Abstract
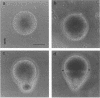
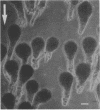
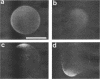
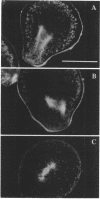
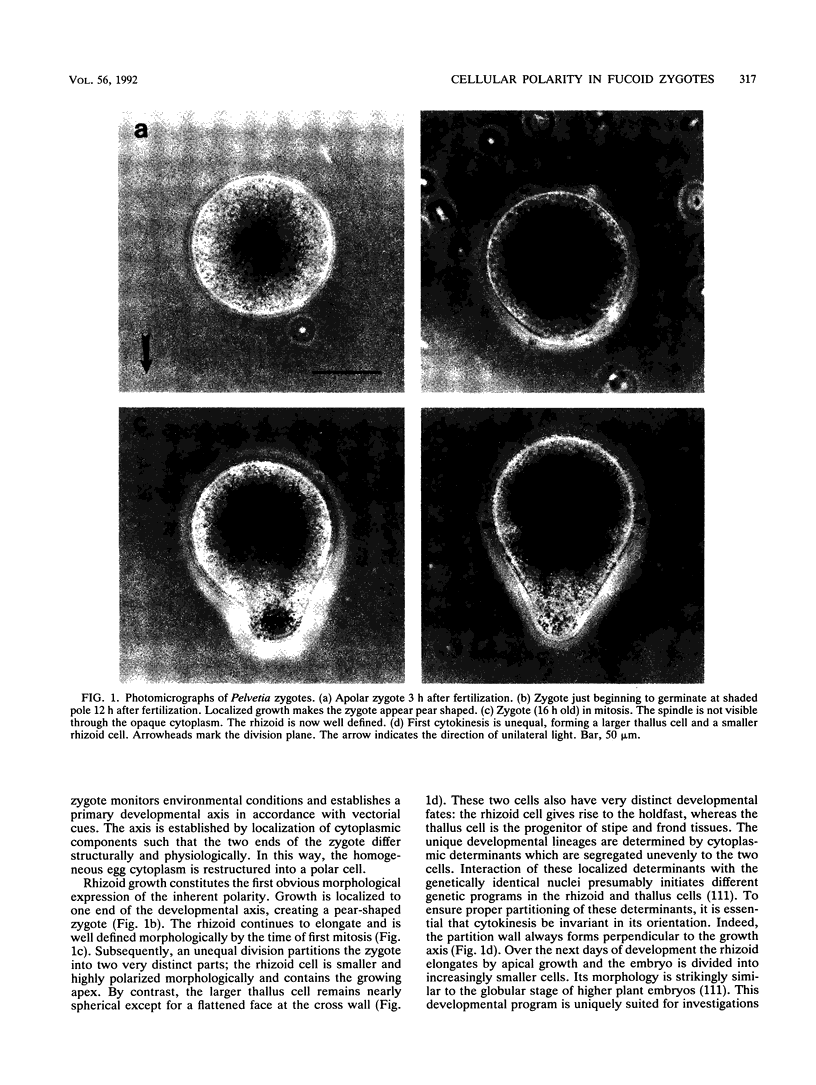
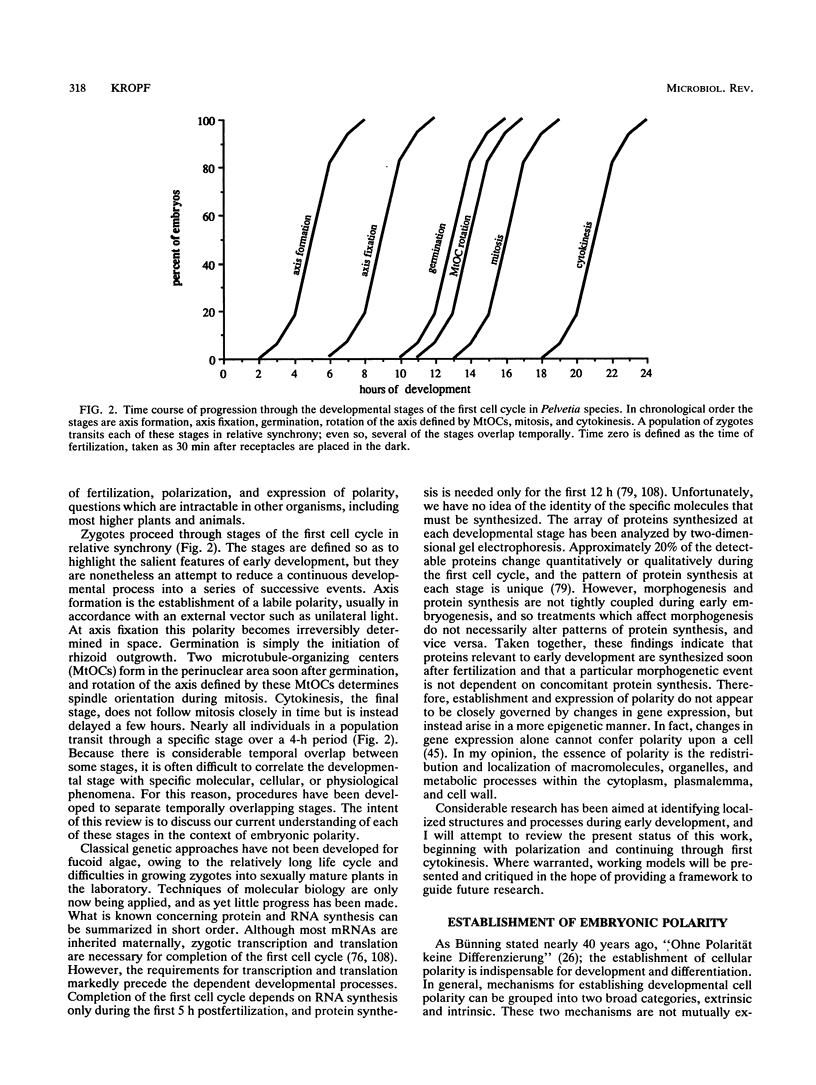
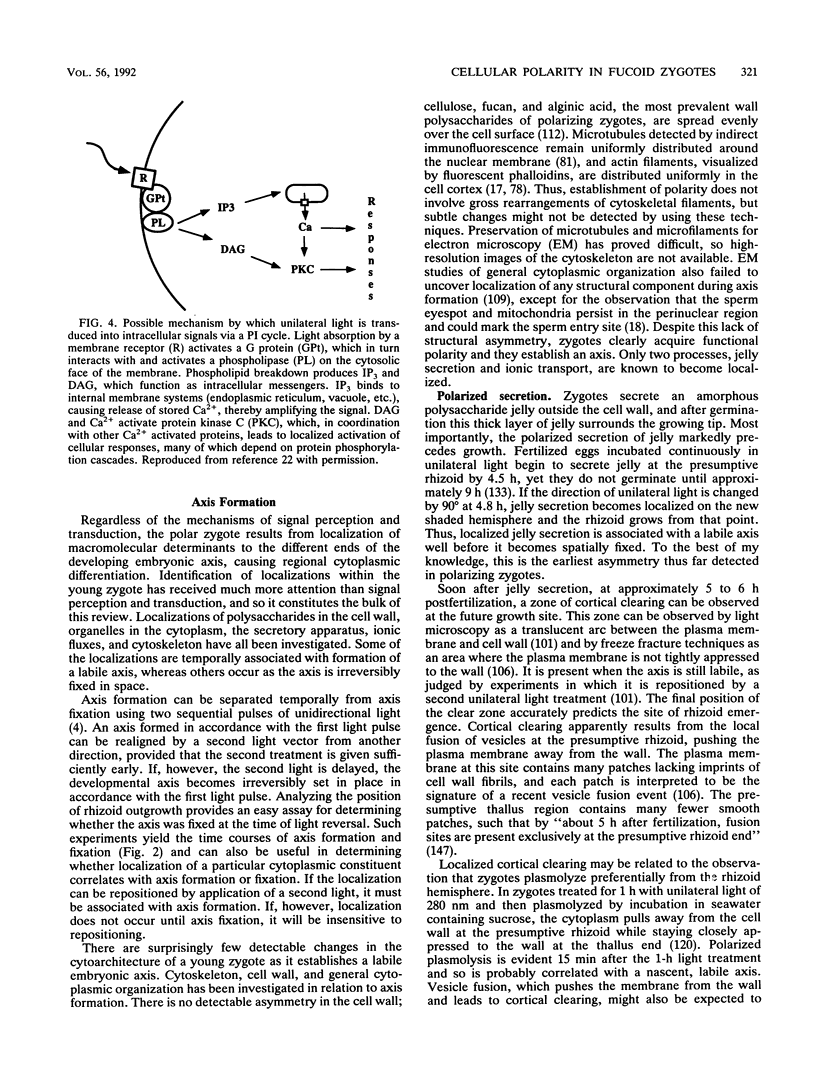
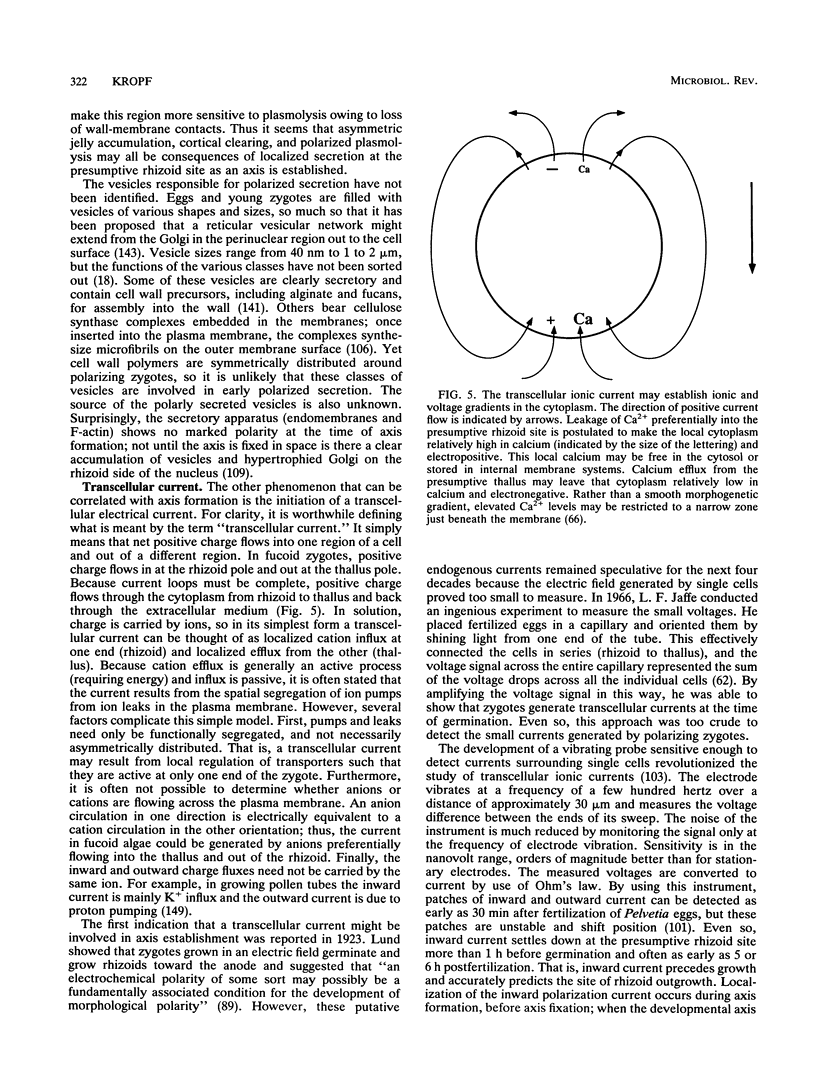
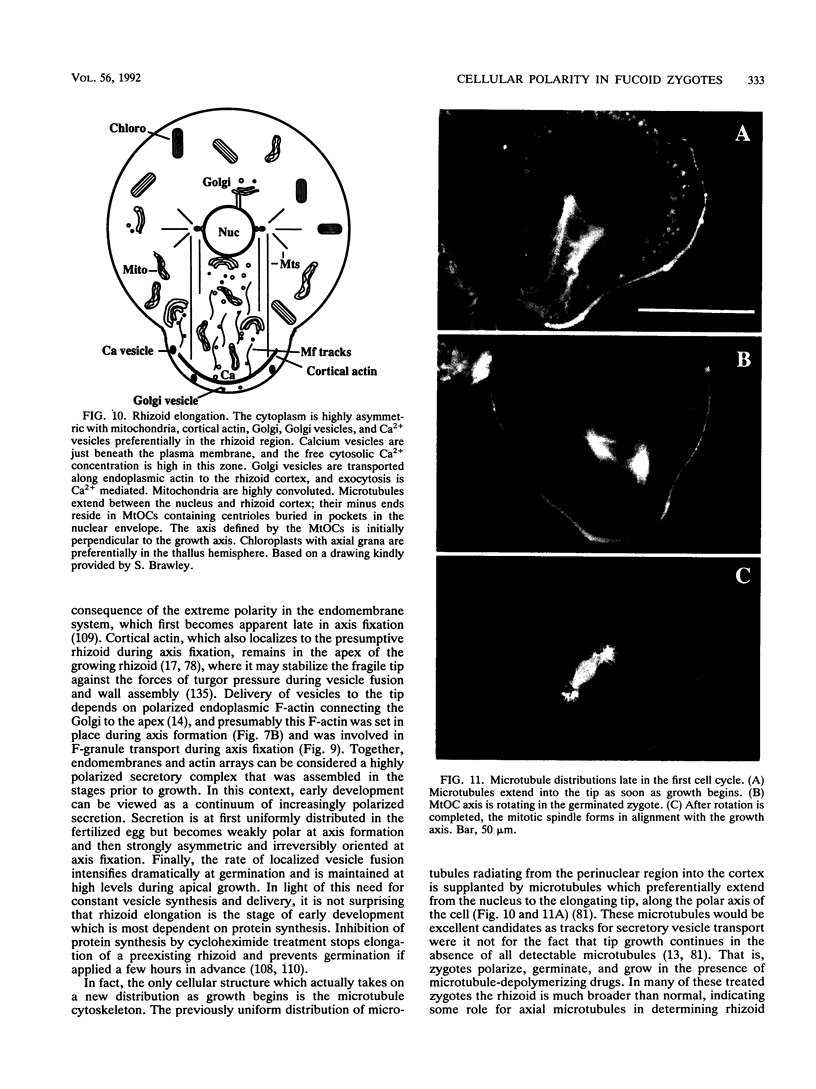
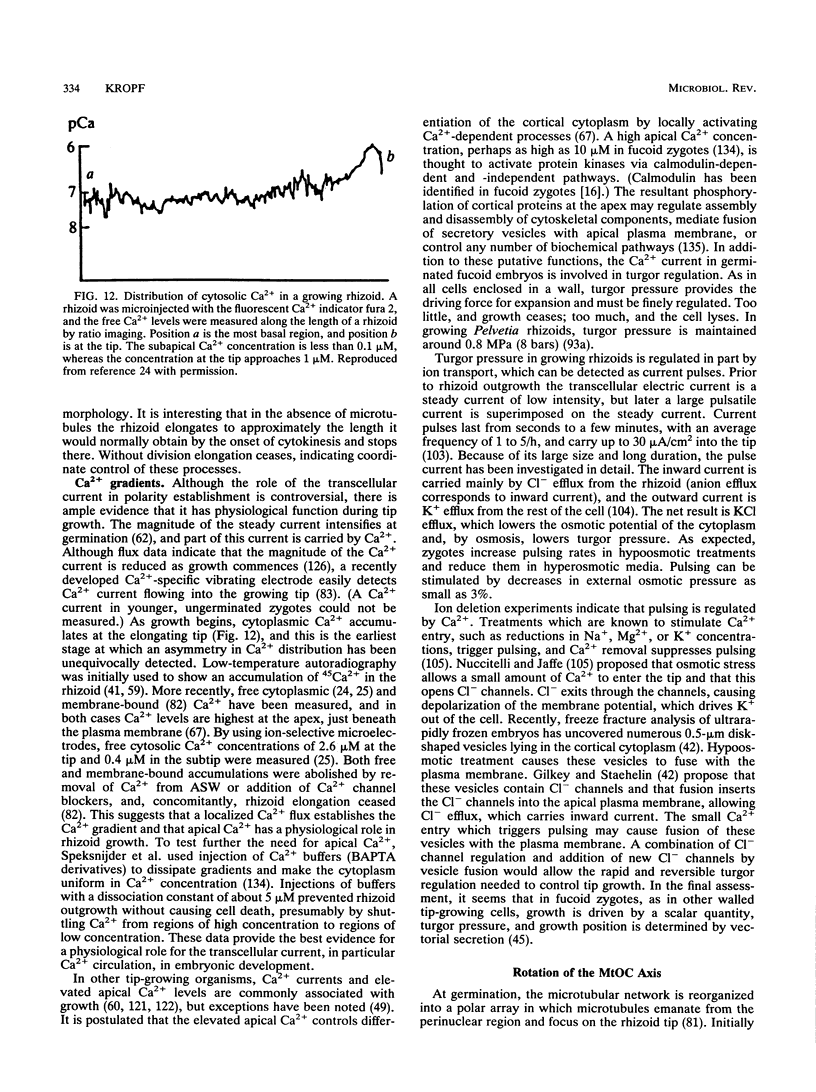
Zygotes of fucoid algae have long been studied as a paradigm for cell polarity. Polarity is established early in the first cell cycle and is then expressed as localized growth and invariant cell division. The fertilized egg is a spherical cell and, by all accounts, bears little or no asymmetry. Polarity is acquired epigenetically a few hours later in the form of a rhizoid/thallus axis. The initial stage of polarization is axis selection, during which zygotes monitor environment gradients to determine the appropriate direction for rhizoid formation. In their natural setting in the intertidal zone, sunlight is probably the most important polarizing vector; rhizoids form away from the light. The mechanism by which zygotes perceive environmental gradients and transduce that information into an intracellular signal is unknown but may involve a phosphatidylinositol cycle. Once positional information has been recorded, the cytoplasm and membrane are reorganized in accordance with the vectorial information. The earliest detectable asymmetries in the polarizing zygote are localized secretion and generation of a transcellular electric current. Vesicle secretion and the inward limb of the current are localized at the presumptive rhizoid. The transcellular current may establish a cytoplasmic Ca2+ gradient constituting a morphogenetic field, but this remains controversial. Localized secretion and establishment of transcellular current are sensitive to treatment with cytochalasins, indicating that cytoplasmic reorganization is dependent on the actin cytoskeleton. The nascent axis at first is labile and susceptible to reorientation by subsequent environmental vectors but soon becomes irreversibly fixed in its orientation. Locking the axis in place requires both cell wall and F-actin and is postulated to involve an indirect transmembrane bridge linking cortical actin to cell wall. This bridge anchors relevant structures at the presumptive rhizoid and thereby stabilizes the axis. Approximately halfway through the first cell cycle, the latent polarity is expressed morphologically in the form of rhizoid growth. Elongation is by tip growth and does not appear to be fundamentally different from tip growth in other organisms. The zygote always divides perpendicular to the growth axis, and this is controlled by the microtubule cytoskeleton. Two microtubule-organizing centers on the nuclear envelope rotate such that they align with the growth axis. They then serve as spindle poles during mitosis. Cytokinesis bisects the axial spindle, resulting in a transverse crosswall. Although the chronology of cellular events associated with polarity is by now rather detailed, causal mechanisms remain obscure.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Jacobsen L., Joaquin J., Jaffe L. F. Ionic concentrations in developing Pelvetia eggs. Dev Biol. 1972 Apr;27(4):538–545. doi: 10.1016/0012-1606(72)90191-1. [DOI] [PubMed] [Google Scholar]
- Bentrup F. W., Jaffe L. F. Analyzing the "group effect": rheotropic responses of developing fucus eggs. Protoplasma. 1968;65(1):25–35. doi: 10.1007/BF01666369. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Brawley S. H., Quatrano R. S. Sulfation of fucoidin in Fucus embryos. IV. Autoradiographic investigations of fucoidin sulfation and secretion during differentiation and the effect of cytochalasin treatment. Dev Biol. 1979 Dec;73(2):193–205. doi: 10.1016/0012-1606(79)90063-0. [DOI] [PubMed] [Google Scholar]
- Brawley S. H., Quatrano R. S., Wetherbee R. Fine-structural studies of the gametes and embryo of Fucus vesiculosus L. (Phaeophyta). I. Fertilization and pronuclear fusion. J Cell Sci. 1976 Mar;20(2):233–254. doi: 10.1242/jcs.20.2.233. [DOI] [PubMed] [Google Scholar]
- Brawley S. H., Quatrano R. S., Wetherbee R. Fine-structural studies of the gametes and embryo of Fucus vesiculosus L. (Phaeophyta). III. Cytokinesis and the multicellular embryo. J Cell Sci. 1977 Apr;24:275–294. doi: 10.1242/jcs.24.1.275. [DOI] [PubMed] [Google Scholar]
- Brawley S. H., Roberts D. M. Calmodulin-binding proteins are developmentally regulated in gametes and embryos of fucoid algae. Dev Biol. 1989 Feb;131(2):313–320. doi: 10.1016/s0012-1606(89)80004-1. [DOI] [PubMed] [Google Scholar]
- Brawley S. H., Robinson K. R. Cytochalasin treatment disrupts the endogenous currents associated with cell polarization in fucoid zygotes: studies of the role of F-actin in embryogenesis. J Cell Biol. 1985 Apr;100(4):1173–1184. doi: 10.1083/jcb.100.4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley S. H. The fast block against polyspermy in fucoid algae is an electrical block. Dev Biol. 1991 Mar;144(1):94–106. doi: 10.1016/0012-1606(91)90482-i. [DOI] [PubMed] [Google Scholar]
- Brawley S. H., Wetherbee R., Quatrano R. S. Fine-structural studies of the gametes and embryo of Fucus vesiculosus L. (Phaeophyta). II. The cytoplasm of the egg and young zygote. J Cell Sci. 1976 Mar;20(2):255–271. doi: 10.1242/jcs.20.2.255. [DOI] [PubMed] [Google Scholar]
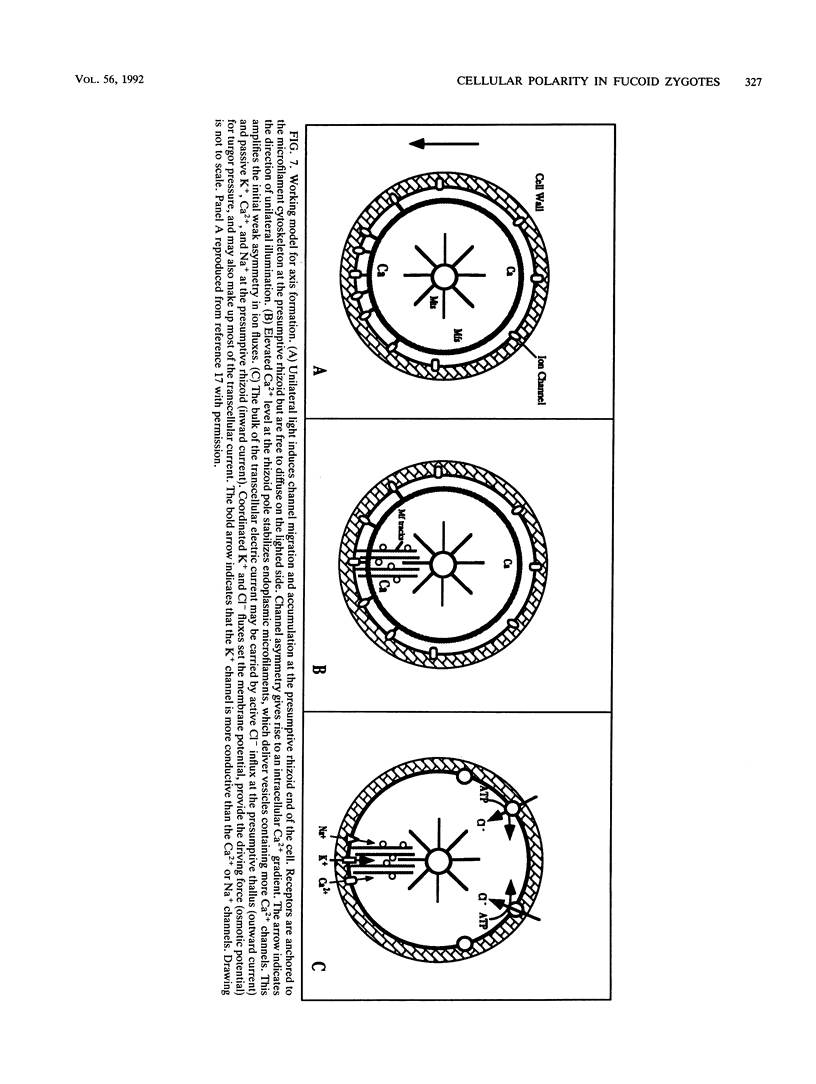
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Callow M. E., Coughlan S. J., Evans L. V. The role of Golgi bodies in polysaccharide sulphation in Fucuszygotes. J Cell Sci. 1978 Aug;32:337–356. doi: 10.1242/jcs.32.1.337. [DOI] [PubMed] [Google Scholar]
- Callow M. E., Evans L. V., Bolwell G. P., Callow J. A. Fertilization in brown algae. I. SEM and other observations on Fucus serratus. J Cell Sci. 1978 Aug;32:45–54. doi: 10.1242/jcs.32.1.45. [DOI] [PubMed] [Google Scholar]
- Cooper J. A. The role of actin polymerization in cell motility. Annu Rev Physiol. 1991;53:585–605. doi: 10.1146/annurev.ph.53.030191.003101. [DOI] [PubMed] [Google Scholar]
- Crayton M. A., Wilson E., Quatrano R. S. Sulfation of fucoidan in Fucus embryos. II. Separation from initiation of polar growth. Dev Biol. 1974 Jul;39(1):164–167. doi: 10.1016/s0012-1606(74)80018-7. [DOI] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Drubin D. G. Development of cell polarity in budding yeast. Cell. 1991 Jun 28;65(7):1093–1096. doi: 10.1016/0092-8674(91)90001-f. [DOI] [PubMed] [Google Scholar]
- Gerencser G. A., White J. F., Gradmann D., Bonting S. L. Is there a Cl- pump? Am J Physiol. 1988 Nov;255(5 Pt 2):R677–R692. doi: 10.1152/ajpregu.1988.255.5.R677. [DOI] [PubMed] [Google Scholar]
- Gerisch G., Noegel A. A., Schleicher M. Genetic alteration of proteins in actin-based motility systems. Annu Rev Physiol. 1991;53:607–628. doi: 10.1146/annurev.ph.53.030191.003135. [DOI] [PubMed] [Google Scholar]
- Gilroy S., Read N. D., Trewavas A. J. Elevation of cytoplasmic calcium by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature. 1990 Aug 23;346(6286):769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- Griffing L. R., Quatrano R. S. Isoelectric focusing of plant cell membranes. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4804–4808. doi: 10.1073/pnas.81.15.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. To shape a cell: an inquiry into the causes of morphogenesis of microorganisms. Microbiol Rev. 1990 Dec;54(4):381–431. doi: 10.1128/mr.54.4.381-431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogsett W. E., Quatrano R. S. Isolation of Polysaccharides Sulfated during Early Embryogenesis in Fucus. Plant Physiol. 1975 Jan;55(1):25–29. doi: 10.1104/pp.55.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogsett W. E., Quatrano R. S. Sulfation of fucoidin in Fucus embryos. III. Required for localization in the rhizoid wall. J Cell Biol. 1978 Sep;78(3):866–873. doi: 10.1083/jcb.78.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A. A. Centrosome movement in the early divisions of Caenorhabditis elegans: a cortical site determining centrosome position. J Cell Biol. 1989 Sep;109(3):1185–1193. doi: 10.1083/jcb.109.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A. A., White J. G. Determination of cell division axes in the early embryogenesis of Caenorhabditis elegans. J Cell Biol. 1987 Nov;105(5):2123–2135. doi: 10.1083/jcb.105.5.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAFFE L. F. Tropistic responses of zygotes of the Fucaceae to polarized light. Exp Cell Res. 1958 Oct;15(2):282–299. doi: 10.1016/0014-4827(58)90031-4. [DOI] [PubMed] [Google Scholar]
- Jaffe L. A., Weisenseel M. H., Jaffe L. F. Calcium accumulations within the growing tips of pollen tubes. J Cell Biol. 1975 Nov;67(2PT1):488–492. doi: 10.1083/jcb.67.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F. Electrical currents through the developing fucus egg. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1102–1109. doi: 10.1073/pnas.56.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F. Electrophoresis along cell membranes. Nature. 1977 Feb 17;265(5595):600–602. doi: 10.1038/265600a0. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. Localization in the developing Fucus egg and the general role of localizing currents. Adv Morphog. 1968;7:295–328. doi: 10.1016/b978-1-4831-9954-2.50012-4. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F., Neuscheler W. On the mutual polarization of nearby pairs of fucaceous eggs. Dev Biol. 1969 Jun;19(6):549–565. doi: 10.1016/0012-1606(69)90037-2. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F., Robinson K. R., Nuccitelli R. Local cation entry and self-electrophoresis as an intracellular localization mechanism. Ann N Y Acad Sci. 1974;238:372–389. doi: 10.1111/j.1749-6632.1974.tb26805.x. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Koehler L. D., Linskens H. F. Incorporation of protein and RNA precursors into fertilized Fucus eggs. Protoplasma. 1967;64(2):209–212. doi: 10.1007/BF01250389. [DOI] [PubMed] [Google Scholar]
- Kohno T., Shimmen T. Accelerated sliding of pollen tube organelles along Characeae actin bundles regulated by Ca2+. J Cell Biol. 1988 May;106(5):1539–1543. doi: 10.1083/jcb.106.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
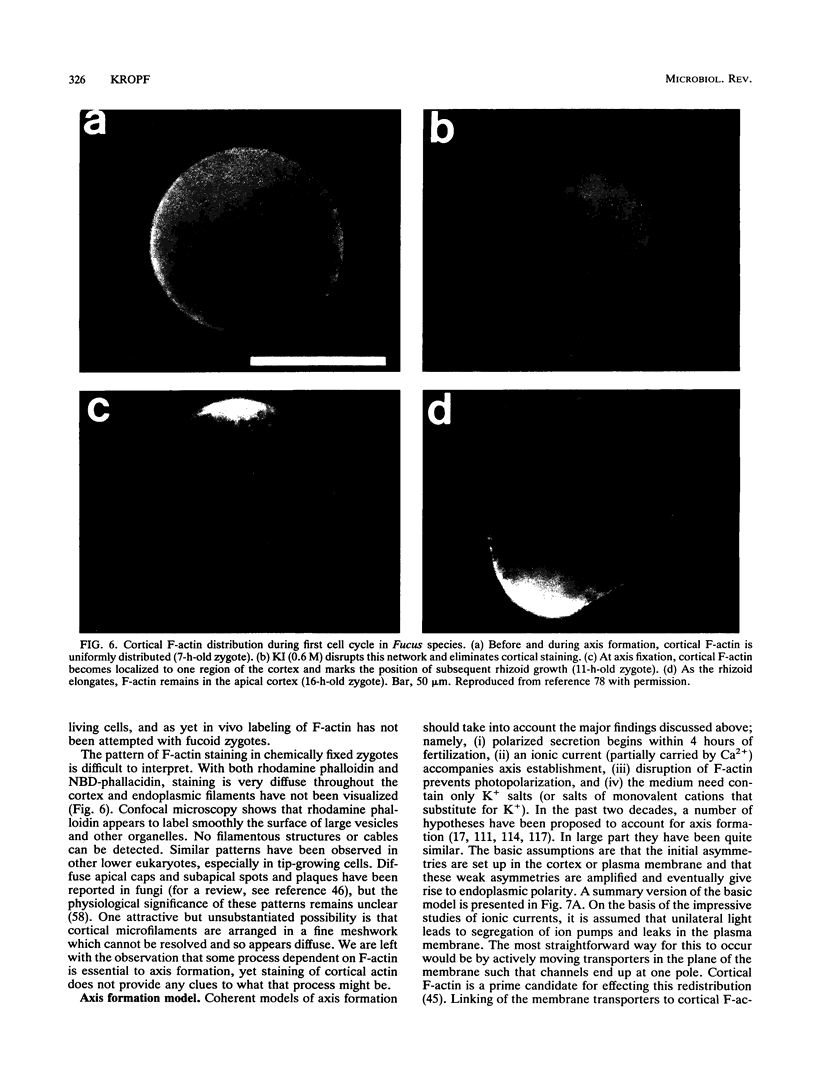
- Kropf D. L., Berge S. K., Quatrano R. S. Actin Localization during Fucus Embryogenesis. Plant Cell. 1989 Feb;1(2):191–200. doi: 10.1105/tpc.1.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf D. L., Hopkins R., Quatrano R. S. Protein synthesis and morphogenesis are not tightly linked during embryogenesis in Fucus. Dev Biol. 1989 Aug;134(2):451–461. doi: 10.1016/0012-1606(89)90118-8. [DOI] [PubMed] [Google Scholar]
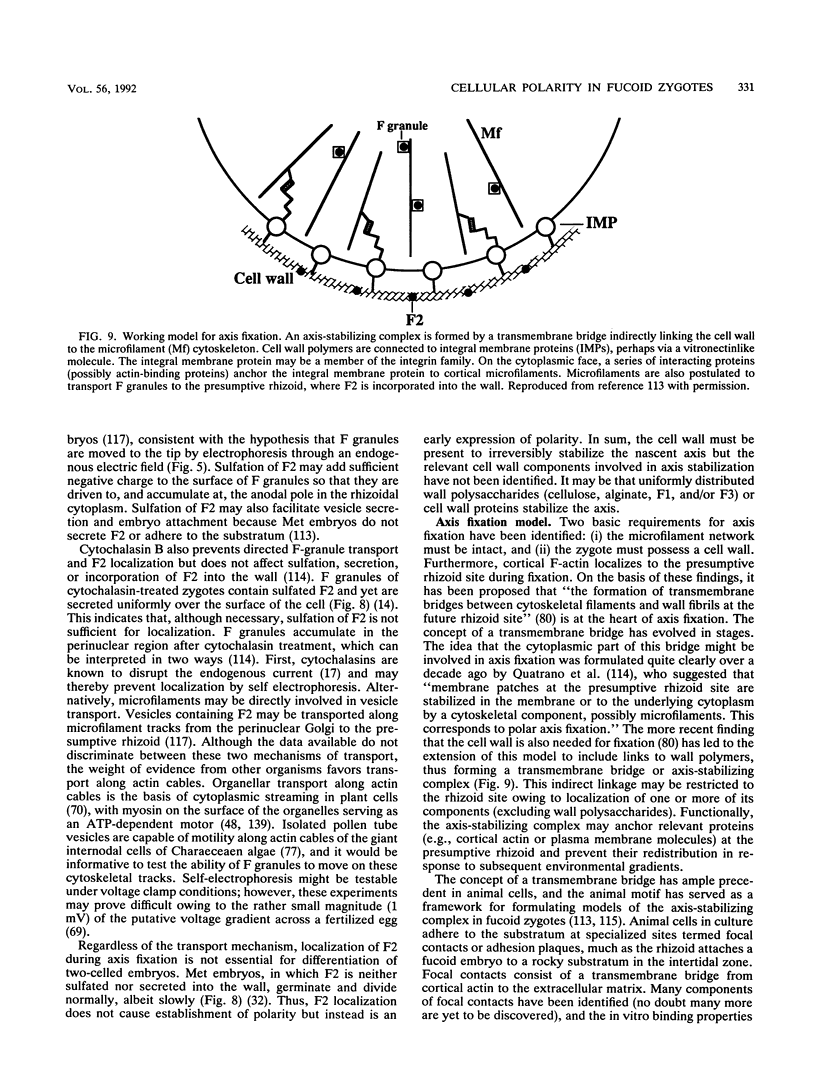
- Kropf D. L., Kloareg B., Quatrano R. S. Cell wall is required for fixation of the embryonic axis in Fucus zygotes. Science. 1988 Jan 8;239(4836):187–190. doi: 10.1126/science.3336780. [DOI] [PubMed] [Google Scholar]
- Kühtreiber W. M., Jaffe L. F. Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. J Cell Biol. 1990 May;110(5):1565–1573. doi: 10.1083/jcb.110.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehle L. Phosphatidyl inositol metabolism and its role in signal transduction in growing plants. Plant Mol Biol. 1990 Oct;15(4):647–658. doi: 10.1007/BF00017839. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Pattern formation during animal development. Science. 1991 Apr 12;252(5003):234–241. doi: 10.1126/science.1672778. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Jaffe L. F. Cells without cytoplasmic movements respond to cytochalasin. Dev Biol. 1973 Jan;30(1):206–208. doi: 10.1016/0012-1606(73)90058-4. [DOI] [PubMed] [Google Scholar]
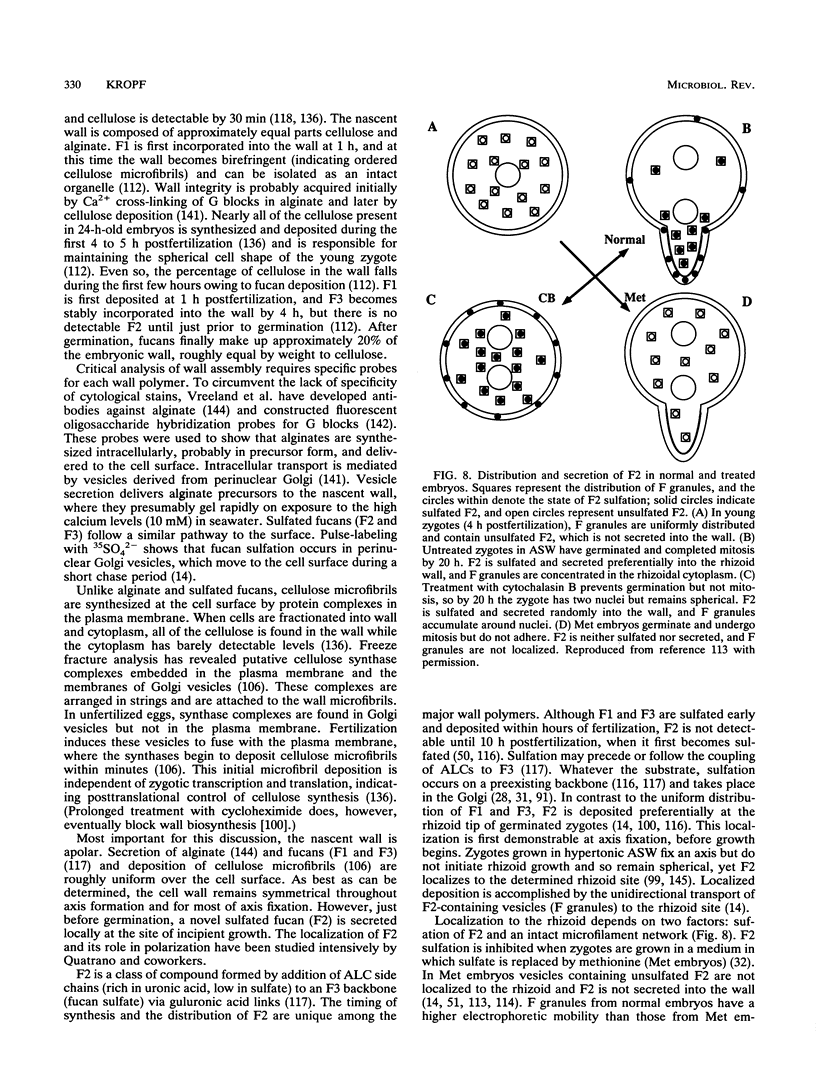
- Novotny A. M., Forman M. The relationship between changes in cell wall composition and the establishment of polarity in Fucus embryos. Dev Biol. 1974 Sep;40(1):162–173. doi: 10.1016/0012-1606(74)90116-x. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R., Jaffe L. F. Spontaneous current pulses through developing fucoid eggs. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4855–4859. doi: 10.1073/pnas.71.12.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R., Jaffe L. F. The ionic components of the current pulses generated by developing fucoid eggs. Dev Biol. 1976 Apr;49(2):518–531. doi: 10.1016/0012-1606(76)90193-7. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R. Oöplasmic segregation and secretion in the Pelvetia egg is accompanied by a membrane-generated electrical current. Dev Biol. 1978 Jan;62(1):13–33. doi: 10.1016/0012-1606(78)90089-1. [DOI] [PubMed] [Google Scholar]
- Poo M., Robinson K. R. Electrophoresis of concanavalin A receptors along embryonic muscle cell membrane. Nature. 1977 Feb 17;265(5595):602–605. doi: 10.1038/265602a0. [DOI] [PubMed] [Google Scholar]
- Quatrano R. S. An ultrastructural study of the determined site of rhizoid formation in Fucus zygotes. Exp Cell Res. 1972 Jan;70(1):1–12. doi: 10.1016/0014-4827(72)90174-7. [DOI] [PubMed] [Google Scholar]
- Quatrano R. S., Brian L., Aldridge J., Schultz T. Polar axis fixation in Fucus zygotes: components of the cytoskeleton and extracellular matrix. Dev Suppl. 1991;1:11–16. [PubMed] [Google Scholar]
- Quatrano R. S., Crayton M. A. Sulfation of fucoidan in Fucus embryos. I. Possible role in localization. Dev Biol. 1973 Jan;30(1):29–41. doi: 10.1016/0012-1606(73)90045-6. [DOI] [PubMed] [Google Scholar]
- Quatrano R. S., Griffing L. R., Huber-Walchli V., Doubet R. S. Cytological and biochemical requirements for the establishment of a polar cell. J Cell Sci Suppl. 1985;2:129–141. doi: 10.1242/jcs.1985.supplement_2.7. [DOI] [PubMed] [Google Scholar]
- Quatrano R. S. Rhizoid formation in Fucus zygotes: dependence on protein and ribonucleic acid syntheses. Science. 1968 Oct 25;162(3852):468–470. doi: 10.1126/science.162.3852.468. [DOI] [PubMed] [Google Scholar]
- Quatrano R. S. Separation of processes associated with differentiation of two-celled Fucus embryos. Dev Biol. 1973 Jan;30(1):209–213. doi: 10.1016/0012-1606(73)90059-6. [DOI] [PubMed] [Google Scholar]
- Quatrano R. S., Stevens P. T. Cell wall assembly in fucus zygotes: I. Characterization of the polysaccharide components. Plant Physiol. 1976 Aug;58(2):224–231. doi: 10.1104/pp.58.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K. R., Cone R. Polarization of fucoid eggs by a calcium ionophore gradient. Science. 1980 Jan 4;207(4426):77–78. doi: 10.1126/science.207.4426.77. [DOI] [PubMed] [Google Scholar]
- Robinson K. R., Jaffe L. F. Ion movements in a developing fucoid egg. Dev Biol. 1973 Dec;35(2):349–361. doi: 10.1016/0012-1606(73)90029-8. [DOI] [PubMed] [Google Scholar]
- Robinson K. R., Jaffe L. F. Polarizing fucoid eggs drive a calcium current through themselves. Science. 1975 Jan 10;187(4171):70–72. doi: 10.1126/science.1167318. [DOI] [PubMed] [Google Scholar]
- Saddler H. D. The membrane potential of Acetabularia mediterranea. J Gen Physiol. 1970 Jun;55(6):802–821. doi: 10.1085/jgp.55.6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L. C., Wang C. S., Walling L. L., Lord E. M. A Homolog of the Substrate Adhesion Molecule Vitronectin Occurs in Four Species of Flowering Plants. Plant Cell. 1991 Jun;3(6):629–635. doi: 10.1105/tpc.3.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter R. L., Guggino S. E., Lonergan T. A., Galston A. W. The effects of blue and far red light on rhythmic leaflet movements in samanea and albizzia. Plant Physiol. 1981 May;67(5):965–968. doi: 10.1104/pp.67.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Meiners S., Cheresh D. A. RGD-dependent linkage between plant cell wall and plasma membrane: consequences for growth. J Cell Biol. 1989 May;108(5):1955–1965. doi: 10.1083/jcb.108.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speksnijder J. E., Miller A. L., Weisenseel M. H., Chen T. H., Jaffe L. F. Calcium buffer injections block fucoid egg development by facilitating calcium diffusion. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6607–6611. doi: 10.1073/pnas.86.17.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens P. T., Quatrano R. S. Cell wall assembly in Fucus zygotes. II. Cellulose synthesis and deposition is controlled at the post-translational level. Dev Biol. 1978 Feb;62(2):518–525. doi: 10.1016/0012-1606(78)90233-6. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Oldberg A., Hayman E. G., Pierschbacher M. D., Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J. 1985 Oct;4(10):2519–2524. doi: 10.1002/j.1460-2075.1985.tb03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. J., Hepler P. K., Scordilis S. P. Immunochemical and immunocytochemical identification of a myosin heavy chain polypeptide in Nicotiana pollen tubes. J Cell Sci. 1989 Apr;92(Pt 4):569–574. doi: 10.1242/jcs.92.4.569. [DOI] [PubMed] [Google Scholar]
- Weisenseel M., Jaffe L. F. Membrane potential and impedance of developing fucoid eggs. Dev Biol. 1972 Apr;27(4):555–574. doi: 10.1016/0012-1606(72)90193-5. [DOI] [PubMed] [Google Scholar]