photoreaction is to retain the proteins that bind to the nanofibers in the hydrogels. After washing, we release the proteins from the hydrogels with the addition of laemeli buffer to a final concentration of 2x.17 After being boiled for 4 minutes and centrifuged at 14000 rpm for 3 minutes, the sample was loaded onto SDS-PAGE gel for analysis. As shown in Fig. 2 (see Fig. S2 for the full scope of the SDS-PAGE gel), with the increase of the concentrations of Triton, most of the proteins desorb from the hydrogels. Silver stain reveals that the intensities of the bands of the non-specifically-absorbed proteins decrease with the increase of the Triton concentration (e.g., the band indicated by (<)). However, as shown in lanes 2 and 3 of Fig. 2, the intensities of two protein bands (within the molecular weight range of 37 to 75 kDa, indicated by (*)) remain almost constant when the concentrations of the Triton are 0.6% and 0.3%, implying that these two bands correspond to the proteins interacting specifically with the nanofibers of 1.
Figure 2.
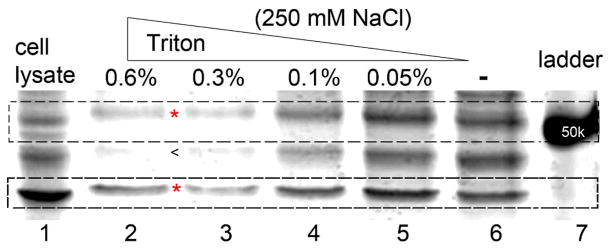
Silver staining shows that different washing conditions alter the ratio of proteins binding on the supramolecular hydrogel. Under the harshest washing condition, the hydrogels afford two major bands (highlighted by * in dash line boxes) while other bands fade away, indicating the specific binding between those proteins and hydrogels. The ratio of those two bands (top/bottom) is 3:7 in lane 2.
To identify the proteins that interact with the molecular nanofibers of 1, the protein bands with 37–75 kDa is analyzed by in-gel digestion18 and tandem MS analysis.19 Fig. 3A shows the proteins that have the sequence coverage higher than 15% from the tandem MS analysis. The sequence coverage of the proteins suggests that the molecular nanofibers interact with tubulins, actins, and several other proteins, which agrees well the molecular weights of the proteins in the bands of 55 kDa and 42 kDa.2
Figure 3.
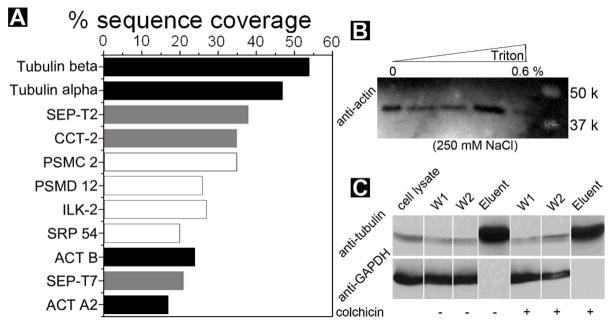
(A) Tandem MS analysis indicates the most abundant hydrogel-bound cytosolic proteins by sequence coverage. Black: proven binding partners; grey partners that bind to cytoskeleton protein/fibers; white proteins that were identified with no known association with the verified proteins. Validation of protein-nanofibers interactions: (B) cell lysate was incubated with the hydrogel and thoroughly washed at the conditions shown on the top and subjected to Western blotting with antibody to actin; (C) Cell lysate was incubated with the hydrogel and thoroughly washed at the condition of 250 mM of NaCl, 0.6% triton, w/o colchicines, and subjected to Western blotting with antibodies to tubulin and GAPDH (another abundant protein expressed inside mammalian cells).
Based on the proteomic analysis (Fig. 3A), we use Western blot20 to confirm some of the protein hits. As shown in Figure 3B, the protein bands at 42 kDa clearly contains actins. This result also explains the observation of SEP-T7 in the MS spectra because SEP-T7 can co-precipitate with actin.21 As a frequently used control in protein analysis, Western blot of GAPDH protein22 in Fig. 3C validates that the washing step successfully remove nonspecific bound proteins from the hydrogel of 1. As shown in Fig. 3C, Western blot proves the existence of tubulins in the band of 55 kDa. The addition of 6 μM of colchicine (which depolymerizes microtubules) hardly changes the binding results, suggesting that the nanofibers bind both tubulins and microtubules. This result also agrees with the high sequence coverage of SEP-T2 because SEP-T2 can co-localize with microtubules.23 While the nature of the interactions between the nanofibers and the other hits in Fig. 3A remain to be elucidated, we speculate that a chaperonin (CCT-2) may interact with the peptidic nanofibers of 1 because it binds to damaged or unfolded proteins.
In summary, this work indicates that the aggregates of small molecules can selectively interact with certain protein targets. Although the increase of the binding specificity of the nanofibers toward a particular protein target relies on the molecular structure of the small molecules, the negative result of anti-GAPDH stain in Fig. 3C indicates that the interaction between tubulins and the nanofibers of 1 unlikely is nonspecific. Because it is easy to incorporate other bioactive molecules (e.g., taxol) to hdyrogelators via the C-terminal16 or the ε-amino group of lysine residue24 of molecules like 1, this approach also allows the study self-assembled small molecule-protein interaction. Thus, this supramolecular hydrogel pull-down assay offers a facile way for elucidating the correlations between the protein targets and the aggregates of the small molecules, which ultimately contribute to establish the molecular foundation for the functions and applications of aggregates (e.g., nanofibers) of small molecules in cellular environment.
Supplementary Material
Acknowledgments
This work was partially supported by NIH (R01CA142746) and HFSP (RGP0056/2008). We thank EM facilities of Brandeis University for TEM. M. J. C. L. acknowledges the HHMI predoctoral fellowship.
Footnotes
Electronic Supplementary Information (ESI) available: rheological test, hydrogel washing conditions, additional electrophoresis results, and synthesis. See DOI:10.1039/b000000x/
Notes and references
- 1.Whitesides GM, Grzybowski B. Science. 2002;295:2418–2421. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 2.Lodish H, Berk A, Kaiser CA, Krieger M, Scott MP, Bretscher A, Ploegh H, Matsudaira P. Molecular Cell Biology. 6. W. H. Freeman; New York: 2007. [Google Scholar]
- 3.Querfurth HW, LaFerla FM. New Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 4.Caron P, Beckers A, Cullen DR, Goth MI, Gutt B, Laurberg P, Pico AM, Valimaki M, Zgliczynski W. J Clin Endocrinol Metab. 2002;87:99–104. doi: 10.1210/jcem.87.1.8153. [DOI] [PubMed] [Google Scholar]
- 5.Glover JR, Lindquist S. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- 6.Szostak JW. Philos Trans R Soc B-Biol Sci. 2011;366:2894–2901. doi: 10.1098/rstb.2011.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]; Koga S, Williams DS, Perriman AW, Mann S. Nat Chem. 2011;3:720–724. doi: 10.1038/nchem.1110. [DOI] [PubMed] [Google Scholar]
- 8.Whitesides GM, Mathias JP, Seto CT. Science. 1991;254:1312–1319. doi: 10.1126/science.1962191. [DOI] [PubMed] [Google Scholar]
- 9.Lehn JM. Angew Chem Int Ed. 1988;27:89–112. [Google Scholar]
- 10.Wang ZH, Wang HM, Zheng WT, Zhang J, Zhao Q, Wang SF, Yang ZM, Kong DL. Chem Commun. 2011;47:8901–8903. doi: 10.1039/c1cc11564b. [DOI] [PubMed] [Google Scholar]; Kobayashi H, Friggeri A, Koumoto K, Amaike M, Shinkai S, Reinhoudt DN. Organic Lett. 2002;4:1423–1426. doi: 10.1021/ol025519+. [DOI] [PubMed] [Google Scholar]; Estroff LA, Addadi L, Weiner S, Hamilton AD. Org & Biomol Chem. 2004;2:137–141. doi: 10.1039/b309731e. [DOI] [PubMed] [Google Scholar]; Estroff LA, Hamilton AD. Chem Rev. 2004;104:1201–1217. doi: 10.1021/cr0302049. [DOI] [PubMed] [Google Scholar]; Ray S, Das AK, Banerjee A. Chem Commun. 2006:2816–2818. doi: 10.1039/b605498f. [DOI] [PubMed] [Google Scholar]; Matsumoto S, Yamaguchi S, Wada A, Matsui T, Ikeda M, Hamachi I. Chem Commun. 2008:1545–1547. doi: 10.1039/b719004b. [DOI] [PubMed] [Google Scholar]; Xiao LX, Liu C, Zhu JH, Pochan DJ, Jia XQ. Soft Matter. 2010;6:5293–5297. doi: 10.1039/C0SM00511H. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cui H, Hodgdon TK, Kaler EW, Abezgauz L, Danino D, Lubovsky M, Talmon Y, Pochan DJ. Soft Matter. 2007;3:945–955. doi: 10.1039/b704194b. [DOI] [PubMed] [Google Scholar]; Sadownik JW, Ulijn RV. Chem Commun. 2010;46:3481–3483. doi: 10.1039/c001982h. [DOI] [PubMed] [Google Scholar]; Williams RJ, Smith AM, Collins R, Hodson N, Das AK, Ulijn RV. Nat Nanotech. 2009;4:19–24. doi: 10.1038/nnano.2008.378. [DOI] [PubMed] [Google Scholar]; Wang YJ, Desbat B, Manet S, Aime C, Labrot T, Oda R. J Colloid Interf Sci. 2005;283:555–564. doi: 10.1016/j.jcis.2004.09.003. [DOI] [PubMed] [Google Scholar]; Sangeetha NM, Maitra U. Chem Soc Rev. 2005;34:821–836. doi: 10.1039/b417081b. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Liang G, Xu B. Acc Chem Res. 2008;41:315–326. doi: 10.1021/ar7001914. [DOI] [PubMed] [Google Scholar]
- 12.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matson JB, Stupp SI. Chem Commun. 2012;48:26–33. doi: 10.1039/c1cc15551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiyonaka S, Sada K, Yoshimura I, Shinkai S, Kato N, Hamachi I. Nature Mater. 2004;3:58–64. doi: 10.1038/nmat1034. [DOI] [PubMed] [Google Scholar]
- 15.Suchanek M, Radzikowska A, Thiele C. Nat Methods. 2005;2:261–267. doi: 10.1038/nmeth752. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Kuang Y, Gao YA, Xu B. Langmuir. 2011;27:529–537. doi: 10.1021/la1020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.2010 doi: 10.1101/pdb.rec12340. C. S. H. P. [DOI] [Google Scholar]
- 18.Rosenfeld J, Capdevielle J, Guillemot JC, Ferrara P. Anal Biochem. 1992;203:173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- 19.Aebersold R, Goodlett DR. Chem Rev. 2001;101:269–295. doi: 10.1021/cr990076h. [DOI] [PubMed] [Google Scholar]
- 20.Renart J, Reiser J, Stark GR. Proc Natl Acad Sci USA. 1979;76:3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinoshita M, Kumar S, Mizoguchi A, Ide C, Kinoshita A, Haraguchi T, Hiraoka Y, Noda M. Gene Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- 22.Barber RD, Harmer DW, Coleman RA, Clark BJ. Physiol Genomics. 2005;21:389–395. doi: 10.1152/physiolgenomics.00025.2005. [DOI] [PubMed] [Google Scholar]
- 23.Spiliotis ET, Kinoshita M, Nelson WJ. Science. 2005;307:1781–1785. doi: 10.1126/science.1106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao Y, Kuang Y, Guo ZF, Guo ZH, Krauss IJ, Xu B. J Am Chem Soc. 2009;131:13576–13577. doi: 10.1021/ja904411z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.