Abstract
Behaviour and physiology are regulated by both environment and social context. A central goal in the study of the social control of behaviour is to determine the underlying physiological, cellular and molecular mechanisms in the brain. The African cichlid fish Astatotilapia burtoni has long been used as a model system to study how social interactions regulate neural and behavioural plasticity. In this species, males are either socially dominant and reproductively active or subordinate and reproductively suppressed. This phenotypic difference is reversible. Using an integrative approach that combines quantitative behavioural measurements, functional genomics and bioinformatic analyses, we examine neural gene expression in dominant and subordinate males as well as brooding females. We confirm the role of numerous candidate genes that are part of neuroendocrine pathways and show that specific co-regulated gene sets (modules), as well as specific functional Gene Ontology categories, are significantly associated with dominance or reproductive state. Finally, even though the dominant and subordinate phenotypes are robustly defined, we find a surprisingly high degree of individual variation in the transcript levels of the very genes that are differentially regulated between these phenotypes. Our results demonstrate the molecular complexity in the brain underlying social behaviour, identify novel targets for future studies, validate many candidate genes, and exploit individual variation in order to gain biological insights.
Keywords: cichlid, microarray, social behaviour, behavior
INTRODUCTION
Brain and behaviour are sculpted by a dynamic interplay between genotype and environment. Changes in social status mediate changes in a wide range of molecular, neuroanatomical, endocrine and behavioural characteristics (Oliveira et al., 2002; Fernald, 2004; Sapolsky, 2005) at different time scales (Hofmann, 2003). Nervous systems respond by modulating neural properties and neuroendocrine responses. Such rapid, short-term changes will often lead to sustained longer-term changes in brain and behaviour through differential gene expression leading to subsequent structural and physiological changes. However, few studies have attempted to determine the molecular underpinnings of socially regulated phenotypes within a comprehensive genomic framework, especially in vertebrates. Here we apply a molecular systems approach in a highly social fish to ask how social context regulates the molecular and physiological substrates that in turn sculpt subsequent behaviour.
The African cichlid fish, Astatotilapia burtoni (formerly: Haplochromis burtoni) has become an important model system to study the mechanisms underlying socially mediated behavioural change. In this species, 20–30% of males are dominant (D), slow growing, brightly coloured and actively defend territories for mating. The remaining subordinate (S) males mimic females by schooling and displaying cryptic colouration, while experiencing faster growth (Fernald, 1977; Hofmann et al., 1999a). Subordinate males show little aggression and territoriality, and, importantly, have regressed gonads and thus are not reproductive (Fernald and Hirata, 1977a; 1977b; Francis et al., 1993). These behavioural and physiological characters are plastic and influenced by the immediate social environment, such that an individual male switches between the dominant and subordinate phenotypes several times during its life depending upon its relative ability to obtain and maintain access to a territory through encounters with other males (Hofmann et al., 1999a). Environmental conditions, availability of territorial shelters, relative body size and physiological condition influence the probability of acquiring and maintaining a territory. The phenotypic switch occurs over a timescale of minutes to days to weeks in both the field and the laboratory (White et al., 2002; Hofmann, 2003; Burmeister et al., 2005).
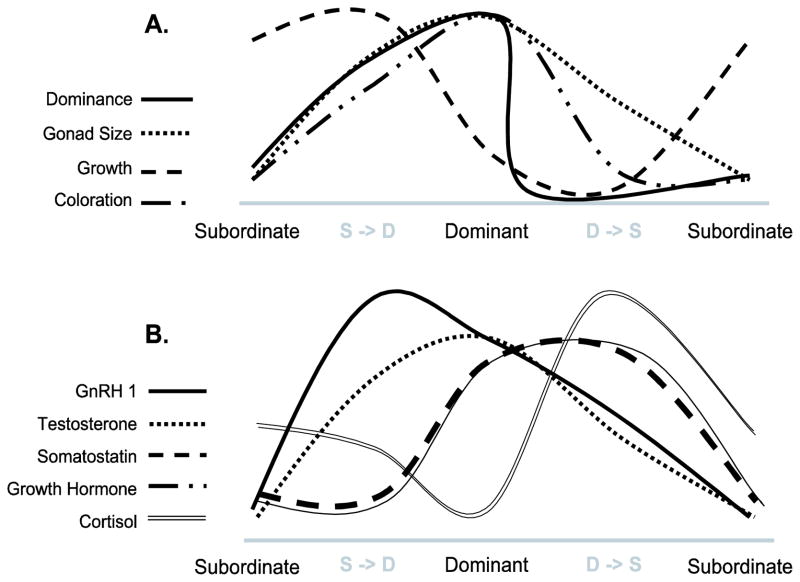
In the laboratory, A. burtoni has been the focus of hormonal and molecular studies related to a broad range of phenotypic traits that are affected by the transition between the two male phenotypes (Fig. 1) (for detailed reviews see: Hofmann and Fernald, 2000; Fernald, 2002; 2004; Hofmann, 2003). Variation in several different components of the endocrine system reflects the complexity underlying the corresponding phenotypic switch between these dramatically different male phenotypes. Neuroendocrine pathways regulating, androgen production (Parikh et al., 2006), growth (Hofmann et al., 1999a; 1999b), and stress response (Fox et al., 1997) change in a complex fashion as animals undergo phenotypic change (Fig. 1). Parikh et al (2006) suggested that the higher levels of testosterone (T) and 11-ketotestosterone (11KT) they measured in dominant males might promote aggressive behaviour, as has been shown using androgen manipulation in other fish species. Production and release of neuropeptides and neuromodulators such as gonadotropin releasing hormone (GnRH1) (White et al., 2002) and somatostatin (Hofmann and Fernald, 2000; Trainor and Hofmann, 2006, 2007) are higher in dominant males. According to White et al (2002), GnRH mRNA levels and GSI (a measure of gonadal development) are positively correlated. The higher level of GnRH in dominant males coincides with acquisition of territory and mating opportunity, as such it similar to GnRH changes in seasonally reproducing species, (Amano et al. 1995, Dawson et al. 2001, Nelson 2005; Hofmann 2006). Fluctuations at the molecular level also co-vary with the observed behavioural switch of male phenotype. In the case of GnRH (Au et al., 2006; Parhar et al., 2005) and somatostatin (Trainor and Hofmann, 2006), specific receptor subtypes are regulated according to social status. Furthermore, the membrane properties of GnRH1-expressing neurons, reduced excitability in subordinate males, corresponds with the differences in peptide release between dominant and subordinate males (Greenwood and Fernald, 2004).
Figure 1.
Schematic representation of A) phenotypic and B) physiological characteristics associated with Dominant and Subordinate male phenotypes in A. burtoni. The graphs are based on the following studies: gonad size: Hofmann and Fernald 2000; growth: Hofmann et al., 1999a; GnRH1: White et al., 2002; testosterone: Francis et al., 1993; somatostatin: Hofmann and Fernald 2000; growth hormone: Hofmann et al 1999b; cortisol: Fox et al., 1997.
Manipulation of the social environment allows experimental control of the phenotypic switch (Francis et al., 1993; White et al., 2002; Burmeister et al., 2005). The ease of experimental manipulation is paired with a wealth of ecological and evolutionary information available for haplochromine cichlids in general (for review, see Kocher, 2004; Salzburger et al., 2005). For example, there is thought to be a trade-off between reproduction and survival, such that territorial males, the sole reproducers, are also subject to higher mortality rates through predation, likely due to their conspicuous colouration (Fernald and Hirata, 1977a; 1977b; Maan et al., 2008). Furthermore, A. burtoni is currently undergoing whole genome sequencing, along with three other African cichlid species (see URL: www.broad.mit.edu/models/tilapia/) and thus offers an unrivaled laboratory-based model system for the genomic analysis of complex and ecologically-relevant phenotypes.
cDNA microarrays, for transcript profiling, have become a powerful tool when applied to species of behavioural, ecological or evolutionary interest (e.g. alternative life histories: Aubin-Horth et al., 2005a; cooperative breeding: Aubin-Horth et al., 2007; social behaviour: Grozinger and Robinson, 2002; Robinson et al., 2005; Whitfield et al., 2003; response to heat stress: Buckley et al., 2006; response to environmental estrogens: Martyniuk et al., 2006; physiology of drug addiction: Rhodes and Crabbe, 2005). In the present study, we employ a microarray platform that contains many known candidate genes as well as ~4000 brain-derived cichlid cDNAs (Renn, Aubin-Horth and Hofmann, 2004). Such a combined candidate gene and genomic strategy allows hypothesis-driven and discovery-based experiments on a single platform. The obviously complex nature of behavioural traits – such as this socially regulated, reversible switch between dominant and subordinate phenotypes – requires a discovery-based approach in order to identify the many genes involved.
In the present study, we analyze gene transcript patterns for reproductively active dominant males and reproductively suppressed subordinate males as well as for brooding females. We then compare the expression pattern for each of the three phenotypes in order to identify gene sets associated with reproduction or dominance behaviour, providing insight into the molecular modularity underlying these phenotypes. Next, we annotate the array features according to Gene Ontology (GO) with the goal of identifying gene regulation within molecular categories free of a priori expectations or experimenter bias. Finally, we examine variation in gene expression patterns between individual animals within a social phenotype and ask whether any of these variable genes are also those that are differentially expressed between the social phenotypes.
RESULTS AND DISCUSSION
Male Dominance Behaviour and Gene Expression Differences
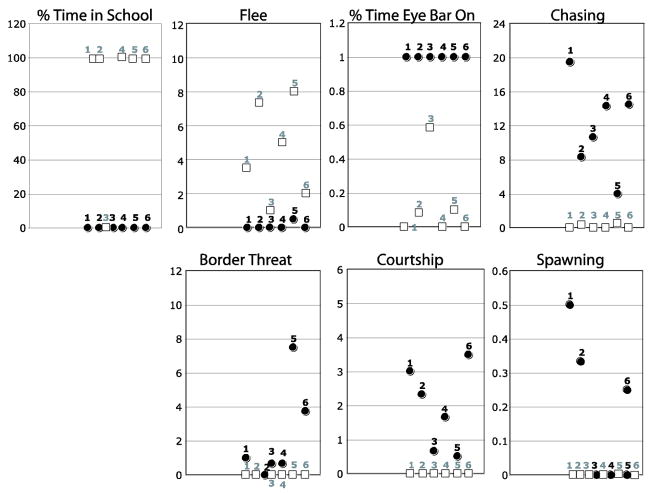
Individually tagged males were placed into community tanks with females such that naturalistic dominance hierarchies were established among the males according to standard protocol (Hofmann et al., 1999a). Regular observations over the course of five weeks identified males of definitive dominant (D) and subordinate (S) phenotypes. The territory holding males (n = 6) behaved more aggressively than subordinate males (n = 6), as reflected by their significantly higher dominance index (DI; D males: 15.7±1.7; S males: −3.5±0.8, t-test p < 0.001) and reproductive index (RI; D males: 2.12±0.57; S males: 0.0±0.0; t-test p = 0.004). The gonadosomatic index (GSI) varied continuously across the two groups showing the expected (yet in this study non-significant) trend for higher GSI in dominant males (GSI, D males: 0.85±0.23; S males: 0.56±0.04%; t-test p = 0.25). There was no significant difference in growth rate over the course of the experiment (GR; D males: 1.25±0.215; S males: 1.39 ±0.285; t-test p = 0.69) or condition factor (residuals) at the time of sacrifice (CF; D males: 0.01±0.053; S males: −0.01 ±0.061; t-test p = 0.58). Note, however, that CF differed marginally when the social communities were established at the beginning of the experiment (D males: 0.03±0.051; S males: −0.03±0.068; t-test p = 0.07), which could suggest that increased condition may be advantageous when ascending to dominance in the first place. Specific behaviours also varied significantly and predictably between the dominant and subordinate males (Fig. 2): increased presence of an eye-bar in dominants (p < 0.001); increased schooling behaviour of subordinates (p = 0.0005); more fleeing behaviour by subordinates (p = 0.004); and more frequent chasing behaviour (p = 0.0003), border threat behaviour (p = 0.08) and sexual behaviour (p = 0.003) by dominants. These results are representative of dominant and subordinate males in A. burtoni as previously described (e.g., Francis et al., 1993; Hofmann and Fernald, 2000; White et al., 2002).
Figure 2.
Social behaviour varied significantly and predictably between the dominant and subordinate males. Y-axis represents (A, C) the percent time or (B, D, E, F) the average number of observed events per 10 minute focal observation. Identified squares, dominants, and circles, subordinates are consistent with figure 5.
For the first investigation of our microarray data, we conducted Bayesian analysis of gene expression (Townsend and Hartl, 2002) by treating the six individuals of each phenotype as biological replicates and calculated gene expression differences at the level of phenotype (dominant, subordinate or brooding female). When comparing the different phenotypes, array features representing 3647 cichlid unique sequences passed quality filters in a sufficient number of hybridizations to be considered in the results. Dominant and subordinate phenotypes showed significant differences in gene expression for 171 genes (Bayesian Posterior Probability, BPP ≥ 0.99) (87 up- regulated; 84 down-regulated with dominance; Supplementary tables 1 & 2). Permutation analysis of the data shows that, at this significance threshold of BPP ≥ 0.99, only ~17% of these 171 genes could be expected to show significant differential expression by chance, a reasonable rate of false positives (Table 1). Therefore, almost five percent of the genes studied were differentially regulated in the brain according to male dominance phenotype. This percentage is considerably smaller than that found in typical honey bee colonies (39%) where nurse and forager phenotypes are not only distinguished by their social role, but also differ in their daily behavioural routines and the environment in which they move about (Whitfield et al., 2003). These results also show phenotype specific regulation of a considerably smaller portion of the genome than the 15% observed difference between alternative mating tactics in a study of Atlantic salmon males, which similarly compared sexually mature and immature males (Aubin-Horth et al., 2005a).
Table 1.
False Discovery Rate estimated by randomization of hybridization ratios both between genes and between arrays using permutation with replacement and resampling for analyses conducted at the level of phenotype and sex. The number of features identified by analysis of the permutated dataset as significant for each threshold is reported as the average (n=5) percentage of the number of features identified at that threshold by analysis of the original dataset.
| Bayesian Probability Threshold | |||||
|---|---|---|---|---|---|
| >=0.99 | >=0.995 | >=0.999 | >=0.9995 | ||
| phenotypes compared | Male Dominant > Male Subordinate | 89 | 61 | 41 | 37 |
| Male Dominant > female | 273 | 218 | 148 | 127 | |
| Male Subordinate > Male Dominant | 84 | 61 | 26 | 23 | |
| Male Subordinate > female | 277 | 220 | 136 | 116 | |
| Female > Dominant male | 205 | 154 | 96 | 78 | |
| Female > Subordinate Male | 219 | 164 | 95 | 83 | |
| Males > Female | 318 | 269 | 174 | 147 | |
| Female > Males | 253 | 211 | 119 | 103 | |
| FDR % | 17.08 | 10.76 | 3 | 2.07 | |
| Sterr | 0.61 | 0.57 | 0.39 | 0.39 | |
Candidate Genes
The microarray was designed to include candidate genes previously studied in the context of social dominance and other behavioural contexts in A. burtoni (Hofmann, 2003; Fernald, 2004). The inclusion of known candidate genes allowed us to test multiple hypotheses and also offered validation of the microarray results by comparison with previous studies for some of these genes (summarized in Fig. 1). For instance, peptidergic neurons in the pre-optic area (POA), and other brain regions, express several neurohormones (e.g., arginine vasotocin, GnRH1, galanin) as well as neurohormone receptors and steroid receptors, which have previously been shown in separate studies to play a role in the regulation of social behaviour. Below, we provide for the first time a combined analysis of these neuroendocrine pathways in A. burtoni.
As predicted from previous studies using ribonuclease protection assays and in situ hybridization, among the three GnRH neuropeptide genes that are expressed in the brain of fish, only GnRH1, the form expressed in the POA (White et al., 1994; White et al., 2002) showed highly significant regulation in the microarray results (BPP = 0.9998) with dominant males having higher levels. Given the small number of cells expressing this neuropeptide (~300 Munz, 1999; Soma et al., 1996), confirmation of GnRH1 regulation by our microarray analysis provides an important cross-validation and confirms the sensitivity of the array even when using whole brain RNA. As predicted from previous studies (White et al., 1994; White et al., 2002), our results also confirmed that the other two forms of GnRH, (midbrain) GnRH2 and GnRH3 (terminal nerve), are not regulated according to male social phenotype (BPP = 0.366; BPP = 0.700 respectively). None of the GnRH receptor subtypes on the array were significantly regulated, although studies have demonstrated their regulation in the pituitary in relationship to sexual maturity and social status (Parhar et al., 2005; Au et al., 2006).
Galanin, a neuropeptide that links metabolic activity and reproduction through regulation of GnRH release (reviewed by Kageyama et al., 2005; Tortorella et al., 2007), was marginally up-regulated in dominant males (BPP = 0.9592). There is considerable evidence from mammals that galanin reduces nociception (Wiesenfeldhallin et al., 1992), increases food intake (Schick et al., 1993) and stress reactivity (Holmes et al., 2002), and that it plays a role in regulation of sexual behaviour, and is itself regulated by GnRH and estrogen (Gabriel, et al., 1993). Specifically, in fish, galanin is thought to play a role in regulation of food intake and is widely distributed in the brain, being localized to the olfactory bulb, telencephalon, hypothalamus, midbrain, and posterior brain (reviewed by Volkoff et al., 2005) as well as the pituitary (Jadhao and Pinelli, 2001) and peripheral tissues (Johnsson et al., 2001). Our results suggest the intriguing possibility that galanin might be up-regulated in dominant males as a response to reduced food intake and constant challenges to their social status by other individuals. Future studies will test these novel hypotheses.
Arginine vasotocin (AVT; represented by multiple clones on the array), the non-mammalian homolog of arginine vasopressin (AVP), was among the most strongly regulated genes in this study, being up regulated in the brains of dominant males (BPP > 0.9999). AVP/AVT has been implicated in the regulation of social behaviour across vertebrates, including aggression and social affiliation (Goodson, 1998; Goodson and Adkins-Regan, 1999; Winslow et al., 1993). In teleost fish, AVT is known to play a role in male mating tactics (peacock blenny: Carneiro et al., 2003; Grober et al., 2002; midshipman: Goodson and Bass, 2001), as well as in the behavioural regulation of sex change and the associated territory acquisition (bluehead wrasse: Semsar and Godwin, 2003; Semsar and Godwin, 2004). AVT is also associated with territorial aggression (damselfish: Santangelo and Bass, 2006) as well as dominant and territorial behaviour in both the male and female of a breeding pair as compared to their subordinate helpers in another cichlid species, the cooperative breeding Neolamprologus pulcher (Aubin-Horth et al., 2007). Our data from A. burtoni suggest a role for AVT in regulation of dominance and are consistent with an in situ hybridization-based study (Greenwood et al., submitted). The AVT V1a receptor, which plays a fundamental role in affiliative behaviors in voles (Lim et al., 2004), was not represented on the array.
The enzyme aromatase, which converts testosterone to estrogen, is important in sex determination (Nakamura and Kobayashi, 2005), sex change in fish (Black et al., 2005; Marsh et al., 2006), and regulation of social behavior (Hallgren et al., 2006). There are two isoforms of aromatase, one localized to the brain and the other to the gonads, and both are represented on the microarray. Here, dominant males showed increased neural expression of the brain form (2 features on the array, BPP = 0.9914; 0.9997), but not the gonad form of aromatase (BPP = 0.3192). This result suggests that the elevated testosterone levels found in dominant males (Parikh et al., 2006) may affect aggression, courtship or dominance through aromatization and subsequent action via estrogen receptors in the brain. In birds, aromatase activity increases during the territorial period and correlates with aggression (e.g. Soma et al., 2003; Silverin et al., 2004). Blocking brain aromatase reduces male courtship in guppies (Hallgren et al., 2006), further suggesting estrogen-mediated neuroendocrine regulation of reproductive behavior for some species. However, studies on gonadal sex change in fish (Black et al., 2005; Marsh et al., 2006) suggest the opposite relationship between brain aromatase and male aggression and thus a more complex mechanism possibly involving differences in receptor expression, binding proteins, or anatomical localization. Estrogen receptors did not show differential regulation on the array, although they may have been expected to, according to Burmeister et al. (2007). The inability to reliably detect differences in receptor gene expression is likely due to small, localized effects that are masked by whole brain gene expression levels.
Novel Genes
We bioinformatically annotated the ESTs obtained from the cichlid cDNA library features represented on the microarray (see methods). Several of the genes thus identified fall into categories that represent candidates likely to play a role in the social regulation of a complex phenotype (Table 2). These genes are considered here as “novel genes”, rather than “candidate genes” because the annotation process does not involve rigorous manual curation of genes a priori that was employed for the candidate genes discussed above. In addition to many genes involved in cellular metabolism that are differentially regulated between the two social phenotypes, we find genes encoding structural proteins, cell-cycle regulators, specific transcription factors, a plethora of neuropeptides, components of the neurosecretory machinery, and neurotransmitter receptors.
Table 2.
Best hits for dominant and subordinate regulated genes grouped according to presumed functional category (see Methods for details). For array feature identification and BPP for differential regulation see supplementary tables 1 & 2.
| Dominant greater than Subordinate | Subordinate great than Dominant | |
|---|---|---|
| Peptides Neurotransmitters and growth hormones | Prolactin 1 Prolactin 2 Somatotropin Somatolactin Proopiomelanocortin AVT GABA - receptor beta subunit Tilapia growth hormone. Glycoprotein alpha GNRH-1 Brain aromatase |
Orphan nuclear hormone receptor Kainate Receptor Cholecystokinin C-type natriuretic peptide |
| Granins | Chromogranin Neurogranin Secretogranin II |
|
| Cell Structure | Beta actin Beta tubulin Septin 7 Elongation Factor 1a |
Clasp2 -regulation of microtubule dynamics |
| Synaptic Vesicle | similar to Synaptophysin Q5RCZ2_PONPY - secretory vesicle electron transport |
|
| Transcription factor | ETS family efr1 | bHLH HLH |
| Axonal Growth | Neuromodulin Neuroserpin |
|
| Cell Cycle regulation | Schip-1 Pdcd4 |
Rbm5 protein putative tumor suppressor |
| cellular metabolic enzymes | Enolase 1 Enolase 2 Uridine kinase Lactate dehydrogenase |
many ATPase’s, NADH dehybdrogenase and oxidoreductase Glutathion S-transferase Glutamine synthetase Betaine aldehyde dehydrogenase Leucine carboxyl methyltransferase |
Genes coding for structural proteins such as tubulin and actin, proteins that bind scaffold elements, such as septin 7 and ELF-1a, were more highly expressed in dominant males reflecting the observed differences in soma size between dominant and subordinate for preoptic neurons expressing GnRH1 (Francis et al., 1993) and somatostatin (Hofmann and Fernald, 2000). Furthermore, genes involved in axonal growth, neuromodulin (also known to play a role in modeling of sex-specific brain regions: Simerly, 2002) and neuroserpin (Miranda and Lomas, 2006), also to be up-regulated in dominant males. Taken together, the regulation of this gene set strongly suggests increased neuronal re-wiring in dominant males not previously reported and possibly similar in scale to the massive remodeling of neural circuits seen in seasonal accession to territoriality and mating accompanied by increased testosterone levels in song birds (Devoogd and Nottebohm, 1981; reviewed in Arnold, 1992). It is particularly intriguing that neuroserpins may play a role in anxiety and sexual behaviours. Specifically, neuroserpin-deficient rats showed decreases in exploratory behaviour along with increases in anxiety and neophobia (Madani et al. 2003) and, in swordtail fish, neuroserpin expression increased in the brain of females exposed to an attractive male compared to females exposed to a non-attractive male (Cummings et al., 2008). This association of neuroserpin with social behavior is intriguing in that it may enable dominant males to approach and interact with novel stimuli such as competitors and potential mates.
Similarly, several cell cycle regulators (Table 2) were significantly regulated in dominant and subordinate phenotypes, suggesting that the extent of neurogenesis and subsequent cell death may also differ between these phenotypes, a hypothesis consistent with the finding that cell proliferation in the brain is correlated with high social status in rainbow trout (Sorensen et al., 2007). While there is currently no other evidence for plasticity of this kind in neuroanatomical structures outside the pre-optic area (POA) in A. burtoni, gross neuroanatomical differences that correspond to species typical reproductive strategies have been identified in other cichlid species (Pollen et al., 2007).
Genes encoding neuropeptides and protein hormones that have not been previously studied in this system were perhaps the most striking, though not unexpected, class of genes regulated according to social status. In addition to the neuropeptides GnRH1, AVT and galanin discussed above, we found somatotropin/growth hormone (GH), prolactin, somatolactin (all members of the GH family of genes), as well as proopiomelanocortin (POMC) to be up-regulated in dominant males. Interestingly, a similar pattern of endocrine gene regulation (GH, prolactin, somatolactin, POMC) is observed in Atlantic salmon, such that the expression profile for the early maturing “sneaker” male compared to for reproductive dominant males, suggesting conserved function of these pathways (Aubin-Horth et al., 2005a). We found cholecystokinin (CCK) and natriuretic peptide to be up-regulated in subordinate males.
Since the activity of pituitary somatotrophes is associated with testis maturity and is stimulated by high levels of GnRH in several fish species (reviewed by Legac et al., 1993; Yu and Peter, 1991), we suggest that the increased expression of growth-related genes in dominant males is likely related to gonad maturation. Somatolactin, which thus far has been found only in teleost fish, is involved in both growth (Forsyth and Wallis, 2002) and colour change (Fukamachi and Sugimoto, 2004): two plastic traits associated with social dominance in A. burtoni. Importantly, the observed increase in expression of GH is consistent with the previous finding that circulating GH levels are higher in dominant males (Hofmann et al, 1999b). Additionally, the Growth Hormone-Releasing Hormone (GHRH)/GH axis facilitates territorial behaviour in A. burtoni (Hofmann et al, 1999b; Trainor and Hofmann, 2006; note that GHRH was not represented on the array). Finally, antagonists of the neuropeptide somatostatin (which inhibits GH production and release in the pituitary; this gene was not represented on the microarray) inhibited aggressive behaviour in A. burtoni males without affecting sexual behaviour (Trainor and Hofmann, 2006).
In addition to neuropeptide genes, we found many genes involved in production, maturation, release and reception of neuropeptides and neurotransmitters to be differentially expressed between social phenotypes. For example, secretory granule proteins, such as a homolog of synaptophysin as well as members of the granin family of acidic proteins, were up-regulated in dominant males. These are notably found in a wide variety of endocrine and neuro-endocrine cells (for reviews see Gerst, 1999; Helle, 2004) and the regulation pattern found here may simply be a consequence of increased neuroendocrine activity in dominant males.
While not many neurotransmitter receptors were present on the array, there is one very intriguing result to report: A GABA-(A) receptor was up-regulated in dominant males whereas at least two subunits of the kainate-type glutamate receptor were up-regulated in subordinate males. Clearly, these differences were not predicted nor do we have, at this point, a clear understanding of their specific roles in regulating social dominance. However, the fact that these receptors are affected by (or affect) social phenotypes likely reflects the interconnected nature of neurotransmitter systems and suggests that regulation at all levels of neural circuits may underlie the transition from one social phenotype to another. Interestingly, an antagonistic relationship between GABA and kainate signaling has been suggested in the regulation of reproductive physiology in other systems (e.g., Sagrillo et al., 1996; Chu and Moenter, 2005; Clarkson and Herbison, 2006; Liu et al., 2006; see discussion in Molecular Modules below).
Molecular Functions, Biological Processes and Cellular Locations
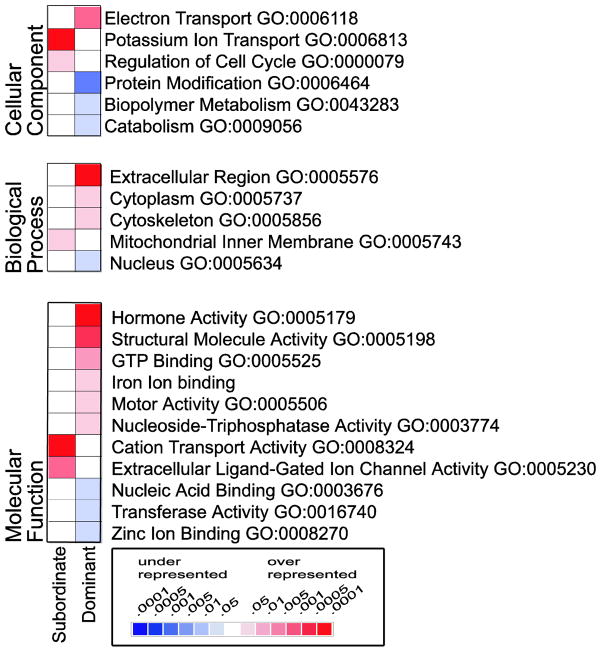
The Gene Ontology annotation scheme applied to the cichlid microarray allows rigorous statistical analysis for over- and under-representation of particular molecular functions, biological process, and cellular locations in the genes that are differentially expressed in each male type. Despite the biased nature of the GO terms due to their origin and application in model organisms and directed research, this tool offers a mechanism for statistical analysis of microarray results according to function (Shaw et al., 1999). These terms, unlike specific gene names, avoid experimenter bias and cross-referencing between experiments and even between species, to relate experimental results between organisms and platforms. Of the 171 features regulated according to male social phenotype, GO terms could be applied to 85. Analysis at all GO levels revealed 22 categories that are statistically over- or under-represented among the genes that are regulated by social phenotype (p ≤ 0.05; compared to their representation among all genes above threshold) (Fig. 3). Using permutation analysis, we determined that only five GO terms were expected to show significant over- or under-representation by chance alone (hyper geometric test p ≤ 0.05, only l GO term at p ≤ 0.01). Importantly, this unbiased statistical approach confirms our observation discussed earlier that cytoskeleton/structural molecules as well as hormone signaling are up-regulated in dominant males (Table 3). Furthermore, cation/potassium transport pathways appear to be important building blocks for each male phenotype. In dominant males, the biological processes of GTP binding, iron ion binding and motor activity were significantly enriched, whereas in subordinate males potassium ion transport, regulation of cellular cation transport and ligand-gated ion channel function were activated. Though difficult to interpret directly, this bioinformatic approach results in a considerable data reduction, facilitates comparisons across species and platforms, and provides a framework of hypotheses for future studies on the molecular underpinnings of socially regulated brain function.
Figure 3.
Analysis, at all Gene Ontology (GO) levels, revealed 22 categories that were statistically over- (red) or under- (blue) represented. The three separate GO vocabularies (molecular function, biological process and cellular location) provide overlapping information, as many of the genes are annotated according to each. P-values represent uncorrected results for the hypergeometric test. GO number and name are according to 200605 releases.
Table 3.
Gene Ontology terms that were significantly over- or under-represented among the genes regulated according to male phenotype. Each of the three independent ontologies is given. Significance is determined to be p<0.05 for the uncorrected hypergeometric p-value. TC number refers to “tentative contig” number assigned in release 1.0 A. burtoni cichlid gene index (compbio.dfci.harvard.edu).
| BIOLOGICAL PROCESS | |||
|---|---|---|---|
| GO: 0006118 Electron Transport D>S overrepresented p=1.01E-03 8 of 55 | |||
| TC400 | 0.9991 | Q5RCZ2_PONPY | similar to secretory vesicle-specific electron transport protein |
| HH_NB_Harvardcol_000005749 | 0.9914 | cloned fragment | Brain Aromatase |
| HH_AB_BRAIN2000_000003171 | 0.9999 | Q803D7_BRARE | Sb:cb825 protein (Fragment). |
| HH_AB_BRAIN2000_000001582 | 0.9997 | Q9DEZ3_ORENI | Brain aromatase (Fragment). |
| HH_AB_BRAIN2000_000001970 | 0.9925 | Q6GLW8_XENLA | Hypothetical protein. |
| TC59 | 0.9988 | Q94SU5_9TELE | Cytochrome c oxidase polypeptide II |
| TC2 | >0.9999 | COX1_GADMO | NADH dehydrogenase 1 |
| TC9 | >0.9999 | CYB_TROMR | cytochrome b family |
| GO:0006813 Potassium Ion Transport S>D overrepresented p=4.83E-04 5 of 25 | |||
| TC15 | >0.9999 | Q90279_CARAU | Kainate receptor alpha subunit. |
| TC34 | 0.9972 | Q7ZU25_BRARE | Atp1a1a.1 protein. |
| TC16 | 0.998 | Q90278_CARAU | Kainate receptor beta subunit. |
| TC17 | >0.9999 | Q90278_CARAU | Kainate receptor beta subunit. |
| TC139 | 0.9909 | Q9DGL2_BRARE | Na+/K+ ATPase beta subunit |
| G0:0000079 Regulation of Cell Cycle S>D overrepresented p=4.76E-02 3 of 30 | |||
| TC109 | 0.9971 | SERF2_HUMAN | Small EDRK-rich factor 2 |
| HH_AB_BRAIN2000_000001151 | 0.9919 | RAS_CARAU | Ras-like protein |
| HH_AB_BRAIN2000_000005191 | 0.9965 | RBM5_HUMAN | RNA-binding protein 5 |
| GO:0006464 Protein Modification D>S underrepresented p=1.7E-02 none of 148 | |||
| GO:0043283 Biopolymer metabolism D>S underrepresented p=3.47E-02 only 2 of 161 | |||
| TC42 | 0.9999 | PAB1_HUMAN | Polyadenylate-binding protein 1 |
| HH_AB_BRAIN2000_000004166 | 0.9992 | Q6NX86_BRARE | High mobility group box 1. |
| GO:0009056 Catabolism D>S underrepresented p=3.38E-02 none of 81 | |||
| CELLULAR COMPONENT | |||
| GO:0005737 Cytoplasm D>S overrepresented p=1.27E-02 23 annotated genes out of 383 analyzed | |||
| TC40 | 0.9997 | Q8AVI3_XENLA | Arbp-prov protein. |
| TC236 | >0.9999 | LDHB_FUNHE | L-lactate dehydrogenase B chain |
| HH_AB_BRAIN2000_000001815 | 0.993 | Q9I921_CARAU | Beta actin. |
| TC2 | >0.9999 | COX1_GADMO | NADH dehydrogenase 1 |
| TC28 | >0.9999 | Q9DFT6_9PERC | Beta tubulin. |
| TC32 | 0.9998 | Q6PC12_BRARE | Enolase 1 (Alpha). |
| HH_AB_BRAIN2000_000001970 | 0.9925 | Q6GLW8_XENLA | Hypothetical protein. |
| TC348 | >0.9999 | RB6A_HUMAN | Ras-related protein Rab-6A |
| TC118 | 0.9998 | Q9DG82_BRARE | Prosaposin. |
| TC291 | 0.9987 | ACT2_FUGRU | Actin, cytoplasmic 2 (Beta-actin 2). |
| HH_AB_BRAIN2000_000002804 | 0.9995 | Q6P4P6_BRARE | Zgc:73142 protein (HMP19 protein). |
| TC18 | >0.9999 | Q8UW60_ORENI | Elongation factor 1a. |
| TC42 | 0.9999 | PAB1_HUMAN | Polyadenylate-binding protein 1 |
| TC399 | 0.9999 | Q802G7_BRARE | Selenoprotein M. |
| HH_AB_BRAIN2000_000003171 | 0.9999 | Q803D7_BRARE | Sb:cb825 protein (Fragment). |
| TC37 | 0.9944 | Q6GQM9_BRARE | Enolase 2. |
| HH_AB_BRAIN2000_000003205 | 0.9947 | Q6GNE7_XENLA | MGC82852 protein ribosome receptor activity |
| HH_AB_BRAIN2000_000003279 | 0.9905 | Q9YHD1_ANGAN | Vacuolar-type H+ transporting ATPase B2 subunit. |
| TC59 | 0.9988 | Q94SU5_9TELE | Cytochrome c oxidase polypeptide II |
| TC48 | 0.9934 | Q9DFT6_9PERC | Beta tubulin. |
| TC9 | >0.9999 | CYB_TROMR | cytochrome b family |
| HH_AB_BRAIN2000_000003055 | 0.9989 | Q5U4S4_XENLA | LOC495448 protein. |
| GO:0005856 Cytoskeleton D>S overrepresented p= 2.85E-02 7 of 77 | |||
| TC291 | 0.9987 | ACT2_FUGRU | Actin, cytoplasmic 2 (Beta-actin 2). |
| HH_AB_BRAIN2000_000001469 | 0.9967 | SEPT7_MOUSE | Septin 7 |
| TC28 | >0.9999 | Q9DFT6_9PERC | Beta tubulin. |
| TC19 | >0.9999 | TBA6_HUMAN | Alpha-tubulin 6 |
| TC12 | >0.9999 | ACT1_FUGRU | cytoplasmic 1 (Beta-actin 1). |
| HH_AB_BRAIN2000_000001815 | 0.993 | Q9I921_CARAU | Beta actin. |
| TC48 | 0.9934 | Q9DFT6_9PERC | Beta tubulin. |
| GO:0005576 Extracellular Region D>S overrepresented p=5.25E-06 15 of 106 analyzed | |||
| HH_AB_BRAIN2000_000004166 | 0.9992 | Q6NX86_BRARE | High mobility group box 1. |
| TC49 | >0.9999 | SOMA_ORENI | Somatotropin precursor |
| HH_AB_BRAIN2000_000003361 | 0.99 | O70534_RAT | ZOG protein. |
| TC124 | 0.9998 | Q6ZM74_BRARE | Similar to synaptophysin |
| HH_AB_BRAIN2000_000005345 | >0.9999 | Q9W7F7_OREMO | Proopiomelanocortin. |
| HH_AB_Stanfordcol_000005677 | 0.9998 | cloned fragment | Arginine vasotocin |
| TC290 | 0.9986 | GBRA5_HUMAN | GABA receptor alpha-5 subunit |
| TC118 | 0.9998 | Q9DG82_BRARE | Prosaposin. |
| TC194 | >0.9999 | NEUS_CHICK | Neuroserpin precursor |
| HH_AB_BRAIN2000_000001923 | >0.9999 | PRL2_OREMO | Prolactin-2 precursor |
| TC325 | >0.9999 | PRL1_OREMO | Prolactin-1 precursor |
| TC331 | >0.9999 | SOML_PAROL | Somatolactin precursor |
| TC310 | 0.9987 | Q8JHD7_HAPBU | Arginine vasotocin |
| HH_AB_Stanfordcol_000005722 | >0.9999 | cloned fragment | Arginine vasotocin |
| TC134 | >0.9999 | Q9DEI5_OREMO | Glycoprotein alpha subunit |
| GO:0005743 Mitochondrial Inner Membrane S>D overrepresented p=3.07E-02 4 of 44 | |||
| TC36 | 0.9929 | Q8JHI0_BRARE | Solute carrier family 25 member 5protein |
| TC13 | >0.9999 | Q8M522_HAPBU | NADH dehydrogenase |
| TC21 | 0.9998 | Q8HLY8_9SMEG | NADH-ubiquinone oxidoreductase |
| TC4 | >0.9999 | Q7YDM6_9LABR | ATP synthase 6. |
| GO:0005634 Nucleus D>S underrepresented p=2.3E-02 only 5 of 275 | |||
| TC12 | >0.9999 | ACT1_FUGRU | cytoplasmic 1 (Beta-actin 1). |
| TC32 | 0.9998 | Q6PC12_BRARE | Enolase 1 (Alpha). |
| HH_AB_BRAIN2000_000001906 | 0.9910 | O42448_ONCMY | Id2 protein. |
| HH_AB_BRAIN2000_000003955 | 0.9919 | Q5MBG6_BRARE | ETS family transcription factor efr1. |
| HH_AB_BRAIN2000_000004166 | 0.9992 | Q6NX86_BRARE | High mobility group box 1. |
| MOLECULAR FUNCTION | |||
| GO:0005525 GTP Binding D>S overrepresented p=5.23E-03 7 out of 56 | |||
| HH_AB_BRAIN2000_000001469 | 0.9967 | SEPT7_MOUSE | Septin 7 |
| TC18 | >0.9999 | Q8UW60_ORENI | Elongation factor 1a. |
| TC19 | >0.9999 | TBA6_HUMAN | Alpha-tubulin 6 |
| HH_AB_BRAIN2000_000005599 | 0.9959 | Q6PBW0_BRARE | Hypothetical protein zgc:73218. |
| TC28 | >0.9999 | Q9DFT6_9PERC | Beta tubulin. |
| TC48 | 0.9934 | Q9DFT6_9PERC | Beta tubulin. |
| TC348 | >0.9999 | RB6A_HUMAN | Ras-related protein Rab-6A |
| GO:0005179 Hormone Activity D>S overrepresented p=1.08E-09 9 of 16 | |||
| TC49 | >0.9999 | SOMA_ORENI | Somatotropin precursor |
| HH_AB_BRAIN2000_000001923 | >0.9999 | PRL2_OREMO | Prolactin-2 precursor |
| TC325 | >0.9999 | PRL1_OREMO | Prolactin-1 precursor |
| HH_AB_BRAIN2000_000005345 | >0.9999 | Q9W7F7_OREMO | Proopiomelanocortin. |
| TC331 | >0.9999 | SOML_PAROL | Somatolactin precursor |
| TC310 | 0.9987 | Q8JHD7_HAPBU | Arginine vasotocin |
| TC134 | >0.9999 | Q9DEI5_OREMO | Glycoprotein alpha subunit |
| HH_AB_Stanfordcol_000005722 | >0.9999 | cloned fragment | Arginine vasotocin |
| HH_AB_Stanfordcol_000005677 | 0.9998 | cloned fragment | Arginine vasotocin |
| GO:0005506 iron ion binding D>S overrepresented p=1.05E-02 5 of 35 | |||
| TC400 | 0.9991 | Q5RCZ2_PONPY | similar to secretory vesicle-specific electron transport protein |
| HH_NB_Harvardcol_000005749 | 0.9914 | cloned fragment | Brain Aromatase |
| HH_AB_BRAIN2000_000001582 | 0.9997 | Q9DEZ3_ORENI | Brain aromatase (Fragment). |
| TC2 | >0.9999 | COX1_GADMO | NADH dehydrogenase 1 |
| TC9 | >0.9999 | CYB_TROMR | cytochrome b family |
| GO:0003374 Motor Activity D>S overrepresented p=1.91E-02 3 of 15 | |||
| TC291 | 0.9987 | ACT2_FUGRU | Actin, cytoplasmic 2 (Beta-actin 2). |
| TC12 | >0.9999 | ACT1_FUGRU | cytoplasmic 1 (Beta-actin 1). |
| HH_AB_BRAIN2000_000001815 | 0.993 | Q9I921_CARAU | Beta actin. |
| GO:001711 Nucleoside-Triphosphatase Activity D>S overrepresented p=2.50E-02 7 of 75 | |||
| TC18 | >0.9999 | Q8UW60_ORENI | Elongation factor 1a. |
| TC19 | >0.9999 | TBA6_HUMAN | Alpha-tubulin 6 |
| HH_AB_BRAIN2000_000005599 | 0.9959 | Q6PBW0_BRARE | Hypothetical protein zgc:73218. |
| TC28 | >0.9999 | Q9DFT6_9PERC | Beta tubulin. |
| HH_AB_BRAIN2000_000003279 | 0.9905 | Q9YHD1_ANGAN | Vacuolar-type H+ transporting ATPase B2 subunit. |
| TC48 | 0.9934 | Q9DFT6_9PERC | Beta tubulin. |
| TC348 | >0.9999 | RB6A_HUMAN | Ras-related protein Rab-6A |
| GO:0005198 Structural Molecule Activity D>S overrepresented p=6.53E-04 11 of 93 | |||
| TC40 | 0.9997 | Q8AVI3_XENLA | Arbp-prov protein. |
| TC236 | >0.9999 | LDHB_FUNHE | L-lactate dehydrogenase B chain |
| TC291 | 0.9987 | ACT2_FUGRU | Actin, cytoplasmic 2 (Beta-actin 2). |
| HH_AB_BRAIN2000_000001469 | 0.9967 | SEPT7_MOUSE | Septin 7 |
| TC19 | >0.9999 | TBA6_HUMAN | Alpha-tubulin 6 |
| TC12 | >0.9999 | ACT1_FUGRU | cytoplasmic 1 (Beta-actin 1). |
| HH_AB_BRAIN2000_000005599 | 0.9959 | Q6PBW0_BRARE | Hypothetical protein zgc:73218. |
| TC28 | >0.9999 | Q9DFT6_9PERC | Beta tubulin. |
| HH_AB_BRAIN2000_000001815 | 0.993 | Q9I921_CARAU | Beta actin. |
| HH_AB_BRAIN2000_000003205 | 0.9947 | Q6GNE7_XENLA | MGC82852 protein ribosome receptor activity |
| TC48 | 0.9934 | Q9DFT6_9PERC | Beta tubulin. |
| GO:0008324 Cation Transporter Activity S>D overrepresented p=2.84E-04 9 of 84 | |||
| TC13 | >0.9999 | Q8M522_HAPBU | NADH dehydrogenase |
| TC15 | >0.9999 | Q90279_CARAU | Kainate receptor alpha subunit. |
| TC1 | >0.9999 | NU1M_BRARE | NADH-ubiquinone oxidoreductase chain 1 |
| TC21 | 0.9998 | Q8HLY8_9SMEG | NADH-ubiquinone oxidoreductase |
| TC4 | >0.9999 | Q7YDM6_9LABR | ATP synthase 6. |
| TC34 | 0.9972 | Q7ZU25_BRARE | Atp1a1a.1 protein. |
| TC16 | 0.998 | Q90278_CARAU | Kainate receptor beta subunit. |
| TC17 | >0.9999 | Q90278_CARAU | Kainate receptor beta subunit. |
| TC139 | 0.9909 | Q9DGL2_BRARE | Na+/K+ ATPase beta subunit |
| GO:0005230 Extracellular Ligand-Gated Ion Channel activity S>D overrepresented p=2.78E-03 3 of 11 | |||
| TC15 | >0.9999 | Q90279_CARAU | Kainate receptor alpha subunit. |
| TC16 | 0.998 | Q90278_CARAU | Kainate receptor beta subunit. |
| TC17 | >0.9999 | Q90278_CARAU | Kainate receptor beta subunit. |
| GO:0003676 Nucleic Acid Binding D>S underrepresented p=3.47E-02 only 6 of 280 | |||
| TC18 | >0.9999 | Q8UW60_ORENI | Elongation factor 1a. |
| TC32 | 0.9998 | Q6PC12_BRARE | Enolase 1 (Alpha). |
| TC40 | 0.9997 | Q8AVI3_XENLA | Arbp-prov protein. |
| TC42 | 0.9999 | PAB1_HUMAN | Polyadenylate-binding protein 1 |
| HH_AB_BRAIN2000_000003955 | 0.9919 | Q5MBG6_BRARE | ETS family transcription factor efr1. |
| HH_AB_BRAIN2000_000004166 | 0.9992 | Q6NX86_BRARE | High mobility group box 1. |
| GO:0016740 Transferase Activity D>S underrepresented p=1.25E-02 only 1 of 150 | |||
| HH_AB_BRAIN2000_000004040 | 0.9925 | UCKL1_HUMAN | Uridine/cytidine kinase-like 1. |
| GO:0008270 Zinc Ion Binding D>S underrepresented p=1.13E-02 none of 106 | |||
Molecular Modules Underlying Dominance and Reproduction
The notion that biological entities (e.g., cognitive tasks, developmental programs, neural circuits, metabolic pathways) operate as functional and discrete (i.e., largely non-overlapping) units, or modules, is not new (Fodor, 1983; Redies and Puelles, 2001; Schlosser and Wagner, 2004; Op de Beeck et al., 2008). In molecular systems biology, a module can simply be defined as a set of co-regulated genes or proteins (Segal et al., 2004). Many such modules may serve as building blocks for assembly of more complex processes. To date, most studies in this area have primarily been concerned with molecular and cellular networks and pathways in simple unicellular systems (Hartwell et al., 1999; Wolf and Arkin, 2003). However, the ultimate challenge in the biology of complex systems is the integration across many levels of biological organization, from molecules to whole organisms in an ever-changing environment. In the following, we make an initial attempt at such an integration of genomic data with physiological and behavioural phenotypes to provide a comprehensive conceptual framework for understanding phenotypically plastic traits. Specifically, we examine male dominance phenotypes in terms of molecular modules of socially controlled traits such as aggression, territoriality, reproduction and growth.
Because we also obtained neural expression profiles of brooding females, we examined variation in transcript levels in relation to sex. 571 genes were regulated according to sex (318 male up-regulated, 253 female up-regulated; Table 1), a number that is far greater than that for dominance phenotypes within males (171 genes or ca. 5%) and suggests that while the switch between dominance phenotypes is multifaceted (see Fig. 1), the difference in brain gene expression profiles between males and females is even more dramatic, affecting ca. 16% of the genes on the array. Interestingly, this proportion is comparable to the 15% observed difference between alternative mating tactics in males of Atlantic salmon (Aubin-Horth et al., 2005a). A considerable fraction of the 171 genes associated with male social phenotypes were also regulated according to sex (Supp. Table 1 and 2). However, among the social status-regulated genes, there were similar proportions of female-enriched and male-enriched genes (Fig. 4). Among the 87 dominant up-regulated genes, were 11 female-enriched and 20 male-enriched genes (hypergeometric test; p = 0.09), and among the 84 subordinate up-regulated genes there were 16 female-enriched genes, and 21 male-enriched genes (hypergeometric test; p = 0.13). In other words, subordinate males do not appear to be molecularly feminized nor are dominant fish simply “super-males”. This result makes sense in light of the reproductive state of these animals. Although female behaviour is, in many ways, similar to that of subordinate males, both brooding females and dominant males are reproductively active. Furthermore, just as the metabolic demands of maintaining a territory are associated with reduced growth in dominant males (Hofmann et al., 1999a), mouth-brooding females starve while incubating their offspring and exhibit a marked reduction in body mass (Mrowk, 1984). Subordinate males, on the other hand, do not reproduce and metabolic energy is directed toward growth (Hofmann et al., 1999a). Thus, the eleven genes (including synaptophysin, neuroserpin and GABA-receptor) up-regulated in both dominant males and brooding females may be part of a module facilitating reproduction and/or reducing growth, and may not necessarily be involved in dominance behaviour per se.
Figure 4.
Venn diagram depicting the relationship of sexually regulated and socially regulated genes. These relationships subdivide the gene classes to indicate modules of gene expression that potentially underlie reproduction (orange), submissive behaviour (lavender), and super-male dominance (green) and opposing super-male dominance (teal). Numbers indicate total unique sequences, and unsequenced array features. Gene names given represent best hit blast annotation for available sequences. The Venn diagram indicates regulation at a BPP of ≥0.99, (the specific BPP, down to 0.80, for regulation is indicated in supplementary tables 1 and 2)
Twenty of the 318 male-enriched genes were even more highly expressed in dominant compared to subordinate males. We suggest that these genes, which include several structural proteins (actin, tubulin, ELF-1) and hormones (GnRH-I, AVT, somatolactin, POMC) (Sup. Table 1) are part of a “super-male” module, which likely plays a role in the aggressive and/or sexual behaviours typical of the dominant male (Fig. 4). Even though we know from previous studies using other approaches that the absolute levels of GnRH1 (Francis et al., 1993; White et al., 2002) and GH (Hofmann et al., 1999b) are higher in dominant males, it is important to note that cDNA microarrays measure only relative transcript levels. Future studies will have to determine whether these genes must be activated in order to produce a dominant phenotype or whether their expression needs to be suppressed for the subordinate phenotype to occur. Following a similar rationale, we suggest that the 21 male-enriched genes (including NeuroD and Kainate receptor) that were further up-regulated in subordinate males relative to dominant males represent a masculinizing module in some sense. In this case, reduced expression would be critical for the manifestation of behaviours linked to aspects of social dominance and reproduction. Consequently, the 21 genes in this masculinizing module could be considered to be complementary to the “super-male” module. Finally, since brooding females and subordinate males all display submissive behaviors and very little aggression, it is tempting to suggest that the 16 genes (including natriuretic peptide) expressed in a similar pattern in these two (Fig. 4). phenotypes
The opposing pattern of regulation for receptor expression in two classic neurotransmitter systems that we observed between male social phenotypes (see above) is also maintained in relation to sex. The gene encoding a GABA-(A) receptor was up-regulated in dominant males and also in females, whereas the kainate-type glutamate receptor was up-regulated in males in general and particularly so in subordinate males. There is a wealth of research that ties the GABA-(A) receptor to the regulation of hypothalamic-pituitary-gonadal axis via integration of steroid feedback to GnRH neurons (for review see Sagrillo et al., 1996). In mammals, GABA has mixed inhibitory and excitatory effects on the release of GnRH due in part to a developmental switch from GABA-(A) receptor depolarization to hyperpolarization (Clarkson and Herbison, 2006). In fishes, depending on species, GABA has either excitatory or inhibitory effects on GnRH release (Trudeau et al., 2000). Interestingly, glutamate-controlled GABA release has been implicated in GnRH regulation (Chu and Moenter, 2005; Clarkson and Herbison, 2006). The kainate system has also been proposed to underlie observed sex differences in the mechanisms of the neural glucocorticoid/stress response. Female mice show less atrophy of hippocampal neurons in response to elevated glucocorticoid levels possibly due to the increased expression of NMDA, AMPA, and kainate glutamate receptor subtypes (Liu et al., 2006). In A. burtoni, the increased kainate receptor expression seen in subordinate males could similarly provide a neuroprotective effect against the elevated cortisol levels seen in subordinate males during specific social situations (Fox et al., 1997). Future pharmacological and neurohistochemical experiments will elucidate the mechanistic interactions of these neurotransmitter systems in relation to sex and social behaviour.
Taken together, our molecular systems analysis supports the notion that transcript patterns may indeed be organized in a modular fashion and can be strongly associated with behavioural and/or physiological traits associated with social phenotypes or sex in either concordant or contrasting ways. Additionally, we can exploit expression variation between phenotypes for tentatively annotating gene function and predicting functional roles of these genes.
Individual Variation in Gene Expression
To appreciate the importance of variation in gene expression for phenotypic plasticity, we need to evaluate the expression differences between individuals. To determine the extent to which individuals of the same phenotype differ in their expression profiles, we estimated transcript levels for each individual separately. Individual profiles were then clustered for similarity according to the estimated transcript levels using the gene list that had been identified as significantly regulated between any two phenotypes (BPP ≥ 0.99) (Euclidian distance matrix based on resampling for bootstrap confidence levels) (Fig. 5).
Figure 5.
Hierarchical clustering of (left) phenotypes and (right) individuals (based on expression profiles for the genes regulated at level of phenotype). The genes have been ordered according to K-means clustering. The heatmaps (red – up-regulated, green down-regulated) show estimated gene expression levels. Heat values are relative only within, not across genes. The numbers identify individual males consistent with figure 2. Confidence values at the nodes were obtained by bootstrap analysis (1000 permutations with resampling).
Similarities in expression profiles of individuals
Sex was a strong factor in the clustering of individual expression profiles, as five of the six females clustered separately from all males. While males of same social phenotype tended to have more similar brain expression profiles, two dominant individuals clustered with the subordinate males. The analysis of behavioural data of these males did not suggest any obvious difference from other dominant males (see Fig. 2). It is unlikely (since we controlled for age and size and based on our observations) that these two dominant males were experiencing dominance for their first time. Also, at the level of behaviour, these two dominant males were not similar to each other. While male 5 did show the fewest chases and the most border threats (Fig. 1) of all dominant males, the behaviors of male 4 were not in any way different from those of the other dominant males. While it is possible that there is more than one molecular substrate for constructing and maintaining a dominant male phenotype, the expression profiles of these two dominant males are no more similar to each other than to subordinate males.
When individual profiles were clustered according to estimated transcript levels using all genes on the array that passed filtering for every fish, rather than only those genes that showed significant regulation, similar results were obtained (data not shown). While the bootstrapped confidence values were lower, the same four dominant males formed a cluster suggesting that gene regulation according to sex and according to social status account for the greatest variation among individuals in this study. Principal component analysis corroborated this conclusion (Supplementary Fig. 1).
Variation among individuals within and between male phenotypes
While overall the clustering resulted in a robust separation of the three phenotypes according to sex and social status, the individual variation, already apparent at this level, prompted further inquiry into expression variation between individuals of the same phenotype. In voles, expression patterns of oxytocin receptor for females and vasopressin receptor for males is correlated with individual variation in social and anxiety-related behaviours (Olazabal and Young, 2006). Similarly, male mice show individual variation in estrogen receptor distribution that correlates with aggressive behaviour (Trainor et al., 2006). Previous studies in A. burtoni and other cichlid species have also reported strong covariation patterns within a social phenotype, between the expression of candidate genes and specific phenotype measures (somatostatin correlated with aggression: Trainor and Hofmann, 2006; AVT correlated with hormone titers: Aubin-Horth et al., 2007). We therefore asked whether significant differences in gene expression existed between individuals of the same phenotype. Furthermore, we tested whether significant differences in gene expression between individuals of the same phenotype could be found among those genes that are differentially regulated between phenotypes.
In order to investigate the degree of individual variation in gene expression, we determined the number of genes that varied significantly in expression between individuals of the same phenotype and compared it to the number that varied between individuals of different phenotypes. Although statistical power was lower because we had only four technical replicates per individual as opposed to six biological replicates in the analyses above (Clark and Townsend, 2007), we were able to measure the average number of genes significantly regulated (BPP ≥ 0.99) in each possible pairwise comparison of two individuals within and between phenotypes. For intra-phenotype variations among males, an average of 82.8 (s.e. ± 4.5; 2.3% of all genes analyzed) genes varied between any two dominant males while a mean of 92.3 (s.e. ± 4.3; 2.6%) genes varied between any two subordinate males. The identity of these genes was substantially different for each pairwise comparison, such that 38% of all the array features varied in at least one intra-phenotype comparison. Interestingly, for inter-phenotype variation between individual males an average of 132 (s.e. ± 8.4; 3.6%) genes varied between any two males of differing social phenotype, which was not significantly different from, the intra-phenotype variation (t-test p = 0.13). Although it is difficult to set an equivalent threshold for significant variation between individuals and between phenotypes, this high degree of individual variation is consistent with other studies that have examined genome-scale individual differences in gene expression to study the molecular basis of natural variation. Whitehead and Crawford (2006) found that 69% of the metabolic pathway genes showed significant variation between individuals within a population, while only 12% were significantly regulated between populations adapted to different temperatures. Similarly, in yeast, up to 50% of the expressed genes show significantly different levels of expression among individual strains (Brem et al., 2002). Not only do we find a similar number of genes to be regulated between individuals of the same or different phenotypes, we find no significant difference in coefficient of variation of expression level for sets of these individually regulated genes. This result indicates that absolute gene expression levels vary between individuals of the same phenotype as much as between phenotypes.
While one might expect low variation within a phenotype for those genes that define that phenotype, an alternative hypothesis posits that those genes that define the social phenotype vary across individuals displaying that phenotype in a manner associated with variation in physiological and behavioural traits (for examples see Trainor and Hofmann, 2006; Aubin-Horth et al., 2007; Cummings et al., 2008). In support of this notion, we found a statistically significant over-representation of intra-phenotype regulated genes among those that were regulated by social status (Table 4). About 72% of the 87 genes up-regulated in the dominant phenotype were also significantly regulated among individuals within phenotype (41 among dominant and 39 among subordinate, 24 of which are shared). Similarly, 64% of the 84 genes that were up-regulated in the subordinate phenotype were also significantly regulated among individuals (47 among dominant and 52 among subordinate, 38 of which are shared). This variation cannot be explained by technical variation in array hybridizations: for a given animal, an array feature must show consistent results across four dye-reversed hybridizations before it can be identified as regulated across individuals according to our statistical analysis. Rather, our results show that even considerable and potentially important within-phenotype variation in gene expression can give rise to reliable and readily identifiable between-phenotype differences. Future integrative studies will help determine whether the observed variation between individuals is caused by or causes subtle phenotypic differences, or represents a dramatic, alternative molecular mechanisms for constructing the same phenotype.
Table 4.
Enrichment of intra-phenotype gene expression variation is significant for genes that are both up-regulated and down-regulated for that phenotype in comparison to the other phenotype. Hypergeometric test.
| Regulated by Phenotype | |||
|---|---|---|---|
| Dominant | Subordinate | ||
| Regulated by Individual | 87 | 84 | |
| w/in dominant | 824 | 41 (p=2.9E-07) | 47 (p=2.76E-11) |
| w/in subordinate | 925 | 39 (p=4.3E-05) | 52 (p=1.2E-12) |
(3598 total genes analyzed)
Summary
By including candidate genes on the microarray, we have validated the discovery-based approach that identifies the gene expression patterns for reproductively active dominant males and reproductively suppressed subordinate males as well as for brooding females. While the regulation of neuroendocrine genes was predicted from previous research, the unexpected and novel discovery of opposing roles for two classic neurotransmitter systems opens exciting new avenues for future research. Furthermore, the increased neuronal remodeling activity in dominant males suggests that the known neuroanatomical changes observed in preoptic GnRH and somatostatin neurons may extend to other cell types and/or additional brain regions. Using the Gene Ontology (GO) framework to interrogate the data for statistical significance reinforces the importance of hormonal regulation and highlights the hitherto underappreciated roles of cytoskeletal components in addition to neurotransmitter pathways. Interestingly, expression profiles vary across individuals within a male phenotype for roughly the same number of genes and with similar magnitudes of transcript abundance as seen between male phenotypes. Specifically, there is a surprisingly high level of variation among those genes that molecularly define the very phenotypes in the first place. Taken together, our genome-scale analysis of molecular systems in the brain has identified complex patterns of gene expression that are associated with a socially regulated switch in behavioural phenotype.
Methods
Animals
Fish derived from a wild-caught stock population were kept in aquaria under conditions mimicking their natural environment (Fernald and Hirata, 1977b): pH 8.0, 28 C water temperature and 12:12 light: dark cycle. Gravel substrate and terra cotta shelters allowed the establishment and maintenance of territories necessary for reproduction (Fernald and Hirata, 1977a). Fish were fed every morning with cichlid pellets and flakes. All work was carried out in compliance with Animal Care and Use Guidelines.
Behavioural experiments
Males
For the behavioural experiments males were marked (coloured bead combinations attached near the dorsal fin). Nine groups of 2 – 3 males with 2 – 3 gravid females were established in one half of a 30 gallon aquarium. Each group was visually isolated from neighboring fish. Ten-minute behavioural observations were made approximately twice every week for five weeks by direct observation of chasing, threat, display, border threat, courtship, flee, and schooling (as described by Fernald, 1977). Behavioural measures were used to calculate a Dominance Index (DI) as the sum of all aggressive behaviours (threat, chasing, border threat and carousel) minus the number of submissive behaviour (flee) and Reproductive Index (RI) as the total of all reproductive behaviours (court, dig, spawn). On the final day of the experiment, males of each status were taken for gene expression profiling only if they had continuously expressed either dominant or subordinate phenotype for the past 28 days. Six mouth-brooding females were also selected for expression profiling. The eighteen fish used in for gene expression analysis were obtained from a total of seven different groups on the basis of stable phenotype.
For the purpose of hybridization design the animals were treated as equivalent within phenotype, though comparisons between each can be made indirectly (see below). Close inspection of the behavioral and microarray data did not reveal any co-variation between animals derived from the same social group. Standard length, body mass, and gonad mass were measured for each fish to calculate the gonadosomatic index (GSI) as gonad mass/body mass. Condition factor (CF), was calculated based on the residuals from the regression of body mass on standard length for before and after the experiment (r2 = 0.95; p = 2.79 × 10−6), and growth rate (GR) was calculated as the relative change in standard length over the course of the experiment. Whole brains were dissected and stored in RNAlater (Ambion) within 5 minutes of initial tank disturbance.
The array used in this study has numerous redundant features, i.e. two or more features represent the same gene. We exploited this property of our array for quality control purposes and to assess the sensitivity of the approach. For example, the array includes four independent features that represent the neuropeptide GnRH1 (two previously cloned cDNAs and two obtained independently from the cDNA library) known to be expressed in only ~300 neurons and up-regulated in dominant males (Davis and Fernald, 1990; White et al., 2002). All four features were similarly significantly differentially expressed, being up-regulated with dominance and thus serve as both a biological and a technical control demonstrating the sensitivity even with whole brain RNA.
Microarray Analysis
RNA extraction
Brains were homogenized (Tissue tearor, Biospec products) and total RNA was extracted according to standard Trizol (Invitrogen) and phase lock gel (Eppendorf) protocols. RNA integrity was determined on the Bioanalyzer (Agilent) and spectrophotometer (Agilent) prior to indirect RNA labeling protocol, starting with 2 μg of total RNA according to Renn et al. (2004). Briefly, each RNA sample was labeled twice, once with Cy3 and once with Cy5. After purification from unincorporated label, each was divided in half, combined with the appropriate samples and every individual was compared to two individuals from each of the other two phenotypes in a balanced loop design incorporating a dye-reversal (see annotation of submitted data at GEO for details). Targets were hybridized to the brain-specific cDNA array from Astatotilapia burtoni (Renn et al., 2004) at 65 degrees Celsius for 12 – 16 hours. Arrays were scanned on an Axon 4000B arrays scanner (Genepix 4.0; Molecular Devices). The loop design allows for the direct comparison of samples of interest thus offering greater statistical power (Churchill, 2002) with fewer replicates.
After filtering for bad feature morphology, hybridization artifacts and low intensity (< 2 standard deviation above local background), raw data was imported into R software (v1.0 R-Development team, 2004) and normalized using the Linear Models for Microarray Data package (LIMMA v1.6.6; Smyth et al., 2004). Background-subtracted mean intensities were normalized using a within array printtip-lowess normalization and used to calculate ratios for a Bayesian analysis of gene expression levels (BAGEL v3.6; Townsend and Hartl, 2002). BAGEL takes advantage of additional information obtained from transitive comparisons of individuals in loop designs experiments (Townsend and Hartl, 2002; Churchill, 2002) Genes represented by more than one feature on the array were only counted as significant if at least one representative passed significance threshold and the full complement survived Fisher’s test of combining probabilities from multiple tests of significance (Sokal and Rohlf, 1995, pg 794). All raw and processed data are available at NCBI’s GEO database (www.ncbi.nlm.nih.gov/projects/geo/) sample numbers GSM267785 - 819 of series GSE10624.
Functional Annotation
The clone templates for PCR amplification were end sequenced (Salzburger et al., 2008) resulting in 4258 expressed sequence tags (EST), 3670 of which were deemed to be of high quality and have been submitted to GenBank (accession number CN472211 - CN468542) and are maintained at the Dana-Farber Cancer Institute as a GeneIndex (http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=a_burtoni) such that 1280 clones combine into 399 tentative contigs (TC) leaving 2381 singleton sequences for a total of 2780 unique sequences. Half (49.5%) of the sequences (1408 out of 2842) could be annotated with a “best hit” at a threshold of e −12 or better according to BLAST alignment to uniprot (version 5.2). Gene Ontology terms were applied to 869 unique cichlid genes by transitive annotation, meaning the GO annotations for a cichlid gene’s best hit were collected and used here for further analysis. In order to avoid species bias we collected GO terms from all genes with the same name as the best hit annotation. GO annotations also include confidence codes. The less reliable annotations derive from”inferred sequence otation similarity (ISS)” (IEA)” (The Gene Ontology those annotations when excluded Consortium, 2000), therefore, we transitively applying GO terms to the ESTs represented on the A. burtoni microarray. The resulting gene ontology (GO) graphs (referred to as directed acyclic graphs DAGs) were then “slimmed” to 183 terms and a total of 4,102 total annotations. For the “slimming” process, the leaf-most nodes that were selected to contain a minimum of 10 annotated cichlid sequences and parent nodes were retained only if an additional 10 cichlid sequences were annotated at that level.
Over- and under-representation of GO terms for a regulated set of genes was determined in Cytoscape (Shannon et al., 2003) using the Biological Network Gene Ontology tool, BiNGO (Maere et al., 2005), which relies upon hypergeometric statistical significance. As GO categories are highly non-independent, the statistical treatment of these terms is still under discussion (Ge et al., 2003). Also, due to the small number of genes for each ontology term and the relatively small number of genes that are regulated, there is less statistical power to identify significantly under-represented GO terms. For these reasons, we use GO analysis as a hypothesis-generating tool and report only uncorrected hypergeometric p-values.
Clustering
Prior to clustering, features representing replicate ESTs were collapsed by combining probabilities from multiple tests of significance (Sokal and Rohlf, 1995; p 794) and the average expression level was determined for each set of features. A hierarchical clustering analysis was applied to the list of genes that were significantly regulated according to dominant, subordinate and brooding female phenotypes. The estimated gene expression levels were used to obtain the dissimilarity matrix applying Euclidian distance measure, which integrates effects of amplitude of ratios as well as direction (correlation) in patterns. Clustering analysis of gene expression patterns of each individual was performed using the hclust function in R software v2.0.1. Clustering was based on dissimilarity measures obtained using the dist functions in the stats package. The consensus tree and bootstrap confidence values for each tree node were obtained with the consensus function in the maanova package (Wu et al., 2002). The consensus tree and confidence values were calculated as the proportion of trees obtained with bootstrapped datasets that agreed with the original tree. Each bootstrapped tree was based on the Euclidian distance matrix calculated for each of 1000 permutated gene expression profile datasets obtained by resampling with replacement. Alternate clustering methods, and different measures of distance are available and are similarly appropriate for gene expression analysis. Hierarchical clustering, based upon all features on the full array rather than only regulated genes, provided a similar tree with reduced confidence values at each node (not shown). The heatmap function and colour options in the package gplots were used to visualize clusters of gene expression, the z-transformed expression ratios were grouped by k-means function in the stats package of R and ordered as such while the samples were ordered according to the consensus hierarchical cluster. Gene Ontology terms provide a means to address the possible functional relationship of a cluster of genes that are coordinately regulated. However, no statistically significant over- or under representation of Gene Ontology terms was seen for any of the gene groups identified by the k-means clusters according to a hypergeometric test (not shown).
Supplementary Material
Supplementary Table 1. Array feature or Tentative Contig (TC) identification for genes significantly up-regulated in dominant males relative to subordinate males. Bayesian posterior probability is given for regulation according to social status as well as according to sex (M>F and F>M).
Supplementary Table 2. Array feature or Tentative Contig (TC) identification for genes significantly up-regulated in subordinate males relative to dominant males. Bayesian posterior probability is given for regulation according to social status as well as according to sex (M>F and F>M).
Supplementary Figure 1. Principal Component Analysis according to estimated gene expression level for all genes that passed filtering. The first PC, x-axis, separates males from females. with a gradient Dominant (circles)-Subordinate (squares)-Female (triangles)). PC2 separates individuals by dominance but not by sex. Analysis based on log2 estimated expression levels for individuals was conducted in R using prcomp command.
Acknowledgments
We are grateful to Josiah Altschuler and Melinda Snitow for animal care, Sarah Annis for assistance with the behavioural experiments, Christian Landry for programming and photography, Amir Karger for bioinformatics advice, and Victoria Zero, Brian Dias, Lin Huffman and Kim Hoke for comments on earlier versions of this manuscript. We thank the Hofmann laboratory and the members of the Bauer Center for Genomics Research for stimulating discussions. This research was supported by an NRSA post-doctoral fellowship to SCPR, a postdoctoral fellowship from FQRNT (Fonds Québécois de la Recherche sur la Nature et les Technologies) and a postdoctoral fellowship from the Natural Science and Engineering Research Council of Canada (NSERC) to NAH, and by National Institutes of Health grant NIGMS GM068763, the Bauer Center for Genomics Research (HAH).
Footnotes
Authors’ contributions:
All authors contributed to the design, implementation, analysis and presentation of this experimental data set.
References
- Amano M, Hyodo S, Kitamura S, Ikuta K, Suzuki Y, Urano A, Aida K. Short photoperiod accelerates preoptic and ventral telencephalic salmon GnRH synthesis and precocious maturation in underyearling male masu salmon. General and Comparative Endocrinology. 1995;99:22–27. doi: 10.1006/gcen.1995.1080. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Hormonally-Induced Alterations in Synaptic Organization in the Adult Nervous-System. Experimental Gerontology. 1992;27:99–110. doi: 10.1016/0531-5565(92)90032-u. [DOI] [PubMed] [Google Scholar]
- Au TM, Anna KG, Fernald RD. Differential social regulation of two pituitary gonadotropin-releasing hormone receptors. Behavioural Brain Research. 2006;170:342–346. doi: 10.1016/j.bbr.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Aubin-Horth N, Desjardins JK, Martei YM, Balshine S, Hofmann HA. Masculinized dominant females in a cooperatively breeding species. Molecular Ecology. 2007;16:1349–1358. doi: 10.1111/j.1365-294X.2007.03249.x. [DOI] [PubMed] [Google Scholar]
- Aubin-Horth N, Landry CR, Letcher BH, Hofmann HA. Alternative life histories shape brain gene expression profiles in males of the same population. Proceedings of the Royal Society B-Biological Sciences. 2005a;272:1655–1662. doi: 10.1098/rspb.2005.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin-Horth N, Letcher BH, Hofmann HA. Interaction of rearing environment and reproductive tactic on gene expression profiles in Atlantic Salmon. Journal of Heredity. 2005b;96:261–278. doi: 10.1093/jhered/esi030. [DOI] [PubMed] [Google Scholar]
- Black MP, Balthazart J, Baillien M, Grober MS. Socially induced and rapid increases in aggression are inversely related to brain aromatase activity in a sex-changing fish, Lythrypnus dalli. Proceedings of the Royal Society B-Biological Sciences. 2005;272:2435–2440. doi: 10.1098/rspb.2005.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem RB, Yvert G, Clinton R, Kruglyak L. Genetic dissection of transcriptional regulation in budding yeast. Science. 2002;296:752–755. doi: 10.1126/science.1069516. [DOI] [PubMed] [Google Scholar]
- Buckley BA, Gracey AY, Somero GN. The cellular response to heat stress in the goby Gillichthys mirabilis: a cDNA microarray and protein-level analysis. Journal of Experimental Biology. 2006;209:2660–2677. doi: 10.1242/jeb.02292. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Jarvis ED, Fernald RD. Rapid Behavioral and Genomic Responses to Social Opportunity. PLOS Biology. 2005;3:1996–2004. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Kailasanath V, Fernald RD. Social dominance regulates androgen and estrogen receptor gene expression. Hormones and Behavior. 2007;51:164–170. doi: 10.1016/j.yhbeh.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro LA, Oliveira RF, Canario AVM, Grober MS. The effect of arginine vasotocin on courtship behaviour in a blenniid fish with alternative reproductive tactics. Fish Physiology and Biochemistry. 2003;28:241–243. [Google Scholar]
- Churchill GA. Fundamentals of experimental design for cDNA microarrays. Nature Genetics. 2002;32:490–495. doi: 10.1038/ng1031. [DOI] [PubMed] [Google Scholar]
- Chu ZG, Moenter SM. Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: A possible local feedback circuit. Journal of Neuroscience. 2005;25:5740–5749. doi: 10.1523/JNEUROSCI.0913-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TA, Townsend JP. Quantifying variation in gene expression. Molecular Ecology. 2007;16:2613–2616. doi: 10.1111/j.1365-294X.2007.03354.x. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Molecular and Cellular Endocrinology. 2006;254:32–38. doi: 10.1016/j.mce.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Cummings ME, Larkins-Ford J, Reilly CRL, Wong RY, Ramsey M, Hofmann HA. Sexual and social stimuli elicit rapid and contrasting genomic responses. Proceedings of the Royal Society, B. 2008;275:393–402. doi: 10.1098/rspb.2007.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MR, Fernald RD. Social control of neuronal soma size. Journal of Neurobiology. 1990;21:1180–1188. doi: 10.1002/neu.480210804. [DOI] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. Journal of Biological Rhythms. 2001;16:365–80. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Devoogd T, Nottebohm F. Gonadal-Hormones Induce Dendritic Growth in the Adult Avian Brain. Science. 1981;214:202–204. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Quantitative behavioural observations of Haplochromis burtoni under semi-natural conditions. Animal Behaviour. 1977;25:643–653. [Google Scholar]
- Fernald RD. Social regulation of the brain: sex, size and status. Genetics and Biology of Sex Determination. 2002;244:169–186. [PubMed] [Google Scholar]
- Fernald RD. Social influences on the brain. Hormones and Behavior. 2004;46:129–130. [Google Scholar]
- Fernald RD, Hirata NR. Field study of Haplochromis burtoni: Quantitative behavioral observations. Animal Behaviour. 1977a;25:964–975. [Google Scholar]
- Fernald RD, Hirata NR. Field Study of Haplochromis burtoni: Habitats and co-habitants. Environmental Biology of Fishes. 1977b;2:299–308. [Google Scholar]
- Fodor JA. The Modularity of Mind. The MIT Press; Cambridge, MA: 1983. [Google Scholar]
- Forsyth IA, Wallis M. Growth hormone and prolactin-molecular and functional evolution. Journal of Mammary Gland Biology and Neoplasia. 2002;7:291–312. doi: 10.1023/a:1022804817104. [DOI] [PubMed] [Google Scholar]
- Fox HE, White SA, Kao MHF, Fernald RD. Stress and dominance in a social fish. Journal of Neuroscience. 1997;17:6463–6469. doi: 10.1523/JNEUROSCI.17-16-06463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis RC, Soma K, Fernald RD. Social Regulation of the Brain Pituitary-Gonadal Axis. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:7794–7798. doi: 10.1073/pnas.90.16.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukamachi S, Sugimoto M. Identification of the gene for morphological body-color change: Somatolactin. Zoological Science. 2004;21:1224–1224. [Google Scholar]
- Gabriel SM, Koenig JI, Washton DL. Estrogen stimulation of galanin gene-expression and galanin-like immunoreactivity in the rat and its blockade by the estrogen antagonist keoxifene. Regulatory Peptides. 1993;45:407–419. doi: 10.1016/0167-0115(93)90367-h. [DOI] [PubMed] [Google Scholar]
- Ge YC, Dudoit S, Speed TP. Resampling-based multiple testing for microarray data analysis. Test. 2003;12:1–77. [Google Scholar]
- The Gene Ontology Consortium. Gene Ontology: tool for the unification of biology. Nature Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst JE. SNAREs and SNARE regulators in membrane fusion and exocytosis. Cellular and Molecular Life Sciences. 1999;55:707–734. doi: 10.1007/s000180050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Hormones and Behavior. 1998;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) Journal of Neuroendocrinology. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Research Reviews. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Greenwood AK, Fernald RD. Social regulation of the electrical properties of gonadotropin-releasing hormone neurons in a Cichlid fish (Astatotilapia burtoni) Biology of Reproduction. 2004;71:909–918. doi: 10.1095/biolreprod.104.030072. [DOI] [PubMed] [Google Scholar]
- Greenwood AK, Wark AR, Fernald RD, Hofmann HA. Arginine vasotocin mediates both dominant and subordinate behavior via two distinct preoptic pathways. ProcRSoc B. 2008 doi: 10.1098/rspb.2008.0622. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober MS, George AA, Watkins KK, Carneiro LA, Oliveira RF. Forebrain AVT and courtship in a fish with male alternative reproductive tactics. Brain Research Bulletin. 2002;57:423–425. doi: 10.1016/s0361-9230(01)00704-3. [DOI] [PubMed] [Google Scholar]
- Grozinger CM, Robinson GE. Microarray analysis of pheromone-mediated gene expression in the honey bee brain. Integrative and Comparative Biology. 2002;42:1237–1237. [Google Scholar]
- Hallgren SLE, Linderoth M, Olsen KH. Inhibition of cytochrome p450 brain aromatase reduces two male specific sexual behaviours in the male Endler guppy (Poecilia reticulata) General and Comparative Endocrinology. 2006;147:323–328. doi: 10.1016/j.ygcen.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature. 1999;402:C47–52. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- Helle KB. The granin family of uniquely acidic proteins of the diffuse neuroendocrine system: comparative and functional aspects. Biological Reviews. 2004;79:769–794. doi: 10.1017/s146479310400644x. [DOI] [PubMed] [Google Scholar]
- Hofmann HA, Benson ME, Fernald RD. Social status regulates growth rate: Consequences for life-history strategies. Proceedings of the National Academy of Sciences of the United States of America. 1999a;96:14171–14176. doi: 10.1073/pnas.96.24.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann HA, Le Bail PY, Fernald RD. Social control of growth and growth hormone levels in an African cichlid fish. Soc Neursci Abst. 1999b;29:348.16. [Google Scholar]
- Hofmann HA, Fernald RD. Social status controls somatostatin neuron size and growth. Journal of Neuroscience. 2000;20:4740–4744. doi: 10.1523/JNEUROSCI.20-12-04740.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann HA. Functional Genomics of Neural and Behavioral Plasticity. Journal of Neurobiology. 2003;54:272–282. doi: 10.1002/neu.10172. [DOI] [PubMed] [Google Scholar]
- Hofmann HA. GnRH signaling in behavioral plasticity. Current Opinion in Neurobiology. 2006;16:343–350. doi: 10.1016/j.conb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Crawley JN. Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. Journal of Molecular Neuroscience. 2002;18:151–165. doi: 10.1385/JMN:18:1-2:151. [DOI] [PubMed] [Google Scholar]
- Jadhao A, Pinelli C. Galanin-like immunoreactivity in the brain and pituitary of the “four-eyed” fish, Anableps anableps. Cell and Tissue Research. 2001;306:309–318. doi: 10.1007/s004410100445. [DOI] [PubMed] [Google Scholar]
- Johnsson M, Axelsson M, Holmgren S. Large veins in the Atlantic cod (Gadus morhua) and the rainbow trout (Oncorhynchus mykiss) are innervated by neuropeptide-containing nerves. Anatomy and Embryology. 2001;204:109–115. doi: 10.1007/s004290100182. [DOI] [PubMed] [Google Scholar]
- Kageyama H, Takenoya F, Kita T, Hori T, Guan HL, Shioda S. Galanin-like peptide in the brain: effects on feeding energy metabolism and reproduction. Regulatory Peptides. 2005;126:21–26. doi: 10.1016/j.regpep.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Kocher TD. Adaptive evolution and explosive speciation: The cichlid fish model. Nature Reviews Genetics. 2004;5:288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- Legac F, Blaise O, Fostier A, Lebail PY, Loir M, Mourot B, Weil C. Growth-Hormone (GH) and Reproduction - a Review. Fish Physiology and Biochemistry. 1993;11:219–232. doi: 10.1007/BF00004569. [DOI] [PubMed] [Google Scholar]
- Lim MM, Hammock EA, Young LJ. The role of vasopressin in the genetic and neural regulation of monogamy. J Neuroendocrinol. 2004;16:325–32. doi: 10.1111/j.0953-8194.2004.01162.x. [DOI] [PubMed] [Google Scholar]
- Liu HH, Payne HR, Wang B, Brady ST. Gender differences in response of hippocampus to chronic glucocorticoid stress: Role of glutamate receptors. Journal of Neuroscience Research. 2006;83:775–786. doi: 10.1002/jnr.20782. [DOI] [PubMed] [Google Scholar]
- Maan ME, Eshuis B, Haesler MP, Schneider MV, Van Alphen JJM, Seehausen O. Color polymorphism and predation in a Lake Victoria cichlid fish. Copeia. 2008 in press. [Google Scholar]
- Madani R, Kosloz S, Akhmedov A, Cinelli P, Kinter J, Lipp HP, Sonderegger P, Wolfer DP. Impaired explorative behavior in neophobia in genetically modified mice lacking or overexpressing the extracellular serine protease inhibitor neuroserpin. Molecular and Cellular Neuroscience. 2003;23:473–494. doi: 10.1016/s1044-7431(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of Gene Ontology categories in Biological Networks. Bioinformatics. 2005;21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- Marsh KE, Creutz LM, Hawkins MB, Godwin J. Aromatase immunoreactivity in the bluehead wrasse brain, Thalassoma bifasciatum: Immunolocalization and co-regionalization with arginine vasotocin and tyrosine hydroxylase. Brain Research. 2006;1126:91–101. doi: 10.1016/j.brainres.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyniuk CJ, Xiong HL, Crump K, Chiu S, Sardana R, Nadler A, Gerrie ER, Xia XH, Trudeau VL. Gene expression profiling in the neuroendocrine brain of male goldfish (Carassius auratus) exposed to 17 alpha-ethinylestradiol. Physiological Genomics. 2006;27:328–336. doi: 10.1152/physiolgenomics.00090.2006. [DOI] [PubMed] [Google Scholar]
- Miranda E, Lomas DA. Neuroserpin: a serpin to think about. Cellular and Molecular Life Sciences. 2006;63:709–722. doi: 10.1007/s00018-005-5077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrowk A. Brood care motivation and hunger in the mouth brooding cichlid Pseudochrenilabrus multicolor. Behavioural Processes. 1984;9:181–190. doi: 10.1016/0376-6357(84)90039-1. [DOI] [PubMed] [Google Scholar]
- Munz H. GnRH-systems in the forebrain of cichlid fish. European Journal of Morphology. 1999;37:100–102. [PubMed] [Google Scholar]
- Nakamura M, Kobayashi Y. Sex change in coral reef fish. Fish Physiology and Biochemistry. 2005;31:117–122. doi: 10.1007/s10695-006-7595-x. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. An Introduction to Behavioral Endocrinology. 3. Sinauer Associates; Sunderland, MA: 2005. [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AVM. Social modulation of androgen levels in male teleost fish. Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology. 2002;132:203–215. doi: 10.1016/s1096-4959(01)00523-1. [DOI] [PubMed] [Google Scholar]
- Op de Beeck HP, Haushofer J, Kanwisher NG. Interpreting fMRI data: maps, modules and dimensions. Nat Rev Neurosci. 2008;9:123–135. doi: 10.1038/nrn2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhar IS, Ogawa S, Sakuma Y. Three GnRH receptor types in laser-captured single cells of the cichlid pituitary display cellular and functional heterogeneity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2204–2209. doi: 10.1073/pnas.0409494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh VN, Clement TS, Fernald RD. Androgen level and male social status in the African cichlid, Astatotilapia burtoni. Behavioural Brain Research. 2006;166:291–295. doi: 10.1016/j.bbr.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Pollen AA, Dobberfuhl AP, Scace J, Igulu MM, Renn SCP, Shumway CA, Hofmann HA. Environmental Complexity and Social Organization Sculpt the Brain in Lake Tanganyikan Cichlid Fish. Brain Behavior and Evolution. 2007;70:21–39. doi: 10.1159/000101067. [DOI] [PubMed] [Google Scholar]
- Redies C, Puelles L. Modularity in vertebrate brain development and evolution. BioEssays. 2001;23:1100–1111. doi: 10.1002/bies.10014. [DOI] [PubMed] [Google Scholar]
- Renn SCP, Aubin-Horth N, Hofmann HA. Biologically meaningful expression profiling across species using heterologous hybridization to a cDNA microarray. Bmc Genomics. 2004:5. doi: 10.1186/1471-2164-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Crabbe JC. Gene expression induced by drugs of abuse. Current Opinion in Pharmacology. 2005;5:26–33. doi: 10.1016/j.coph.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Grozinger CM, Whitfield CW. Sociogenomics: Social life in molecular terms. Nature Reviews Genetics. 2005;6:257–U16. doi: 10.1038/nrg1575. [DOI] [PubMed] [Google Scholar]
- Sagrillo CA, Grattan DR, McCarthy MM, Selmanoff M. Hormonal and neurotransmitter regulation of GnRH gene expression and related reproductive behaviors. Behavior Genetics. 1996;26:241–277. doi: 10.1007/BF02359383. [DOI] [PubMed] [Google Scholar]
- Salzburger W, Mack T, Verheyen E, Meyer A. Out of Tanganyika: Genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evolutionary Biology. 2005;5:15. doi: 10.1186/1471-2148-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzburger W, Renn SCP, Steinke D, Hofmann HA, Braasch I, Meyer A. Annotation of expressed sequence tags for the East African cichlid fish species Astatotilapia burtoni and evolutionary analyses of cichlid ORFs. BMC Genomics. 2008;9:96. doi: 10.1186/1471-2164-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo N, Bass AH. New insights into neuropeptide modulation of aggression: field studies of arginine vasotocin in a territorial tropical damselfish. Proceedings of the Royal Society B-Biological Sciences. 2006;273:3085–3092. doi: 10.1098/rspb.2006.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Schick RR, Samsai S, Zimmermann JP, Eberl T, Endres C, Schusdziarra V, Classen M. Effect of Galanin on Food-Intake in Rats - Involvement of Lateral and Ventromedial Hypothalamic sites. American J Physiol. 1993;264:R355–R361. doi: 10.1152/ajpregu.1993.264.2.R355. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Wagner GP, editors. Modularity in Development and Evolution. University Of Chicago Press; Chicago, IL: 2004. [Google Scholar]
- Segal E, Friedman, Koller D, Regev A. A module map showing conditional activity of expression modules in cancer. Nature Genetics. 2004;36:1090–1098. doi: 10.1038/ng1434. [DOI] [PubMed] [Google Scholar]
- Semsar K, Godwin J. Social influences on the arginine vasotocin system are independent of gonads in a sex-changing fish. Journal of Neuroscience. 2003;23:4386–4393. doi: 10.1523/JNEUROSCI.23-10-04386.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semsar K, Godwin J. Multiple mechanisms of phenotype development in the bluehead wrasse. Hormones and Behavior. 2004;45:345–353. doi: 10.1016/j.yhbeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Shaw DR, Ashbumer M, Blake JA, Baldarelli RM, Botstein D, Davis AP, Cherry JM, Lewis S, Lutz CM, Richardson JE, et al. Gene Ontology: a controlled vocabulary to describe the function, biological process and cellular location of gene products in genome databases. American Journal of Human Genetics. 1999;65:A419–A419. [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research. 2003;13:2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverin B, Baillien M, Balthazart J. Territorial aggression, circulating levels of testosterone, and brain aromatase activity in free-living pied flycatchers. Hormones and Behavior. 2004;45:225–34. doi: 10.1016/j.yhbeh.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annual Review of Neuroscience. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Smyth GK, Thorne NP, Wettenhall J. User’s Guide. 2004. LIMMA: Linear Models for Microarray Data Version 1.6.6. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry, The principles and practice of statistics in biological research. 3. New York: W.H. Freeman and Company; 1995. [Google Scholar]
- Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: relationship to aggressive and sexual behavior. Journal of Neurobiology. 2003;56:209–21. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- Soma KK, Francis RC, Wingfield JC, Fernald RD. Androgen regulation of hypothalamic neurons containing gonadotropin-releasing hormone in a cichlid fish: Integration with social cues. Hormones and Behavior. 1996;30:216–226. doi: 10.1006/hbeh.1996.0026. [DOI] [PubMed] [Google Scholar]
- Sorensen C, Overli O, Summers CH, Nilsson GE. Social regulation of neurogenesis in teleosts. Brain Behavior and Evolution. 2007;70:239–246. doi: 10.1159/000105487. [DOI] [PubMed] [Google Scholar]
- Tortorella C, Neri G, Nussdorfer GG. Galanin in the regulation of the hypothalamic-pituitary-adrenal axis (Review) International Journal of Molecular Medicine. 2007;19:639–647. [PubMed] [Google Scholar]
- Townsend JP, Hartl DL. Bayesian analysis of gene expression levels: statistical quantification of relative mRNA level across multiple strains or treatments. Genome Biology. 2002;3:research0071.1–0071.16. doi: 10.1186/gb-2002-3-12-research0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Hofmann HA. Somatostatin regulates aggressive behavior in an African cichlid fish. Endocrinology. 2006;147:5119–5125. doi: 10.1210/en.2006-0511. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: How interactions between aromatase and the environment modulate aggression. Frontiers in Neuroendocrinology. 2006;27:170–179. doi: 10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Hofmann HA. Somatostatin and somatostatin receptor gene expression in dominant and subordinate males of an African cichlid fish. Behavioural Brain Research. 2007;179:314–320. doi: 10.1016/j.bbr.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau VL, Spanswick D, Fraser EJ, Lariviere K, Crump D, Chiu S, MacMillan M, Schulz RW. The role of amino acid neurotransmitters in the regulation of pituitary gonadotropin release in fish. Biochemistry and Cell Biology-Biochimie Et Biologie Cellulaire. 2000;78:241–259. [PubMed] [Google Scholar]
- Volkoff H, Canosa LF, Unniappan S, Cerda-Reverter JM, Bernier NJ, Kelly SP, Peter RE. Neuropeptides and the control of food intake in fish. General and Comparative Endocrinology. 2005;142:3–19. doi: 10.1016/j.ygcen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- White SA, Bond CT, Francis RC, Kasten TL, Fernald RD, Adelman JP. A 2nd Gene for Gonadotropin-Releasing-Hormone - cDNA and Expression Pattern in the Brain. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1423–1427. doi: 10.1073/pnas.91.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SA, Nguyen T, Fernald RD. Social regulation of gonadotropin-releasing hormone. Journal of Experimental Biology. 2002;205:2567–2581. doi: 10.1242/jeb.205.17.2567. [DOI] [PubMed] [Google Scholar]
- Whitehead A, Crawford DL. Variation within and among species in gene expression: raw material for evolution. Molecular Ecology. 2006;15:1197–1211. doi: 10.1111/j.1365-294X.2006.02868.x. [DOI] [PubMed] [Google Scholar]
- Whitfield CW, Cziko AM, Robinson GE. Gene expression profiles in the brain predict behavior in individual honey bees. Science. 2003;302:296–299. doi: 10.1126/science.1086807. [DOI] [PubMed] [Google Scholar]
- Wiesenfeldhallin Z, Xu XJ, Langel U, Bedecs K, Hokfelt T, Bartfai T. Galanin-mediated Control of Pain - Enhanced Role after Nerve Injury. Proc Nat Acad Science USA. 1992;89:3334–3337. doi: 10.1073/pnas.89.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A Role for Central Vasopressin in Pair Bonding in Monogamous Prairie Voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wolf DM, Arkin AP. Motifs, Modules and Games in Bacteria. Curr Opinion Microbiol. 2003;6:125–134. doi: 10.1016/s1369-5274(03)00033-x. [DOI] [PubMed] [Google Scholar]
- Wu H, Kerr MK, Cui X, Churchill GA. The analysis of gene expression data: methods and software. Springer; 2002. MAANOVA: A Software Package for the Analysis of Spotted cDNA Microarray Experiments. [Google Scholar]
- Yu KL, Peter RE. Changes in Brain Levels of Gonadotropin-Releasing-Hormone and Serum Levels of Gonadotropin and Growth-Hormone in Goldfish During Spawning. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1991;69:182–188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Array feature or Tentative Contig (TC) identification for genes significantly up-regulated in dominant males relative to subordinate males. Bayesian posterior probability is given for regulation according to social status as well as according to sex (M>F and F>M).
Supplementary Table 2. Array feature or Tentative Contig (TC) identification for genes significantly up-regulated in subordinate males relative to dominant males. Bayesian posterior probability is given for regulation according to social status as well as according to sex (M>F and F>M).
Supplementary Figure 1. Principal Component Analysis according to estimated gene expression level for all genes that passed filtering. The first PC, x-axis, separates males from females. with a gradient Dominant (circles)-Subordinate (squares)-Female (triangles)). PC2 separates individuals by dominance but not by sex. Analysis based on log2 estimated expression levels for individuals was conducted in R using prcomp command.